Form 424B4 Audentes Therapeutics,
Table of Contents
Filed Pursuant to Rule 424(b)(4)
Registration No. 333-219797
The information in this preliminary prospectus supplement is not complete and may be changed. This preliminary prospectus supplement and the accompanying prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion
Preliminary Prospectus Supplement dated January 23, 2018
PROSPECTUS SUPPLEMENT
(To prospectus dated August 23, 2017)
$150,000,000

Common Stock
We are offering shares of our common stock.
Our common stock is listed on The Nasdaq Global Market under the symbol “BOLD.” The last reported sale price of our common stock on The Nasdaq Global Market on January 22, 2018 was $37.31 per share. We are an “emerging growth company” as defined under the federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements.
Investing in the common stock involves risks that are described in the section entitled “Risk Factors” beginning on page S-15 of this prospectus supplement and in the documents incorporated by reference into this prospectus supplement before investing in our securities.
| Per Share |
Total | |||
| Public offering price |
$ | $ | ||
| Underwriting discounts and commissions (1) |
$ | $ | ||
| Proceeds, before expenses, to us |
$ | $ |
| (1) | See the section entitled “Underwriting” for a description of the compensation payable to the underwriters. |
The underwriters may also exercise their option to purchase up to an additional $22,500,000 of shares of our common stock from us, at the public offering price, less the underwriting discounts and commissions, for 30 days after the date of this prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The shares will be ready for delivery on or about , 2018.
Joint Book-Running Managers
| BofA Merrill Lynch | Cowen | Leerink Partners |
Co-Manager
Wedbush PacGrow
The date of this prospectus supplement is , 2018
Table of Contents
Prospectus Supplement
| Page |
||||
| S-1 | ||||
| S-2 | ||||
| S-15 | ||||
| S-18 | ||||
| S-19 | ||||
| S-20 | ||||
| S-21 | ||||
| Material U.S. Federal Income Tax Considerations for Non-U.S. Holders of Common Stock |
S-22 | |||
| S-27 | ||||
| S-34 | ||||
| S-34 | ||||
| S-34 | ||||
| S-34 | ||||
Prospectus
| Page |
||||
| ii | ||||
| 1 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| 12 | ||||
| 19 | ||||
| 21 | ||||
| 22 | ||||
| 23 | ||||
| 26 | ||||
| 26 | ||||
| 26 | ||||
| 26 | ||||
Neither we nor the underwriters have authorized anyone to provide any information or to make any representations other than those contained in this prospectus supplement, the accompanying prospectus or in any free writing prospectuses we have prepared. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference herein and therein, and in any free writing prospectus that we have authorized for use in connection with this offering is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have changed since that date. You should read this prospectus supplement, the accompanying prospectus, the documents incorporated by reference herein and therein, and any free writing prospectus that we have authorized for use in connection with this offering in their entirety before making an
S-i
Table of Contents
investment decision. You should also read and consider the information in the documents to which we have referred you in the sections of this prospectus supplement entitled “Where You Can Find Additional Information” and “Incorporation of Certain Information by Reference.”
For investors outside the United States: Neither we nor the underwriters have done anything that would permit our public offering or possession or distribution of this prospectus supplement or the accompanying prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus supplement or the accompanying prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of our common stock and the distribution of this prospectus supplement or the accompanying prospectus outside of the United States.
S-ii
Table of Contents
ABOUT THIS PROSPECTUS SUPPLEMENT
On August 8, 2017, we filed with the Securities and Exchange Commission, or SEC, a registration statement on Form S-3 (File No. 333-219797) utilizing a shelf registration process relating to the securities described in this prospectus supplement, which registration statement became effective on August 23, 2017. Under this shelf registration process, we may, from time to time, sell common stock and other securities, of which this offering is a part.
This document is in two parts. The first part is the prospectus supplement, including the documents incorporated by reference herein, which describes the specific terms of this offering and also adds to and updates the information contained in the accompanying prospectus and the documents incorporated by reference. The second part, the accompanying prospectus, including the documents incorporated by reference therein, provides more general information, some of which may not apply to this offering. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. Before you invest, you should carefully read this prospectus supplement, the accompanying prospectus and the information incorporated by reference herein and therein, as well as the additional information described in this prospectus supplement under “Where You Can Find Additional Information.” This prospectus supplement may add, update or change information contained in the accompanying prospectus. To the extent that any statement we make in this prospectus supplement is inconsistent with statements made in the accompanying prospectus or any documents incorporated by reference therein, the statements made in this prospectus supplement will be deemed to modify or supersede those made in the accompanying prospectus and such documents incorporated by reference therein.
We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the offering of the common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement must inform themselves about, and observe any restrictions relating to, the offering of the common stock and the distribution of this prospectus supplement outside the United States. This prospectus supplement does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
This prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus supplement or the accompanying prospectus are the property of their respective owners.
Unless the context otherwise indicates, references in this prospectus supplement to “Audentes Therapeutics”, “we”, “our”, “us” and “the Company” refer, collectively, to Audentes Therapeutics, Inc., a Delaware corporation.
We have registered the trademarks “Audentes,” “Audentes Therapeutics” and “Courageous Patients. Bold Effort.” in the European Union and we have trademark applications for each of these trademarks pending with the U.S. Patent and Trademark Office. The Audentes logo and all product names are our common law trademarks. All other service marks, trademarks and tradenames appearing in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names referred to in this prospectus appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames.
S-1
Table of Contents
This summary highlights information about us, this offering and information appearing elsewhere in this prospectus supplement, in the accompanying prospectus and in the documents incorporated by reference herein and therein and does not contain all of the information you should consider in making your investment decision. Before deciding to invest in shares of our common stock, you should read this summary together with the more detailed information, including our consolidated financial statements and the accompanying notes, which are incorporated by reference into this prospectus supplement. You should carefully consider, among other things, the matters discussed in the sections entitled “Risk Factors,” “Selected Consolidated Financial Data,” our consolidated financial statements and the accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” incorporated by reference into this prospectus supplement. Some of the statements in this prospectus supplement and the accompanying prospectus constitute forward-looking statements that involve risks and uncertainties. See “Special Note Regarding Forward-Looking Statements.”
Audentes Therapeutics, Inc.
Overview
We are a clinical stage biotechnology company focused on developing and commercializing gene therapy products for patients living with serious, life-threatening rare diseases caused by single gene defects. We believe that gene therapy has powerful potential to treat these diseases through delivery of a functional copy of the gene to affected cells, resulting in production of the normal protein. We have built a compelling portfolio of product candidates, including AT132 for the treatment of X-Linked Myotubular Myopathy, or XLMTM, AT342 for the treatment of Crigler-Najjar Syndrome, or Crigler-Najjar, AT982 for the treatment of Pompe disease and AT307 for the treatment of the CASQ2 subtype of Catecholaminergic Polymorphic Ventricular Tachycardia, or CASQ2-CPVT. We have initiated a Phase 1/2 clinical study of AT132 and plan to dose the first patient in a Phase 1/2 clinical study of AT342 in the first quarter of 2018. We are conducting IND-enabling preclinical studies for the systemic administration of AT307 for the treatment of CASQ2-CPVT, for which we plan to file an IND in the first quarter of 2018, and AT982 for the treatment of Pompe disease, for which we plan to file an IND in the second quarter of 2018. We maintain full global rights to all our product candidates.
We have developed a proprietary in-house cGMP manufacturing capability that we believe provides us with a core strategic advantage, enabling superior control over development timelines, costs and intellectual property. Our manufacturing facility is located in South San Francisco in a building that we have improved to support our research, process development and manufacturing capabilities in accordance with current Good Manufacturing Practices, or cGMP, requirements. We have established a comprehensive platform for production of our adeno-associated virus vector, or AAV, product candidates and plan continued investment to further optimize our manufacturing capabilities to cost-effectively produce high-quality AAV vectors at both clinical and commercial scale.
