Form 8-K LABORATORY CORP OF AMERI For: Oct 26
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
October 26, 2015
(Date of earliest event reported)
LABORATORY CORPORATION OF
AMERICA HOLDINGS
(Exact Name of Registrant as Specified in its Charter)
Delaware | 1-11353 | 13-3757370 | ||
(State or other jurisdiction of Incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) | ||
358 South Main Street, | ||||
Burlington, North Carolina | 27215 | 336-229-1127 | ||
(Address of principal executive offices) | (Zip Code) | (Registrant’s telephone number including area code) | ||
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
[ ] | Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
[ ] | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
[ ] | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
[ ] | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) | |
Item 7.01 | Regulation FD Disclosure | |
Summary information of the Company dated October 26, 2015.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
LABORATORY CORPORATION OF AMERICA HOLDINGS
Registrant
By: | /s/ F. SAMUEL EBERTS III | |
F. Samuel Eberts III | ||
Chief Legal Officer and Secretary | ||
October 26, 2015

8-K FILED OCTOBER 26, 2015 THIRD QUARTER 2015 SUPPLEMENTAL FINANCIAL INFORMATION

1 FORWARD LOOKING STATEMENT This presentation contains forward-looking statements which are subject to change based on various important factors, including without limitation, competitive actions in the marketplace, adverse actions of governmental and other third-party payers and the results from the Company’s acquisition of Covance. Actual results could differ materially from those suggested by these forward- looking statements. Further information on potential factors that could affect LabCorp’s operating and financial results is included in the Company’s Form 10-K for the year ended December 31, 2014, and the Company’s Form 10-Q for the quarter ended June 30, 2015, including under the heading risk factors, and in the Company’s other filings with the SEC, as well as in the risk factors included in Covance’s filings with the SEC. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change.

2 LabCorp Diagnostics The LabCorp Diagnostics segment includes historical LabCorp business units, excluding its Clinical Trials operations (which are part of the Covance Drug Development segment), and including the Nutritional Chemistry and Food Safety operations acquired as part of the Covance acquisition. Covance Drug Development The Covance Drug Development segment includes historical Covance business units, excluding its Nutritional Chemistry and Food Safety operations (which are part of the LabCorp Diagnostics segment), and including the LabCorp Clinical Trials operations. OPERATING SEGMENT OVERVIEW

