Form 8-K INFINITY PHARMACEUTICALS For: Jan 12
�
�
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
�
�
FORM 8-K
�
�
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): January�12, 2015
�
�
Infinity Pharmaceuticals, Inc.
(Exact name of registrant as specified in charter)
�
�
�
| Delaware | � | 000-31141 | � | 33-0655706 |
| (State or other jurisdiction of incorporation) |
� | (Commission File Number) |
� | (IRS Employer Identification No.) |
�
| 780 Memorial Drive, Cambridge, MA | � | 02139 |
| (Address of principal executive offices) | � | (Zip Code) |
Registrant�s telephone number, including area code: (617)�453-1000
�
�
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
�
| � | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
�
| � | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
�
| � | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
�
| � | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
�
�
�
Forward Looking Statements
This Form 8-K and the exhibits attached hereto contain forward-looking statements of Infinity Pharmaceuticals, Inc. (�Infinity� or the �Company�) that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this Form 8-K and the exhibit attached hereto, are forward-looking statements. The words �anticipate,� �believe,� �estimate,� �expect,� �intend,� �may,� �plan,� �predict,� �project,� �target,� �potential,� �will,� �would,� �could,� �should,� �continue,� �contemplate,� or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about: the Company�s estimate regarding its cash balances for the year ended December�31, 2014 and other expectations regarding its business, plans, prospects and strategies. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that the Company makes due to a number of important factors, including those Risk Factors discussed in Infinity�s quarterly report on Form 10-Q filed with the Securities and Exchange Commission (�SEC�) on November�10, 2014, and its other filings with the SEC. The forward-looking statements in this Form 8-K and the exhibit attached hereto represent the Company�s views as of the date of this Form 8-K. The Company anticipates that subsequent events and development will cause its views to change. However, while it may elect to update these forward-looking statements at some point in the future, it has no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing the Company�s views as of any date subsequent to the date of this Form 8-K.
�
| Item�2.02 | Results of Operations and Financial Condition. |
Although it has not finalized its full financial results for the fourth quarter and fiscal year ended December�31, 2014, the Company announced on January�12, 2015, that it expects to report that it had approximately $330 million in cash, cash equivalents and available-for-sale securities as of December�31, 2014.
The information contained in Item�2.02 of this Form�8-K is unaudited and preliminary, and does not present all information necessary for an understanding of the Company�s financial condition as of December�31, 2014 and its results of operations for the three months and year ended December�31, 2014. The audit of the Company�s consolidated financial statements for the year ended December�31, 2014 is ongoing and could result in changes to the information set forth above.
The information in this Item�2.02 shall not be deemed �filed� for purposes of Section�18 of the Securities Exchange Act of 1934, as amended (the �Exchange Act�) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
�
| Item�7.01 | Regulation FD Disclosure. |
From time to time, the Company intends to conduct meetings with third parties in which its current corporate slide presentation is presented. A copy of this slide presentation, dated January�12, 2015, is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information responsive to Item�7.01 of this Form 8-K and Exhibit 99.1 hereto shall not be deemed �filed� for purposes of Section�18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such a filing.
�
| Item�8.01 | Other Events. |
On January�12, 2015, the Company issued a press release announcing its 2015 business goals and financial guidance. The full text of this press release is filed as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
| Item�9.01 | Financial Statements and Exhibits. |
�
| � | (d) | The following exhibits are included in this report: |
�
| Exhibit No. |
�� | Description |
| 99.1 | �� | Presentation dated January�12, 2015 |
| 99.2 | �� | Press Release dated January�12, 2015 |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
�
| � | � | INFINITY PHARMACEUTICALS, INC. | ||||
| Date: January�12, 2015 | � | � | By: | � | /s/����Lawrence E. Bloch, MD, JD�������� | |
| � | � | � | Lawrence E. Bloch, MD, JD | |||
| � | � | � | EVP, Chief Financial Officer and Chief Business Officer | |||
| Exhibit 99.1 �
|
�
Building a Fully Integrated
Biopharmaceutical Company
January�12, 2015
|
|
�
Forward-Looking Statements
This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Such forward-looking statements include those regarding the Company�s expectations about: its plans to complete patient enrollment in DYNAMO in the first half of 2015 and in DUO in the second half of 2015; its plans to report topline data from DYNAMO in the second half of 2015; its plans to initiate additional studies of duvelisib in 2015; its expectations regarding further development of duvelisib or IPI-443 in RA; its ability to execute on its strategic plans; the potential complementary effects of inhibiting PI3K-delta and PI3K-gamma; the therapeutic potential of PI3K inhibition and duvelisib; and its fiscal year 2014 year-end cash balance and 2015 financial guidance. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the company�s current expectations. For example, there can be no guarantee that Infinity will report data in the time frames it has estimated, that any product candidate Infinity is developing will successfully complete necessary preclinical and clinical development phases, or that development of any of Infinity�s product candidates will continue. Further, there can be no guarantee that Infinity�s strategic collaboration with AbbVie will continue or that any positive developments in Infinity�s product portfolio will result in stock price appreciation. Management�s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: Infinity�s results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; a failure of Infinity and/or AbbVie to fully perform under the strategic collaboration and/or an early termination of the collaboration and license agreement; the content and timing of decisions made by the U.S. FDA and other regulatory authorities, investigational review boards at clinical trial sites and publication review bodies; Infinity�s ability to obtain and maintain requisite regulatory approvals and to enroll patients in its clinical trials; unplanned cash requirements and expenditures; development of agents by Infinity�s competitors for diseases in which Infinity is currently developing or intends to develop its product candidates; and Infinity�s ability to obtain, maintain and enforce patent and other intellectual property protection for any product candidates it is developing. These and other risks which may impact management�s expectations are described in greater detail under the caption �Risk Factors� included in Infinity�s quarterly report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on November�10, 2014, and other filings filed by Infinity with the SEC. Any forward-looking statements contained in this press release speak only as of the date hereof, and Infinity expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
Infinity�s website is http://www.infi.com. Infinity regularly uses its website to post information regarding its business, product development programs and governance. Infinity encourages investors to use www.infi.com, particularly the information in the section entitled �Investors/Media,� as a source of information about Infinity. References to www.infi.com in this presentation are not intended to, nor shall they be deemed to, incorporate information on www.infi.com into this presentation by reference.