Our vision is to become a fully integrated biotechnology company. In pursuit of this goal, we are executing on our core strategic initiatives, which include the advancement of our current product candidates, the continued development of our proprietary in-house manufacturing capabilities, and the expansion of our pipeline. We have assembled a world-class team with expertise in gene therapy, rare disease drug development and commercialization, and biologics manufacturing.
Our mission is to dramatically and positively transform the lives of patients suffering from serious, life- threatening rare diseases with limited or no treatment options. For example, we are developing AT132 to treat XLMTM, a disease for which there are no approved therapies and from which approximately 50% of affected
S-2
Table of Contents
children die in the first 18 months of life. We believe our product candidates have the potential to provide long-lasting benefits, changing the lives of patients with these devastating diseases. Given the available clinical and regulatory pathways, we believe that the rarity and severity of the diseases we target may provide advantages for drug development, including the potential for expedited development and regulatory review, and market exclusivity.
We focus on the treatment of rare diseases caused by single gene, or monogenic, defects in DNA that we believe can be effectively addressed using gene therapy. Conventional approaches such as protein therapeutics attempt to replace the deficient protein, but they do not correct the underlying genetic defect causing the disease. In addition, protein therapeutics often require frequent administration by injection or infusion and often result in sub-optimal safety and efficacy. We believe gene therapy is an ideal treatment modality for diseases caused by monogenic defects. Our portfolio of product candidates employs the use of AAV, a small, non-pathogenic virus that is genetically engineered to function as a delivery vehicle, or vector, and is administered to a patient to introduce a healthy copy of a mutated gene to the body. AAV gene therapy vectors are modified such that they will not cause an infection like a normal virus, but are capable of delivering therapeutic genes into patients’ cells. Vectors derived from AAV have a well-established safety profile in humans and have been shown to effectively deliver genes to the liver, eye, muscle and brain. Preclinical and clinical data demonstrate that AAV vectors are capable of providing durable efficacy with a favorable adverse event profile due at least in part to AAV’s low immunogenic potential. AAV vectors can be described by the serotype, or strain, of the original virus isolate that was used to form the outer shell, or capsid, of the vector. We selected AAV8 and AAV9 as our in-licensed vector capsid serotypes, based on their biological properties, which we believe will translate into positive clinical effect in our target indications.
Our business model is to develop and commercialize a broad portfolio of gene therapy product candidates to treat rare diseases. We use a focused set of criteria to select product candidates that we believe have the best chance of success. These criteria include:
| • | serious, life-threatening rare diseases; |
| • | monogenic diseases with well-understood biology; |
| • | disease characteristics well-suited for treatment with AAV gene therapy technology; |
| • | high potential for meaningful clinical benefit; |
| • | compelling preclinical data; |
| • | clear measures for evaluation in clinical trials; and |
| • | opportunities for expedited development through established regulatory pathways. |
S-3
Table of Contents
We have built a portfolio of gene therapy product candidates and we intend to further expand our portfolio over time. Set forth below is a table summarizing our development programs.
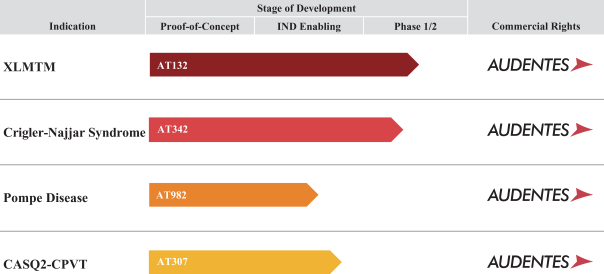
AT132. XLMTM is characterized by extreme muscle weakness, respiratory failure and early death with an estimated 50% mortality rate in the first 18 months of life. The disease is the result of mutations in the MTM1 gene that affect the production of myotubularin, an enzyme required for normal development and function of skeletal muscle. The incidence of XLMTM is estimated to be one in 50,000 male births. Currently, only supportive treatment options, such as ventilator use or a feeding tube, are available. We are developing AT132, an AAV8 vector containing a functional copy of the MTM1 gene, for the treatment of XLMTM. We believe AT132 may provide patients with significantly improved outcomes based on the ability of AAV8 to treat skeletal muscle. Preclinical study results in both canine and murine models of the disease demonstrated dramatic improvements in all outcomes, including histology, muscle strength, respiratory function and survival. Our goal is to achieve these same benefits in XLMTM patients following a single intravenous administration of AT132.
The clinical development of AT132 was initiated with RECENSUS, a retrospective medical chart review designed to characterize the disease course, natural history and unmet medical need in XLMTM. In December 2017, Audentes announced the publication of initial data from 112 boys in RECENSUS. This analysis confirmed and expanded upon the understanding of the significant disease burden of XLMTM on patients, families and the healthcare system. Audentes is also conducting INCEPTUS, a prospective natural history run-in study. The primary objectives of INCEPTUS are to characterize the clinical condition of children with XLMTM, identify subjects for potential enrollment in ASPIRO (the Phase 1/2 clinical study of AT132 in XLMTM), and serve as a longitudinal baseline and within-patient control for ASPIRO. Preliminary data reported from INCEPTUS confirm the significant neuromuscular and respiratory deficits experienced by XLMTM patients, and provide insight into the relevance and sensitivity of assessments used in ASPIRO.
ASPIRO is a multicenter, multinational, open-label, ascending dose, delayed-treatment control study to evaluate the safety and preliminary efficacy of AT132 in approximately 12 XLMTM patients less than five years of age. We initiated ASPIRO in September 2017 and reported interim data from the first dose cohort of ASPIRO patients in January 2018. The interim data includes safety and efficacy assessments comprised of three AT132-treated patients dosed at 1x1014 vector genomes (vg) per kilogram (kg), and one delayed-treatment control patient. As of December 21, 2017, individual patient follow-up ranged from 4 to 12 weeks.
S-4
Table of Contents
There have been a total of eight adverse events, or AEs, reported in ASPIRO, four of which were determined to be serious adverse events, or SAEs. All SAEs occurred in Patient 3, the first of which was a hospitalization one week post-administration due to pneumonia and was deemed not treatment-related. On January 4, 2018, we reported that Patient 3 was also hospitalized at week seven post-administration due to a gastrointestinal infection and elevated troponin level, the latter of which was deemed a probably treatment-related SAE and was responding to treatment with intravenous steroid administration and supportive care.
Subsequent to the December 21, 2017 data cutoff date, the clinical investigator responsible for managing Patient 3 determined that the adverse events that contributed to the week seven hospitalization should be recategorized as three distinct SAEs, related to each other and deemed to be probably treatment-related. These include myocarditis, elevated troponin levels and mildly elevated creatinine kinase levels, each of which have resolved. Patient 3 was clinically stable throughout the hospital admission and has now been discharged from the hospital.
Of the four non-serious AEs, two have been determined to be probably or possibly treatment-related. Patient 1 experienced a mild, clinically asymptomatic exacerbation of a pre-existing elevated bilirubin level, which was deemed possibly treatment-related and resolved with treatment. Patient 2 experienced a clinically asymptomatic elevation in liver enzyme levels toward the end of the protocol-specified prednisolone weaning period, which was deemed to be probably treatment-related and resolved by extending the duration of steroid coverage.
The key assessment of neuromuscular function in this first data set from the first dose cohort is the CHOP-INTEND scale, in which a maximal score of 64 reflects the level of neuromuscular function that a healthy baby is expected to approach by 3-6 months of age. Additional analyses to be reported based on longer term follow-up include the MFM-20 and Bayley-III™ scales of infant and toddler development (fine and gross motor function). Motor developmental milestones are captured within each of the neuromuscular assessments.