3 THIRD QUARTER CONSOLIDATED RESULTS (DOLLARS IN MILLIONS, EXCEPT PER SHARE DATA) (1) Adjusted Operating Income and Adjusted EPS exclude amortization, restructuring and special items (2) See Reconciliation of non-GAAP Financial Measures on slides 15 - 18 The following consolidated results include Covance as of February 19, 2015; prior to February 19, 2015, all consolidated results exclude Covance 3Q15 3Q14 % Change Net Revenue $2,269.9 $1,551.8 46.3% Adjusted Operating Income(1) (2) $385.5 $271.0 42.3% Adjusted Operating Margin 17.0% 17.5% (50 bps) Adjusted EPS(1) (2) $2.07 $1.80 15.0% Operating Cash Flow $288.0 $175.6 64.0% Less: Capital Expenditures ($67.8) ($52.6) 28.9% Free Cash Flow $220.2 $123.0 79.0%

4 YEAR-TO-DATE CONSOLIDATED RESULTS (DOLLARS IN MILLIONS, EXCEPT PER SHARE DATA) (1) Adjusted Operating Income and Adjusted EPS exclude amortization, restructuring and special items (2) See Reconciliation of non-GAAP Financial Measures on slides 15 - 18 (3) Operating cash flow in the first quarter of 2015 is negatively impacted by $153.5 million of non-recurring items related to the Covance acquisition (4) Adjusted for $153.5 million of non-recurring items related to the Covance acquisition in the first quarter of 2015 The following consolidated results include Covance as of February 19, 2015; prior to February 19, 2015, all consolidated results exclude Covance Nine Months Nine Months Ended 9/30/15 Ended 9/30/14 % Change Net Revenue $6,260.9 $4,498.9 39.2% Adjusted Operating Income(1) (2) $1,078.8 $778.3 38.6% Adjusted Operating Margin 17.2% 17.3% (10 bps) Adjusted EPS(1) (2) $5.94 $5.15 15.3% Operating Cash Flow(3) $597.8 $525.3 13.8% Less: Capital Expenditures ($170.7) ($157.2) 8.6% Free Cash Flow $427.1 $368.1 16.0% Free Cash Flow, Excluding Acquisition-Related Charges(4) $580.6 $368.1 57.7%

5 THIRD QUARTER PRO FORMA SEGMENT RESULTS (DOLLARS IN MILLIONS) (1) Adjusted Operating Income excludes amortization, restructuring and special items (2) See Reconciliation of non-GAAP Financial Measures on slides 15 - 18 Pro forma results assume that the acquisition of Covance closed on January 1, 2014 3Q15 3Q14 % Change Net Revenue LabCorp Diagnostics $1,600.9 $1,526.9 4.8% Covance Drug Development $669.0 $652.0 2.6% Total Net Revenue $2,269.9 $2,178.9 4.2% Adjusted Operating Income(1) (2) LabCorp Diagnostics $330.2 $305.6 8.0% Adjusted Operating Margin 20.6% 20.0% 60 bps Covance Drug Development $97.0 $88.4 9.7% Adjusted Operating Margin 14.5% 13.6% 90 bps Unallocated Corporate Expense ($41.7) ($43.1) (3.2%) Total Adjusted Operating Income $385.5 $350.9 9.9% Total Adjusted Operating Margin 17.0% 16.1% 90 bps