2
|
|
�
|
|
�
|
|
�
|
|
�
|
|
�
|
|
�
|
|
�
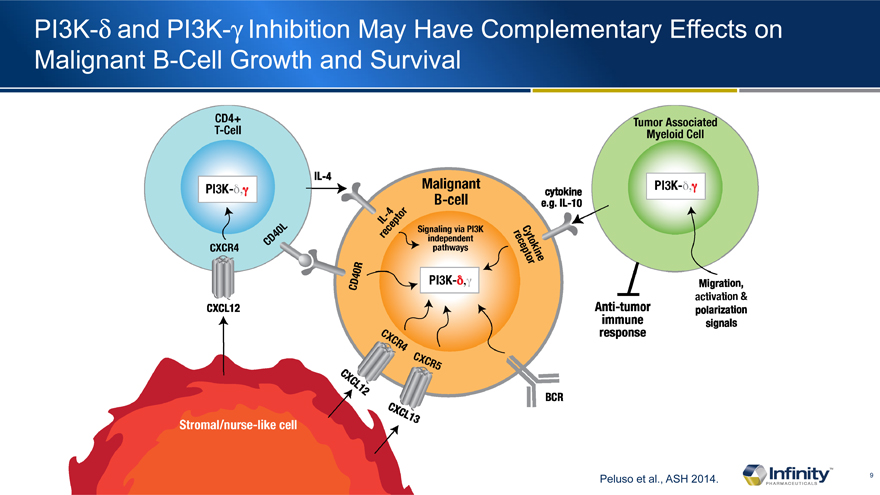
PI3K-d and PI3K-g Inhibition May Have Complementary Effects on Malignant B-Cell Growth and Survival
Peluso et al., ASH 2014.
9
|
|
�
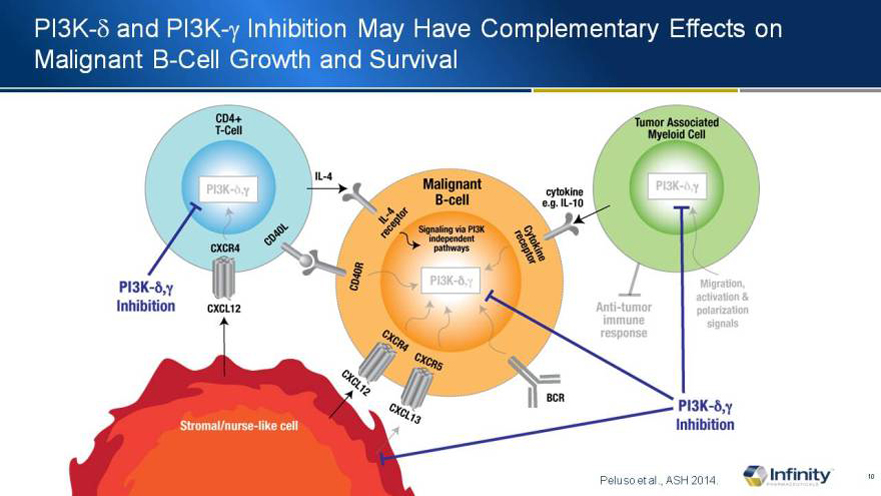
PI3K-d and PI3K-g Inhibition May Have Complementary Effects on Malignant B-Cell Growth and Survival
Peluso et al., ASH 2014.
10
|
|
�
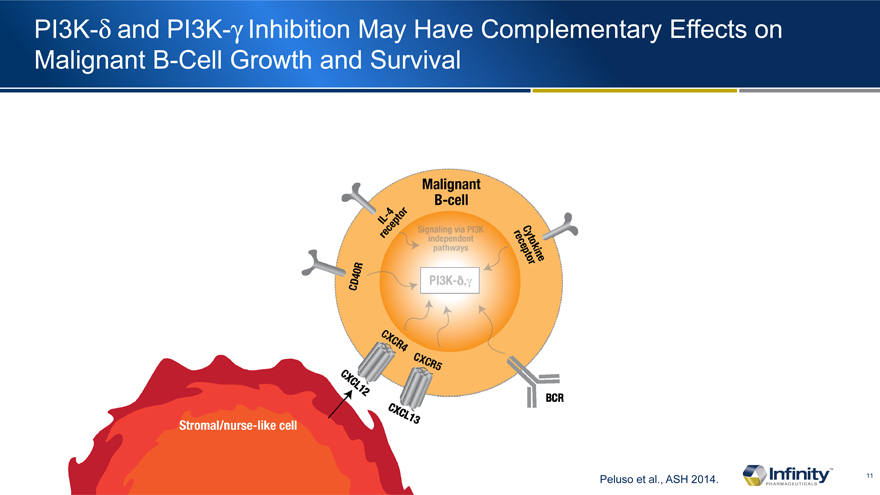
PI3K-d and PI3K-g Inhibition May Have Complementary Effects on Malignant B-Cell Growth and Survival
Peluso et al., ASH 2014.
11
|
|
�
Supported by R/R iNHL Phase 1 data
72% response overall rate (ORR) response 33% complete (CR)
Response rate data for 25 mg BID dose (n=18); Flinn et al. ASH 2014.
|
|
�
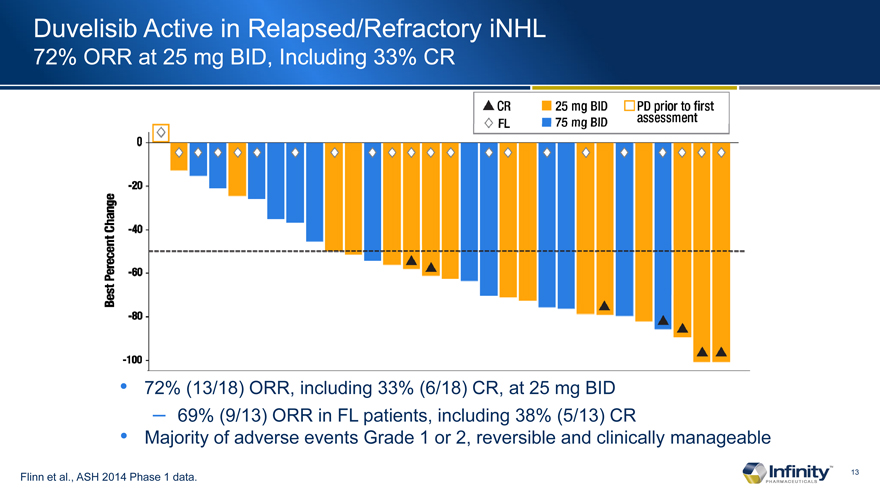
Duvelisib Active in Relapsed/Refractory iNHL
72% ORR at 25 mg BID, Including 33% CR
72% (13/18)�ORR, including 33% (6/18)�CR, at 25 mg BID
� 69% (9/13)�ORR in FL patients, including 38% (5/13)�CR
Majority of adverse events Grade 1 or 2, reversible and clinically manageable
Flinn et al., ASH 2014 Phase 1 data.
