Form 8-K SEQUENOM INC For: Sep 28
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 8-K
Current Report
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 28, 2015
SEQUENOM, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 000-29101 | 77-0365889 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| 3595 John Hopkins Court, San Diego, CA | 92121 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (858) 202-9000
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 2.02 | Results of Operations and Financial Condition. |
On September 28, 2015, Dirk van den Boom, the registrant’s Interim President and Chief Executive Officer, and other members of the registrant’s senior management team, will present at its 2015 investor and analyst day in New York, NY, starting at 2:00 pm Eastern time to provide an overview of and update on the registrant. The presentation, currently posted on the registrant’s website at www.sequenom.com/invest and attached hereto as Exhibit 99.1 and incorporated herein by reference, provides selected preliminary financial and operational results for the year to date and the registrant’s current revenue and cash burn outlook for fiscal year 2015.
The information in this Item 2.02, and Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section. The information in this current report shall not be incorporated by reference into any registration statement or other document filed with the Securities and Exchange Commission, whether filed before or after the date hereof regardless of any general incorporation language in any such filing, unless we expressly set forth in such filing that such information is to be considered “filed” or incorporated by reference therein.
| Item 7.01 | Regulation FD Disclosure. |
The information contained in Item 2.02, Results of Operations and Financial Condition, is incorporated herein by reference in its entirety.
| Item 8.01 | Other Events. |
The information contained in Item 2.02, Results of Operations and Financial Condition, is incorporated herein by reference in its entirety.
Forward-Looking Statements
Statements contained in this Current Report on Form 8-K regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks are described more fully in the registrant’s filings with the Securities and Exchange Commission, including without limitation the registrant’s most recent Quarterly Report on Form 10-Q and other documents subsequently filed with or furnished to the Securities and Exchange Commission. All forward-looking statements contained in this Current Report on Form 8-K speak only as of the date on which they were made. The registrant undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| 99.1 | Presentation of Sequenom, Inc. |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SEQUENOM, INC. | ||||||
| Dated: September 28, 2015 | By: | /s/ Jeffrey D. Linton | ||||
| Name: | Jeffrey D. Linton | |||||
| Title: | Senior Vice President, General Counsel | |||||
| Exhibit 99.1
|
INVESTOR + ANALYST DAY
September 28, 2015
|
|
Welcome
Dirk van den Boom, PhD Interim President and CEO Chief Scientific & Strategy Officer
2
|
|
Forward-looking statements
Except for historical information, matters set forth in this presentation, including statements regarding Sequenom’s plans, potential, opportunities, financial or other expectations, projections, goals, objectives, milestones, strategies, market growth, timelines, product pipeline, clinical studies, product development, and the potential benefits of its products and products under development, are forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995.
These forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially, including the risks and uncertainties associated with Sequenom’s operating performance and financial position, the market demand for and acceptance of Sequenom’s and Sequenom Laboratories’ products and services, research, development and commercialization of new products, reliance upon the collaborative efforts of others, competition, intellectual property rights, government regulation, obtaining or maintaining regulatory approvals, litigation, and other risks detailed in Sequenom’s SEC filings.
These forward-looking statements are based on current information that is likely to change, speak only as of the date hereof, and Sequenom undertakes no obligation to revise or update such statements.
3
|
|
Today’s agenda
Opening remarks
Dirk van den Boom
Corporate vision + overview
Dirk van den Boom
Reproductive health + genetic testing
Mathias Ehrich, Rob Lozuk
Coffee break
Oncology
Mathias Ehrich, Daniel Grosu
Closing remarks
Dirk van den Boom
4
|
|
Speakers
Mathias Ehrich, MD Rob Lozuk Daniel Grosu, MD
Senior Vice President, Senior Vice President, Senior Vice President, Research and Development Commercial Operations Chief Medical Officer
5
|
|
Corporate vision and overview
|
|
Sequenom: positioning for 2020
Prenatal market is rapidly evolving creating both challenges and opportunities
Recent performance does not reflect Sequenom’s potential
Entire organization understands the challenges
Sequenom is focused on driving value, leading innovation, and creating a sustainable business which delivers long-term value to shareholders
Strategic plan is in place to capture value from reproductive health market opportunities and testing beyond traditional noninvasive prenatal testing
We are laying the foundation / building blocks for future growth in oncology
7
|
|
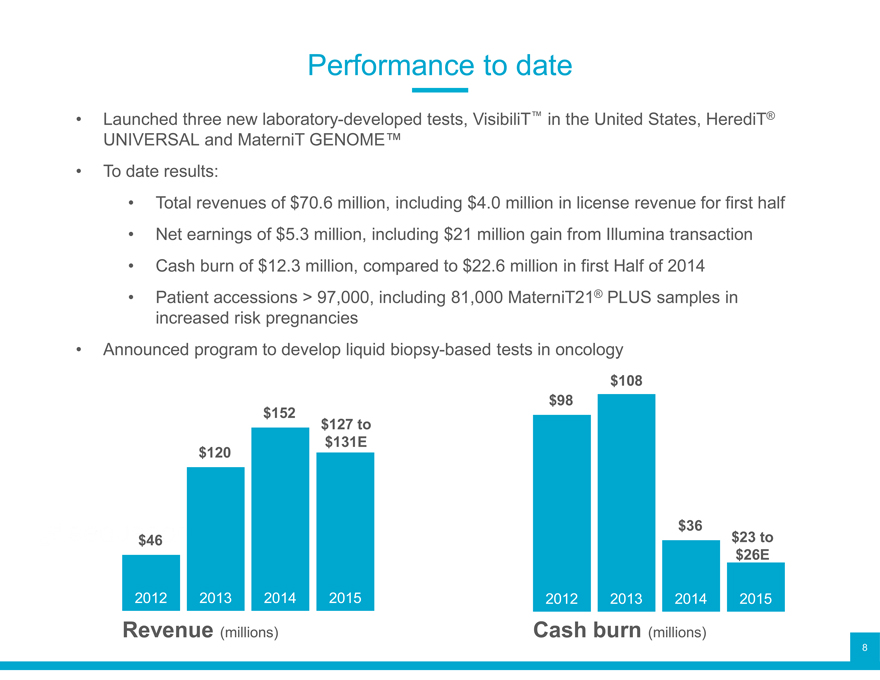
Performance to date
Launched three new laboratory-developed tests, VisibiliT™ in the United States, HerediT®
UNIVERSAL To date results: and MaterniT GENOME™
Total Net earnings revenues of of $5.3 $70.6 million, million, including including $21 $ 4.0 million million gain in from license Illumina revenue transaction for first half
Cash burn of $12.3 million, compared to $22.6 million in first Half of 2014
Patient accessions > 97,000, including 81,000 MaterniT21® PLUS samples in
Announced increased program risk to pregnancies develop liquid biopsy-based tests in oncology
$108 $152 $98 $127 to $120 $131E
$36 $23 to $46 $26E
2012 2013 2014 2015 2012 2013 2014 2015 Revenue (millions) Cash burn (millions)
8
|
|
Providing unique insights into your health all starts with a simple blood draw…
|
|
Our vision
Interpreting the genome to improve your life
|
|
Sequenom’s distinction
Innovator Committed to excellence. Responsible for new standard for noninvasive prenatal testing and the largest new global diagnostic category.
Trusted Peer-reviewed publications and transparency in medical education, billing and commercialization.
Comprehensive Most advanced noninvasive offerings of genetic testing solutions for clinicians and their patients.
Dedicated Customer service, genetic counselors and medical directors. Every patient matters.
11
|
|
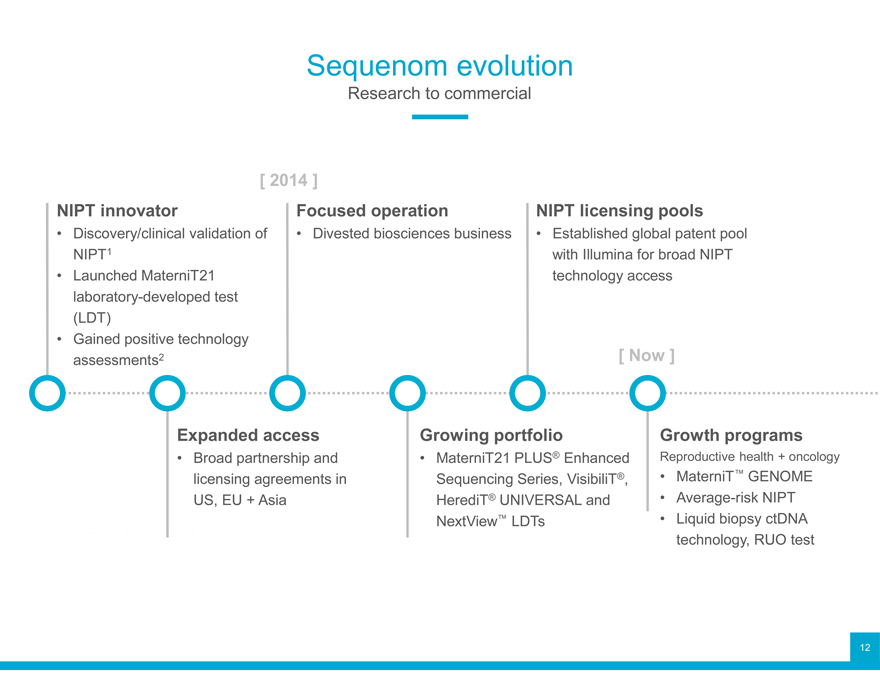
Sequenom evolution