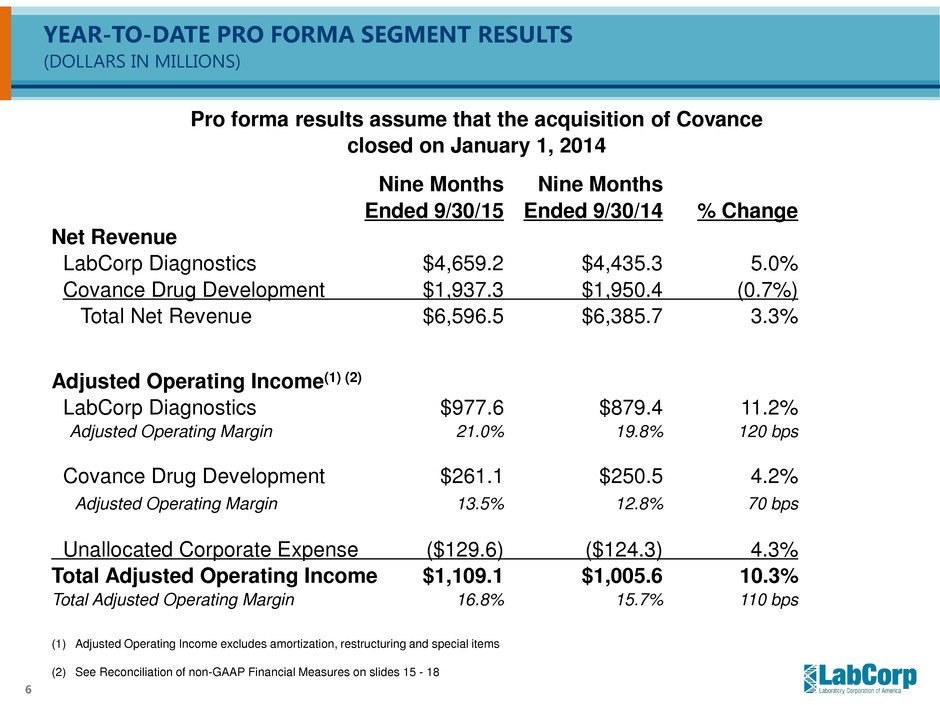
6 YEAR-TO-DATE PRO FORMA SEGMENT RESULTS (DOLLARS IN MILLIONS) (1) Adjusted Operating Income excludes amortization, restructuring and special items (2) See Reconciliation of non-GAAP Financial Measures on slides 15 - 18 Pro forma results assume that the acquisition of Covance closed on January 1, 2014 Nine Months Nine Months Ended 9/30/15 Ended 9/30/14 % Change Net Revenue LabCorp Diagnostics $4,659.2 $4,435.3 5.0% Covance Drug Development $1,937.3 $1,950.4 (0.7%) Total Net Revenue $6,596.5 $6,385.7 3.3% Adjusted Operating Income(1) (2) LabCorp Diagnostics $977.6 $879.4 11.2% Adjusted Operating Margin 21.0% 19.8% 120 bps Covance Drug Development $261.1 $250.5 4.2% Adjusted Operating Margin 13.5% 12.8% 70 bps Unallocated Corporate Expense ($129.6) ($124.3) 4.3% Total Adjusted Operating Income $1,109.1 $1,005.6 10.3% Total Adjusted Operating Margin 16.8% 15.7% 110 bps

7 SELECT FINANCIAL METRICS (DOLLARS IN MILLIONS) 3Q14 4Q14 1Q15 2Q15 3Q15 Total Depreciation $38.9 $40.8 $54.3 $70.5 $72.2 Total Amortization(1) $18.3 $15.4 $32.4 $46.6 $47.1 Total Adjusted EBITDA(2) $314.2 $295.1 $360.1 $463.4 $460.0 Total Debt to Last Twelve Months Adjusted EBITDA(2) (3) 2.5x 2.5x 4.2x 4.0x 3.8x Total Net Debt to Last Twelve Months Adjusted EBITDA(2) (3) 2.0x 2.0x 3.9x 3.6x 3.4x (1) Excludes amortization of deferred financing fees (2) Adjusted EBITDA excludes restructuring and special items (3) Leverage ratios in 2015 include Covance Adjusted EBITDA from the twelve months prior to the relevant period on a pro forma basis The following consolidated results include Covance as of February 19, 2015; prior to February 19, 2015, all consolidated results exclude Covance
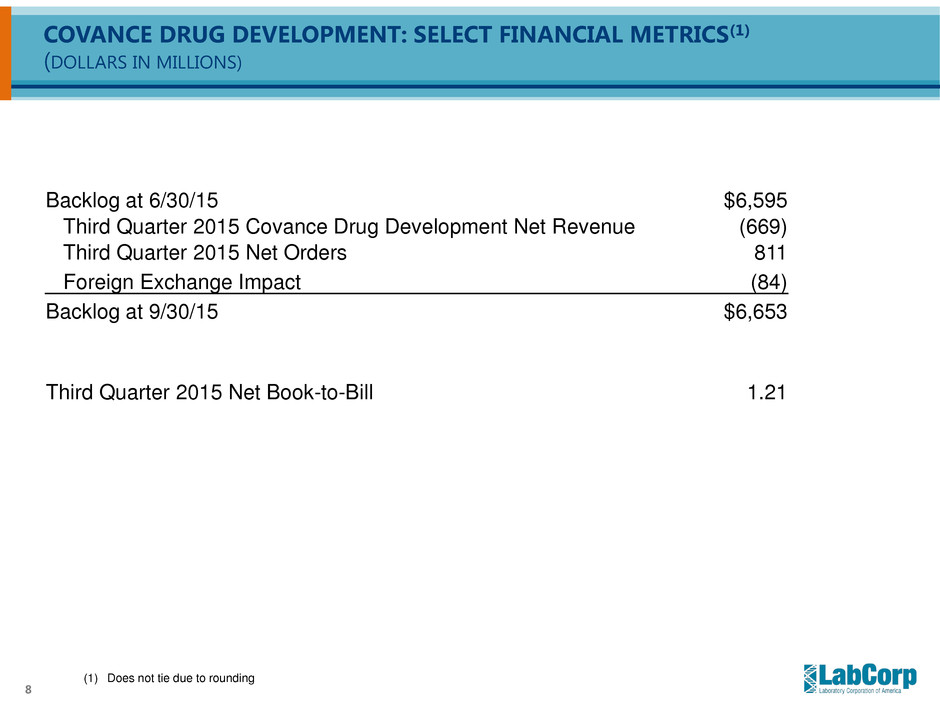
8 COVANCE DRUG DEVELOPMENT: SELECT FINANCIAL METRICS(1) (DOLLARS IN MILLIONS) Backlog at 6/30/15 $6,595 Third Quarter 2015 Covance Drug Development Net Revenue (669) Third Quarter 2015 Net Orders 811 Foreign Exchange Impact (84) Backlog at 9/30/15 $6,653 Third Quarter 2015 Net Book-to-Bill 1.21 (1) Does not tie due to rounding
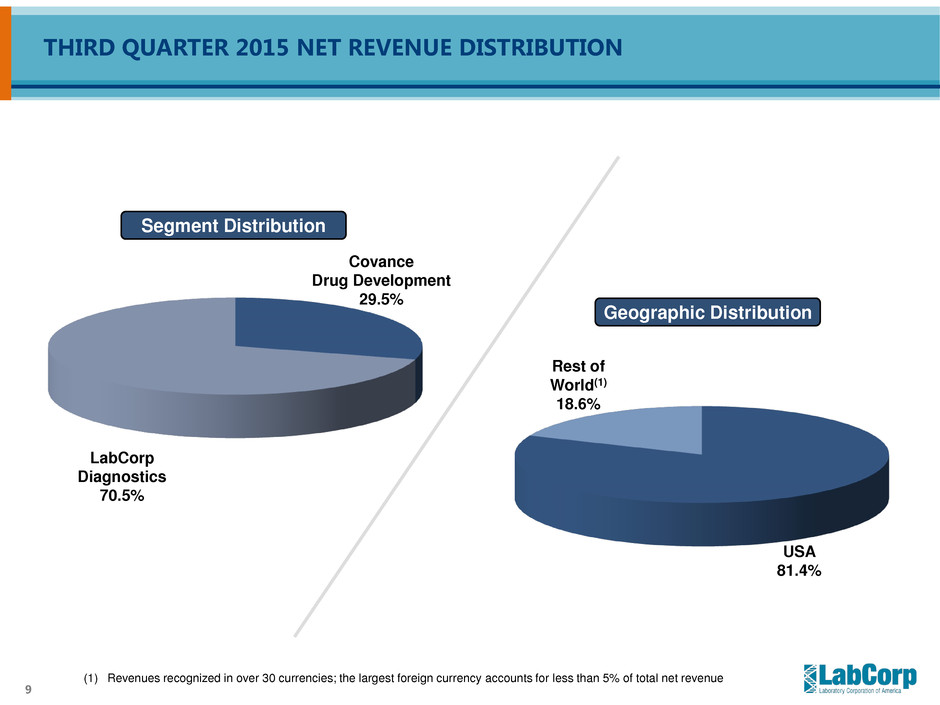
9 (1) Revenues recognized in over 30 currencies; the largest foreign currency accounts for less than 5% of total net revenue Segment Distribution LabCorp Diagnostics 70.5% Covance Drug Development 29.5% USA 81.4% Geographic Distribution Rest of World(1) 18.6% THIRD QUARTER 2015 NET REVENUE DISTRIBUTION
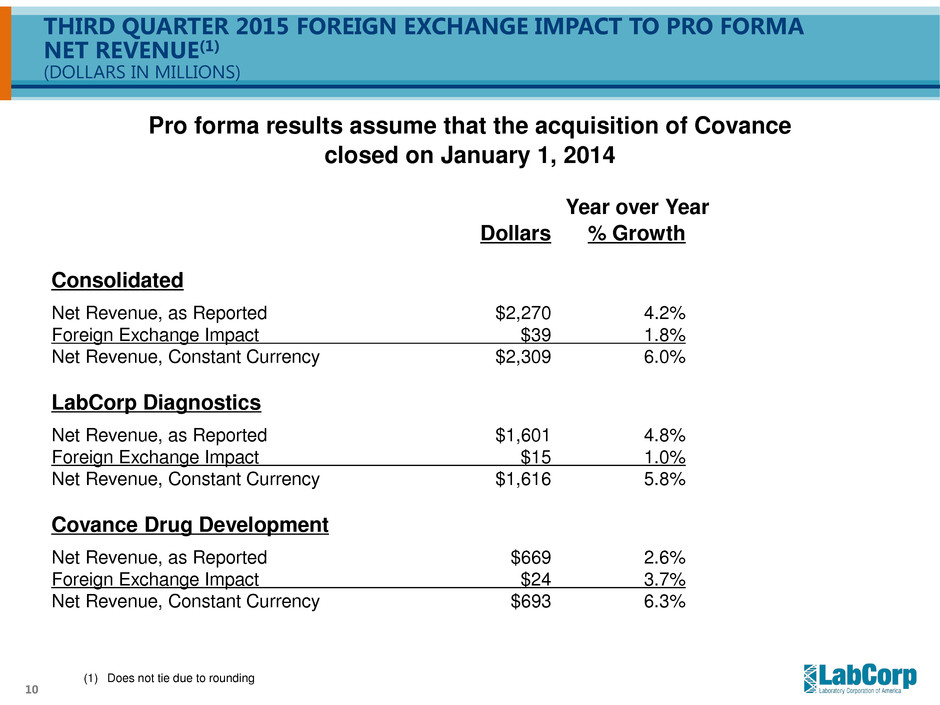