The key assessment of respiratory function in this first data set from the first dose cohort is a measurement of maximal inspiratory pressure, or MIP, for which values ³ 80 cmH20 are considered normal in healthy children less than 5 years of age. Additional analyses to be reported based on longer term follow-up include measurement of maximal expiratory pressure, or MEP, time per day on invasive ventilatory support (tracheostomy) or non-invasive respiratory support (BiPAP), and for those patients who are on 24-hour continuous ventilatory support, an assessment of ability to maintain adequate respiratory function while off a ventilator, termed “respiratory sprinting.”
Patient Interim Data Summaries:
| • | Patient 1: data set includes assessments through week 12 timepoint |
| • | Age 0.8 years (9 months) and on 12 hours of BiPAP per day at baseline |
| • | CHOP-INTEND increased from 29 at baseline to 56 at week 12 |
| • | MIP increased from 33 cmH20 at baseline to 80 cmH20 at week 12 |
| • | No age-appropriate first-year motor milestones were achieved at the baseline assessment; by week 12, Patient 1 had acquired several age appropriate skills, including the ability to control head movements, roll over by himself and sit unassisted for > 5 seconds |
| • | Patient 2: data set includes assessments through week 8 timepoint |
| • | Age 4.1 years and on 17 hours of invasive ventilation per day at baseline |
S-5
Table of Contents
| • | CHOP-INTEND increased from 45 at baseline to 56 at week 8 |
| • | MIP increased from 44 cmH20 at baseline to 77 cmH20 at week 4 |
| • | Patient 3: data set includes assessments through week 4 timepoint |
| • | Age 2.6 years and on continuous (24-hour) invasive ventilation at baseline |
| • | CHOP-INTEND did not change meaningfully from 34 at baseline to 36 at week 4 |
| • | MIP increased from 26 cmH20 at baseline to 44 cmH20 at week 4 |
| • | Patient 4 (delayed-treatment control): data set includes assessments through week 4 timepoint |
| • | Age 4.0 years and on 12 hours of BiPAP per day at baseline |
| • | CHOP-INTEND did not change meaningfully from 49 at baseline to 46 at week 4 |
| • | MIP at baseline was 58 cmH20; MIP was not assessed at week 4 per protocol |
Physicians and caregivers have reported progressive qualitative improvements in disease severity in all treated patients. Ventilator settings (pressure, rate and volume of mechanical ventilation) have been reduced in Patients 1 and 2. All treated patients have demonstrated improvements in airway clearance control, including swallowing and coughing, which is critical to preventing aspiration. By way of example, at baseline Patient 1 required suctioning of the oro-pharyngeal cavity several times per hour, and by week 12 he required no suctioning. In addition, investigators report anecdotally that all treated patients have increased limb and trunk strength, which is an early indicator of gross motor function improvement, and that the velocity and accuracy of their movements have increased. Caregivers also report that patients have increased vocalization, improving their ability to communicate.
AT132 has been granted orphan drug designation in both the United States and European Union, and the FDA has granted Rare Pediatric Disease and Fast Track designations.
AT342. Crigler-Najjar is a rare, congenital autosomal recessive monogenic disease characterized by severely high levels of bilirubin in the blood, which presents a significant risk of irreversible neurological
S-6
Table of Contents
damage and death. The average life expectancy is reported as being 30 years of age with phototherapy. Crigler-Najjar is estimated to affect approximately one in 1,000,000 newborns. Infants with Crigler-Najjar develop severe jaundice shortly after birth resulting in rapid presentation and diagnosis. Crigler-Najjar is caused by mutations in the gene encoding the UGT1A1 (uridine-diphosphate (UDP)-glucuronosyltransferase (UGT) 1A1) enzyme resulting in an inability to convert unconjugated bilirubin to a water-soluble form that can be excreted from the body. Clinical diagnosis is confirmed via genetic testing of the UGT1A1 gene. The current standard of care for Crigler-Najjar is aggressive management of high bilirubin levels with persistent, daily phototherapy, usually for longer than 10 to 12 hours per day using intense fluorescent light focused on the bare skin, while the eyes are shielded. Phototherapy speeds bilirubin decomposition and excretion and lowers serum bilirubin, but wanes in effectiveness as children age due to thickening of the skin and reduction in surface area to body mass ratio. Data from our prospective natural history study LUSTRO demonstrate that persistent phototherapy only reduces bilirubin to levels just below those that are considered to be neurotoxic. In some cases, a liver transplant may be required for survival.
We are developing AT342, an AAV8 vector containing a functional version of the UGT1A1 gene. We have conducted a dose ranging study of AT342 in a Crigler-Najjar knockout mouse model. In this study, a single tail vein injection of AT342 rapidly reduced and normalized bilirubin levels for the duration of the study, an effect that was seen across a range of doses. Previously reported results demonstrate that administration of AAV8-UGT1A1 in newborn Crigler-Najjar mice significantly and durably reduced bilirubin levels, even at UGT1A1 liver expression levels of just five to eight percent of normal. We are advancing AT342 with the goal of administering a single dose that results in a robust, durable reduction in serum bilirubin, a reduction in or elimination of lengthy daily phototherapy, and elimination of the need for a liver transplant. We believe that serum bilirubin levels will be a clinically relevant endpoint and that determination of efficacy of AT342 will be straightforward due to the ease and reliability of measurement.
The IND for AT342 is active, and we have initiated LUSTRO, a prospective natural history-run-in study designed to characterize the disease course, natural history, bilirubin variability and phototherapy usage of patients with Crigler-Najjar. In November 2017, we reported interim data from the LUSTRO study, which confirm the significant risks posed by elevated bilirubin levels in Crigler-Najjar patients and the significant burden on patients and their families that must configure their lives to ensure rigorous adherence to phototherapy of more than 10 to 12 hours per day. We plan to initiate VALENS, the Phase 1/2 clinical study of AT342, in the first quarter of 2018, and to report preliminary clinical data from VALENS in the second quarter of 2018. AT342 has been granted orphan drug designation in both the United States and European Union.
AT982. Pompe disease is a serious, progressive genetic disease characterized by severe muscle weakness, respiratory failure leading to ventilator dependence and, in infants, increased cardiac mass and heart failure. In untreated infants, the disease is often fatal due to cardio-respiratory failure within the first year of life, and in adults the disease is progressive and life-limiting with significant ventilator and wheelchair use. Pompe disease is caused by mutations in the gene encoding the lysosomal enzyme alpha-glucosidase, or GAA, which results in a deficiency of GAA protein and leads to the accumulation of glycogen. The incidence of Pompe disease is approximately one in 40,000 births. The only approved treatment for Pompe disease is enzyme replacement therapy, or ERT, which is a chronic treatment delivered in bi-weekly intravenous infusions. Despite the availability of ERT, significant medical need persists, which is primarily due to the inability of ERT to penetrate key tissues affected by the disease and the immunogenicity of ERT. We believe that gene therapy may address many of these limitations because of the ability of AAV to penetrate relevant tissues in Pompe disease and to produce GAA protein intracellularly, which we expect will result in an improved safety and efficacy profile.