Research to commercial
[ 2014 ]
NIPT innovator Focused operation NIPT licensing pools
Discovery/clinical validation of Divested biosciences business Established global patent pool NIPT1 with Illumina for broad NIPT
Launched MaterniT21 technology access laboratory-developed test (LDT)
Gained positive technology assessments2 [ Now ]
Expanded access Growing portfolio ® Growth programs
Broad partnership and MaterniT21 PLUS Enhanced Reproductive health + oncology licensing agreements in Sequencing Series, VisibiliT®, MaterniT™ GENOME US, EU + Asia HerediT® UNIVERSAL and Average-risk NIPT
NextView™ LDTs • Liquid biopsy ctDNA technology, RUO test
12
|
|
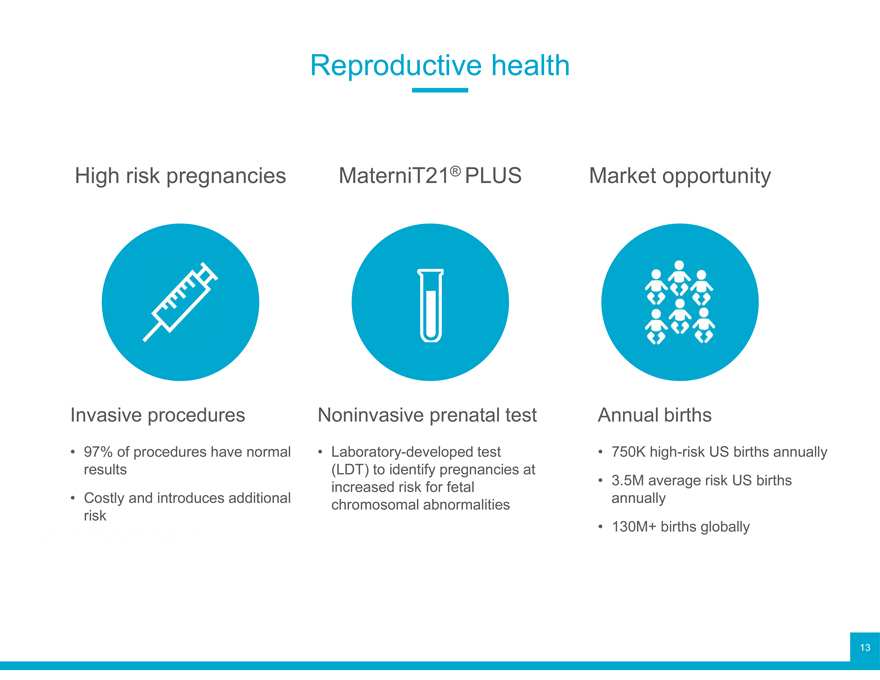
Reproductive health
High risk pregnancies MaterniT21® PLUS Market opportunity
Invasive procedures Noninvasive prenatal test Annual births
97% of procedures have normal Laboratory-developed test 750K high-risk US births annually results (LDT) to identify pregnancies at 3.5M average risk US births
Costly and introduces additional increased risk for fetal annually risk chromosomal abnormalities
130M+ births globally
13
|
|
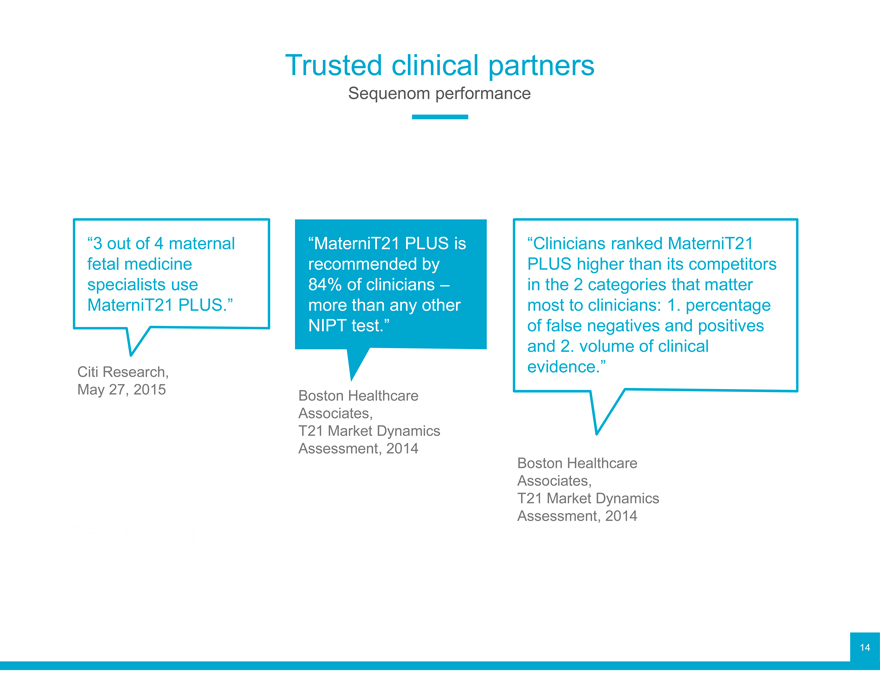
Trusted clinical partners
Sequenom performance
“3 out of 4 maternal “MaterniT21 PLUS is “Clinicians ranked MaterniT21 fetal medicine recommended by PLUS higher than its competitors specialists use 84% of clinicians – in the 2 categories that matter MaterniT21 PLUS.” more than any other most to clinicians: 1. percentage NIPT test.” of false negatives and positives and 2. volume of clinical
Citi Research, evidence.” May 27, 2015 Boston Healthcare Associates, T21 Market Dynamics Assessment, 2014 Boston Healthcare Associates, T21 Market Dynamics Assessment, 2014
14
|
|
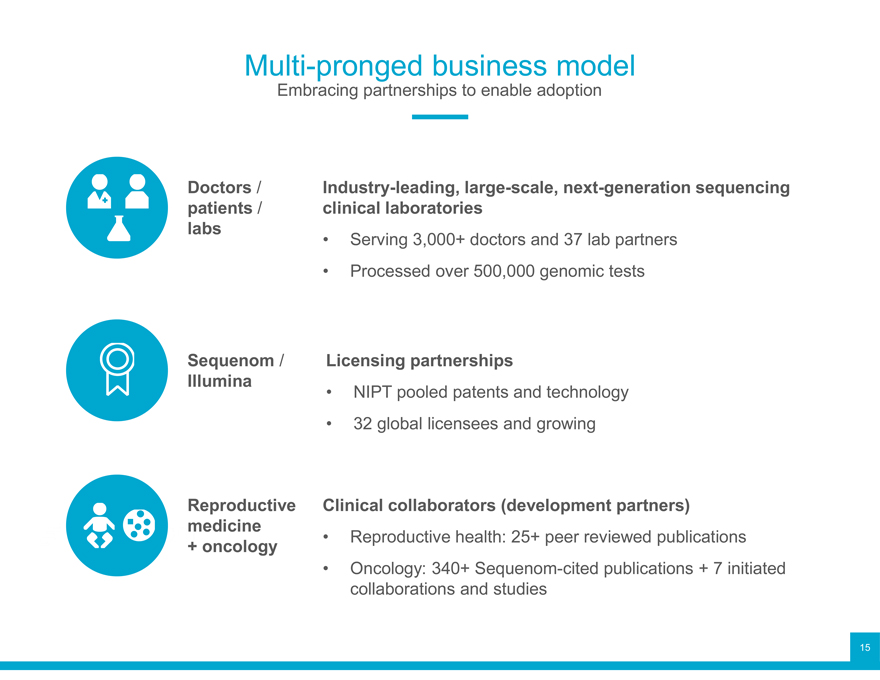
Multi-pronged business model
Embracing partnerships to enable adoption
Doctors / Industry-leading, large-scale, next-generation sequencing patients / clinical laboratories labs • Serving 3,000+ doctors and 37 lab partners
Processed over 500,000 genomic tests
Sequenom / Licensing partnerships
Illumina NIPT pooled patents and technology
32 global licensees and growing
Reproductive Clinical collaborators (development partners) medicine • Reproductive health: 25+ peer reviewed publications
+ oncology
Oncology: 340+ Sequenom-cited publications + 7 initiated collaborations and studies
15
|
|
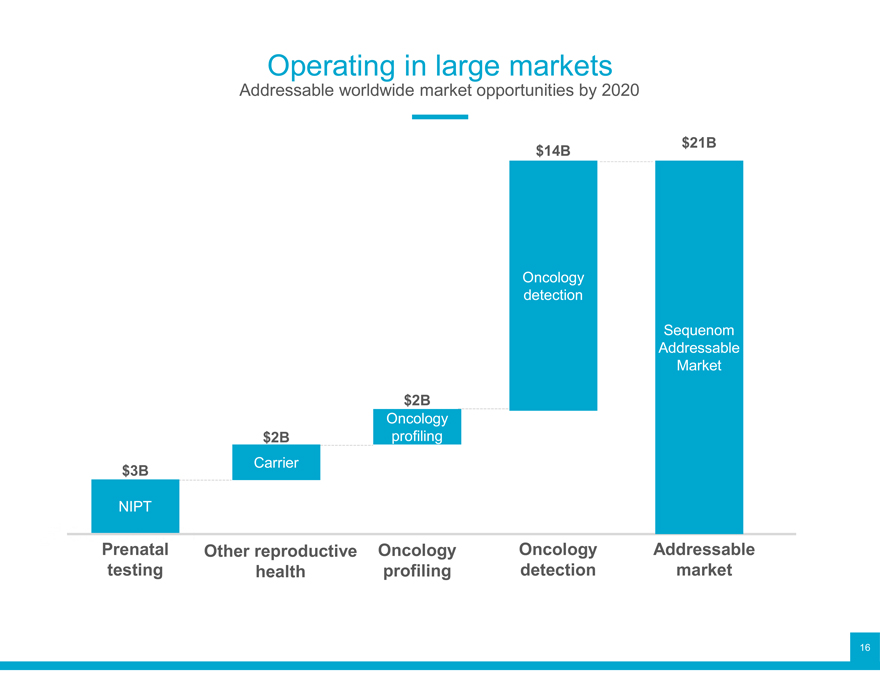
Operating in large markets
Addressable worldwide market opportunities by 2020
$14B $21B
Oncology detection
Sequenom Addressable Market $2B
Oncology Carrier $2B profiling $3B
NIPT
Prenatal Other reproductive Oncology Oncology Addressable testing health profiling detection market
16
|
|
Translating to results
Over $500M revenues by 2020
+$50M/yr NIPT test fees and royalties, with continued adoption of pooled patent globally and NIPT growth in international
Oncology markets
Patents extend beyond 2030
NIPT Liquid biopsy franchise in cancer profiling Royalty and detection Other 2020 revenue drivers reproductive Assumes average risk adoption and health reimbursement in US, growing adoption for whole genome NIPT and liquid biopsy commercialization with coverage and adoption of profiling component
17
|
|
Growth drivers
Products for growth
Reproductive health
Growing desire for complete karyotype level genomic health information
Burgeoning demand for average risk screen for base chromosomal aneuploidies
Broad international growth opportunities
Liquid biopsy in oncology
Therapy profiling / monitoring
Detection / reoccurrence
18
|
|
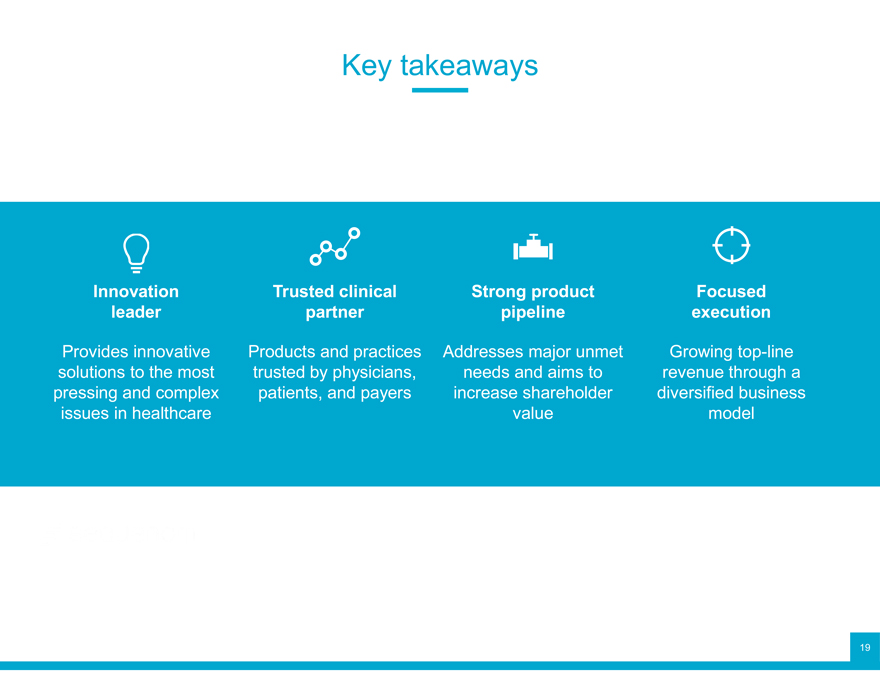
Key takeaways
Innovation Trusted clinical Strong product Focused leader partner pipeline execution
Provides innovative Products and practices Addresses major unmet Growing top-line solutions to the most trusted by physicians, needs and aims to revenue through a pressing and complex patients, and payers increase shareholder diversified business issues in healthcare value model
19
|
|
Reproductive health and genetic testing
Mathias Ehrich, MD Senior Vice President Research and Development
| 1 |
|
|
|
Reproductive health and genetic testing
|
|
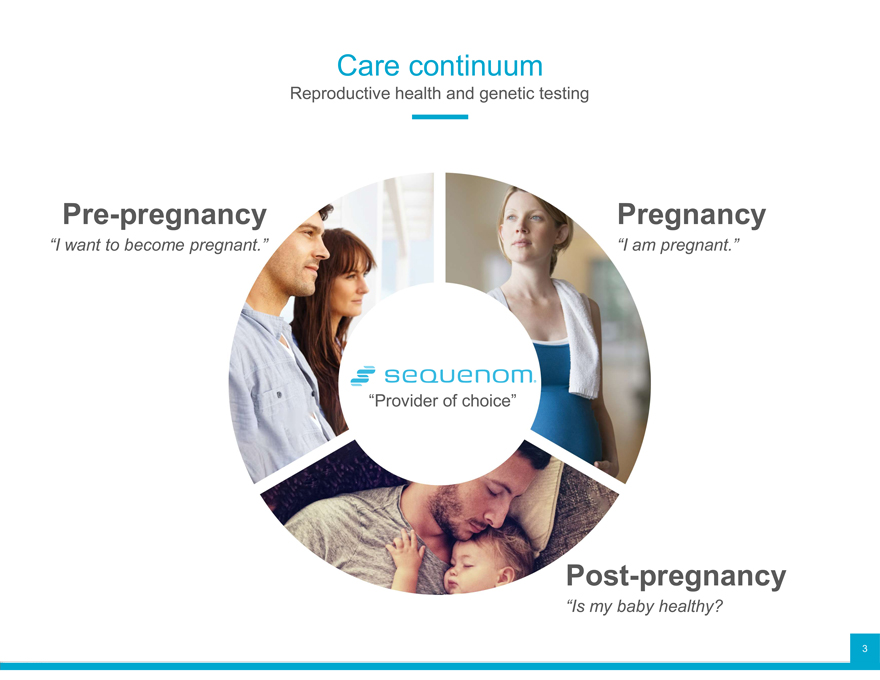
Care continuum
Reproductive health and genetic testing
Pre-pregnancy
“I want to become pregnant.”
Pregnancy
“I am pregnant.”
“Provider of choice”
Post-pregnancy
“Is my baby healthy?
| 3 |
|
|
|
Leaders in noninvasive prenatal testing
Shaping the field
First to show robust clinical validation with over 200 trisomy 21 samples First to create robust, high-throughput clinical laboratory using next generation sequencing First to market in noninvasive prenatal testing using cell-free DNA with breakthrough technology and innovations Industry-leading clinical performance
| 4 |
|
|
|
Leading in scientific breakthroughs
Sequenom accomplishments
Breakthrough innovations
Foundational technology for all current NIPT
Cell-free fetal DNA in maternal blood
Next-generation sequencing of circulating cell-free DNA (ccfDNA) allows for accurate identification of fetal chromosomal abnormalities
Continuous and responsible innovation
Over 225 patents / patent applications added to joint NIPT patent pool with Illumina
| 5 |
|
relationship ID rId2 was not found in the file.
|
|
Leading in innovation
Sequenom accomplishments
R&D advances
Pioneering biochemistry and bioinformatics solutions
Detection of balanced translocations
High accuracy at low no-call rates
Industry leading low false positive rates for reporting of sub-chromosomal events
Genome-wide analysis of deletions / duplications 7 Mb or larger
| 6 |
|
|
|
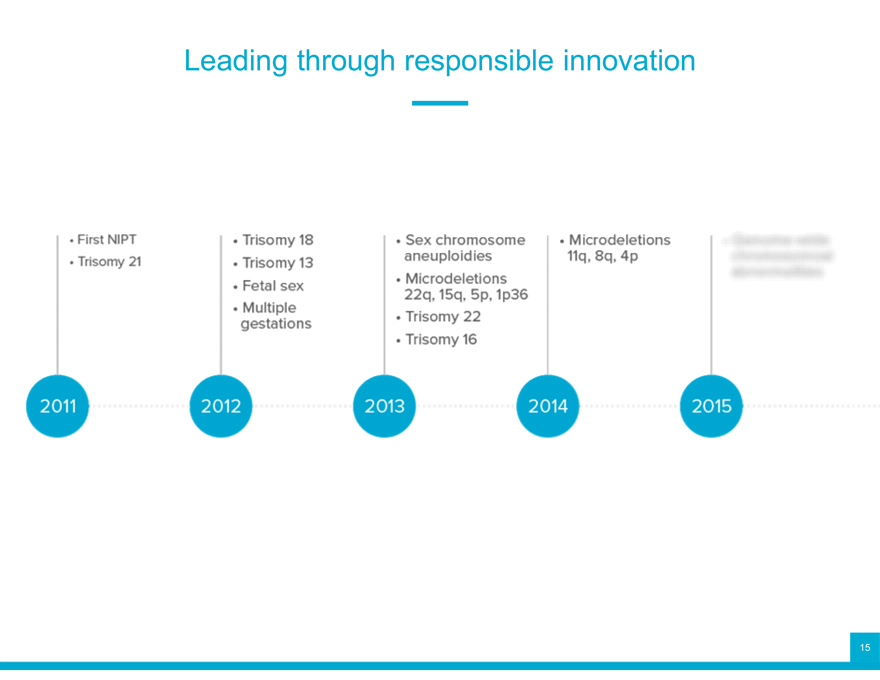
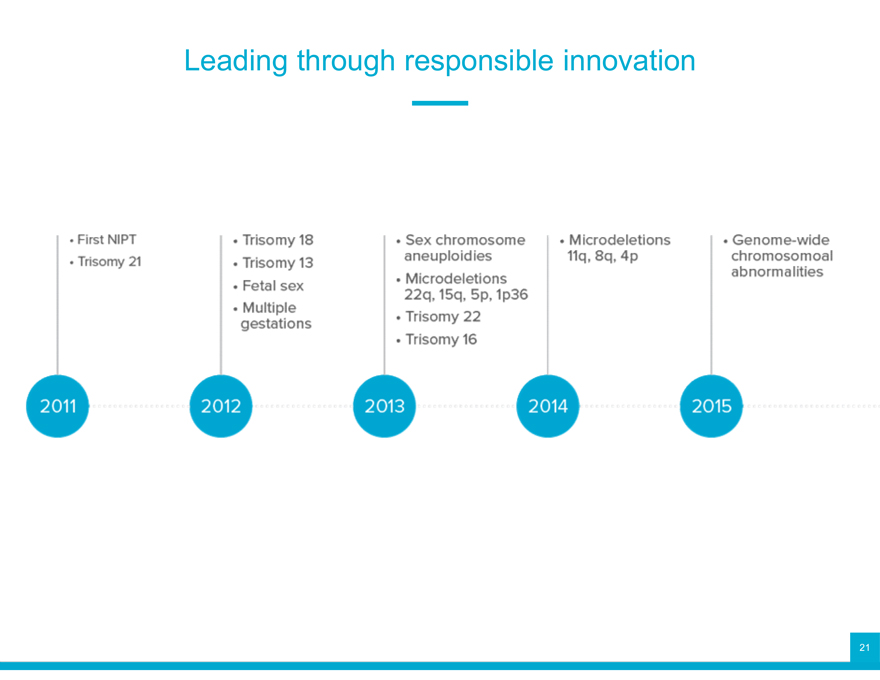
Leading through responsible innovation
Foundational technology
Breakthrough innovations
Leading clinical validation
Leading clinical performance
| 7 |
|
|
|
Leading through responsible innovation
| 8 |
|
|
|
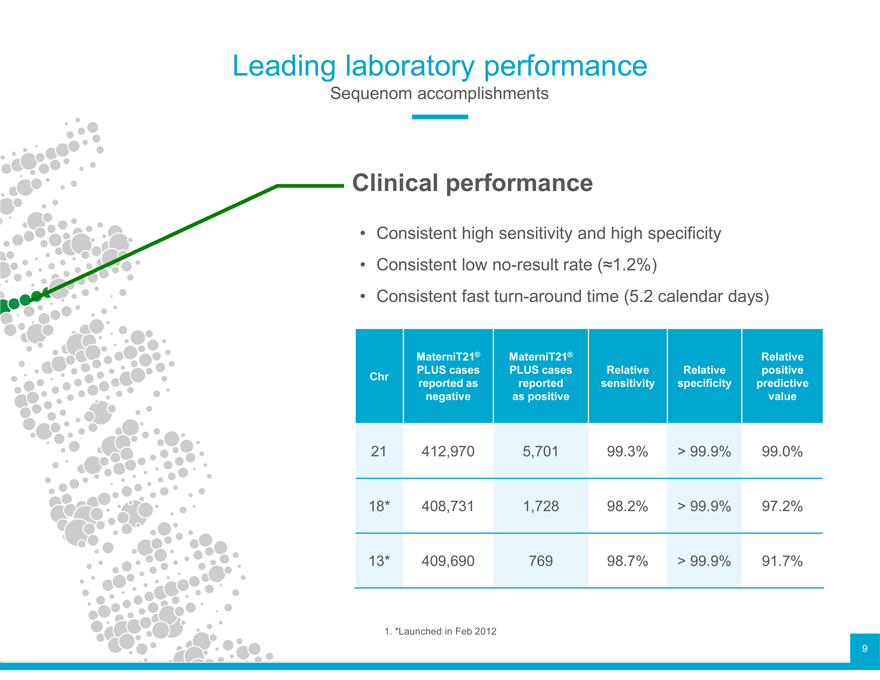
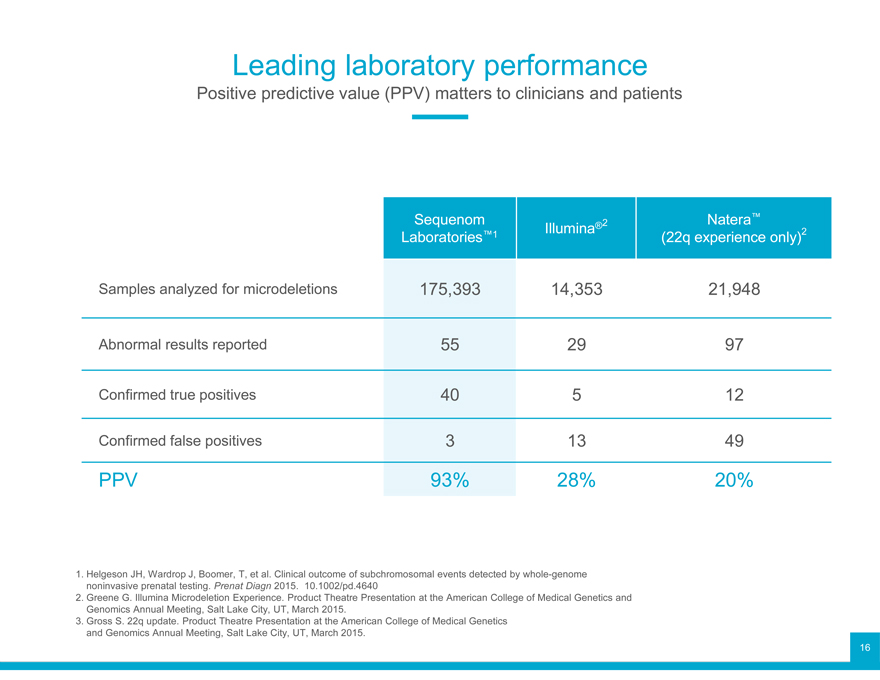
Leading laboratory performance
Sequenom accomplishments
Clinical performance
Consistent Consistent high low no-result sensitivity rate and (?1.2%) high specificity
Consistent fast turn-around time (5.2 calendar days)
MaterniT21® MaterniT21® Relative
Chr PLUSreportedcases as PLUS reported cases sensitivity Relative specificity Relative predictive positive
negative as positive value
21 412,970 5,701 99.3% > 99.9% 99.0%
18* 408,731 1,728 98.2% > 99.9% 97.2%
13* 409,690 769 98.7% > 99.9% 91.7%
1. *Launched in Feb 2012
9
|
|
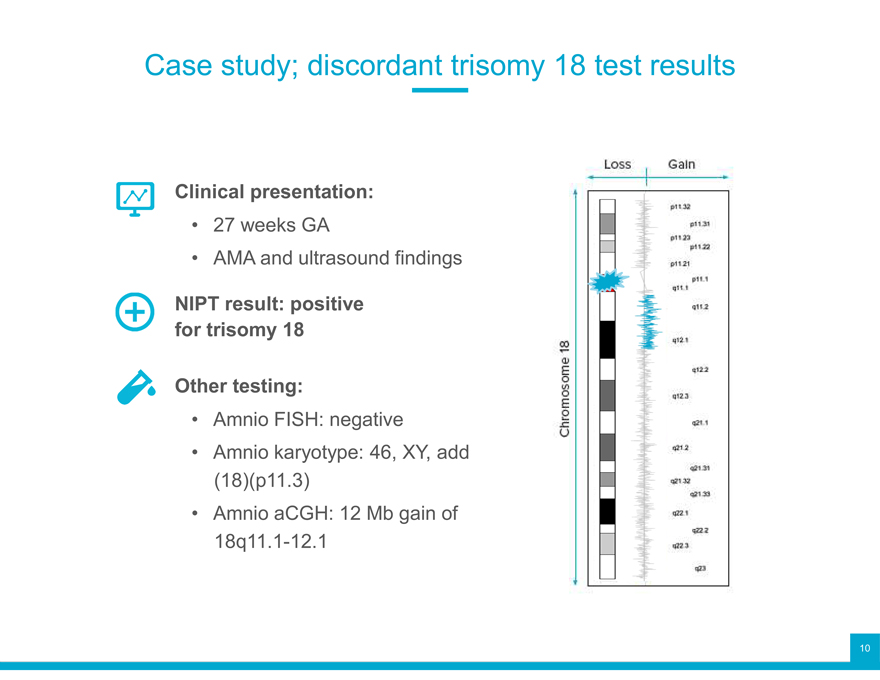
Case study; discordant trisomy 18 test results
Clinical presentation:
27 weeks GA
AMA and ultrasound findings
NIPT result: positive for trisomy 18 Other testing:
Amnio FISH: negative
Amnio karyotype: 46, XY, add (18)(p11.3)
Amnio aCGH: 12 Mb gain of 18q11.1-12.1
10
|
|
“Invasive diagnostic testing for
aneuploidy should be available
for all women, regardless of
maternal age.”