13
|
|
�
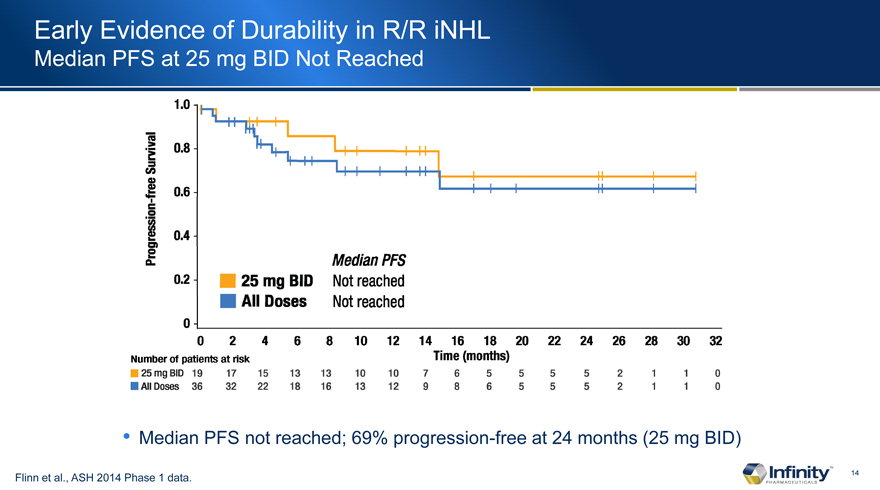
Early Evidence of Durability in R/R iNHL
Median PFS at 25 mg BID Not Reached
Median PFS not reached; 69% progression-free at 24 months (25 mg BID)
Flinn et al., ASH 2014 Phase 1 data.
14
|
|
�
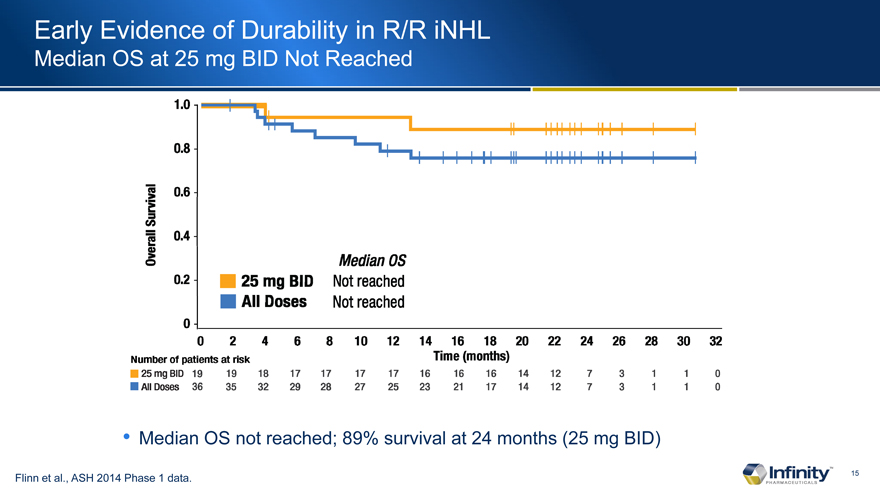
Early Evidence of Durability in R/R iNHL
Median OS at 25 mg BID Not Reached
Median OS not reached; 89% survival at 24 months (25 mg BID)
Flinn et al., ASH 2014 Phase 1 data.
15
|
|
�
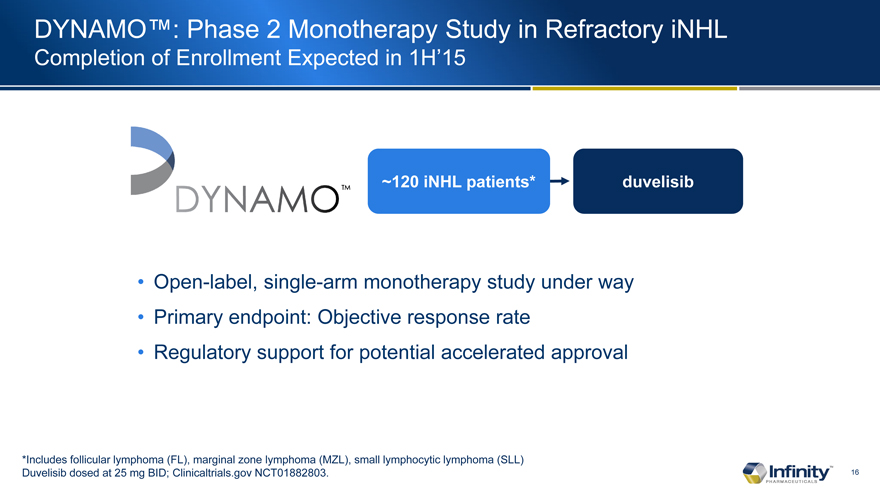
DYNAMO�: Phase 2 Monotherapy Study in Refractory iNHL
Completion of Enrollment Expected in 1H�15
Open-label, single-arm monotherapy study under way
Primary endpoint: Objective response rate
Regulatory support for potential accelerated approval
*Includes follicular lymphoma (FL), marginal zone lymphoma (MZL), small lymphocytic lymphoma (SLL) Duvelisib dosed at 25 mg BID; Clinicaltrials.gov NCT01882803.
16
|
|
�
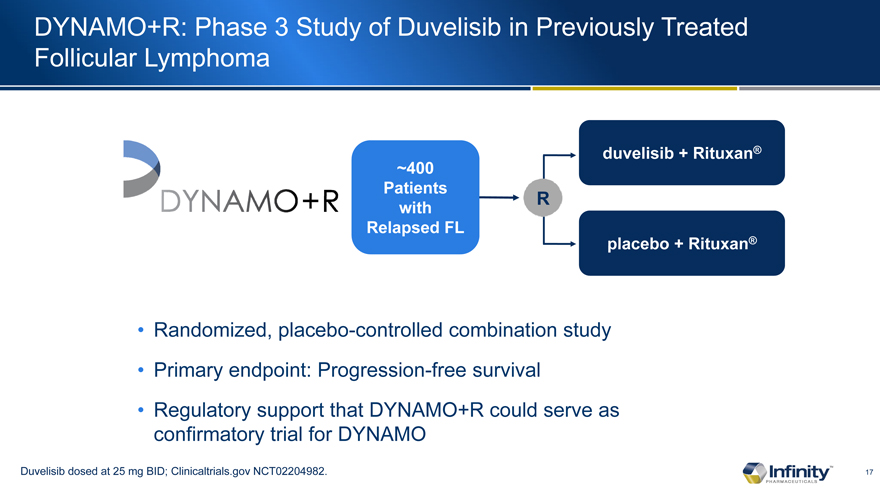
DYNAMO+R: Phase 3 Study of Duvelisib in Previously Treated Follicular Lymphoma
~400 Patients with Relapsed FL
Randomized, placebo-controlled combination study
Primary endpoint: Progression-free survival
Regulatory support that DYNAMO+R could serve as confirmatory trial for DYNAMO
Duvelisib dosed at 25 mg BID; Clinicaltrials.gov NCT02204982.