Recently published studies have described new gene therapy approaches to treating Pompe disease with gene therapy vectors that target tissues beyond skeletal and cardiac muscle, which may be of relevance in the
S-7
Table of Contents
disease. While prior work conducted in collaboration with the University of Florida evaluated candidate vectors designed to target only muscle, we have recently independently initiated a comprehensive construct selection study in the Pompe mouse model, wherein we are evaluating both AAV8 and AAV9 capsid serotypes in novel candidate vectors designed to systemically target GAA expression in a range of tissues, including skeletal and cardiac muscle, motor neurons and the liver. We are evaluating these vectors at multiple doses utilizing a broad battery of neuromuscular function and biochemical assays, and anticipate the full results of this study in the first quarter of 2018. We plan to file an IND for the selected candidate construct in the second quarter of 2018 and to initiate a Phase 1/2 clinical study in Pompe patients in the fourth quarter of 2018. We hold exclusive global rights to both AAV8 and AAV9 in Pompe disease from REGENXBIO, and AT982 has been granted orphan drug designation in both the United States and European Union.
AT307. CASQ2-CPVT is a rare monogenic disease that is characterized by life-threatening arrhythmias that may lead to sudden cardiac death. There are currently only limited treatment options with variable efficacy for patients suffering from CPVT, including beta-blockers and a sodium channel blocker, Flecainide. The autosomal recessive form of the disease is caused by mutations in the calsequestrin 2 gene, or CASQ2 gene, and is characterized by stress-induced heartbeat rhythm changes in an otherwise structurally normal heart. It is estimated that CPVT occurs in one in 10,000 people, with approximately 2% to 5% due to mutations in the CASQ2 gene. This equates to an approximate prevalence of 6,000 affected people in North America, Europe and other addressable markets. Despite treatment with anti-arrhythmia therapies, sympathectomy and implantable cardiac defibrillators, a significant portion of the patients remain symptomatic. We are developing AT307, an AAV9 vector containing a functional version of the CASQ2 gene. Preclinical data in murine models of the disease demonstrated an ability to prevent ventricular tachycardia through restoration of CASQ2 protein expression. We are advancing AT307 with the goal of providing a single administration of AT307 that results in a significant reduction in life-threatening arrhythmic events and a major improvement in quality of life.
We are conducting IND-enabling studies of AT307 and plan to file an IND in the first quarter of 2018. We are also conducting ongoing initiatives around CASQ2-CPVT patient identification and disease burden. In the fourth quarter of 2018 we plan to initiate a Phase 1/2 clinical study to evaluate the safety of AT307 in patients with CASQ2-CPVT and to use the efficacy endpoint of an exercise electrocardiogram, which we believe provides a clear means to evaluate therapeutic benefit. AT307 has been granted orphan drug designation in both the United States and European Union.
Although we believe our product candidates have the potential to provide long-term improvement in patient symptoms with a single administration, we continue to conduct preclinical studies and clinical trials to determine the safety and efficacy profiles of our product candidates. The results of on-going and future studies may be different than the results of our earlier studies and trials. We have not received regulatory approval for any of our product candidates, and in order to obtain regulatory approval and commercialize our product candidates, the FDA or foreign regulatory agencies will need to determine that our product candidates are safe and effective. To date, only one gene therapy product has been approved in the United States and two have been approved in Europe.
We have a focused, passionate team with collective expertise in gene therapy, rare disease drug development and commercialization, and biologics manufacturing. Matthew Patterson, our President, Chief Executive Officer and Co-Founder, is a biotechnology leader with over 20 years of experience at Genzyme Corporation, BioMarin Pharmaceutical, Amicus Therapeutics and our company.
Recent Developments
In August 2017, we entered into an “at-the-market” program and sales agreement with Cowen and Company, LLC, under which the Company may, from time to time, offer and sell common stock having an
S-8
Table of Contents
aggregate offering value of up to $75.0 million, referred to as our “at-the-market” offering. During the three months ended September 30, 2017, the Company sold 1,867,920 shares of common stock under the ATM for aggregate net proceeds of $36.4 million. In October 2017, the Company sold 110,441 additional shares of common stock under the ATM for aggregate net proceeds of $2.7 million. On January 14, 2018, we suspended, and during the duration of this offering we are no longer offering, any shares of our common stock pursuant to the prospectus supplement filed with the SEC on August 8, 2017 pursuant to Rule 424(b)(5) relating to the Sales Agreement with Cowen and Company, LLC, dated as of August 8, 2017.
On January 5, 2018, after evaluating the efficacy and safety profile of the first three patients dosed at 1x1014 vector genomes (vg) per kilogram (kg), we recommended to the independent Data Monitoring Committee, or DMC, of ASPIRO, and the DMC agreed, that we proceed with dosing in ASPIRO by expanding the first dose cohort with an additional three patients and extending the steroid regimen with which we treat patients in ASPIRO. We plan to report the next interim data from ASPIRO in the second quarter of 2018.
While we have not finalized our full financial results for the year ended December 31, 2017, we expect to report that we had approximately $133.6 million of cash, cash equivalents and short-term investments as of December 31, 2017. This amount is preliminary, has not been audited and is subject to change. Additional information and disclosures would be required for a more complete understanding of our financial position and results of operations as of December 31, 2017.
Our Strategy
Our strategy is to leverage the expertise of our team and the transformative potential of gene therapy technology to develop treatments that improve outcomes for patients with serious, life-threatening rare diseases. Key elements of our strategy are:
| • | Focus on serving patients. We take pride in our efforts to harness the transformative potential of gene therapy to improve the lives of patients suffering from devastating rare diseases. We intend to continue to engage with patient advocacy groups to better understand the burden of disease and align our efforts with the needs of patients and caregivers. |
| • | Advance our lead product candidates through clinical development. We have initiated ASPIRO, the Phase 1/2 clinical study of AT132 in XLMTM, and plan to initiate VALENS, the Phase 1/2 clinical study of AT342 for the treatment of Crigler-Najjar in the first quarter of 2018. We are conducting IND-enabling preclinical studies of AT307 to treat CASQ2-CPVT, and AT982 to treat Pompe disease, and plan to file INDs for these programs in the first and second quarter of 2018, respectively. Over time, we plan to develop and commercialize a broad portfolio of gene therapy product candidates to treat serious, life-threatening rare diseases with high unmet medical need. |
| • | Continue to expand our pipeline with additional gene therapy product candidates targeting serious, life-threatening rare diseases. We intend to continue leveraging our expertise and focused selection criteria to expand our pipeline of product candidates. Our relationships with leading academic institutions and other rare disease companies are an important component of our strategy for sourcing additional product candidates. |
| • | Continue to build our proprietary manufacturing capabilities and invest in a state-of-the-art cGMP facility. We believe the quality, reliability and scalability of our gene therapy manufacturing approach will be a core competitive advantage crucial to our long-term success. We manufacture all of the clinical supply for our product candidates at our state-of-the-art, multi-product internal manufacturing facility that has been designed to support commercial licensure by both the U.S. Food and Drug Administration and the European Medicines Agency. |
S-9
Table of Contents
Our Strengths
We believe our leadership position is based on our following strengths:
| • | Rare disease expertise. Led by a management team with over 100 years of combined experience in rare diseases, we are building a fully integrated and industry-leading biotechnology company. Leveraging recent developments in gene therapy, we aim to provide durable and meaningful treatment options to patients suffering from rare monogenic diseases. |
| • | Highly focused selection criteria for development programs. We employ a disciplined approach to select and expand our pipeline of product candidates. We believe the application of our selection criteria enables the efficient, cost-effective and successful development of our product candidates. |
| • | Promising product candidate pipeline. We have built a compelling pipeline, and are currently conducting a Phase 1/2 clinical study of our lead product candidate AT132 for the treatment of X-Linked Myotubular Myopathy, or XLMTM. We have three additional product candidates in development, including AT342 for the treatment of Crigler-Najjar Syndrome, AT982 for the treatment of Pompe disease, and AT307 for the treatment of the CASQ2 subtype of Catecholaminergic Polymorphic Ventricular Tachycardia, or CASQ2-CPVT. |
| • | Proprietary know-how and capabilities. Our proprietary manufacturing capabilities provide a major core strategic advantage, including better control over the cost and timelines of developing our product candidates, superior protection of novel inventions and intellectual property, and expanded possibilities for new programs and partnerships. |