Screening for fetal
chromosomal abnormalities ACOG Practice Bulletin 88, December 2007
Number 77, January 2007
Invasive prenatal testing for
aneuploidy
Number 88, December 2007
11
|
|
Key innovations
12
|
|
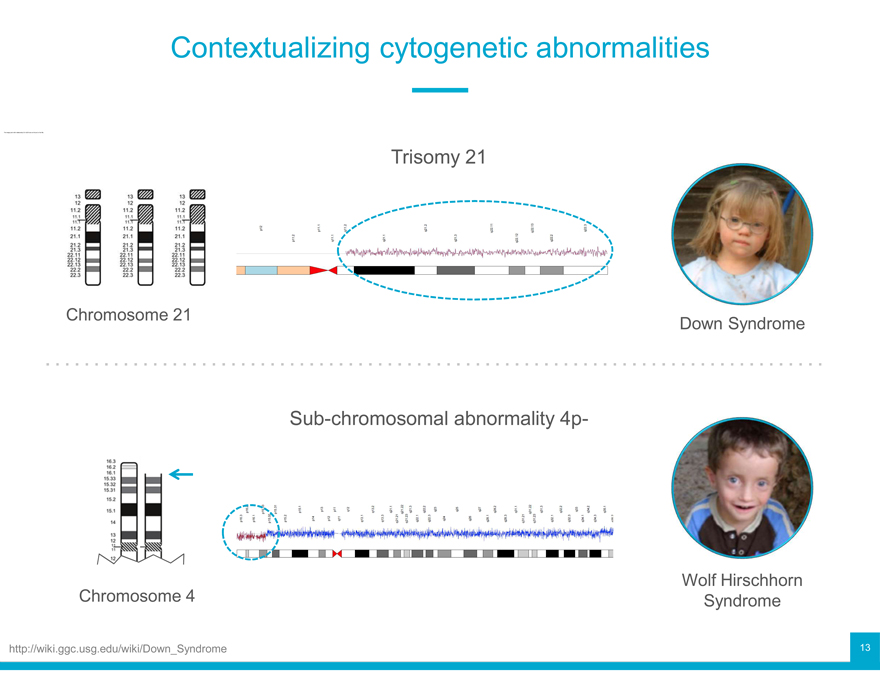
Contextualizing cytogenetic abnormalities
Trisomy 21
Chromosome 21 Down Syndrome
Sub-chromosomal abnormality 4p-
Chromosome 4 Wolf Hirschhorn Syndrome
ggc.usg.edu/wiki/Down_Syndrome 13
|
|
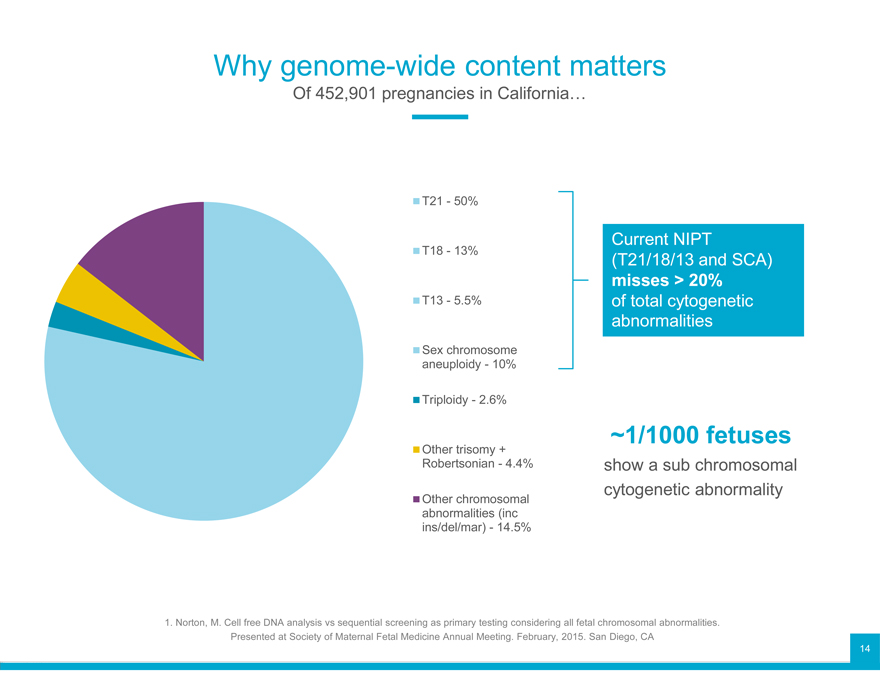
Why genome-wide content matters
Of 452,901 pregnancies in California…
T21—50%
Current NIPT
T18—13% (T21/18/13 and SCA)
misses > 20%
T13—5.5% of total cytogenetic
abnormalities
Sex chromosome
aneuploidy—10%
Triploidy—2.6%
Other trisomy + ~1/1000 fetuses
Robertsonian—4.4% show a sub chromosomal
Other chromosomal cytogenetic abnormality
abnormalities (inc
ins/del/mar)—14.5%
1. Norton, M. Cell free DNA analysis vs sequential screening as primary testing considering all fetal chromosomal abnormalities.
Presented at Society of Maternal Fetal Medicine Annual Meeting. February, 2015. San Diego, CA 14
|
|
Leading through responsible innovation
15
|
|
Leading laboratory performance
Positive predictive value (PPV) matters to clinicians and patients
Laboratories Sequenom™1 Illumina®2 (22q experience Natera™ only)2
Samples analyzed for microdeletions 175,393 14,353 21,948
Abnormal results reported 55 29 97
Confirmed true positives 40 5 12
Confirmed false positives 3 13 49
PPV 93% 28% 20%
1. Helgeson JH, Wardrop J, Boomer, T, et al. Clinical outcome of subchromosomal events detected by whole-genome
noninvasive prenatal testing. Prenat Diagn 2015. 10.1002/pd.4640
2. Greene G. Illumina Microdeletion Experience. Product Theatre Presentation at the American College of Medical Genetics and
Genomics Annual Meeting, Salt Lake City, UT, March 2015.
3. Gross S. 22q update. Product Theatre Presentation at the American College of Medical Genetics
and Genomics Annual Meeting, Salt Lake City, UT, March 2015.
16
|
|
Unmet needs for broad genome-wide coverage
A key opinion leader’s perspective
Ronald J. Wapner, MD
Board-certified in Maternal-Fetal Medicine and Obstetrics and Gynecology New York-Presbyterian/Columbia
17
|
|
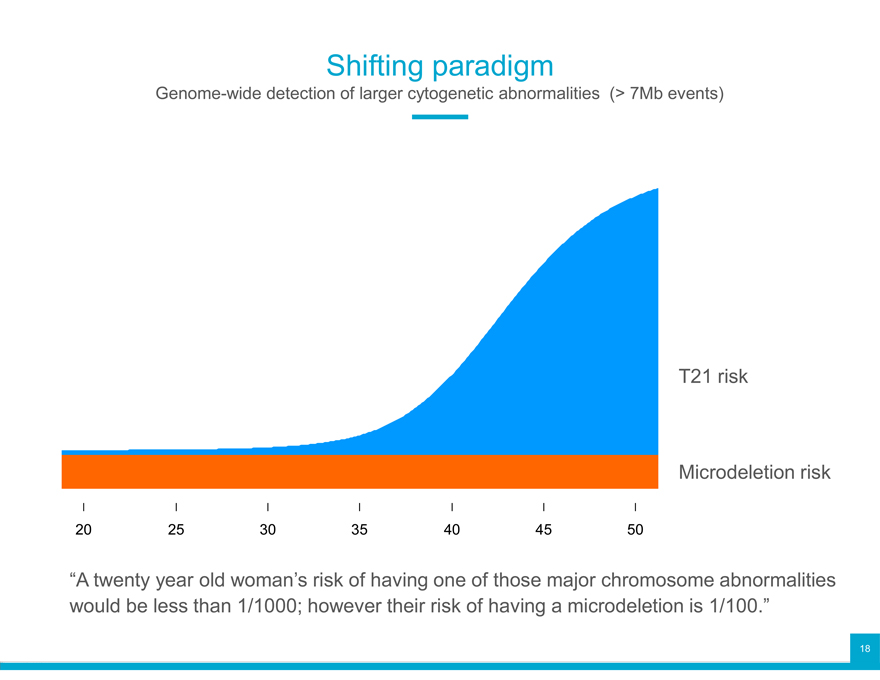
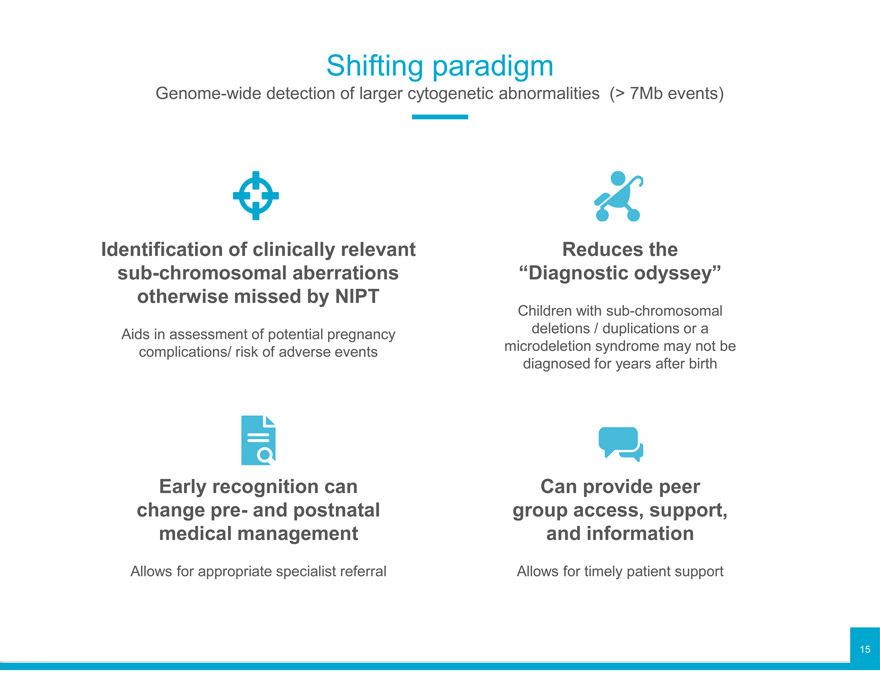
Shifting paradigm
Genome-wide detection of larger cytogenetic abnormalities (> 7Mb events)
T21 risk
Microdeletion risk
20 25 30 35 40 45 50
“A twenty year old woman’s risk of having one of those major chromosome abnormalities
would be less than 1/1000; however their risk of having a microdeletion is 1/100.”
18
relationshipIDrId2wasnotfoundinthefile.
|
|
Shifting paradigm
Genome-wide detection of larger cytogenetic abnormalities (> 7Mb events)
Identification of clinically relevant sub-chromosomal aberrations otherwise missed by NIPT
Aids in assessment of potential pregnancy complications/ risk of adverse events
Early recognition can change pre- and postnatal medical management
Allows for appropriate specialist referral
Reduces the “Diagnostic odyssey”
Children with sub-chromosomal deletions / duplications or a microdeletion syndrome may not be diagnosed for years after birth
Can provide peer group access, support, and information
Allows for timely patient support
15
|
|
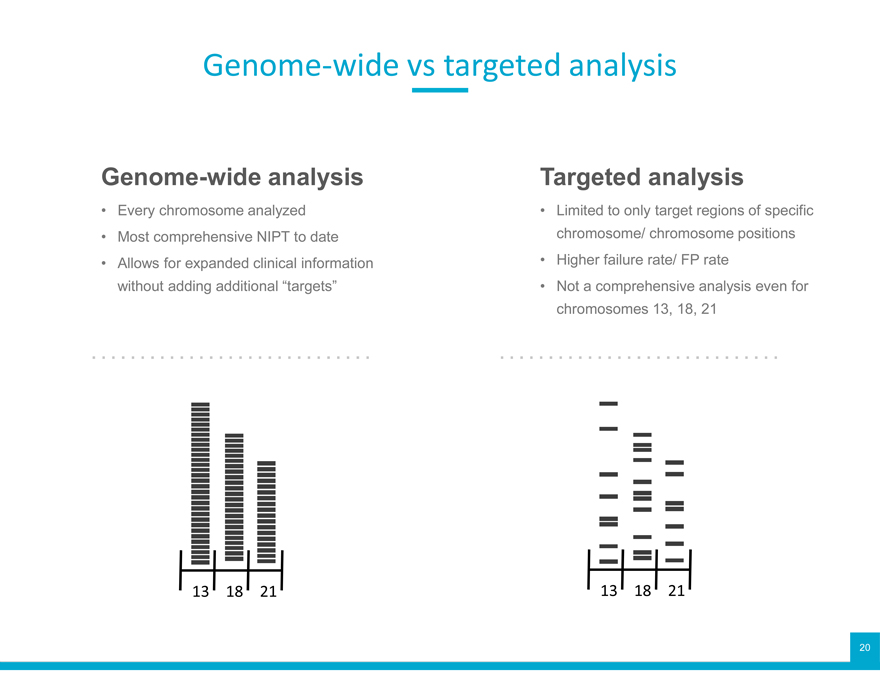
Genome-wide vs targeted analysis
Genome-wide analysis
Every chromosome analyzed
Most comprehensive NIPT to date
Allows for expanded clinical information without adding additional “targets”
Targeted analysis
Limited to only target regions of specific chromosome/ chromosome positions
Higher failure rate/ FP rate
Not a comprehensive analysis even for chromosomes 13, 18, 21
13 18 21 13 18 21
20
|
|
Leading through responsible innovation
21
|
|
Genome-wide content
22
|
|
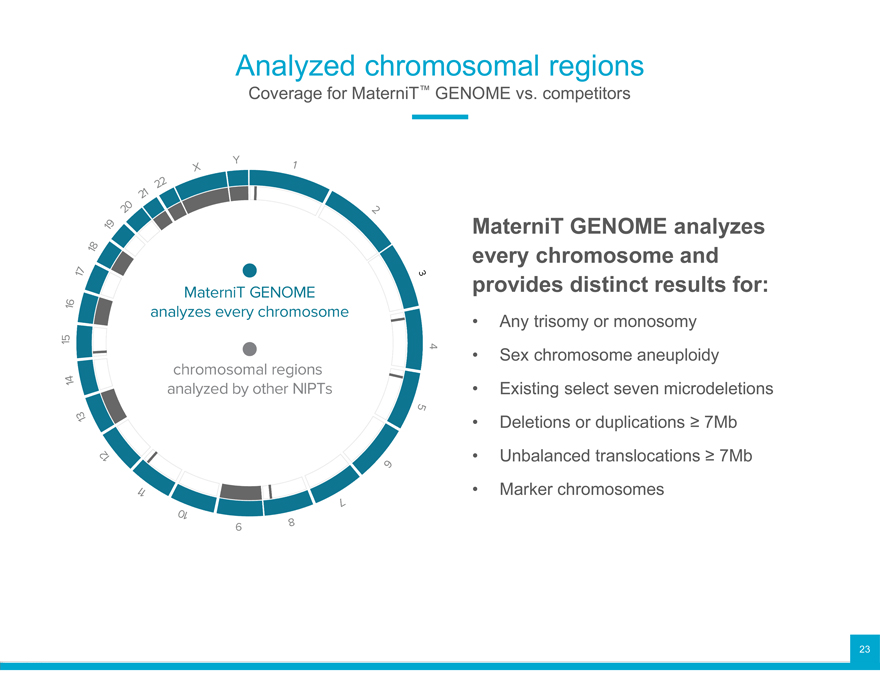
Analyzed chromosomal regions
Coverage for MaterniT™ GENOME vs. competitors
MaterniT GENOME analyzes every chromosome and provides distinct results for:
Any trisomy or monosomy
Sex chromosome aneuploidy
Existing select seven microdeletions
Deletions or duplications ? 7Mb
Unbalanced translocations ? 7Mb
Marker chromosomes
23
|
|
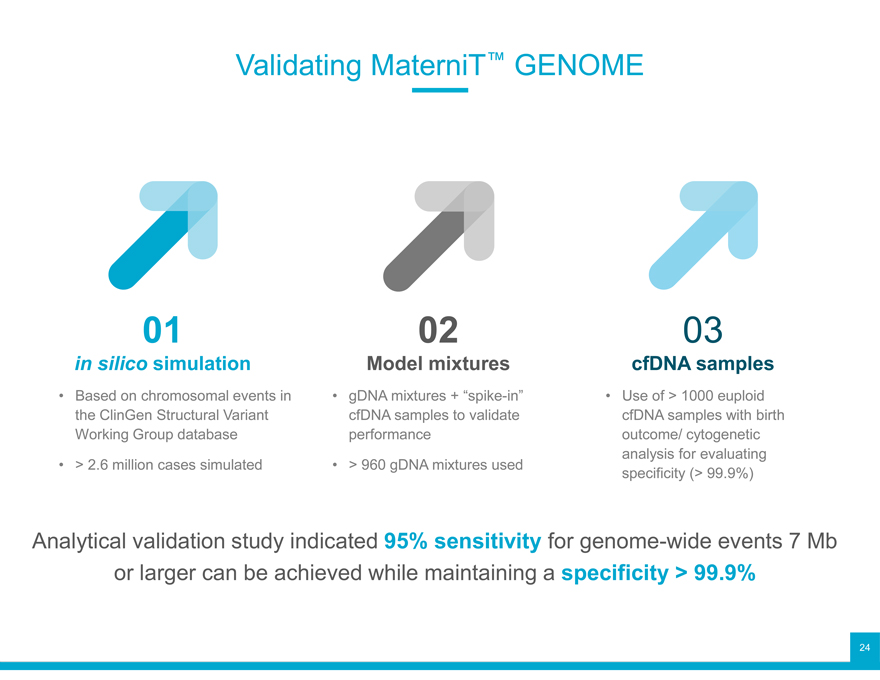
Validating MaterniT™ GENOME
in silico 01simulation
Based on chromosomal events in the ClinGen Structural Variant Working Group database
> 2.6 million cases simulated
02
Model mixtures
gDNA mixtures + “spike-in” cfDNA samples to validate performance
> 960 gDNA mixtures used
03
cfDNA samples
Use of > 1000 euploid cfDNA samples with birth outcome/ cytogenetic analysis for evaluating specificity (> 99.9%)
Analytical validation study indicated 95% sensitivity for genome-wide events 7 Mb or larger can be achieved while maintaining a specificity > 99.9%
24
|
|
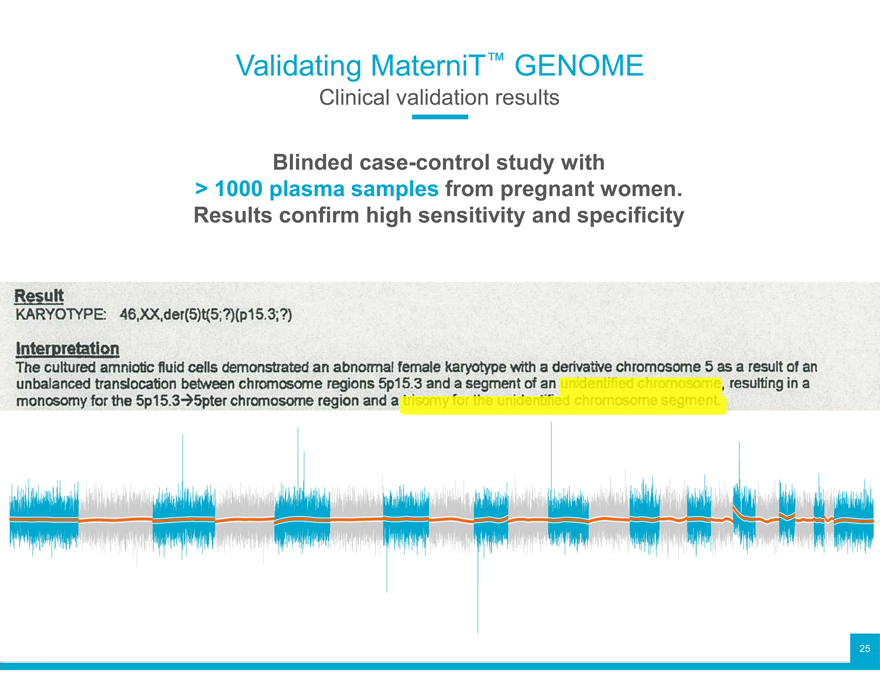
Validating MaterniT™ GENOME
Clinical validation results
Blinded case-control study with > 1000 plasma samples from pregnant women. Results confirm high sensitivity and specificity
25
|
|
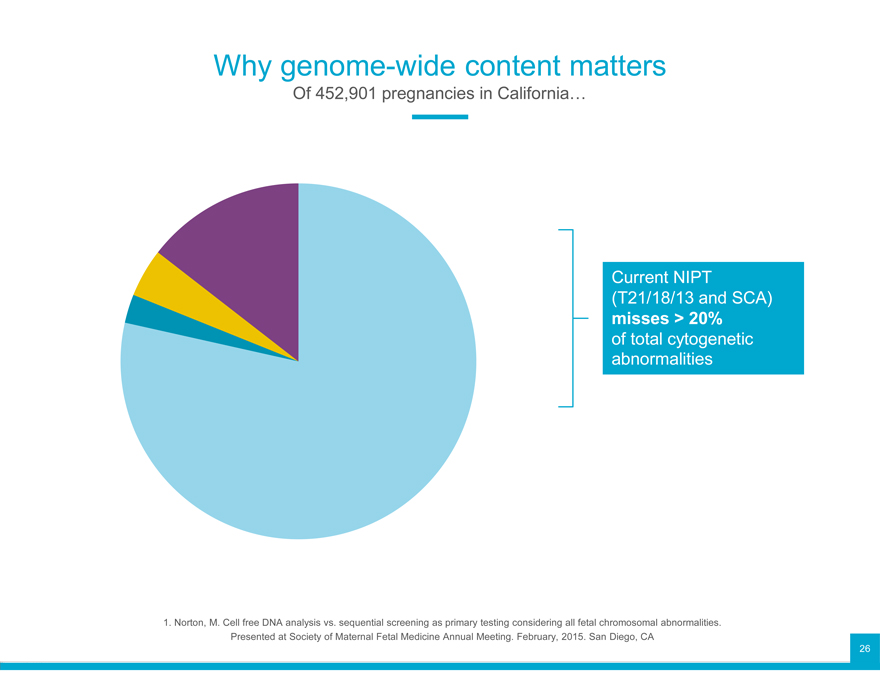
Why genome-wide content matters
Of 452,901 pregnancies in California…
Current NIPT
(T21/18/13 and SCA)
misses > 20%
of total cytogenetic
abnormalities
1. Norton, M. Cell free DNA analysis vs. sequential screening as primary testing considering all fetal chromosomal abnormalities.