duvelisib + Rituxan�
placebo + Rituxan�
17
|
|
�
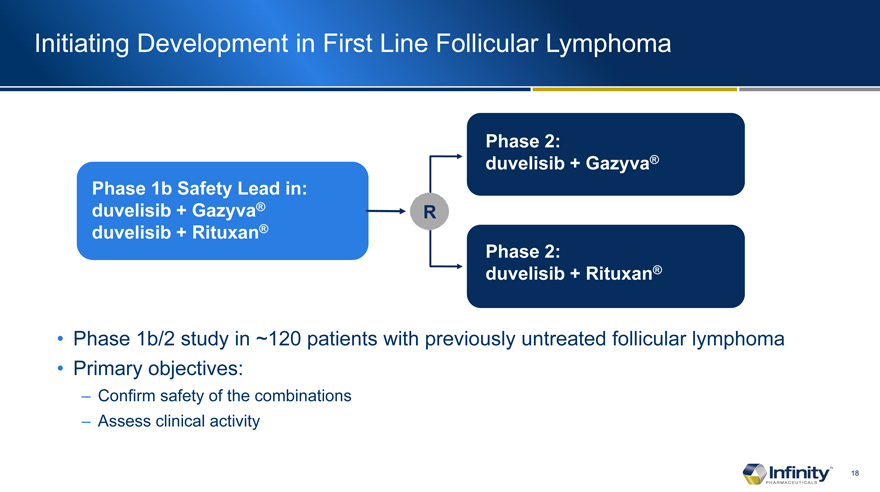
Initiating Development in First Line Follicular Lymphoma
Phase 1b Safety Lead in: duvelisib + Gazyva� duvelisib + Rituxan�
Phase 1b/2 study in ~120 patients with previously untreated follicular lymphoma
Primary objectives:
� Confirm safety of the combinations
� Assess clinical activity
Phase 2: duvelisib + Gazyva�
Phase 2: duvelisib + Rituxan�
18
|
|
�
Supported by R/R CLL Phase 1 data
response 83% nodal
57% (IWCLL), ORR including 1 CR
Response rate data for 25 mg BID dose (n=30); O�Brien et al. ASH 2014.
|
|
�
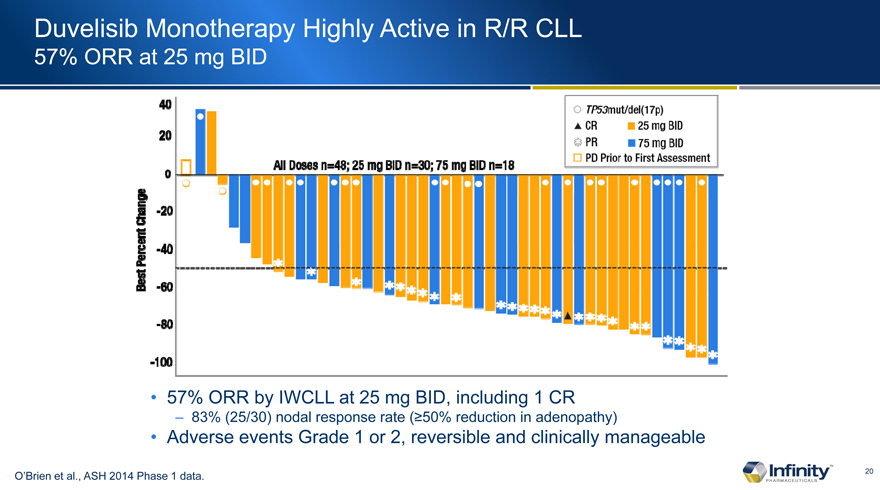
Duvelisib Monotherapy Highly Active in R/R CLL
57% ORR at 25 mg BID
57% ORR by IWCLL at 25 mg BID, including 1 CR
� 83% (25/30)�nodal response rate (�50% reduction in adenopathy)
Adverse events Grade 1 or 2, reversible and clinically manageable
O�Brien et al., ASH 2014 Phase 1 data.
20
|
|
�
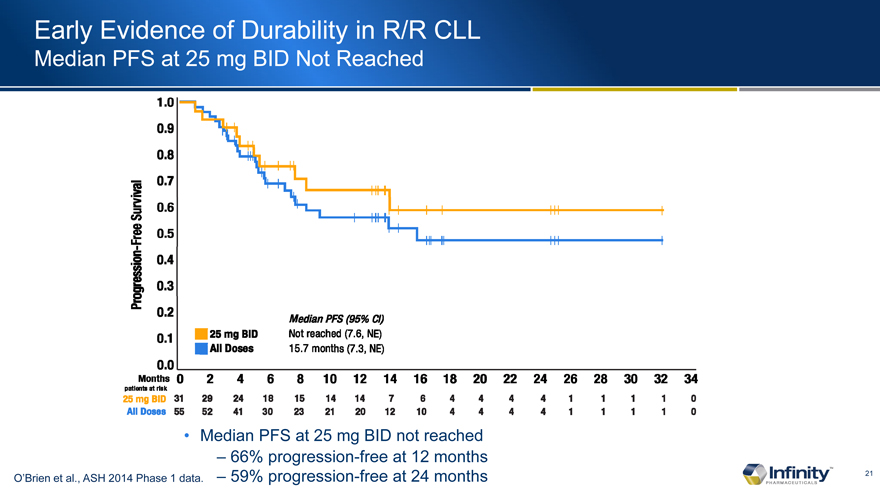
Early Evidence of Durability in R/R CLL
Median PFS at 25 mg BID Not Reached
Median PFS at 25 mg BID not reached