| • | Broad network. We believe our strong relationships with key opinion leaders and patient advocacy groups will support our product development efforts and our potential for future commercial success. Leveraging our collaborations with these parties allows us to better understand the diseases we target and optimize our research, clinical development and commercial plans. |
Risks Related to Our Business
Our business is subject to numerous risks and uncertainties, including those highlighted in and incorporated by reference into the section entitled “Risk Factors” immediately following this prospectus supplement summary. These risks include, but are not limited to, the following:
| • | we have a limited operating history and are very early in our development efforts, and we may be unable to develop, obtain regulatory approval for and ultimately commercialize our product candidates; |
| • | we have only just initiated clinical trials in our lead program, and success in early preclinical or clinical studies may not be indicative of results obtained in later preclinical and clinical studies; |
| • | if we do not achieve our projected development goals in the time frames we announce and expect, the commercialization of our products may be delayed and, as a result, our stock price may decline; |
| • | our product candidates are based on a novel AAV gene therapy technology with which there is little clinical experience, which makes it difficult to predict the time and cost of product candidate development and subsequently obtaining regulatory approval; |
S-10
Table of Contents
| • | ethical and legal concerns about gene therapy and genetic testing may result in additional regulations or restrictions on the development and commercialization of our product candidates; |
| • | even if we complete the necessary clinical trials, we cannot predict when, or if, we will obtain regulatory approval to commercialize a product candidate and the approval may be for a more narrow indication than we seek; |
| • | delays or disruptions in our manufacturing operations may delay or disrupt our development and commercialization efforts; |
| • | we may not be successful in our efforts to build a pipeline of additional product candidates; |
| • | if we are unable to obtain and maintain patent protection for our products and technology, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize products and technology similar or identical to ours, and our ability to successfully commercialize our products and technology may be adversely affected; |
| • | there have been several adverse side effects identified in clinical trials for other gene therapy product candidates in the past, and our product candidates, which are based on gene therapy technology, may cause undesirable and unforeseen side effects or be perceived by the public as unsafe; |
| • | we have a history of operating losses, and we may not achieve or sustain profitability; and |
| • | all of our current product candidates are licensed from or based upon licenses from third parties, and if any of these license or sublicense agreements are terminated or interpreted to narrow our rights, our ability to advance our current product candidates or develop new product candidates based on these technologies will be materially adversely affected. |
Corporate Information
We were incorporated in Delaware in November 2012. Our principal executive offices are located at 600 California Street, 17th Floor, San Francisco, California 94108, and our telephone number is (415) 818-1001. Our website address is www.audentestx.com. The information contained on, or that can be accessed through, our website is not a part of this prospectus. Investors should not rely on any such information in deciding whether to purchase our common stock.
Implications of Being an Emerging Growth Company
As a company with less than $1.07 billion in revenue during our most recently completed fiscal year, we qualify as an “emerging growth company” as defined in Section 2(a) of the Securities Act of 1933, or the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of certain exemptions from various public company reporting requirements, including:
| • | an exemption from compliance with the auditor attestation requirement on the effectiveness of our internal control over financial reporting; |
| • | an exemption from compliance with any requirement that the Public Company Accounting Oversight Board may adopt regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; |
S-11
Table of Contents
| • | reduced disclosure about our executive compensation arrangements; and |
| • | exemptions from the requirements to obtain a non-binding advisory vote on executive compensation or a stockholder approval of any golden parachute arrangements. |
We may take advantage of these exemptions for up to five years after our initial public offering or such earlier time that we are no longer an emerging growth company. Accordingly, the information contained and incorporated by reference herein may be different than the information you receive from other public companies in which you hold stock. We would cease to be an emerging growth company upon the earliest to occur of: the last day of the fiscal year in which we have more than $1.07 billion in annual revenue; the date we qualify as a “large accelerated filer,” with at least $700 million of equity securities held by non-affiliates; the issuance, in any three- year period, by us of more than $1.0 billion in non-convertible debt securities; and the last day of the fiscal year ending after the fifth anniversary of our initial public offering.
The JOBS Act also permits us, as an emerging growth company, to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies and thereby allows us to delay the adoption of those standards until those standards would apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
S-12
Table of Contents
The Offering
| Common stock offered by us |
shares. |
| Common stock to be outstanding immediately after this offering |
shares. |
| Option to purchase additional shares |
We have granted to the underwriters the option, exercisable for 30 days, to purchase up to additional shares of our common stock. |
| Use of proceeds |
We currently intend to use the net proceeds from this offering to advance our full pipeline of product candidates, including: AT132 for the treatment of XLMTM through the interim six-month results from the ongoing ASPIRO trial; AT342 for the treatment of Crigler-Najjar through the interim six-month results from the planned VALENS trial; AT982 for the treatment of Pompe disease through the initiation of a Phase 1/2 clinical trial in the fourth quarter of 2018; AT307 for the treatment of CASQ2-CPVT through the initiation of a Phase 1/2 clinical trial in the fourth quarter of 2018; to improve our internal manufacturing capabilities; and for working capital and other general corporate purposes. See the section entitled “Use of Proceeds.” |
| Risk factors |
You should read the section entitled “Risk Factors” and other information included in this prospectus supplement for a discussion of factors you should consider carefully before deciding to invest in shares of our common stock. |
| Nasdaq symbol |
“BOLD” |
The number of shares of our common stock to be outstanding following this offering is based on 29,733,838 shares of our common stock outstanding as of September 30, 2017 and excludes:
| • | 110,441 shares of our common stock issued pursuant to sales made through our “at-the-market” offering after September 30, 2017; |
| • | 3,689,020 shares of our common stock issuable upon the exercise of outstanding options as of September 30, 2017, with a weighted-average exercise price of approximately $9.92 per share; |
| • | 1,536,311 shares of our common stock issuable upon the exercise of outstanding options granted after September 30, 2017, with a weighted-average exercise price of approximately $27.48 per share; |
| • | 9,914 shares of our common stock issuance upon the exercise of an outstanding warrant, with an exercise price of $15.13 per share; |
| • | 317,437 shares of common stock reserved for future issuance under our stock-based compensation plans as of September 30, 2017, consisting of (i) 107,437 shares of common stock reserved for future issuance under our 2016 Equity Incentive Plan as of September 30, 2017 (consisting of 1,643,748 shares reserved as of September 30, 2017, reduced by 1,536,311 shares underlying stock options granted after September 30, 2017) and (ii) 210,000 shares of common stock reserved for future issuance under our 2016 Employee Stock Purchase Plan; and |
S-13
Table of Contents
| • | 1,495,069 shares of additional common stock reserved for future issuance under our 2016 Equity Incentive Plan pursuant to the automatic annual increase in the number of shares reserved under the plan of 5% of the total shares outstanding that took place January 1, 2018. |
Unless otherwise noted, the information in this prospectus supplement assumes no exercise of outstanding options or warrants and no exercise of the underwriters’ option to purchase additional shares.
S-14
Table of Contents
Investing in our common stock involves a high degree of risk. You should consider carefully the risks and uncertainties described below and in our Quarterly Report on Form 10-Q for the year ended September 30, 2017, which is incorporated by reference into this prospectus supplement, together with all of the other information included in or incorporated by reference into this prospectus supplement or the accompanying prospectus, including the consolidated financial statements, the notes thereto and the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the documents incorporated by reference into this prospectus supplement before deciding whether to invest in shares of our common stock. The risks and uncertainties described below and incorporated by reference into this prospectus supplement are not the only ones we face. Additional risks and uncertainties that we are unaware of or that we deem immaterial may also become important factors that adversely affect our business. If any of the following risks actually occur, our business, financial condition, results of operations and future prospects could be materially and adversely affected. In that event, the market price of our stock could decline, and you could lose part or all of your investment.