Presented at Society of Maternal Fetal Medicine Annual Meeting. February, 2015. San Diego, CA
26
|
|
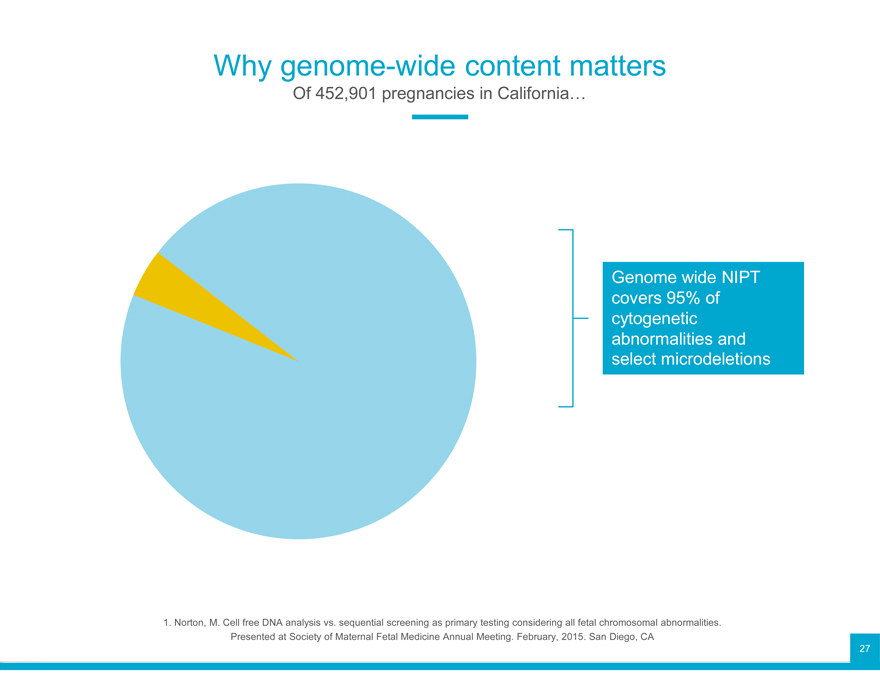
Why genome-wide content matters
Of 452,901 pregnancies in California…
Genome wide NIPT covers 95% of cytogenetic abnormalities and select microdeletions
1. Norton, M. Cell free DNA analysis vs. sequential screening as primary testing considering all fetal chromosomal abnormalities.
Presented at Society of Maternal Fetal Medicine Annual Meeting. February, 2015. San Diego, CA
27
|
|
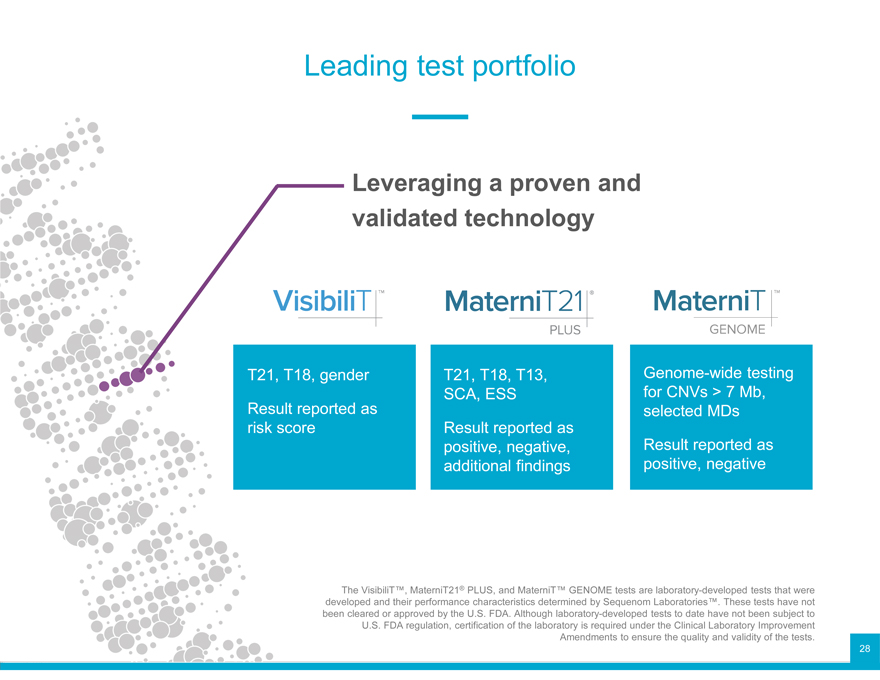
Leading test portfolio
Leveraging a proven and validated technology
T21, T18, gender T21, T18, T13, Genome-wide testing
SCA, ESS for CNVs > 7 Mb,
Result reported as selected MDs
risk score Result reported as
positive, negative, Result reported as
additional findings positive, negative
The VisibiliT™, MaterniT21® PLUS, and MaterniT™ GENOME tests are laboratory-developed tests that were
developed and their performance characteristics determined by Sequenom Laboratories™. These tests have not
been cleared or approved by the U.S. FDA. Although laboratory-developed tests to date have not been subject to
U.S. FDA regulation, certification of the laboratory is required under the Clinical Laboratory Improvement
Amendments to ensure the quality and validity of the tests. 28
|
|
Take-home messages
Shaping the future of NIPT
Best-in-class technology
Robust product portfolio spanning high content needs and cost effective requirements
Continuous and responsible innovation
29
|
|
Reproductive health and genetic testing
Rob Lozuk Senior Vice President Commercial Operations
1
|
|
Commercial execution
|
|
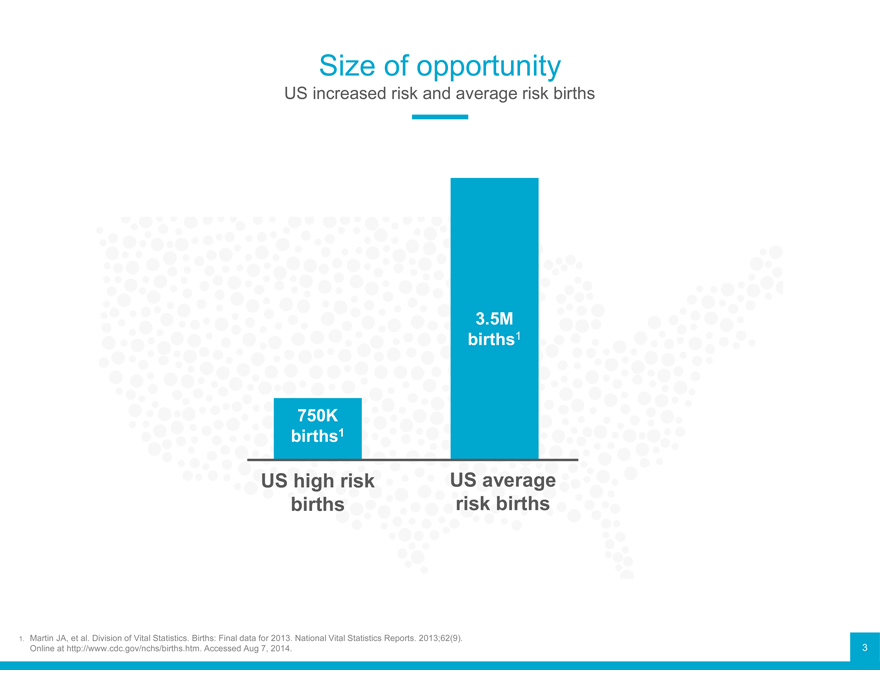
Size of opportunity
US increased risk and average risk births
3.5M births1
750K births1
US high risk US average births risk births
1. Martin JA, et al. Division of Vital Statistics. Births: Final data for 2013. National Vital Statistics Reports. 2013;62(9). 3 Online at http://www.cdc.gov/nchs/births.htm. Accessed Aug 7, 2014.
|
|
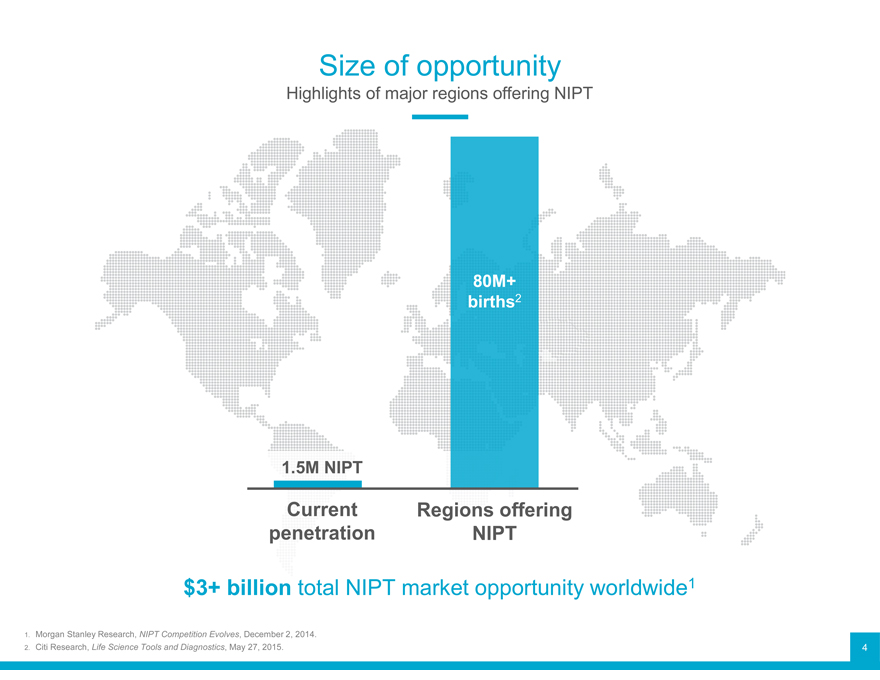
Size of opportunity
Highlights of major regions offering NIPT
80M+ births2
1.5M NIPT
Current Regions offering penetration NIPT
$3+ billion total NIPT market opportunity worldwide1
1. 2. Morgan Stanley Research, NIPT Competition Evolves, December 2, 2014.