10 THIRD QUARTER 2015 FOREIGN EXCHANGE IMPACT TO PRO FORMA NET REVENUE(1) (DOLLARS IN MILLIONS) Year over Year Dollars % Growth Consolidated Net Revenue, as Reported $2,270 4.2% Foreign Exchange Impact $39 1.8% Net Revenue, Constant Currency $2,309 6.0% LabCorp Diagnostics Net Revenue, as Reported $1,601 4.8% Foreign Exchange Impact $15 1.0% Net Revenue, Constant Currency $1,616 5.8% Covance Drug Development Net Revenue, as Reported $669 2.6% Foreign Exchange Impact $24 3.7% Net Revenue, Constant Currency $693 6.3% Pro forma results assume that the acquisition of Covance closed on January 1, 2014 (1) Does not tie due to rounding

11 YEAR-TO-DATE 2015 FOREIGN EXCHANGE IMPACT TO PRO FORMA NET REVENUE(1) (DOLLARS IN MILLIONS) Year over Year Dollars % Growth Consolidated Net Revenue, as Reported $6,597 3.3% Foreign Exchange Impact $116 1.8% Net Revenue, Constant Currency $6,713 5.1% LabCorp Diagnostics Net Revenue, as Reported $4,659 5.0% Foreign Exchange Impact $36 0.8% Net Revenue, Constant Currency $4,695 5.8% Covance Drug Development Net Revenue, as Reported $1,937 (0.7%) Foreign Exchange Impact $80 4.1% Net Revenue, Constant Currency $2,017 3.4% Pro forma results assume that the acquisition of Covance closed on January 1, 2014 (1) Does not tie due to rounding

12 2015 FINANCIAL GUIDANCE Excluding the impact of amortization, restructuring and special items, guidance for 2015 is: Prior Guidance (assumes foreign exchange rates effective as of June 30, 2015) Current Guidance (assumes foreign exchange rates effective as of September 30, 2015) Total net revenue growth: 40% - 42%(1) Approximately 41%(2) LabCorp Diagnostics net revenue growth: 3.5% - 5.5%(3) 4.5% - 5.5%(4) Covance Drug Development net revenue growth: (1.5%) – 0.5%(5) (0.5%) - 0.5%(6) Adjusted EPS: $7.75 - $8.00 $7.80 - $7.95 Operating cash flow: $990 Million - $1,015 Million(7) $970 Million - $995 Million(7) Capital expenditures: $270 Million - $295 Million $250 Million - $275 Million Free cash flow: $695 Million - $745 Million(7) $695 Million - $745 Million(7) Free cash flow, excluding net non-recurring acquisition items: $815 Million - $865 Million(8) $815 Million - $865 Million(8) (1) Net revenue growth was adjusted for approximately 190 basis points of negative currency impact. (2) Net revenue growth is adjusted for approximately 220 basis points of negative currency impact. (3) Net revenue growth was adjusted for approximately 70 basis points of negative currency impact. (4) Net revenue growth is adjusted for approximately 90 basis points of negative currency impact. (5) Net revenue growth versus full year 2014 net revenue, and was adjusted for approximately 320 basis points of negative currency impact. (6) Net revenue growth versus full year 2014 net revenue, and is adjusted for approximately 350 basis points of negative currency impact. (7) Negatively impacted by approximately $120 million of net non-recurring items related to the Covance acquisition (8) Adjusted for $120 million of net non-recurring items related to the Covance acquisition