Risks Related to Our Financial Position
Comprehensive tax reform bills could increase the tax burden on our orphan drug programs and adversely affect our business and financial condition.
The U.S. government has recently enacted comprehensive tax legislation that includes significant changes to the taxation of business entities. These changes include, among others, (i) a permanent reduction to the corporate income tax rate, (ii) a partial limitation on the deductibility of business interest expense, (iii) a shift of the U.S. taxation of multinational corporations from a tax on worldwide income to a territorial system (along with certain rules designed to prevent erosion of the U.S. income tax base) and (iv) a one-time tax on accumulated offshore earnings held in cash and illiquid assets, with the latter taxed at a lower rate.
Further, the newly enacted comprehensive tax legislation, among other things, reduces the orphan drug credit from 100% to 50% of qualifying expenditures. When and if we become profitable, this reduction in tax credits may result in an increased federal income tax burden on our orphan drug programs as it may cause us to pay federal income taxes earlier under the revised tax law than under the prior law and, despite being partially off-set by a reduction in the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, may increase our total federal tax liability attributable to such programs.
Notwithstanding the reduction in the corporate income tax rate, the overall impact of this tax reform is uncertain, and our business and financial condition could be adversely affected. In addition, it is uncertain if and to what extent various states will conform to the newly enacted federal tax law. This prospectus supplement does not discuss any such tax legislation or the manner in which it might affect purchasers of our common stock. We urge our stockholders to consult with their legal and tax advisors with respect to any such legislation and the potential tax consequences of investing in our common stock.
Risks Related to Our Business
The insurance coverage and reimbursement status of newly-approved gene therapy products is uncertain. We may not be able to obtain or maintain adequate coverage and reimbursement for our product candidate(s), if approved.
It is difficult to predict what third-party payors will decide with respect to coverage and reimbursement for our product candidates, as gene and cell therapies are novel products that are generally anticipated to establish premium pricing and are initially intended as a one-time single administration. In the United States, Luxturna, a gene therapy product manufactured by Spark Therapeutics, Inc., was approved for marketing by the FDA in
S-15
Table of Contents
December 2017. In October 2017, the FDA approved Yescarta, an additional CAR-T cell therapy product manufactured by Kite Pharma, Inc. Kymriah, a CAR-T cell therapy product manufactured by Novartis AG, was approved for marketing by the FDA in August 2017. While there is no body of established pricing and reimbursement practices for these novel gene and cell therapy products, and no uniform policy of coverage and reimbursement exists among third-party payors, these products may establish a pricing and reimbursement precedent for our product candidates, if approved. The Centers for Medicare and Medicaid Services, or CMS, administers the Medicare and Medicaid programs, which are increasingly used as models for how private payors develop their coverage and reimbursement policies, but coverage and reimbursement for products can differ significantly from payor to payor. It is difficult to predict what the CMS will decide with respect to coverage and reimbursement for a fundamentally novel gene therapy product such as ours, or how the CMS’s decision will affect our ability to obtain coverage and adequate reimbursement from other third-party payors, if any of our product candidates receive FDA approval. Moreover, reimbursement agencies in the European Union may be more conservative than the CMS. For example, several cancer drugs have been approved for reimbursement in the United States but have not been approved for reimbursement in certain European Union Member States.
Investors should not place undue reliance on the results of preclinical experiments or interim results from ASPIRO, our ongoing Phase 1/2 clinical study of AT132 to treat XLMTM, since preliminary data may not be predictive of future results that we plan will form the basis of our global regulatory approval packages, and our product candidates may not receive regulatory approval.
As of the December 21, 2017 data cutoff, the first dose cohort of ASPIRO, an ongoing Phase 1/2 clinical study of AT132 in XLMTM patients, included three patients with XLMTM dosed at 1x1014 vector genomes (vg) per kilogram (kg), and one delayed-treatment control patient. There have been a total of eight AEs reported in ASPIRO, four of which were determined to be SAEs. All SAEs occurred in Patient 3, the first of which was a hospitalization one week post-administration due to pneumonia and was deemed not treatment related.
On January 4, 2018, we reported that Patient 3 was also hospitalized at week seven post-administration due to a gastrointestinal infection and elevated troponin levels, the latter of which was deemed a probably treatment-related SAE and was responding to treatment with intravenous steroid administration and supportive care.
On January 11, 2018, the clinical investigator responsible for managing Patient 3 determined that the adverse events that contributed to the week seven hospitalization should be recategorized as three distinct SAEs, related to each other and deemed to be probably treatment-related. These include myocarditis, elevated troponin levels and mildly elevated creatinine kinase levels, each of which have resolved. Patient 3 was clinically stable throughout the hospital admission and has now been discharged from the hospital.
Of the four non-serious AEs, two have been determined to be probably or possibly treatment-related. Patient 1 experienced a mild, clinically asymptomatic exacerbation of a pre-existing elevated bilirubin level, which was deemed possibly treatment-related and resolved with treatment. Patient 2 experienced a clinically asymptomatic elevation in liver enzyme levels toward the end of the protocol-specified prednisolone weaning period, which was deemed to be probably treatment-related and was controlled by extending the duration of steroid coverage. However, investors should not place undue reliance on the results from completed preclinical studies or interim results from ASPIRO, since they do not ensure that other clinical study data will be comparable, in terms of safety, tolerability, efficacy or other factors the FDA and other regulators will consider in determining whether to approve a BLA for AT132. In addition, data obtained from preclinical and clinical studies are subject to varying interpretations. If the FDA were to interpret data from ASPIRO differently than we do, the clinical development or regulatory approval of our product candidates may be delayed, limited or prevented. While we believe that our ASPIRO Phase 1/2 clinical study is appropriately designed to evaluate the safety, tolerability and preliminary efficacy of AT132 in XLMTM patients, the FDA may disagree with us, require additional information or studies to be conducted, or impose conditions that could delay, restrict or halt our ongoing ASPIRO study or other planned clinical activities.
S-16
Table of Contents
The final dataset, upon which global regulatory decisions will be based, will differ from the datasets previously disclosed. Reasons for these differences may include, but are not limited to:
| • | the December 21, 2017 interim dataset includes a small sample size with an initial cohort of three treated patients and one delayed-treatment control patient, and results from longer term follow-up in this first dose cohort, or results from future cohorts, may be reinterpreted or may not be replicated; |
| • | not all patients may demonstrate improvement; |
| • | patients may discontinue their involvement in ASPIRO for a number of reasons, including disease progression following a response or a lack of clinical benefit, or despite positive outcomes, could become lost to follow up. Any such discontinuations will impact the amount of data we may collect over time; |
| • | additional time and patient accrual provide new opportunities to capture new adverse events and further characterize the safety and efficacy of AT132; and |
| • | the precise composition of the final dataset is subject to additional regulatory feedback, which is expected closer to the time of a BLA, or equivalent, and the advice may vary by regulatory authority. |
As a result, the efficacy and safety profile of AT132 has not been established, and may differ from any interim dataset publicly disclosed. Moreover, regulatory approvals will be based on the final efficacy and safety databases, and as such, we can give no assurance that AT132 or any of our product candidates will receive regulatory approval.
Risks Related to this Offering
Our management will have broad discretion over the use of the proceeds we receive in this offering and might not apply the proceeds in ways that increase the value of your investment.