Citi Research, Life Science Tools and Diagnostics, May 27, 2015. 4
|
|
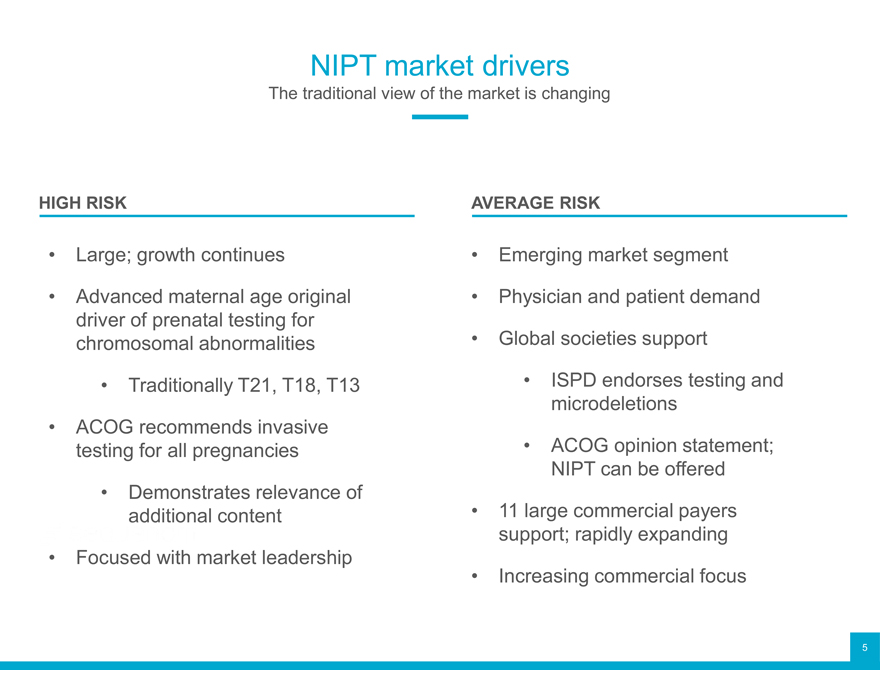
NIPT market drivers
The traditional view of the market is changing
HIGH RISK AVERAGE RISK
Large; growth continues Emerging market segment
Advanced maternal age original Physician and patient demand
driver of prenatal testing for
chromosomal abnormalities Global societies support
Traditionally T21, T18, T13 ISPD endorses testing and
ACOG recommends invasive microdeletions
testing for all pregnancies ACOG opinion statement;
Demonstrates relevance of NIPT can be offered
additional content 11 large commercial payers
support; rapidly expanding
Focused with market leadership Increasing commercial focus
5
|
|
Market dynamics
NIPT future evolution
6
|
|
Positioned to serve the MFM + OB customer groups
Growing our reproductive health franchise
Large whole-genome Broad and differentiated sequencing footprint product portfolio
Comprehensive Focused commercial customer solutions organization
Trusted partner Enabling the global market
7
|
|
Large whole-genome sequencing footprint
Broad and differentiated product portfolio Comprehensive customer solutions Focused commercial organization Trusted partners Enabling the global market
|
|
Large whole-genome sequencing footprint
Sequenom Laboratories
CAP-accredited First clinical Capacity to
+ CLIA-certified laboratory to accommodate molecular operationalize significant diagnostics and scale NGS growth laboratories
9
|
|
Large whole-genome sequencing footprint
Broad and differentiated product portfolio
Comprehensive customer solutions Focused commercial organization Trusted partners Enabling the global market
|
|
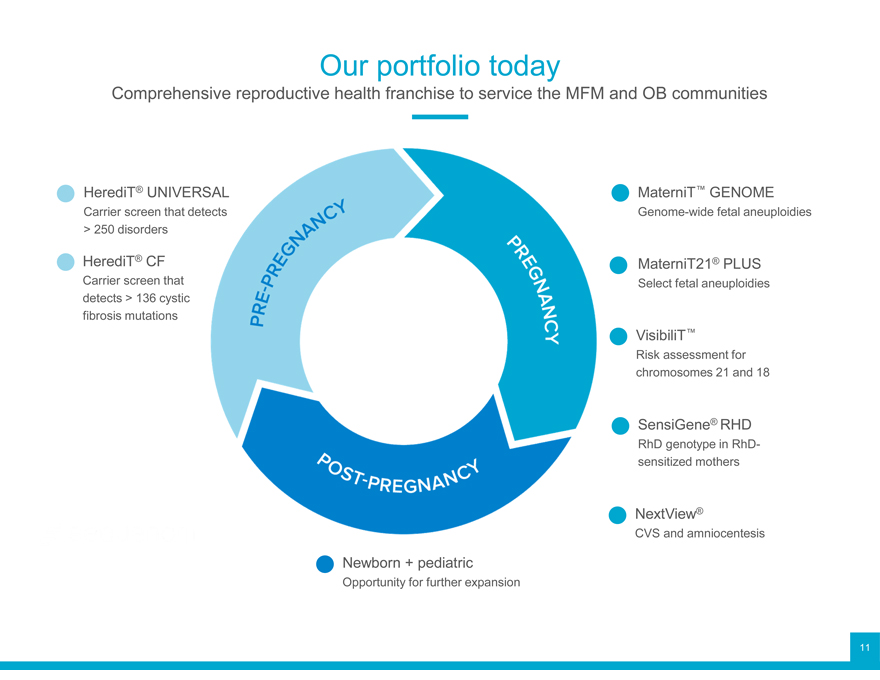
Our portfolio today
Comprehensive reproductive health franchise to service the MFM and OB communities
Carrier HerediT screen ® UNIVE that d Genome-wide MaterniT™ GENOME fetal aneuploidies
> 250 disorders
HerediT Carrier screen ® CF that MaterniT21® PLUS
Select fetal aneuploidies detects > 136 cystic fibrosis mutations VisibiliT Risk assessment ™ for chromosomes 21 and 18 SensiGene RhD genotype ® in RHD RhD-sensitized mothers CVS NextView and amniocentesis ®
Newborn + pediatric
Opportunity for further expansion
11
|
|
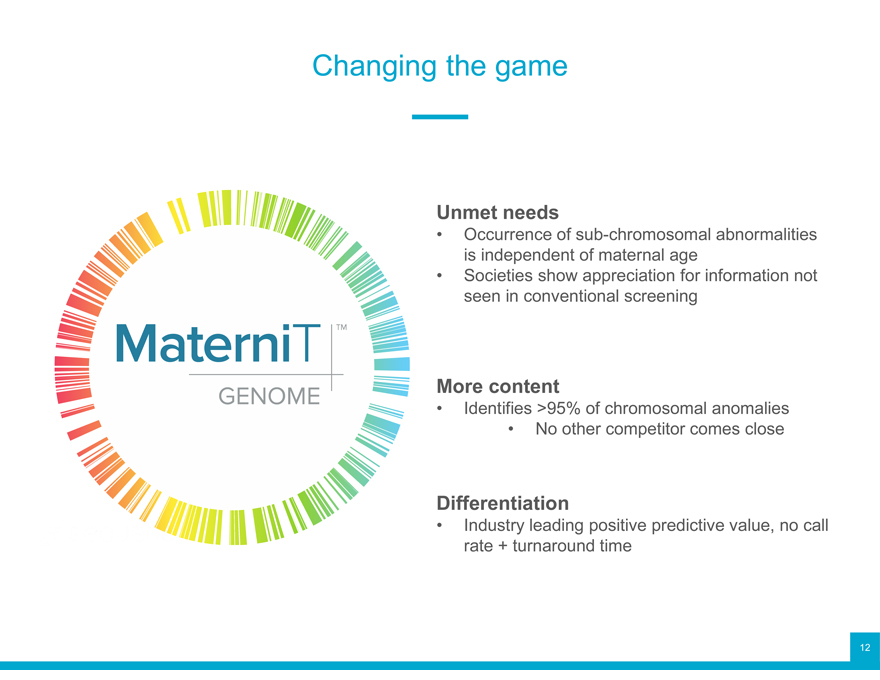
Changing the game
Unmet needs
Occurrence of sub-chromosomal abnormalities is independent of maternal age
Societies show appreciation for information not seen in conventional screening
More content
Identifies >95% of chromosomal anomalies
No other competitor comes close
Differentiation
Industry leading positive predictive value, no call rate + turnaround time
12
|
|
Large whole-genome sequencing footprint Broad and differentiated product portfolio
Comprehensive customer solutions
Focused commercial organization Trusted partners Enabling the global market
|
|
Patient experience
Our focus is multi-faceted and a high priority in the business
14
|
|
Large whole-genome sequencing footprint Broad and differentiated product portfolio Comprehensive customer solutions
Focused commercial organization
Trusted partners Enabling the global market
|
|
MFM commercial organization
Historical approach to the market was successful
110+ f nnel Product portfolio
Logistics Reproductive health tests NPS survey score 92 addressing MFM needs (-100 to +100 scale)1
Billing
Customer service MaterniT21 MaterniT GENOME PLUS
National Genetic counselors accounts
Thre FMs use MaterniT21, more than any other NIPT2
1. Net promoter annual survey 2014; Key question: How likely are you to refer Sequenom Laboratories’ products and services to a friend or colleague?
2. Footnote: Citi Research, Life Science Tools and Diagnostics, May 27, 2015 16
|
|
Broad NIPT adoption via OB/GYN channel
17
|
|
Organization structured by customer segment
MFM and OBGYN dedicated team
Grow reach, frequency
110+ fi nel Product portfolio + utilization Scale Structured as market by call dictates point OBGYN Universal products carrier Simplify product mix
Laser focus on screening + align to customer continued improvements VisibiliT channel needs on customer experience MaterniT 21 PLUS and patient journey
MFM products Leverage + improve
MaterniT GENOME MFM learnings; drive in-network status Invasive MaterniT testing 21 PLUS
18
|
|
Large whole-genome sequencing footprint Broad and differentiated product portfolio Comprehensive customer solutions Focused commercial organization
Trusted partners
Enabling the global market
|
|
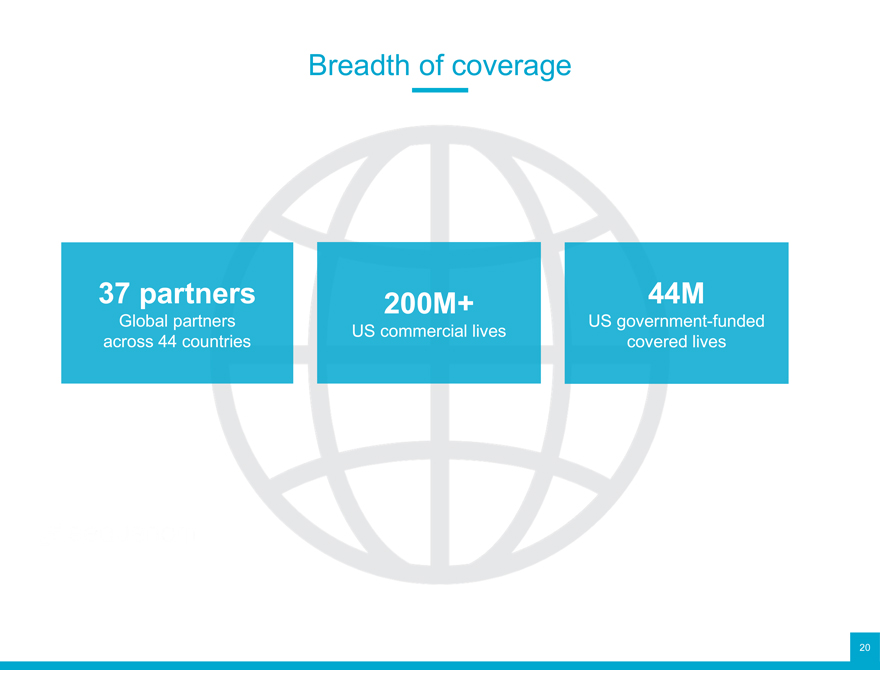
Breadth of coverage
37 partners 200M+ 44M
Global partners US commercial lives US government-funded across 44 countries covered lives
20
|
|
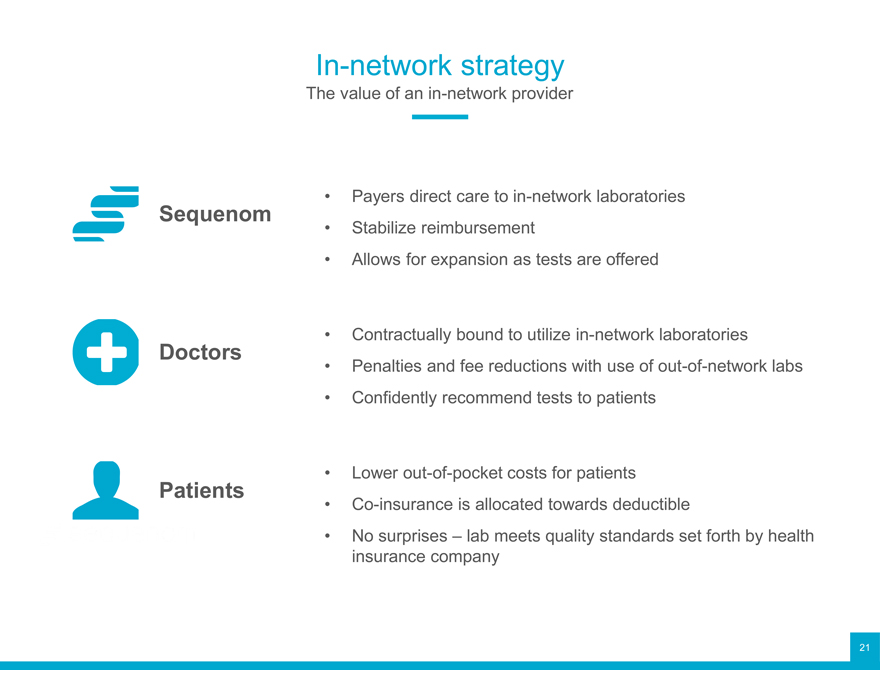
In-network strategy
The value of an in-network provider
Sequenom Stabilize Payers direct reimbursement care to in-network laboratories
Allows for expansion as tests are offered
Doctors Penalties Contractually and bound fee reductions to utilize with in-network use of out-of-network laboratories labs
Confidently recommend tests to patients
Patients Co-insurance Lower out-of-pocket is allocated costs towards for patients deductible
No surprises – lab meets quality standards set forth by health insurance company
21
|
|
Large whole-genome sequencing footprint Broad and differentiated product portfolio Comprehensive customer solutions Focused commercial organization Trusted partners
Enabling the global market
|
|
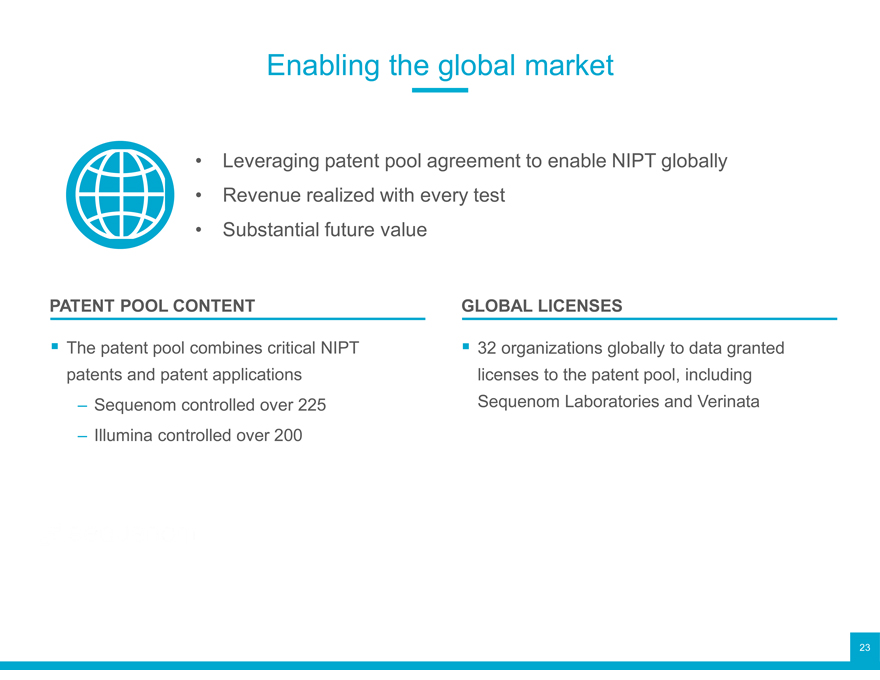
Enabling the global market
Leveraging patent pool agreement to enable NIPT globally
Revenue realized with every test
Substantial future value
PATENT POOL CONTENT GLOBAL LICENSES
The patent pool combines critical NIPT ? 32 organizations globally to data granted patents and patent applications licenses to the patent pool, including
Sequenom controlled over 225 Sequenom Laboratories and Verinata
Illumina controlled over 200
23
|
|
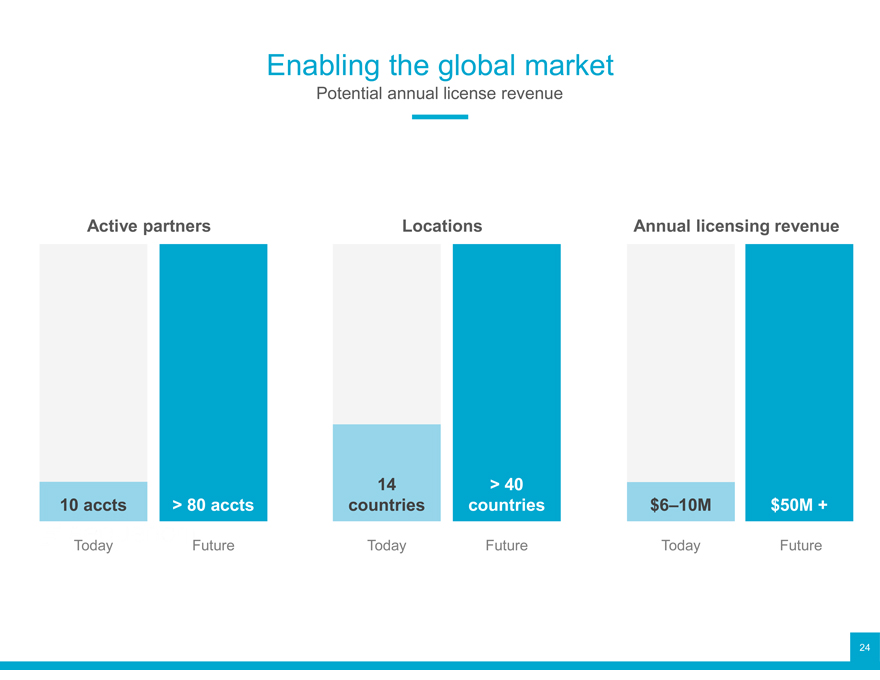
Enabling the global market
Potential annual license revenue
Active partners Locations Annual licensing revenue
14 > 40
10 accts > 80 accts countries countries $6–10M $50M +
Today Future Today Future Today Future
24
|
|
Key takeaways
Well positioned for future growth
Unparalleled in-network footprint
Expansive product portfolio and commercial focus that aligns to customer needs Commitment to the patient experience Numerous revenue streams that monetize the rapidly expanding global marketplace
25
|
|
Oncology
Mathias Ehrich, MD Senior Vice President Research and Development
1
|
|
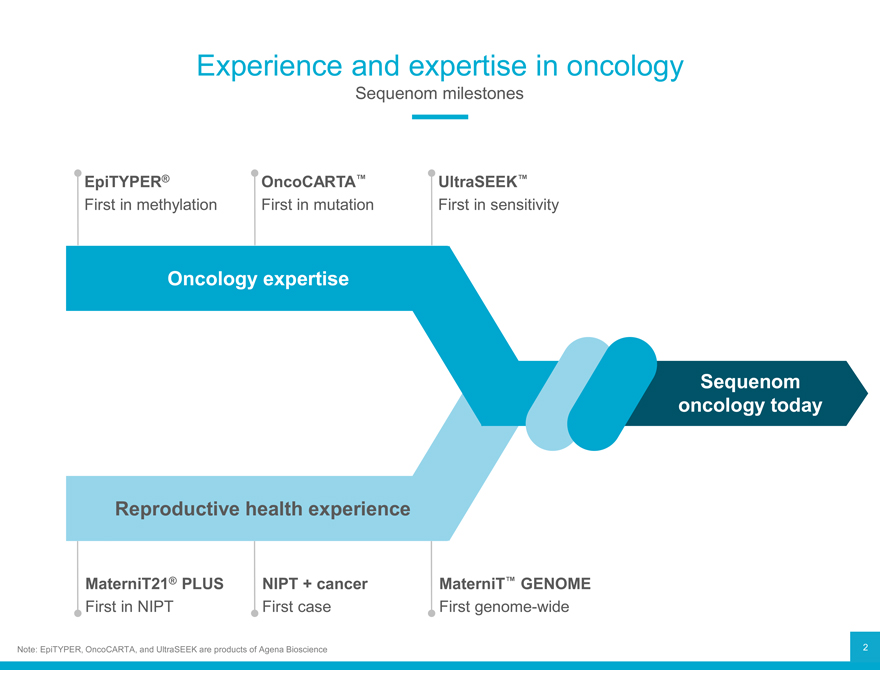
Experience and expertise in oncology
Sequenom milestones
EpiTYPER® OncoCARTA™ UltraSEEK™
First in methylation First in mutation First in sensitivity
Oncology expertise
Sequenom
oncology today
Reproductive health experience
MaterniT21® PLUS NIPT + cancer MaterniT™ GENOME
First in NIPT First case First genome-wide
Note: EpiTYPER, OncoCARTA, and UltraSEEK are products of Agena Bioscience 2
|
|
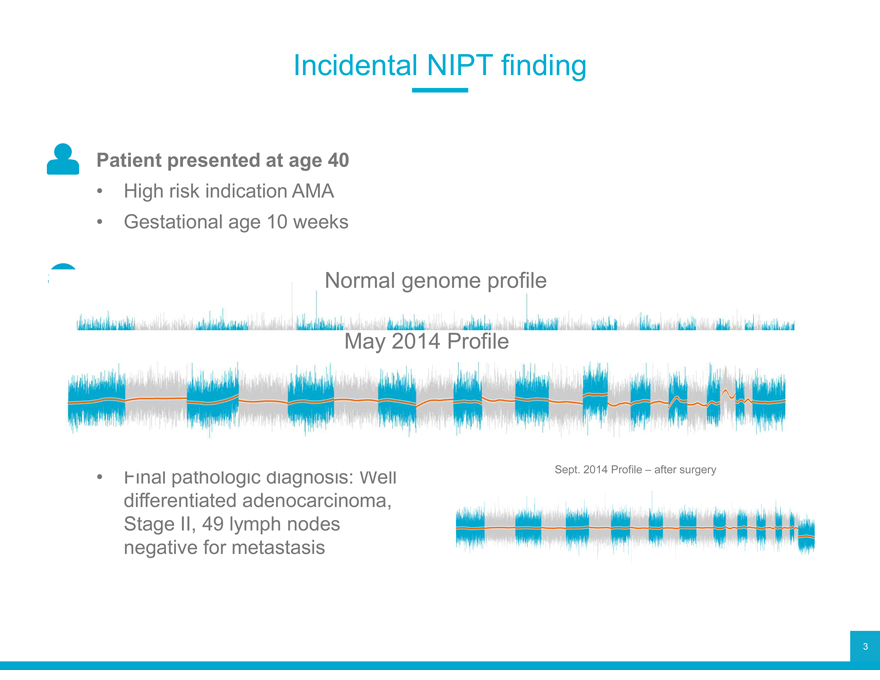
Incidental NIPT finding
Patient presented at age 40
High risk indication AMA
Gestational age 10 weeks
Normal genome profile May 2014 Profile
Sept. 2014 Profile – after surgery
differentiated adenocarcinoma, Stage II, 49 lymph nodes negative for metastasis
3
|
|
Patient story
Video
Eunice Lee, M.D.