� 66% progression-free at 12 months
O�Brien et al., ASH 2014 Phase 1 data. � 59% progression-free at 24 months
21
|
|
�
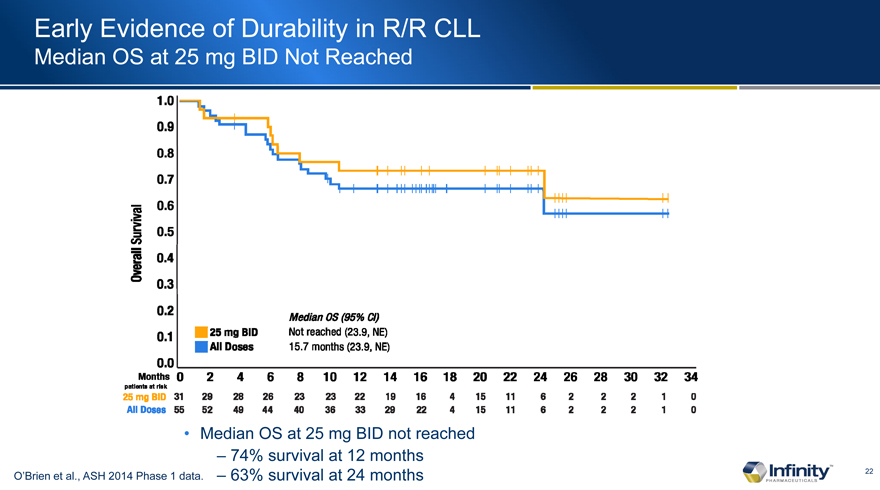
Early Evidence of Durability in R/R CLL
Median OS at 25 mg BID Not Reached
Median OS at 25 mg BID not reached
� 74% survival at 12 months
O�Brien et al., ASH 2014 Phase 1 data. � 63% survival at 24 months
22
|
|
�
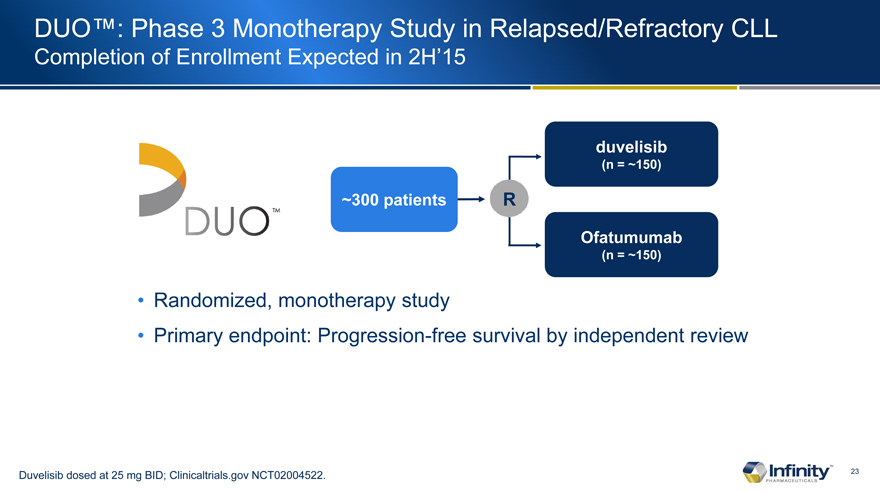
DUO�: Phase 3 Monotherapy Study in Relapsed/Refractory CLL
Completion of Enrollment Expected in 2H�15
~300 patients
duvelisib
(n = ~150)
Ofatumumab
(n = ~150)
Randomized, monotherapy study
Primary endpoint: Progression-free survival by independent review
Duvelisib dosed at 25 mg BID; Clinicaltrials.gov NCT02004522.
23
|
|
�
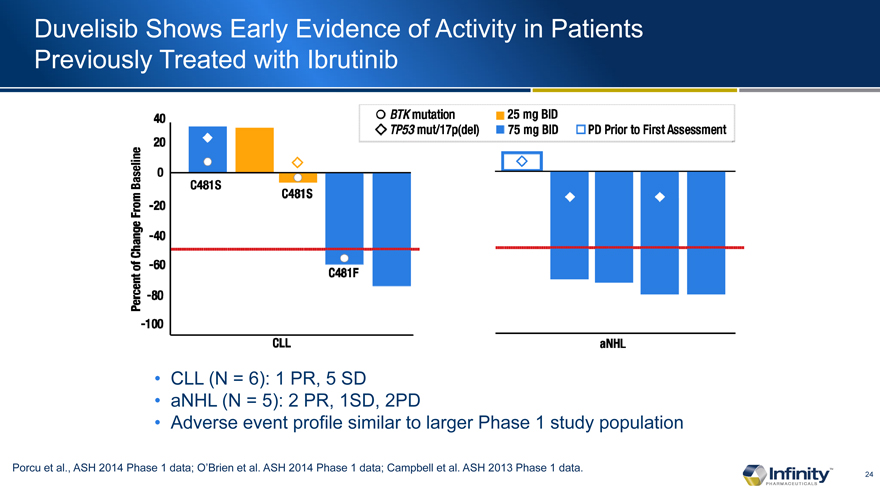
Duvelisib Shows Early Evidence of Activity in Patients Previously Treated with Ibrutinib
CLL (N = 6): 1 PR, 5 SD
aNHL (N = 5): 2 PR, 1SD, 2PD
Adverse event profile similar to larger Phase 1 study population
Porcu et al., ASH 2014 Phase 1 data; O�Brien et al. ASH 2014 Phase 1 data; Campbell et al. ASH 2013 Phase 1 data.
