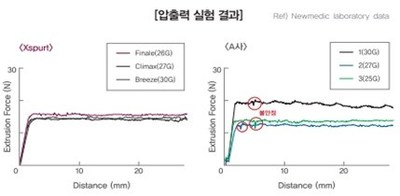
Safety and excellent Korea HA filler provided by Forest Hills Lab at Cosmoprof of Asia Digital Week - Scheduled to obtain CE in July 2021
HONG KONG, Feb. 9, 2021 /PRNewswire/ -- Xspurt will be launched by Forest Hills Lab at Cosmoprof Asia Digital Week and scheduled to obtain CE in July 2021. Xspurt is non-animal based, crosslinked dermal filler using BDDE for linking agent. Xsputy product is obtained through their unique UPHEC technology, making it safe and non-carcinogenic for humans. Xspurt contains less than 1.2EU/mL of endotoxins with no BDDE residue detected and it exceeds the highest level of HA purity.
Xspurt is completely free of all animal products thus reducing the risk of an immunogenic reaction.
Thorough quality control and GMP management have minimized the causes of side effects that can occur in the manufacturing process.
1) Safe materials
The product contains high purity hyaluronic acid for ophthalmic applications made by Shiseido, a Japanese brand.
2) Thorough sterilization
Thorough sterilization processes have lowered the standard level of endotoxin (EU/ml) in their products to 0.5EU/ml or less (cf. the European standard: 12.6EU/ml)
3) Strict purification
The residues of BDDE which is used in the cross-linking process have not been detected in the quality inspection, minimizing side effects and maximizing safety.
Xspurt's ZERO (Zero BDDE & Reliable Operation) Technology, no BDDE residues have been detected in Xspurt products before and after sterilization, minimizing side effects and maximizing safety. BDDE is a chemical used to prevent the fast degradation of hyaluronic acid fillers. Thorough purification processes are required because BDDE residues remaining after cross-linking can cause side effects.
Forest Hills Partners Hong Kong Limited ("FHP"), a Hong Kong company, established R&D, sales and marketing functions for easy expansion into China and other regional markets to leverage Korean technological prowess, brand reputation and attention to quality. FHP is manufacturing and selling cosmeceutical products primarily developed from Korea, ranging across HA filler, meso serum, medical device and other related cosmetic supplements. The management team possesses in-depth market experiences in producing the best Korean technologies for filler products. Mstone Partners adds value through a global network in the healthcare sectors and therapeutic drug development experiences, including FHL's network with MeCox CureMed. FHP strives to being there high-quality and innovative products to all corners of the world in an affordable and seamless fashion.
For product enquiry, please contact
Cheukyin Chow - Sales & Marketing Assistant ManagerTel: +852-21550487Email: [email protected] Website: www.foresthillslab.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/safety-and-excellent-korea-ha-filler-provided-by-forest-hills-lab-at-cosmoprof-of-asia-digital-week---scheduled-to-obtain-ce-in-july-2021-301224563.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/safety-and-excellent-korea-ha-filler-provided-by-forest-hills-lab-at-cosmoprof-of-asia-digital-week---scheduled-to-obtain-ce-in-july-2021-301224563.html
SOURCE Cosmoprof Asia
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- NB Bancorp, Inc. Reports First Quarter 2024 Financial Results
- MiMedia Enters Into Market-Making Agreement with Independent Trading Group
- South Florida City Launches Groundbreaking Initiative to Reduce Gun Violence and Foster Peace
Create E-mail Alert Related Categories
PRNewswire, Press ReleasesSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share