4
|
|
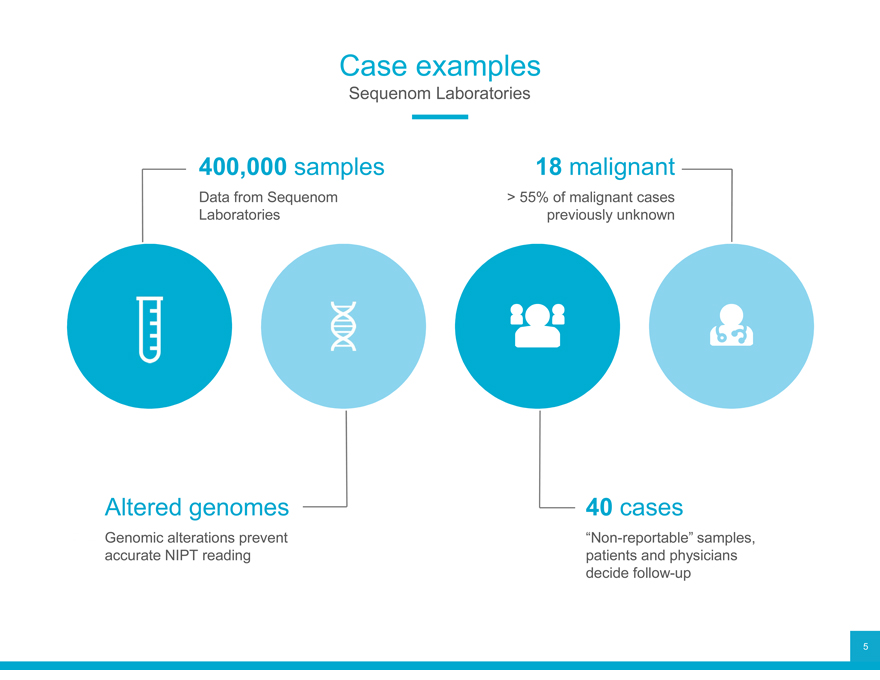
Case examples
Sequenom Laboratories
400,000 Data from Sequenom samples > 55% 18 of malignant malignant cases Laboratories previously unknown
Altered Genomic alterations genomes prevent 40 “Non-reportable” cases samples, accurate NIPT reading patients and physicians decide follow-up
5
|
|
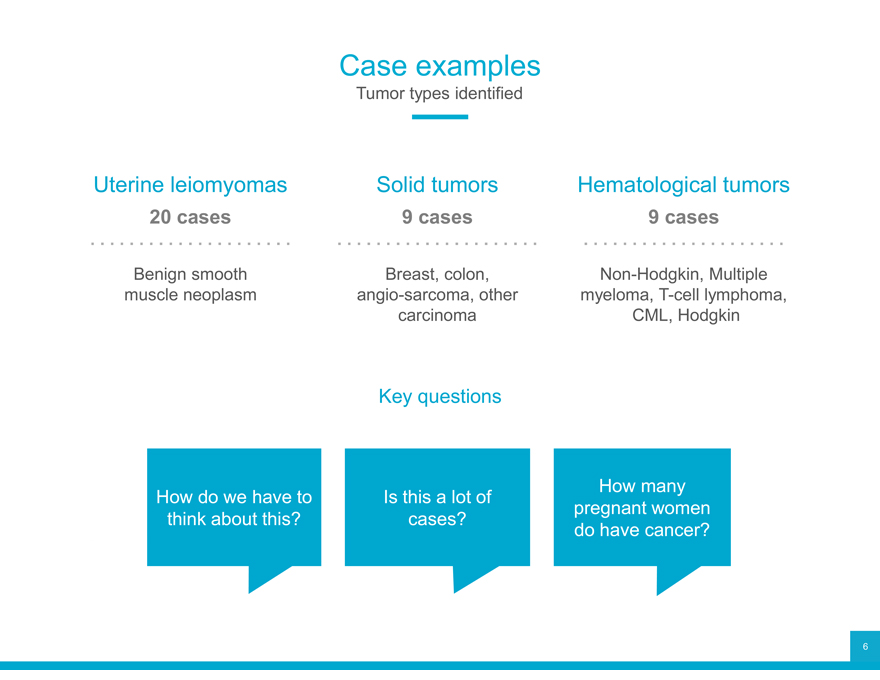
Case examples
Tumor types identified
Uterine leiomyomas Solid tumors Hematological tumors
20 cases 9 cases 9 cases
Benign smooth Breast, colon, Non-Hodgkin, Multiple muscle neoplasm angio-sarcoma, other myeloma, T-cell lymphoma, carcinoma CML, Hodgkin
Key questions
How do we have to Is this a lot of How many think about this? cases? pregnant women do have cancer?
6
|
|
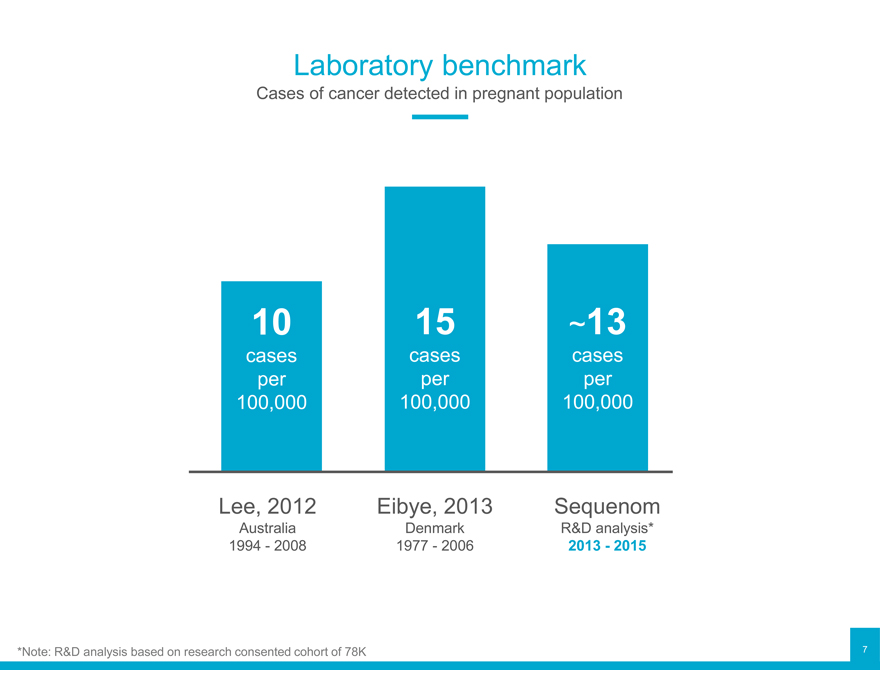
Laboratory benchmark
Cases of cancer detected in pregnant population
10 15 ~13
cases cases cases per per per 100,000 100,000 100,000
Lee, 2012 Eibye, 2013 Sequenom
Australia Denmark R&D analysis* 1994—2008 1977—2006 2013—2015
*Note: R&D analysis based on research consented cohort of 78K
7
|
|
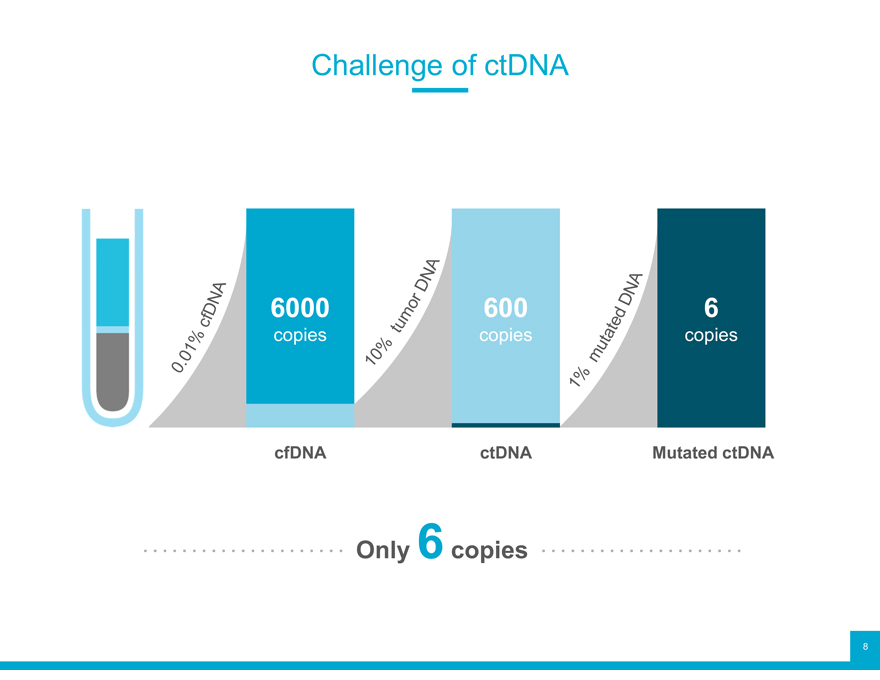
Challenge of ctDNA
6000 600 6
copies copies copies
cfDNA ctDNA Mutated ctDNA
Only 6copies
8
|
|
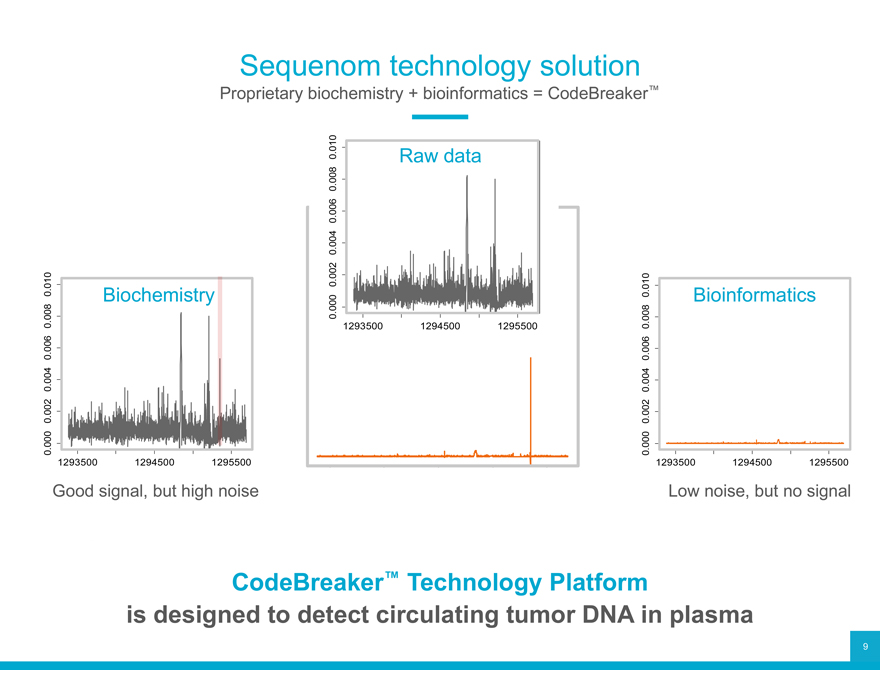
Sequenom technology solution
Proprietary biochemistry + bioinformatics = CodeBreaker™
0.010 Raw data
0.008
0.010 0.006 CodeBreaker™
0.004
0.008 0.002
0.010 Biochemistry 0.010 Bioinformatics
0.008 0.000 0.008
0.006 1293500 1294500 1295500
0.006 0.004 0.006 0.004 0.004 0.002 0.002 0.002 0.000 0.000
1293500 1294500 1295500 0.000 1293500 1294500 1295500
Good signal, but high noise 1293500 1294500 1295500 Low noise, but no signal
CodeBreaker™ Technology Platform is designed to detect circulating tumor DNA in plasma
9
|
|
How do we do it?