24
|
|
�
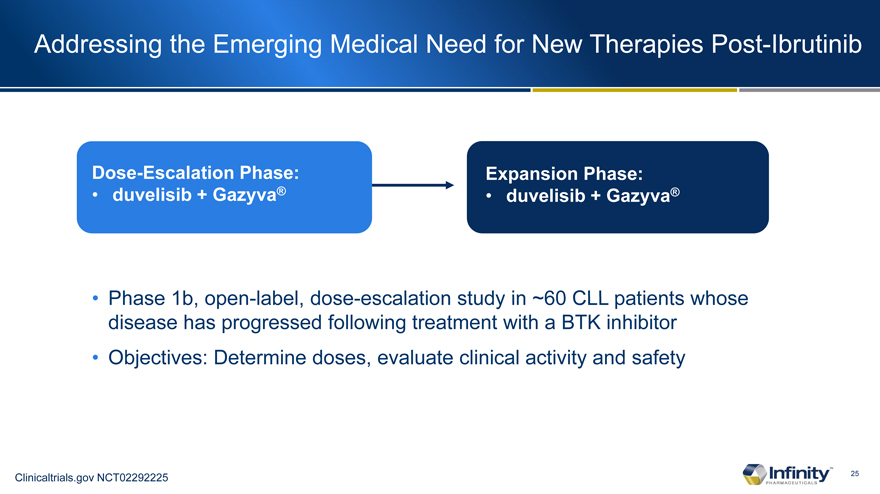
Addressing the Emerging Medical Need for New Therapies Post-Ibrutinib
Dose-Escalation Phase: � duvelisib + Gazyva�
Expansion Phase: � duvelisib + Gazyva�
Phase 1b, open-label, dose-escalation study in ~60 CLL patients whose disease has progressed following treatment with a BTK inhibitor
Objectives: Determine doses, evaluate clinical activity and safety
Clinicaltrials.gov NCT02292225
25
|
|
�
|
|
�
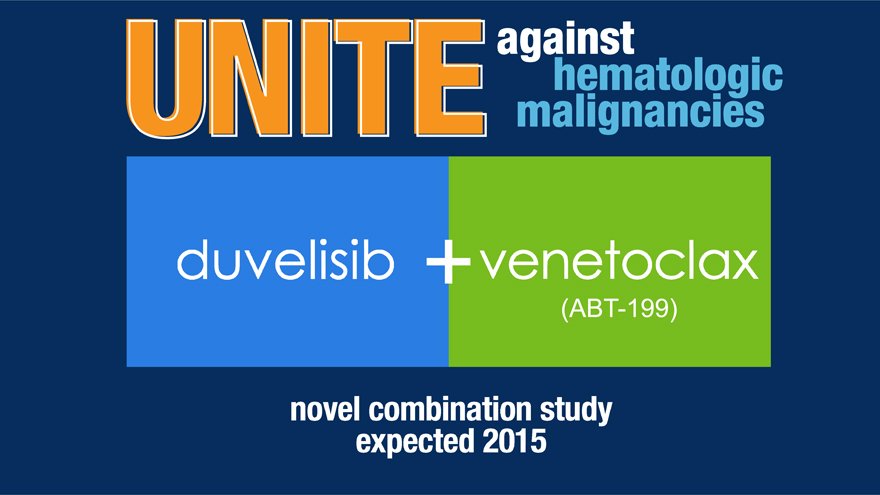
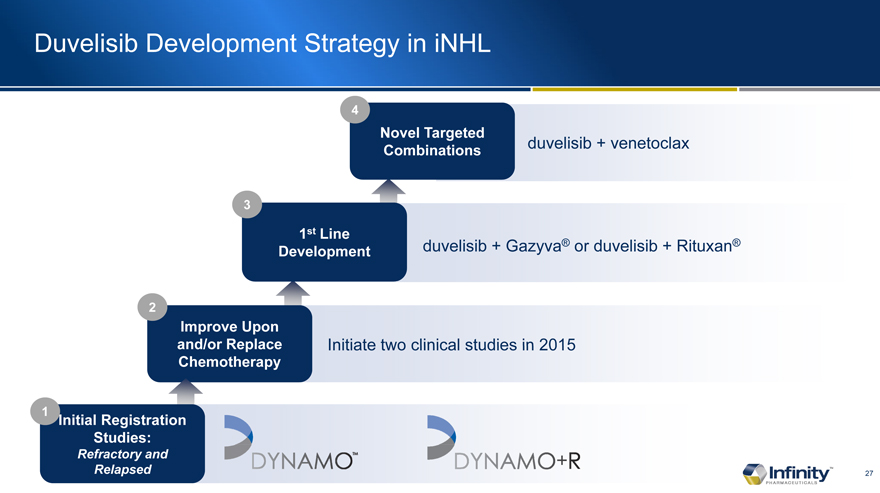
Duvelisib Development Strategy in iNHL
duvelisib + Gazyva� or duvelisib + Rituxan�
duvelisib + venetoclax
Initiate two clinical studies in 2015
27
|
|
�
Novel Ta Combinations
1st Line Development
Improve Upon and/or Replace Chemotherapy
Initial Registration Studies:
Refractory and Relapsed
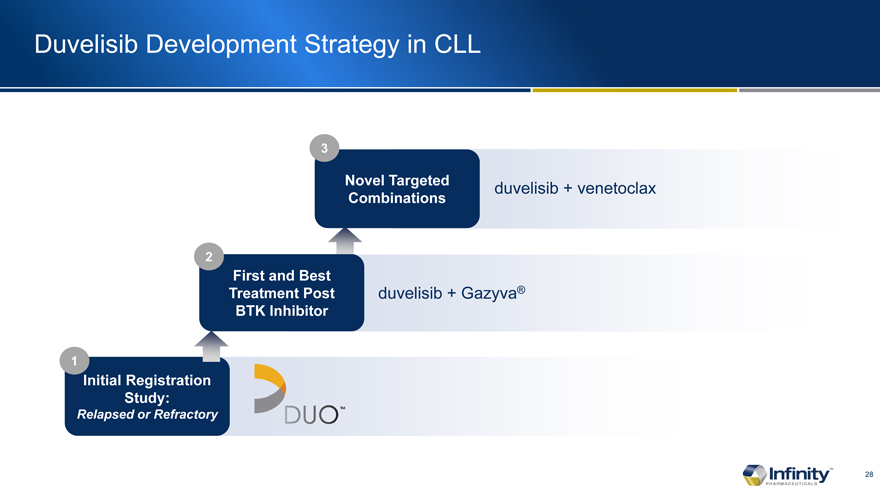
Duvelisib Development Strategy in CLL
Novel Targeted Combinations
First and Treatment BTK
Initial Registration Study:
Relapsed or Refractory
duvelisib + venetoclax
28
|
|
�
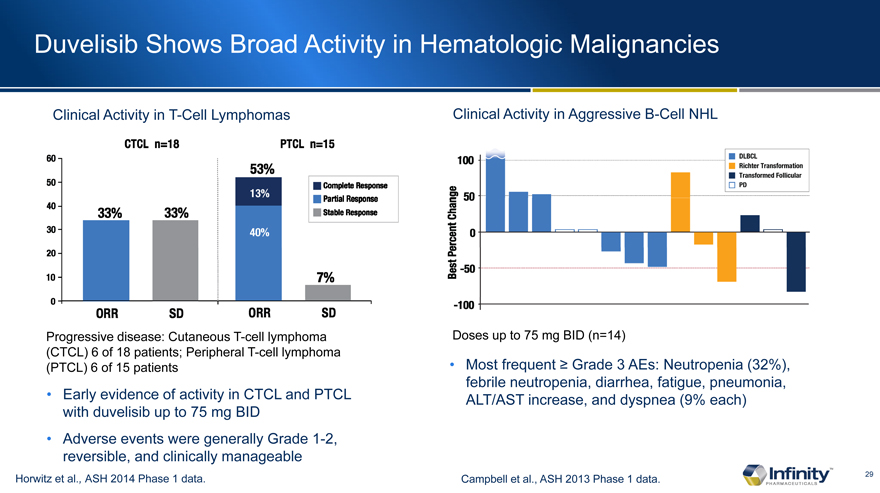
Duvelisib Shows Broad Activity in Hematologic Malignancies
Clinical Activity in T-Cell Lymphomas
Progressive disease: Cutaneous T-cell lymphoma (CTCL) 6 of 18 patients; Peripheral T-cell lymphoma (PTCL) 6 of 15 patients
Early evidence of activity in CTCL and PTCL with duvelisib up to 75 mg BID
Adverse events were generally Grade 1-2, reversible, and clinically manageable
Horwitz et al., ASH 2014 Phase 1 data.