13 2014 PRO FORMA SEGMENT NET REVENUE RECONCILIATION (DOLLARS IN MILLIONS) (1) Adjustments include the removal of LabCorp’s legacy clinical trial services business and the addition of Covance’s nutritional chemistry and food safety business. (2) Adjustments include the addition of LabCorp’s legacy clinical trial services business and the removal of Covance’s nutritional chemistry and food safety business. 1Q14 2Q14 3Q14 4Q14 FY14 LabCorp as reported $1,431 $1,516 $1,552 $1,513 $6,012 Adjustments(1) (17) (22) (25) (26) (89) LabCorp Diagnostics $1,414 $1,494 $1,527 $1,487 $5,922 Covance as reported $620 $639 $627 $634 $2,521 Adjustments(2) 17 22 25 26 89 Covance Drug Development $637 $661 $652 $660 $2,610

14 2014 PRO FORMA SEGMENT ADJUSTED OPERATING INCOME RECONCILIATION (DOLLARS IN MILLIONS) (1) Adjustments include the removal of unallocated corporate expenses and LabCorp’s legacy clinical trial services business, as well as the addition of Covance’s nutritional chemistry and food safety business. Unallocated corporate expenses in 2014 were: Q1 ($33.5 million), Q2 ($35.5 million), Q3 ($36.3 million), Q4 ($35.1 million) and full-year ($140.4 million). (2) Adjustments include the removal of unallocated corporate expenses and Covance’s nutritional chemistry and food safety business, as well as the addition of LabCorp’s legacy clinical trial services business. Unallocated corporate expenses in 2014 were: Q1 ($5.7 million), Q2 ($6.4 million), Q3 ($6.8 million), Q4 ($7.8 million) and full-year ($26.7 million). 1Q14 2Q14 3Q14 4Q14 FY14 LabCorp as reported $210.8 $253.4 $252.8 $234.4 $951.4 Amortization 21.0 22.0 18.3 15.4 76.7 Adjustments(1) 33.1 33.5 34.5 31.1 132.2 LabCorp Diagnostics $264.9 $308.9 $305.6 $280.9 $1,160.3 Covance as Reported $71.0 $76.1 $79.7 $77.7 $304.4 Adjustments(2) 6.3 8.6 8.8 12.0 35.8 Covance Drug Development $77.3 $84.7 $88.5 $89.7 $340.2

15 RECONCILIATION OF NON-GAAP FINANCIAL MEASURES The following consolidated results include Covance as of February 19, 2015; prior to February 19, 2015, all consolidated results exclude Covance Adjusted Operating Income 2015 2014 2015 2014 Operating Income 306.9$ 241.4$ 759.5$ 691.4$ Acquisition-related costs 1.4 - 118.0 - Restructuring and other special charges 26.4 5.8 59.9 15.4 Consulting fees 3.7 5.5 15.2 10.2 Amortization of intangibles and other assets 47.1 18.3 126.2 61.3 Adjusted operating income 385.5$ 271.0$ 1,078.8$ 778.3$ Adjusted EPS Diluted earnings per common share 1.49$ 1.59$ 3.24$ 4.53$ Restructuring and special items 0.27 0.08 1.83 0.18 Amortization expense 0.31 0.13 0.87 0.44 Adjusted EPS 2.07$ 1.80$ 5.94$ 5.15$ Three Months Ended September 30, Nine Months Ended September 30, LABORATORY CORPORATION OF AMERICA HOLDINGS Reconciliation of Non-GAAP Financial Measures (in millions, except per share data)