EGFR T G Mutation Challenge
The trick is not to find a needle in a
haystack
Detecting mutations is not difficult –
they are readily visible
The challenge is to differentiate
sequencing errors from true
mutations
CodeBreaker™
Codebreaker organizes the
sequence reads according to their
sequencing properties
This allows us to identify true
mutations and discard wrong ones
with high precision
10
|
|
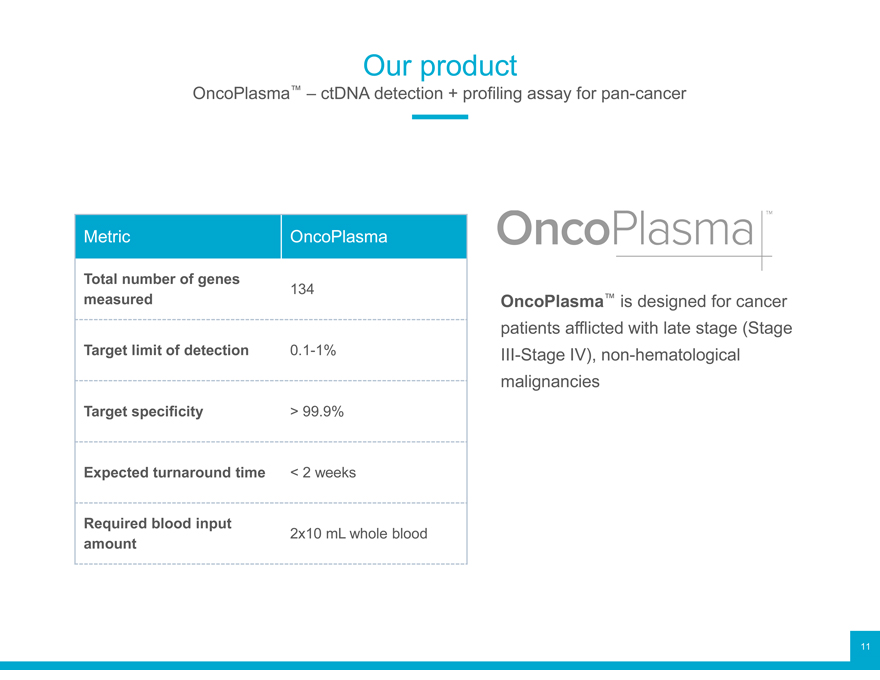
Our product
OncoPlasma™ – ctDNA detection + profiling assay for pan-cancer
Metric OncoPlasma
Total number of genes 134
measured OncoPlasma™ is designed for cancer
patients afflicted with late stage (Stage
Target limit of detection 0.1-1% III-Stage IV), non-hematological
malignancies
Target specificity > 99.9%
Expected turnaround time < 2 weeks
Required blood input 2x10 mL whole blood
amount
11
|
|
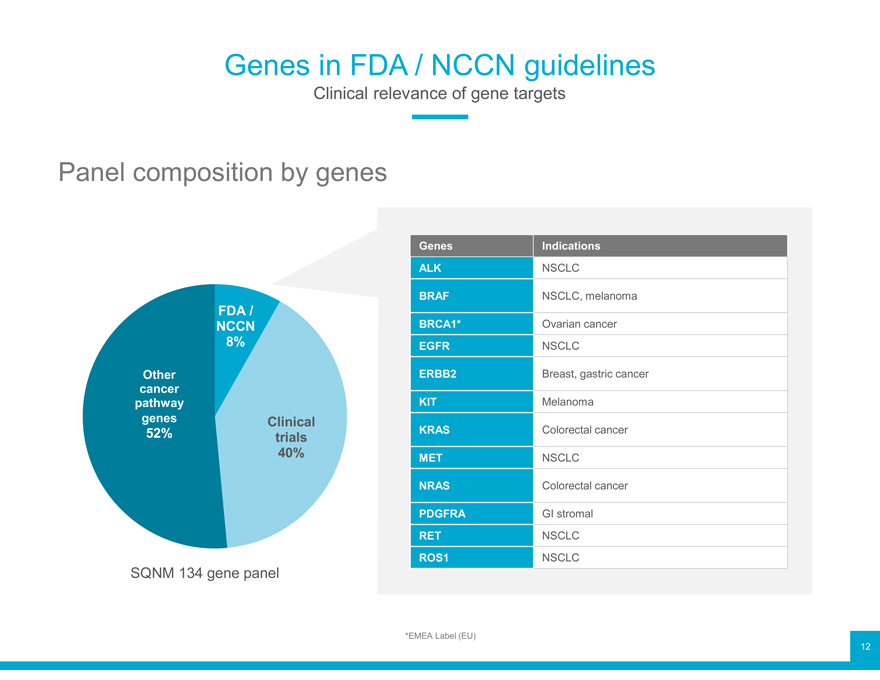
Genes in FDA / NCCN guidelines
Clinical relevance of gene targets
Panel composition by genes
Genes Indications
ALK NSCLC
FDA / BRAF NSCLC, melanoma
NCCN BRCA1* Ovarian cancer
8% EGFR NSCLC
Other ERBB2 Breast, gastric cancer
cancer
pathway KIT Melanoma
genes 52% Clinical trials KRAS Colorectal cancer
40% MET NSCLC
NRAS Colorectal cancer
PDGFRA GI stromal
RET NSCLC
SQNM 134 gene panel ROS1 NSCLC
*EMEA Label (EU) 12
12
|
|
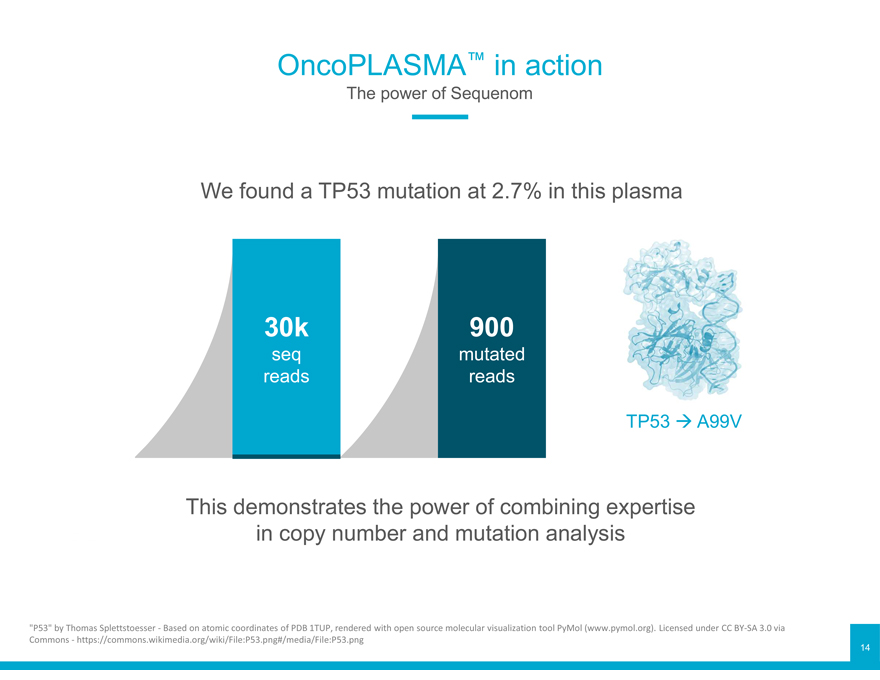
OncoPLASMA™ in action
The power of Sequenom
We asked a simple question: Can we find mutations in the cancer cases we have seen in the lab?
We used the same library that was used for NIPT and ran it with OncoPLASMA.
13
|
|
OncoPLASMA™ in action
The power of Sequenom
We found a TP53 mutation at 2.7% in this plasma
30k 900
seq mutated reads reads
TP53 A99V
This demonstrates the power of combining expertise in copy number and mutation analysis
“P53” by Thomas Splettstoesser -Based on atomic coordinates of PDB 1TUP, rendered with open source molecular visualization toolPyMol (www.pymol.org). Licensed under CC BY-SA 3.0 via Commons -https://commons.wikimedia.org/wiki/File:P53.png#/media/File:P53.png
14
|
|
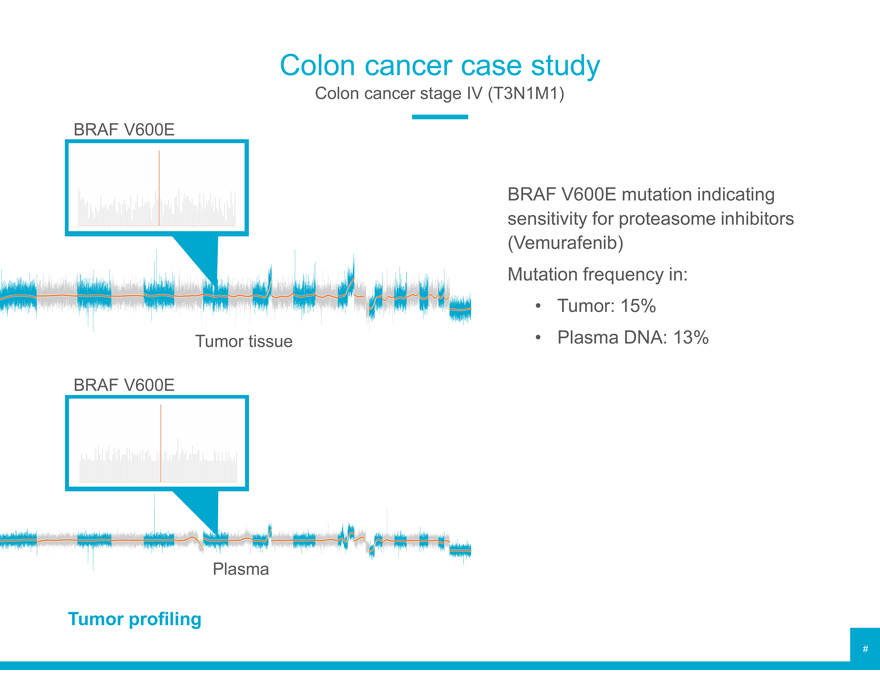
Colon cancer case study
Colon cancer stage IV (T3N1M1) BRAF V600E
BRAF V600E mutation indicating sensitivity for proteasome inhibitors (Vemurafenib) Mutation frequency in:
Tumor: 15% Tumor tissue Plasma DNA: 13%
BRAF V600E
Plasma
Tumor profiling
#
|
|
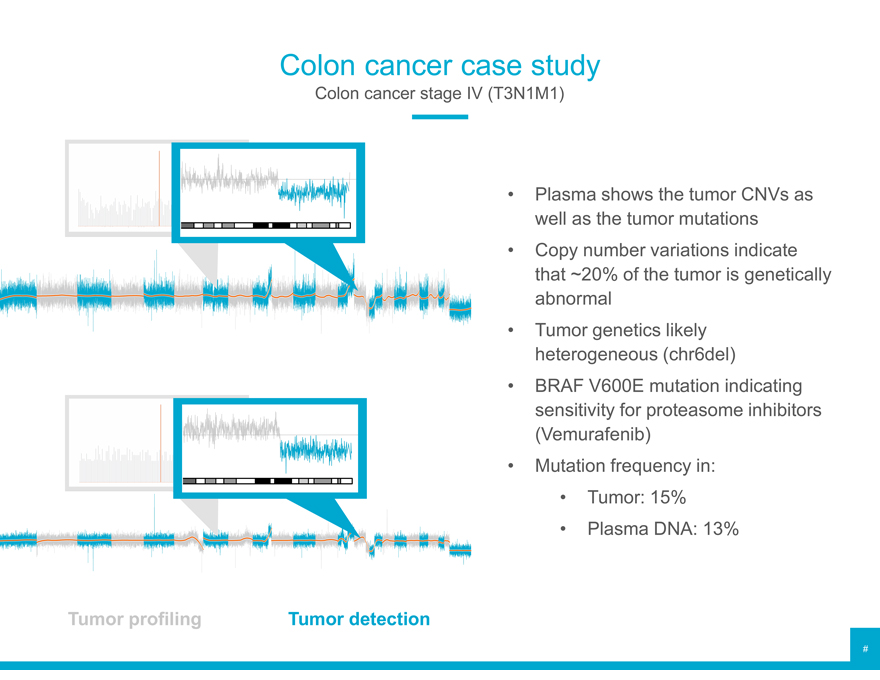
Colon cancer case study
Colon cancer stage IV (T3N1M1)
Plasma shows the tumor CNVs as well as the tumor mutations
Copy number variations indicate that ~20% of the tumor is genetically abnormal
Tumor genetics likely heterogeneous (chr6del)
BRAF V600E mutation indicating sensitivity for proteasome inhibitors (Vemurafenib)
Mutation frequency in:
Tumor: 15%
Plasma DNA: 13%
Tumor profiling Tumor detection
#
|
|
Market opportunity for early detection cancer
Copy number variations and mutations are important CNVs Mutation Copy number analysis is unique to Sequenom
17
|
|
Market opportunity for early detection cancer
The addition of DNA methylation analysis can provide superior results CNVs Mutation Sequenom has world-leading expertise in DNA methylation analysis:
Tech + application development Methylation Functional research in developmental biology
Fetal fraction pioneer
Fetal methylome
Methylation biomarker discovery in lung cancer, colon cancer, AML and pan-cancer
18
|
|
Market opportunity for early detection cancer
These programs can potentially be targeted towards CNVs Mutation • General population screening for colon cancer
Follow-up testing for node positive CT scan in lung cancer screening Methylation Or general cancer recurrence monitoring
19
|
|
Another cancer case study
What plasma can do in early detection
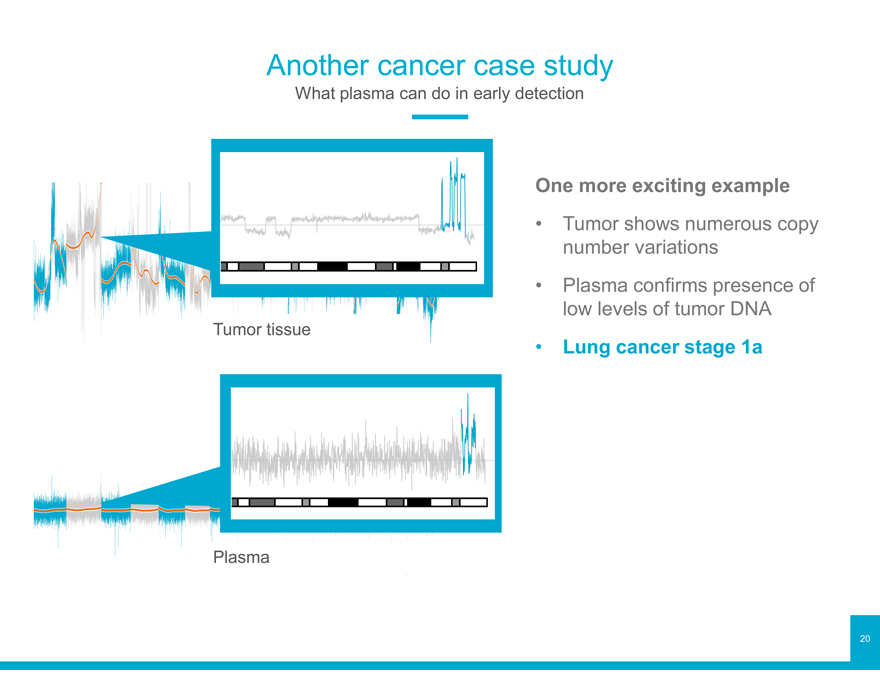
One more exciting example
Tumor shows numerous copy number variations
Plasma confirms presence of low levels of tumor DNA
Tumor tissue • Lung cancer stage 1a
Plasma
20
|
|
Oncology
Daniel Grosu, MD Senior Vice President, Chief Medical Officer
1
|
|
Sequenom liquid biopsy collaboration
A key opinion leader’s perspective
Eric Topol, M.D.
Chief Academic Officer, Scripps Health Director, Scripps Translational Science Institute
2
|
|
Diversity of cancer
One name… many diseases
Glioblastoma Lung adenocarcinoma Breast
200+
types of cancer
Melanoma Colorectal Ovarian Uterine
3
|
|
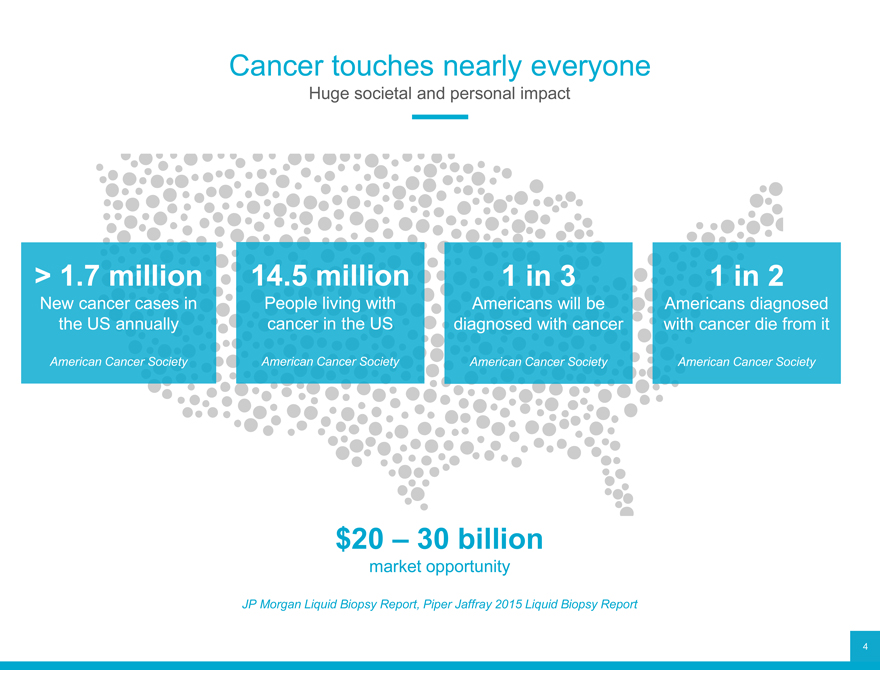
Cancer touches nearly everyone
Huge societal and personal impact
> 1.7 million 14.5 million 1 in 3 1 in 2
New cancer cases in People living with Americans will be Americans diagnosed the US annually cancer in the US diagnosed with cancer with cancer die from it
American Cancer Society American Cancer Society American Cancer Society American Cancer Society
$20 – 30 billion
market opportunity
JP Morgan Liquid Biopsy Report, Piper Jaffray 2015 Liquid Biopsy Report
4
|
|
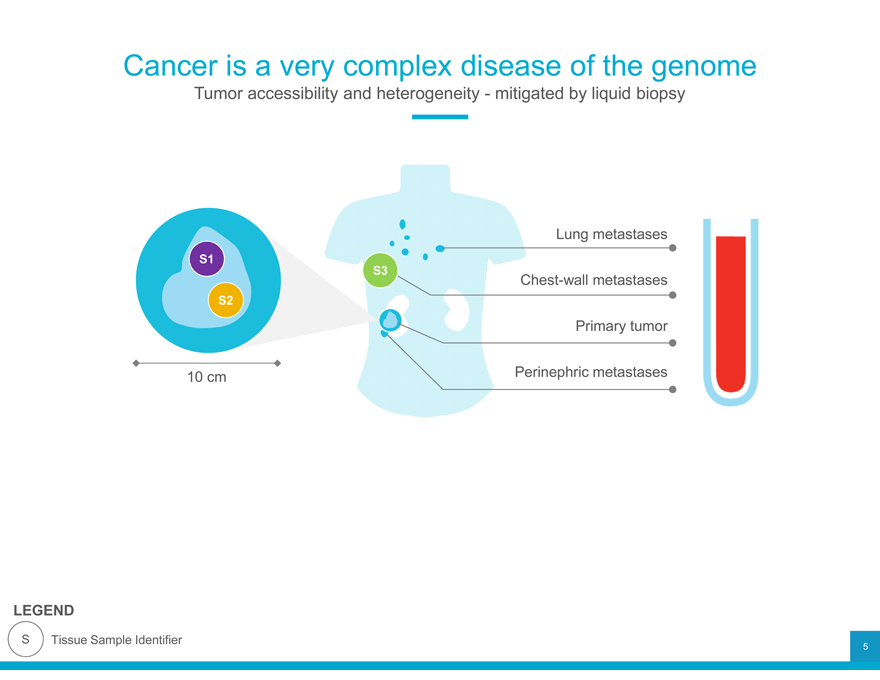
Cancer is a very complex disease of the genome
Tumor accessibility and heterogeneity—mitigated by liquid biopsy
Lung metastases
S1
S3 Chest-wall metastases
S2
Primary tumor 10 cm Perinephric metastases
LEGEND
S Tissue Sample Identifier
5
|
|
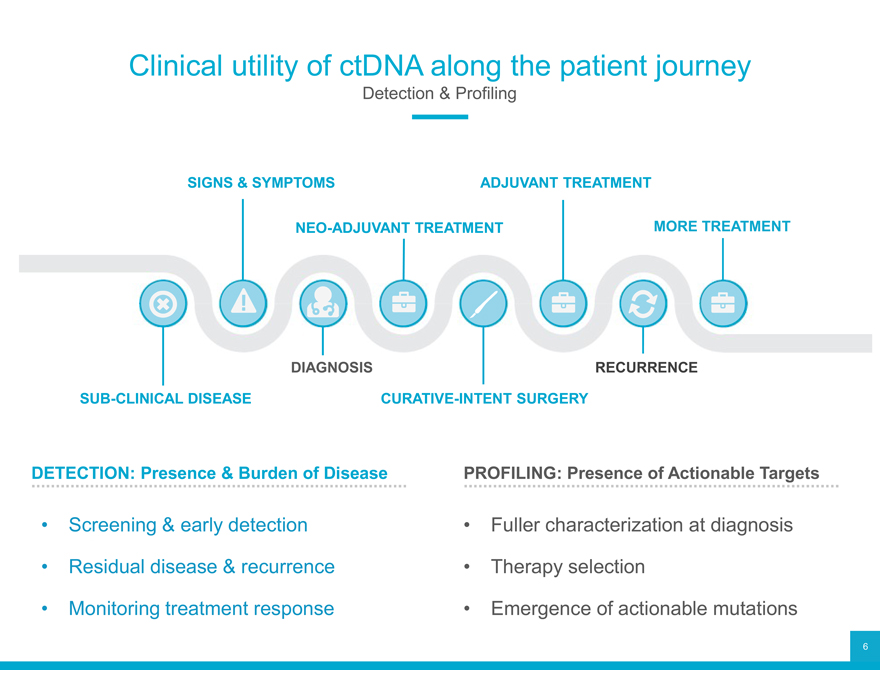
Clinical utility of ctDNA along the patient journey
Detection & Profiling
SIGNS & SYMPTOMS ADJUVANT TREATMENT
NEO-ADJUVANT TREATMENT MORE TREATMENT
DIAGNOSIS RECURRENCE