Clinical Activity in Aggressive B-Cell NHL
Doses up to 75 mg BID (n=14)
Most frequent Grade 3 AEs: Neutropenia (32%), febrile neutropenia, diarrhea, fatigue, pneumonia, ALT/AST increase, and dyspnea (9% each)
Campbell et al., ASH 2013 Phase 1 data.
29
|
|
�
|
|
�
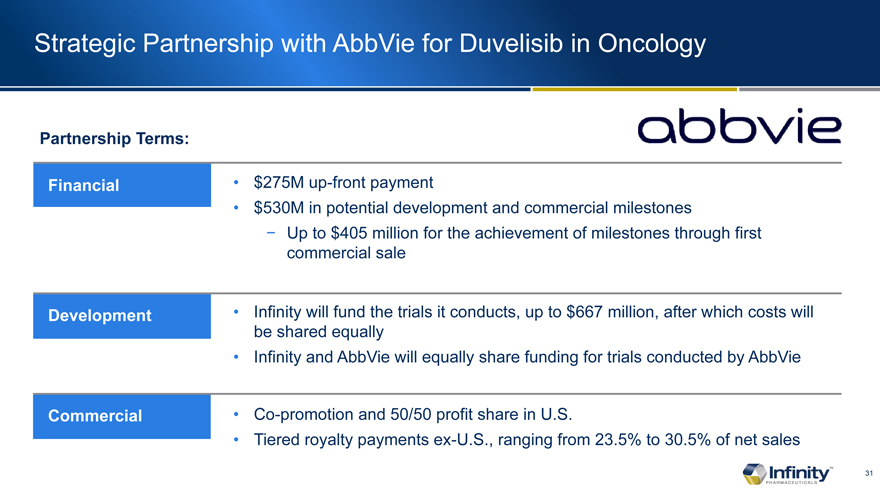
Strategic Partnership with AbbVie for Duvelisib in Oncology
Partnership Terms:
Financial
Development
Commercial
$275M up-front payment
$530M in potential development and commercial milestones
Up to $405 million for the achievement of milestones through first commercial sale
Infinity will fund the trials it conducts, up to $667 million, after which costs will be shared equally
Infinity and AbbVie will equally share funding for trials conducted by AbbVie
Co-promotion and 50/50 profit share in U.S.
Tiered royalty payments ex-U.S., ranging from 23.5% to 30.5% of net sales
31
|
|
�
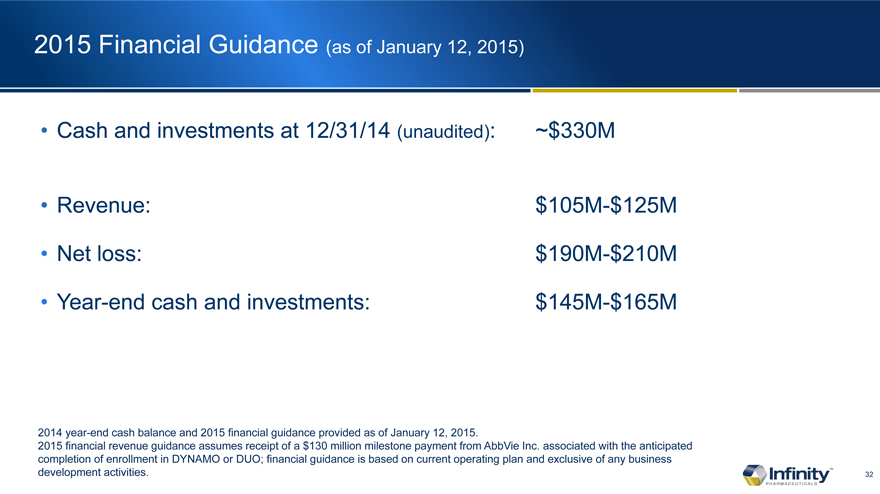
2015 Financial Guidance (as of January�12, 2015)
Cash and investments at 12/31/14 (unaudited):
Revenue:
Net loss:
Year-end cash and investments:
2014 year-end cash balance and 2015 financial guidance provided as of January�12, 2015.
2015 financial revenue guidance assumes receipt of a $130 million milestone payment from AbbVie Inc. associated with the anticipated completion of enrollment in DYNAMO or DUO; financial guidance is based on current operating plan and exclusive of any business development activities.
~$330M
$105M-$125M $190M-$210M $145M-$165M
32
|
|
�
Anticipated 2015 Milestones
Novel Duvelisib Combinations
Initiate first clinical study of duvelisib in combination with venetoclax*
Duvelisib in iNHL
Complete enrollment in DYNAMOTM in 1H�15 Report topline data from DYNAMO in 2H�15 Initiate 2 additional clinical studies
Duvelisib in CLL
Complete enrollment in DUOTM in 2H�15
*This study will be initiated by AbbVie.
33
|
|
�
Building a Fully Integrated Biopharmaceutical Company
www.infi.com
Exhibit 99.2
�

Contact:
Infinity Pharmaceuticals, Inc.
Jaren Irene Madden, 617-286-6264 (mobile)
http://www.infi.com
INFINITY PROVIDES KEY 2015 GOALS AND FINANCIAL GUIDANCE
� Completion of Patient Enrollment in DYNAMOTM and DUOTM Expected in 2015 �
� Topline Data from DYNAMO Anticipated in the Second Half of 2015 �
� Three Clinical Studies of Duvelisib Expected to Begin in 2015 �
� Company to Present at JPMorgan 33rd Annual Healthcare Conference Today at 11:30 a.m. PT �
San Francisco, Cal. � January�12, 2015 � Infinity Pharmaceuticals, Inc. (NASDAQ: INFI) today announced its anticipated pipeline goals and provided financial guidance for 2015. Infinity anticipates that it will report top line data from DYNAMOTM, a Phase 2 study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma (iNHL), in the second half of 2015 following the completion of patient enrollment in the first half of the year. Infinity also expects to complete enrollment of DUOTM, a Phase 3 study of duvelisib in patients with relapsed/refractory chronic lymphocytic leukemia (CLL), in the second half of 2015. During the year, Infinity expects three company-sponsored clinical studies of duvelisib to be initiated, including the first clinical study of duvelisib in combination with venetoclax (ABT-199). Duvelisib is an investigational oral inhibitor of phosphoinositide-3-kinase (PI3K)-delta and PI3K-gamma being developed jointly with AbbVie in oncology.