16 RECONCILIATION OF NON-GAAP FINANCIAL MEASURES The following consolidated results include Covance as of February 19, 2015; prior to February 19, 2015, all consolidated results exclude Covance Free Cash Flow: 2015 2014 2015 2014 Net cash provided by operating activities 288.0$ 175.6$ 597.8$ 525.3$ Less: Capital expenditures (67.8) (52.6) (170.7) (157.2) Free cash flow 220.2$ 123.0$ 427.1$ 368.1$ Free Cash Flow, Excluding Acquisition Related Charges: Net cash provided by operating activities 288.0$ 175.6$ 597.8$ 525.3$ Add back: Acquisition related charges - - 153.5 - Net cash provided by operating activities, excluding acquisition related charges 288.0$ 175.6$ 751.3$ 525.3$ Less: Capital expenditures (67.8) (52.6) (170.7) (157.2) Free cash flow, excluding acquisition related charges 220.2$ 123.0$ 580.6$ 368.1$ LABORATORY CORPORATION OF AMERICA HOLDINGS Reconciliation of Non-GAAP Financial Measures (in millions, except per share data) Three Months Ended September 30, Nine Months Ended September 30,
17 1) During the third quarter of 2015, the Company recorded net restructuring and special items of $26.4 million. The charges included $24.4 million in severance and other personnel costs along with $2.3 million in facility-related costs associated with facility closures and general integration initiatives. The Company reversed previously established reserves of $0.3 million in unused facility-related costs. The Company also recorded $3.7 million in consulting expenses relating to fees incurred as part of its Covance integration costs, along with $1.4 million in short-term equity retention arrangements relating to the acquisition of Covance (all recorded in selling, general and administrative expenses). In addition, the Company recorded a non-cash loss of $2.3 million, upon the dissolution of one of its equity investments (recorded in other, net in the accompanying Consolidated Statements of Operations). The after tax impact of these charges decreased net earnings for the quarter ended September 30, 2015, by $27.7 million and diluted earnings per share by $0.27 ($27.7 million divided by 102.9 million shares). During the first two quarters of 2015, the Company recorded net restructuring and other special charges of $33.5 million. The charges included $9.5 million in severance and other personnel costs along with $9.8 million in costs associated with facility closures and general integration initiatives. The Company reversed previously established reserves of $0.6 million in