SUB-CLINICAL DISEASE CURATIVE-INTENT SURGERY
DETECTION: Presence & Burden of Disease PROFILING: Presence of Actionable Targets
Screening & early detection Fuller characterization at diagnosis
Residual disease & recurrence Therapy selection
Monitoring treatment response Emergence of actionable mutations
6
|
|
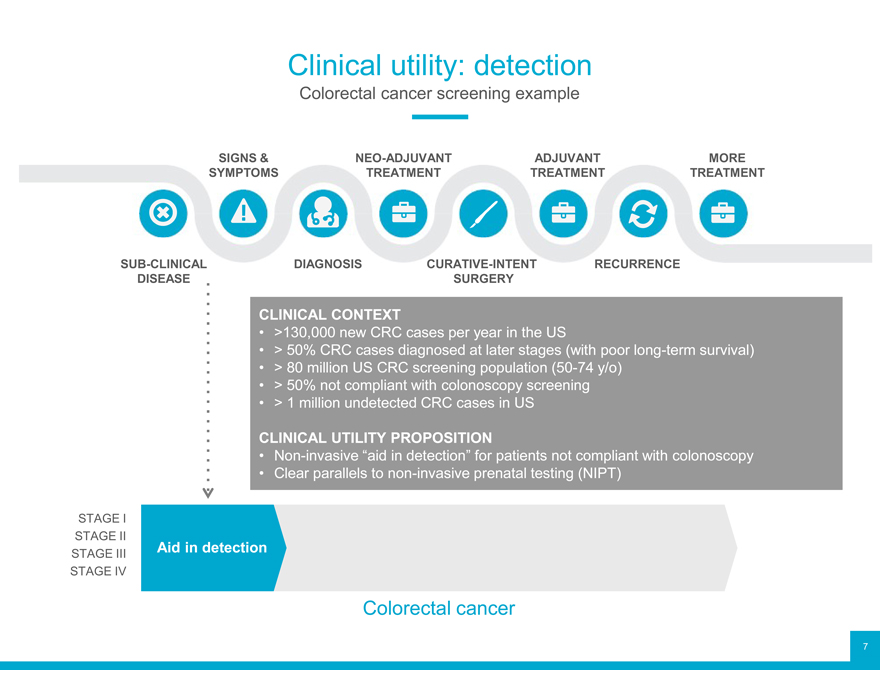
Clinical utility: detection
Colorectal cancer screening example
SIGNS & NEO-ADJUVANT ADJUVANT MORE SYMPTOMS TREATMENT TREATMENT TREATMENT
SUB-CLINICAL DIAGNOSIS CURATIVE-INTENT RECURRENCE DISEASE SURGERY
CLINICAL CONTEXT
>130,000 new CRC cases per year in the US
> 50% CRC cases diagnosed at later stages (with poor long-term survival)
> 80 million US CRC screening population (50-74 y/o)
> 50% not compliant with colonoscopy screening
> 1 million undetected CRC cases in US
CLINICAL UTILITY PROPOSITION
Non-invasive “aid in detection” for patients not compliant with colonoscopy
Clear parallels to non-invasive prenatal testing (NIPT)
STAGE I
STAGE II Aid in detection
STAGE III STAGE IV
Colorectal cancer
7
|
|
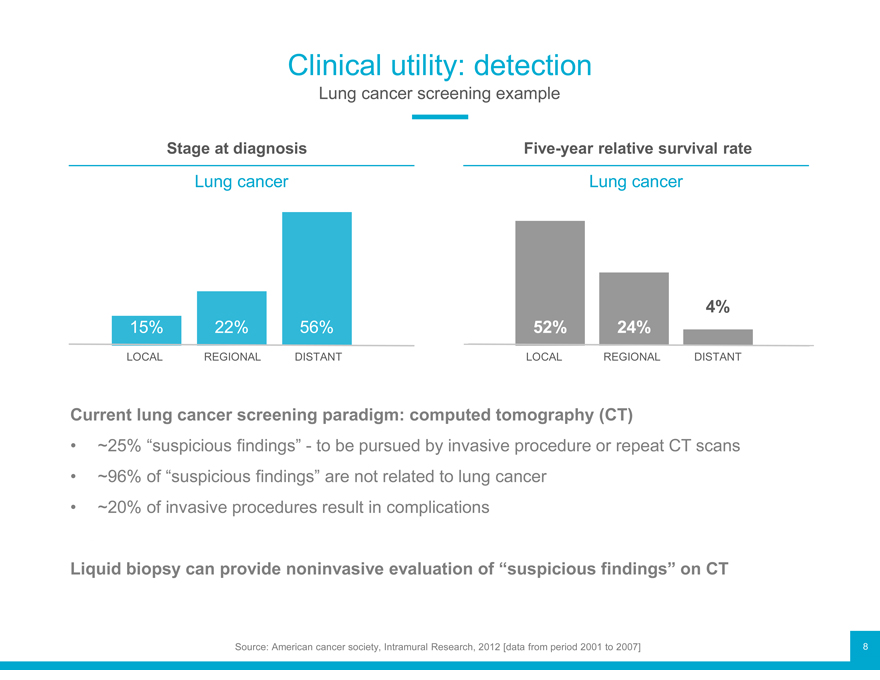
Clinical utility: detection
Lung cancer screening example
Stage at diagnosis Five-year relative survival rate
Lung cancer Lung cancer
4%
15% 22% 56% 52% 24%
LOCAL REGIONAL DISTANT LOCAL REGIONAL DISTANT
Current lung cancer screening paradigm: computed tomography (CT)
~25% “suspicious findings”—to be pursued by invasive procedure or repeat CT scans
~96% of “suspicious findings” are not related to lung cancer
~20% of invasive procedures result in complications
Liquid biopsy can provide noninvasive evaluation of “suspicious findings” on CT
Source: American cancer society, Intramural Research, 2012 [data from period 2001 to 2007]
8
|
|
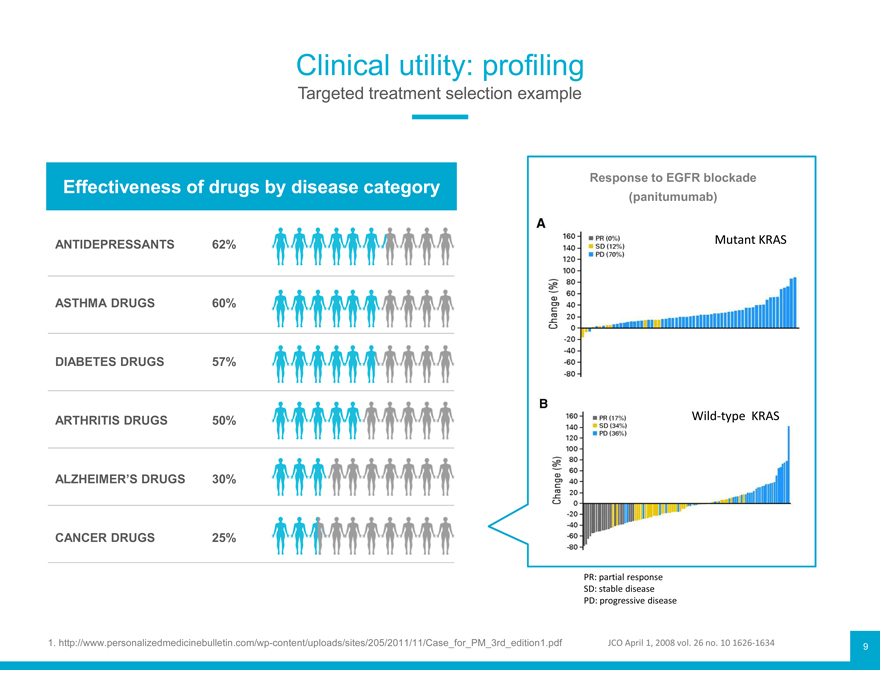
Clinical utility: profiling
Targeted treatment selection example
Effectiveness of drugs by disease category Response (panitumumab) to EGFR blockade
ANTIDEPRESSANTS 62% Mutant KRAS
ASTHMA DRUGS 60%
DIABETES DRUGS 57%
ARTHRITIS DRUGS 50% Wild-type KRAS
ALZHEIMER’S DRUGS 30%
CANCER DRUGS 25%
PR: partial response
SD: stable disease
PD: progressive disease
1. http://www.personalizedmedicinebulletin.com/wp-content/uploads/sites/205/2011/11/Case_for_PM_3rd_edition1.pdf JCO April 1, 2008 vol. 26 no. 10 1626-1634
9
|
|
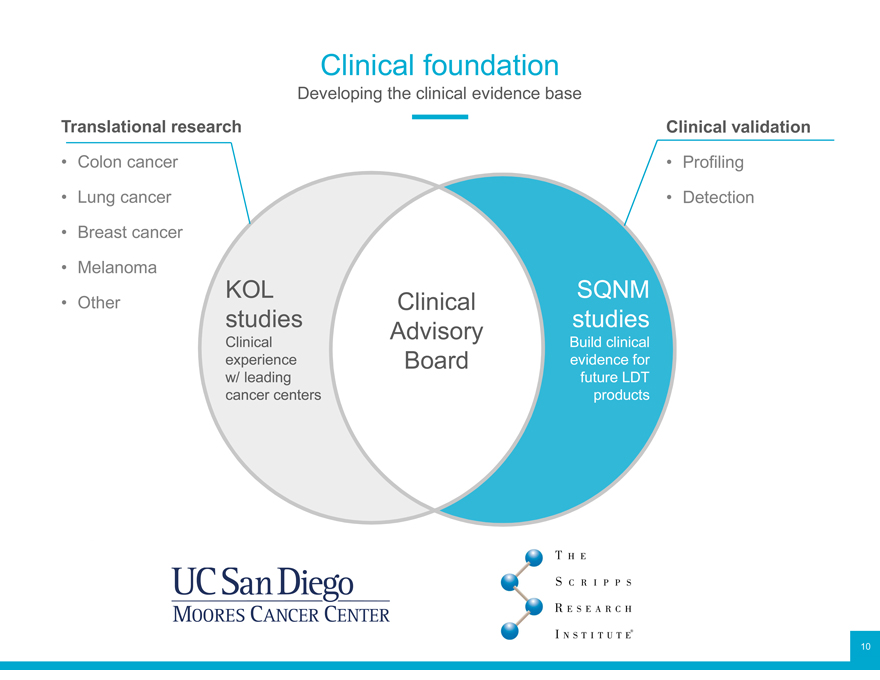
Clinical foundation
Developing the clinical evidence base
Translational research Clinical validation
Colon cancer Profiling
Lung cancer Detection
Breast cancer
Melanoma KOL SQNM
Other studies Clinical studies
Clinical Advisory Build clinical experience Board evidence for w/ leading future LDT cancer centers products
10
|
|
Upcoming liquid biopsy RUO test
Pan-cancer profiling for actionable genomic alterations
OncoPlasma family of Sequenom ™ is poised ctDNA to tests be the first of a
Aimed at molecular profiling of later-stage solid tumors where biopsy is not feasible or cannot provide sufficient material for NGS panel testing
11
|
|
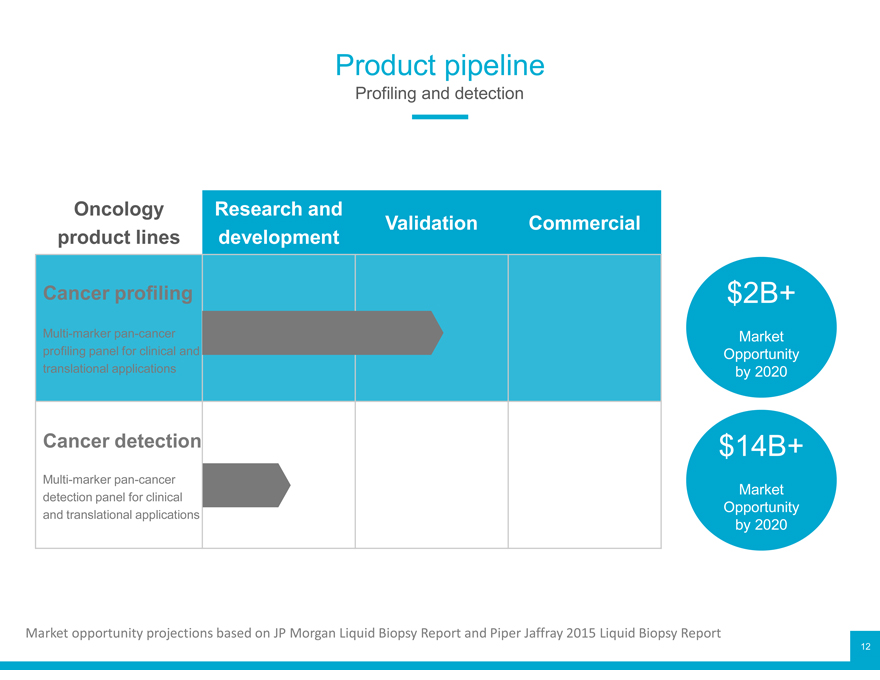
Product pipeline
Profiling and detection
Oncology Research and
product lines development Validation Commercial
Cancer profiling $2B+
Multi-marker pan-cancer Market
Opportunity
translational applications by 2020
Cancer detection $14B+
Multi-marker pan-cancer Market
detection panel for clinical Opportunity
and translational applications by 2020
Market opportunity projections based on JP Morgan Liquid Biopsy Report and Piper Jaffray2015 Liquid Biopsy Report
12
|
|
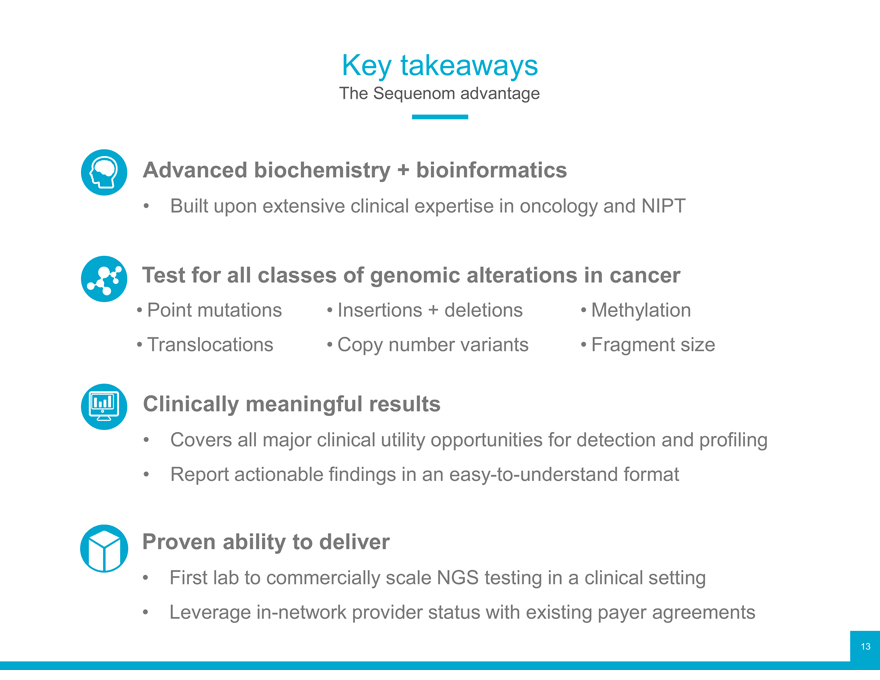
Key takeaways
The Sequenom advantage
Advanced biochemistry + bioinformatics
Built upon extensive clinical expertise in oncology and NIPT
Test for all classes of genomic alterations in cancer
Point mutations Insertions + deletions Methylation Translocations Copy number variants Fragment size
Clinically meaningful results
Covers all major clinical utility opportunities for detection and profiling
Report actionable findings in an easy-to-understand format
Proven ability to deliver
First lab to commercially scale NGS testing in a clinical setting
Leverage in-network provider status with existing payer agreements
13
|
|
Wrap-up
Dirk van den Boom, PhD Interim President and CEO Chief Scientific & Strategy Officer
1
|
|
Positioning for Growth
Positioned to capitalize on Average Risk market
MaterniT™ GENOME paves the way for comprehensive information
Opportunity for proprietary and potentially “kitable” assays in Oncology
Significant opportunity in oncology through circulating tumor DNA detection and profiling
New opportunities are leveraging established commercial infrastructure, contracts and in-network strategy
2
|
|
Dirk van den Boom, PhD Carolyn Beaver
Interim President and CEO Senior Vice President, Chief Scientific & Strategy Officer Chief Financial Officer
Mathias Ehrich, MD Rob Lozuk Daniel Grosu, MD
Senior Vice President, Senior Vice President, Senior Vice President, Research and Development Commercial Operations Chief Medical Officer
3
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Statement of Oi Creditors Regarding Fallback Bid
- UBS hires financial advisor team led by Christopher Toregas in Los Angeles
- RETRANSMISSION: ProcureAM Celebrates Earth Day with Expense Reduction on Natural Disaster Recovery ETF
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share