Our management will have broad discretion to use the net proceeds from this offering, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will be relying on the judgment of our management regarding the application of these proceeds. You will not have the opportunity to influence our decisions on how to use the proceeds, and we may not apply the net proceeds of this offering in ways that increase the value of your investment. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure by our management to apply these funds effectively could harm our business. Pending their use, we intend to invest the net proceeds from this offering in marketable securities that may include investment-grade interest-bearing securities, money market accounts, certificates of deposit, commercial paper and guaranteed obligations of the U.S. government. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
If you purchase our common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
You will suffer immediate and substantial dilution in the net tangible book value of our common stock you purchase in this offering. Assuming a public offering price of $ per share, the last reported sale price of our common stock on The Nasdaq Global Market on , purchasers of common stock in this offering will experience immediate dilution of $ per share in net tangible book value of our common stock. In the past, we issued options, warrants and other securities to acquire common stock at prices below the public offering price. To the extent these outstanding securities are ultimately exercised, investors purchasing common stock in this offering will sustain further dilution. See “Dilution” for a more detailed description of the dilution to new investors in the offering.
S-17
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement and the documents incorporated by reference into this prospectus supplement, including the sections entitled “Prospectus Supplement Summary,” “Risk Factors,” and “Use of Proceeds,” contain forward-looking statements. Forward-looking statements include all statements that are not historical facts and can be identified by the words “believe,” “may,” “will,” “potentially,” “estimate,” “continue.” “anticipate,” “intend,” “could,” “would,” “project,” “plan” “expect,” and similar expressions that convey uncertainty of future events or outcomes.
These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those described in and incorporated by reference into “Risk Factors” and elsewhere in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein. Moreover, we operate in a very competitive and rapidly changing environment, and new risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties, and assumptions, the forward-looking events and circumstances discussed in this prospectus supplement may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus supplement to conform these statements to actual results or to changes in our expectations, except as required by law.
You should read this prospectus supplement, the accompanying prospectus and the documents incorporated by reference into this prospectus supplement and the documents that we reference in this prospectus supplement and have filed with the Securities and Exchange Commission, or SEC, as exhibits to the registration statement of which this prospectus supplement is a part, with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
S-18
Table of Contents
Unless otherwise indicated, information contained in or incorporated by reference into this prospectus supplement and the accompanying prospectus concerning our industry and the markets in which we operate is based on information from various sources, including independent industry publications. In presenting this information, we have also made assumptions based on such data and other similar sources, and on our knowledge of, and our experience to date in, the potential markets for our product candidates. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in and incorporated by reference into the section entitled “Risk Factors” and in the documents incorporated by reference into this prospectus supplement. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
S-19
Table of Contents
We estimate that we will receive net proceeds of approximately $140.6 million from the sale of $150.0 million of the shares of our common stock offered in this offering, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their option to purchase up to $22.5 million of additional shares in full, we estimate that the net proceeds of the shares we sell in this offering will be approximately $161.7 million.
We currently intend to use the net proceeds from this offering to advance our full pipeline of product candidates, including: AT132 for the treatment of XLMTM through the interim six-month results from the ongoing ASPIRO trial; AT342 for the treatment of Crigler-Najjar through the interim six-month results from the planned VALENS trial; AT982 for the treatment of Pompe disease through the initiation of a Phase 1/2 clinical trial in the fourth quarter of 2018; AT307 for the treatment of CASQ2-CPVT through the initiation of a Phase 1/2 clinical trial in the fourth quarter of 2018; to improve our internal manufacturing capabilities; and for working capital and other general corporate purposes.
We estimate that our current cash, cash equivalents, short-term investments and the net proceeds from this offering will be sufficient for us to fund our operating expenses and capital expenditure requirements through 2019.
The expected use of the net proceeds from this offering represents our intentions based upon our current plans and business conditions. As of the date of this prospectus supplement, we cannot predict with any certainty all of the particular uses for the net proceeds or the amounts that we will actually spend on the uses set forth above. We may use a portion of the net proceeds for the acquisition of, or investment in, technologies, intellectual property or businesses that complement our business, although we have no present commitments or agreements to this effect.
The amounts and timing of our future expenditures and the extent of product candidate development may vary significantly depending on numerous factors, including the status, results and timing of our current preclinical studies and clinical trials we may commence in the future, product approval process with the FDA and other regulatory agencies, our current collaborations and any new collaborations we may enter into with third parties and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
The expected net proceeds of this offering will not be sufficient for us to fund any of our product candidates through regulatory approval, and we will need to raise substantial additional capital to complete the development and commercialization of our product candidates.
Pending their use as described above, we intend to invest the net proceeds from this offering in marketable securities that may include investment-grade interest-bearing securities, money market accounts, certificates of deposit, commercial paper and guaranteed obligations of the U.S. government.
S-20
Table of Contents
If you invest in our common stock, your ownership interest will be diluted to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock immediately after this offering.
As of September 30, 2017, our net tangible book value was approximately $163.6 million, or $5.50 per share of common stock. Net tangible book value per share represents the amount of our tangible assets less our liabilities divided by the total number of shares of our common stock outstanding as of September 30, 2017.
After giving effect to the sale and issuance of shares of our common stock at an assumed public offering price of $ per share, the last reported sale price of our common stock on The Nasdaq Global Market on , and after deducting estimated underwriting discounts and commissions and estimated offering expenses, our net tangible book value as of September 30, 2017 would have been approximately $ million, or $ per share of our common stock. This represents an immediate increase in net tangible book value of $ per share to our existing stockholders and an immediate dilution of $ per share to investors purchasing shares in this offering, as follows:
| Assumed public offering price per share |
$ | |||||||
| Net tangible book value per share as of September 30, 2017 |
$ | 5.50 | ||||||
| Increase in net tangible book value per share attributable to new investors in this offering |
||||||||
|
|
|
|||||||
| As adjusted net tangible book value per share after this offering |
||||||||
|
|
|
|||||||
| Dilution in net tangible book value per share to investors in this offering |
$ | |||||||
|
|
|
If the underwriters exercise their option to purchase additional shares in full, our as adjusted net tangible book value per share after this offering would be $ per share, and the dilution in net tangible book value per share to new investors in this offering would be $ per share.
The number of shares of our common stock to be outstanding after this offering excludes:
| • | 110,441 shares of our common stock issued pursuant to sales made through our “at-the-market” offering after September 30, 2017; |
| • | 3,689,020 shares of our common stock issuable upon the exercise of outstanding options as of September 30, 2017, with a weighted-average exercise price of approximately $9.92 per share; |
| • | 1,536,311 shares of our common stock issuable upon the exercise of outstanding options granted after September 30, 2017, with a weighted-average exercise price of approximately $27.48 per share; |
| • | 9,914 shares of our common stock issuance upon the exercise of an outstanding warrant, with an exercise price of $15.13 per share; |
| • | 317,437 shares of common stock reserved for future issuance under our stock-based compensation plans as of September 30, 2017, consisting of (i) 107,437 shares of common stock reserved for future issuance under our 2016 Equity Incentive Plan as of September 30, 2017 (consisting of 1,643,748 shares reserved as of September 30, 2017, reduced by 1,536,311 shares underlying stock options granted after September 30, 2017) and (ii) 210,000 shares of common stock reserved for future issuance under our 2016 Employee Stock Purchase Plan; and |
| • | 1,495,069 shares of additional common stock reserved for future issuance under our 2016 Equity Incentive Plan pursuant to the automatic annual increase in the number of shares reserved under the plan of 5% of the total shares outstanding that took place January 1, 2018. |
S-21
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS OF COMMON STOCK
This section summarizes the material U.S. federal income tax considerations relating to the acquisition, ownership and disposition of our common stock by “non-U.S. holders” (as defined below) pursuant to this offering. This summary does not provide a complete analysis of all potential U.S. federal income tax considerations relating thereto. The information provided below is based upon provisions of the Internal Revenue Code of 1986, as amended (the “Code”), Treasury regulations promulgated thereunder, administrative rulings and judicial decisions currently in effect. These authorities may change at any time, possibly retroactively, or the Internal Revenue Service, or IRS, might interpret the existing authorities differently. In either case, the tax considerations of owning or disposing of our common stock could differ from those described below. As a result, we cannot assure you that the tax consequences described in this discussion will not be challenged by the IRS or will be sustained by a court if challenged by the IRS.