unused facility-related costs. In addition, the Company recorded asset impairments of $14.8 million relating to lab and customer service applications that will no longer be used. The Company also recorded $11.6 million of consulting expenses relating to fees incurred as part of its Project LaunchPad business process improvement initiative as well as Covance integration costs. In addition, the Company also expensed $2.9 million in short-term equity retention arrangements relating to the acquisition of Covance. During the first quarter of 2015, the Company recorded $166.0 million of one-time costs associated with its acquisition of Covance. The costs included $79.5 million of Covance employee equity awards, change in control payments and short-term retention arrangements that were accelerated or triggered by the acquisition transaction (recorded in selling, general and administrative expenses in the accompanying Consolidated Statements of Operations). The acquisition costs also included advisor and legal fees of $33.9 million (recorded in selling, general and administrative expenses in the accompanying Consolidated Statements of Operations), $15.2 million of deferred financing fees associated with the Company’s bridge loan facility as well as a make-whole payment of $37.4 million paid to call Covance’s private placement debt outstanding at the purchase date (both amounts recorded in interest expense in the accompanying Consolidated Statements of Operations). The after tax impact of these charges decreased net earnings for the nine months ended September 30, 2015, by $182.5 million and diluted earnings per share by $1.83 ($182.5 million divided by 99.7 million shares). RECONCILIATION OF NON-GAAP FINANCIAL MEASURES - FOOTNOTES
18 2) During the third quarter of 2014, the Company recorded net restructuring and special items of $5.8 million. The charges included $4.6 million in severance and other personnel costs along with $1.6 million in facility-related costs associated with facility closures and general integration initiatives. The Company reversed previously established reserves of $0.2 in unused severance and $0.2 million in unused facility-related costs. In addition, the Company recorded $5.5 million in consulting expenses relating to fees incurred as part of its comprehensive enterprise-wide cost structure review as well as legal fees associated with its LipoScience acquisition (all such fees recorded in selling, general and administrative expenses). The after tax impact of these combined charges decreased net earnings for the quarter ended September 30, 2014, by $7.0 million and diluted earnings per share by $0.08 ($7.0 million divided by 86.5 million shares). During the