�In 2015, Infinity plans to report topline data from DYNAMO, our registration focused study in iNHL, which we hope will support establishing duvelisib as the first-in-class PI3K-delta,gamma inhibitor for the treatment of blood cancers,� stated Adelene Q. Perkins, Infinity�s president and chief executive officer. �Based on the high level of clinical activity of duvelisib reported to date, as well as preclinical evidence suggesting that inhibition of PI3K-delta and PI3K-gamma may have complementary effects on B-cell growth and survival, we believe duvelisib has the potential to become the best oral therapy for the treatment of iNHL. We also plan to complete patient enrollment in DUO, our registration-focused study in CLL in the second half of 2015.�
�We are also excited to announce that, as an important component of our strategy to achieve better patient outcomes through the combination of novel therapies, the first clinical study of duvelisib in combination with venetoclax will be initiated this year. The three new trials of duvelisib that we expect to begin this year represent a further expansion of DUETTSTM, our global development program designed to evaluate the therapeutic potential of duvelisib in patients with blood cancer across multiple indications and lines of therapy,� Ms.�Perkins continued.
On January�8, 2015, Infinity announced that a Phase 2 study of duvelisib with background methotrexate in patients with moderate-to-severe rheumatoid arthritis (RA) did not meet its primary endpoint at any of the dose levels tested. Infinity will not proceed with further clinical development in RA with duvelisib or IPI-443, its second PI3K-delta,gamma inhibitor. Infinity expects that any further development of IPI-443 in other inflammatory diseases would be conducted through out-licensing or partnering efforts.
2015 Program Goals
Infinity anticipates that the following development milestones will be achieved in 2015:
Novel Duvelisib Combinations
�
| � | � | Initiate first clinical study of duvelisib in combination with venetoclax |
Duvelisib in iNHL
�
| � | � | Complete enrollment in DYNAMO during the first half of 2015 |
�
| � | � | Report topline data from DYNAMO during the second half of 2015 |
�
| � | � | Initiate two additional clinical studies |
Duvelisib in CLL
�
| � | � | Complete enrollment in DUO during the second half of 2015 |
2015 Financial Guidance
Infinity expects to report that it ended 2014 with approximately $330 million in cash and investments (unaudited) and plans to report its fourth quarter and full-year 2014 financial results in late February. The company is providing the following guidance today with respect to its 2015 financial outlook:
�
| � | � | Revenue: Infinity expects revenue for 2015 to range from $105 million to $125 million based on the anticipation of a $130 million milestone payment from AbbVie associated with the completion of enrollment of either DYNAMO or DUO in 2015. |
�
| � | � | Net Loss: Infinity expects net loss for 2015 to range from $190 million to $210 million. |
�
| � | � | Cash and Investments: Infinity expects to end 2015 with a year-end cash and investments balance ranging from $145 million to $165 million. This year-end cash and investments forecast is based on the company�s current operating plan and exclusive of any business development activities. |
Infinity to Present at J.P. Morgan 33rd Annual Healthcare Conference
Infinity will present at the J.P. Morgan 33rd Annual Healthcare Conference in San Francisco on Monday, January�12, 2015, at 11:30�a.m. PT (2:30 p.m. ET). A live webcast of Infinity�s presentation will be accessible on the �investors/media� section of the company�s website, www.infi.com. The presentation will be archived for 30 days following the event. Members of Infinity�s management team will also participate in a breakout question and answer session following the presentation. This breakout session will be webcast live but will not be archived.
About Duvelisib
Duvelisib is an investigational inhibitor of Class I phosophoinositide-3-kinase (PI3K)-delta and PI3K-gamma that is being jointly developed by Infinity Pharmaceuticals, Inc. and AbbVie Inc. The PI3K pathway is known to play a critical role in regulating the growth and survival of certain types of blood cancers. Duvelisib is designed to block the growth and survival of tumor cells by inhibiting PI3K-delta and PI3K-gamma signaling. The investigational agent is being evaluated in registration-focused studies, including DYNAMOTM, a Phase 2 study in patients with refractory indolent non-Hodgkin lymphoma, DYNAMO+R, a Phase 3 study in patients with previously treated follicular lymphoma, and DUOTM, a Phase 3 study in patients with relapsed/refractory chronic lymphocytic leukemia. Duvelisib is an investigational compound and its safety and efficacy have not been evaluated by the U.S. Food and Drug Administration or any other health authority.
About Infinity Pharmaceuticals, Inc.
Infinity is an innovative biopharmaceutical company dedicated to discovering, developing and delivering best-in-class medicines to people with difficult-to-treat diseases. Infinity combines proven scientific expertise with a passion for developing novel small molecule drugs that target emerging disease pathways. For more information on Infinity, please refer to the company�s website at www.infi.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Such forward-looking statements include those regarding the Company�s expectations about: its plans to complete patient enrollment in DYNAMO in the first half of 2015 and in DUO in the second half of 2015; its plans to report topline data from DYNAMO in the second half of 2015; its plans to initiate additional studies of duvelisib in 2015; its expectations regarding further development of duvelisib or IPI-443 in RA; its ability to execute on its strategic plans; the potential complementary effects of PI3K-delta and PI3K-gamma; the therapeutic potential of PI3K inhibition and duvelisib; and its fiscal year 2014 year-end cash balance and 2015 financial guidance. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the company�s current expectations. For example, there can be no guarantee that Infinity will report data in the time frames it has estimated, that any product candidate Infinity is developing will successfully complete necessary preclinical and clinical development phases, or that development of any of Infinity�s product candidates will continue. Further, there can be no guarantee that Infinity�s strategic collaboration with�AbbVie�will continue or that any positive developments in Infinity�s product portfolio will result in stock price appreciation. Management�s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: Infinity�s results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; a failure of Infinity and/or�AbbVie�to fully perform under the strategic collaboration and/or an early termination of the collaboration and license agreement; the content and timing of decisions made by the U.S.�FDA�and other regulatory authorities, investigational review boards at clinical trial sites and publication review bodies; Infinity�s ability to obtain and maintain requisite regulatory approvals and to enroll patients in its clinical trials; unplanned cash requirements and expenditures; development of agents by Infinity�s competitors for diseases in which Infinity is currently developing or intends to develop its product candidates; and Infinity�s ability to obtain, maintain and enforce patent and other intellectual property protection for any product candidates it is developing.�These and other risks which may impact management�s expectations are described in greater detail under the caption �Risk Factors� included in Infinity�s quarterly report on Form 10-Q filed with the�Securities and Exchange Commission (SEC) on�November 10, 2014, and other filings filed by Infinity with the�SEC. Any forward-looking statements contained in this press release speak only as of the date hereof, and Infinity expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
###
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Reticulate Micro Unveils VALOR™ ESA Product Line
- Applied DNA Awarded Contract by HDT Bio For Rapid Vaccine Development Program
- AM Best Revises Issuer Credit Rating Outlook to Positive for Shinkong Insurance Company Limited
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share