first two quarters of 2014, the Company recorded net restructuring and special items of $14.3 million. The charges included $5.3 million in severance and other personnel costs along with $5.0 million in costs associated with facility closures and general integration initiatives. The Company reversed previously established reserves of $0.2 million in unused severance and $0.5 million in unused facility-related costs. In addition, the Company recorded $4.7 million in consulting expenses (recorded in selling, general and administrative expenses) relating to fees incurred as part of its comprehensive enterprise-wide cost structure review as well as one-time CFO transition costs. The after tax impact of these combined charges decreased net earnings for the nine months ended September 30, 2014, by $15.8 million and diluted earnings per share by $0.18 ($15.8 million divided by 86.5 million shares). 3) The Company continues to grow the business through acquisitions and uses Adjusted EPS Excluding Amortization as a measure of operational performance, growth and shareholder returns. The Company believes adjusting EPS for amortization provides investors with better insight into the operating performance of the business. For the quarters ended September 30, 2015 and 2014, intangible amortization was $47.1 million and $18.3 million, respectively ($32.9 million and $11.3 million net of tax, respectively) and decreased EPS by $0.31 ($32.9 million divided by 102.9 million shares) and $0.13 ($11.3 million divided by 86.5 million shares), respectively. For the nine months ended September 30, 2015 and 2014, intangible amortization was $126.2 million and $61.3 million, respectively ($86.5 million and $37.8 million net of tax, respectively) and decreased EPS by $0.87 ($86.5 million divided by 99.7 million shares) and $0.44 ($37.8 million divided by 86.5 million shares), respectively. 4) During the first quarter of 2015, the Company's operating cash flows were reduced due to payment of $153.5 million in acquisition-related charges. These payments were comprised of $75.5 million in legal and advisor fees, $40.6 million in accelerated Covance employee equity awards, and $37.4 million in make-whole payments triggered by calling Covance private placement notes outstanding at the time of the transaction. RECONCILIATION OF NON-GAAP FINANCIAL MEASURES - FOOTNOTES
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Labcorp Announces First-of-Its-Kind Test for Early Indication of Neurodegenerative Diseases and Brain Injuries Using a Blood Draw
- Vaxxinity Issues Shareholder Letter
- Rise48 Equity Celebrates Milestone 50th Multifamily Acquisition
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share