This summary does not address the tax considerations arising under the laws of any non-U.S., state or local jurisdiction, or under U.S. federal gift and estate tax laws, except to the limited extent provided below. In addition, this discussion does not address tax considerations applicable to an investor’s particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
| • | banks, insurance companies or other financial institutions; |
| • | partnerships or entities or arrangements treated as partnerships or other pass-through entities for U.S. federal tax purposes (or investors in such entities); |
| • | corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | persons subject to the alternative minimum tax or medicare contribution tax; |
| • | tax-exempt organizations or tax-qualified retirement plans; |
| • | controlled foreign corporations or passive foreign investment companies; |
| • | persons who acquired our common stock as compensation for services; |
| • | dealers in securities or currencies; |
| • | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; |
| • | persons that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below); |
| • | certain former citizens or long-term residents of the United States; |
| • | persons who hold our common stock as a position in a hedging transaction, “straddle,” “conversion transaction” or other risk reduction transaction; |
| • | persons who do not hold our common stock as a capital asset within the meaning of Section 1221 of the Code (generally, for investment purposes); or |
| • | persons deemed to sell our common stock under the constructive sale provisions of the Code. |
S-22
Table of Contents
In addition, if a partnership or entity classified as a partnership for U.S. federal income tax purposes is a beneficial owner of our common stock, the tax treatment of a partner in the partnership or an owner of the entity will depend upon the status of the partner or other owner and the activities of the partnership or other entity. Accordingly, this summary does not address tax considerations applicable to partnerships that hold our common stock, and partners in such partnerships should consult their tax advisors.
INVESTORS CONSIDERING THE PURCHASE OF OUR COMMON STOCK SHOULD CONSULT THEIR OWN TAX ADVISORS REGARDING THE APPLICATION OF THE U.S. FEDERAL INCOME AND ESTATE TAX LAWS TO THEIR PARTICULAR SITUATIONS AND THE CONSEQUENCES OF FOREIGN, STATE OR LOCAL LAWS, AND TAX TREATIES.
Non-U.S. Holder Defined
For purposes of this summary, a “non-U.S. holder” is any holder of our common stock, other than a partnership, that is not:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized under the laws of the United States, any state therein or the District of Columbia; |
| • | a trust if it (1) is subject to the primary supervision of a U.S. court and one of more U.S. persons have authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person; or |
| • | an estate whose income is subject to U.S. income tax regardless of source. |
If you are a non-U.S. citizen who is an individual, you may, in many cases, be deemed to be a resident alien, as opposed to a nonresident alien, by virtue of being present in the United States for at least 31 days in the calendar year and for an aggregate of at least 183 days during a three-year period ending in the current calendar year. For these purposes, all the days present in the current year, one-third of the days present in the immediately preceding year, and one-sixth of the days present in the second preceding year are counted. Resident aliens are subject to U.S. federal income tax as if they were U.S. citizens. Such an individual is urged to consult his or her own tax advisor regarding the U.S. federal income tax consequences of the ownership or disposition of our common stock.
Dividends
We do not expect to declare or make any distributions on our common stock in the foreseeable future and the terms of our credit facility currently restrict our ability to pay dividends. If we do pay dividends on shares of our common stock, however, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that is applied against and reduces, but not below zero, a non-U.S. holder’s adjusted tax basis in shares of our common stock. Any remaining excess will be treated as gain realized on the sale or other disposition of our common stock. See “—Sale of Common Stock.”
Any dividend paid to a non-U.S. holder on our common stock that is not effectively connected with a non-U.S. holder’s conduct of a trade or business in the United States will generally be subject to U.S. withholding tax at a 30% rate. The withholding tax might apply at a reduced rate under the terms of an applicable income tax treaty between the United States and the non-U.S. holder’s country of residence subject to the discussion below regarding the Foreign Account Tax Compliance Act. You should consult your tax advisors
S-23
Table of Contents
regarding your entitlement to benefits under a relevant income tax treaty. Generally, in order for us or our paying agent to withhold tax at a lower treaty rate, a non-U.S. holder must certify its entitlement to treaty benefits. A non-U.S. holder generally can meet this certification requirement by providing a Form W-8BEN or Form W- 8BEN-E (or any successor form) or appropriate substitute form to us or our paying agent. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to the agent. The holder’s agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. If you are eligible for a reduced rate of U.S. federal withholding tax under an income tax treaty, you may obtain a refund or credit of any excess amounts withheld by filing an appropriate claim for a refund with the IRS in a timely manner.
Dividends received by a non-U.S. holder that are effectively connected with a U.S. trade or business conducted by the non-U.S. holder, and if required by an applicable income tax treaty between the United States and the non-U.S. holder’s country of residence, are attributable to a permanent establishment maintained by the non-U.S. holder in the United States, are not subject to U.S. withholding tax. To obtain this exemption, a non-U.S. holder must provide us or our paying agent with an IRS Form W-8ECI properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. In addition to being taxed at graduated tax rates, dividends received by corporate non-U.S. holders that are effectively connected with a U.S. trade or business of the corporate non-U.S. holder may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable tax treaty.
Sale of Common Stock
Subject to the discussions below regarding Backup Withholding and Information Reporting and the Foreign Account Tax Compliance Act, non-U.S. holders will generally not be subject to U.S. federal income tax on any gains realized on the sale, exchange or other disposition of our common stock unless:
| • | the gain (i) is effectively connected with the conduct by the non-U.S. holder of a U.S. trade or business and (ii) if required by an applicable income tax treaty between the United States and the non-U.S. holder’s country of residence, is attributable to a permanent establishment maintained by the non-U.S. holder in the United States (in which case the special rules described below apply); |
| • | the non-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of the sale, exchange or other disposition of our common stock, and certain other requirements are met (in which case the gain would be subject to a flat 30% tax, or such reduced rate as may be specified by an applicable income tax treaty, which may be offset by U.S. source capital losses, even though the individual is not considered a resident of the United States); or |
| • | the rules of the Foreign Investment in Real Property Tax Act, or FIRPTA, treat the gain as effectively connected with a U.S. trade or business. |
The FIRPTA rules may apply to a sale, exchange or other disposition of our common stock if we are, or were within the shorter of the five-year period preceding the disposition and the non-U.S. holder’s holding period, a “U.S. real property holding corporation,” or USRPHC. In general, we would be a USRPHC if interests in U.S. real estate comprised at least half of the value of our business assets. We do not believe that we are a USRPHC and we do not anticipate becoming one in the future. Even if we become a USRPHC, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as U.S. real property interests only if beneficially owned by a non-U.S. holder that actually or constructively owned more than 5% of our outstanding common stock at any time within the five-year period preceding the disposition.
If any gain from the sale, exchange or other disposition of our common stock, (i) is effectively connected with a U.S. trade or business conducted by a non-U.S. holder and (ii) if required by an applicable
S-24
Table of Contents
income tax treaty between the United States and the non-U.S. holder’s country of residence, is attributable to a permanent establishment maintained by such non-U.S. holder in the United States, then the gain generally will be subject to U.S. federal income tax at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. If the non-U.S. holder is a corporation, under certain circumstances, that portion of its earnings and profits that is effectively connected with its U.S. trade or business, subject to certain adjustments, generally would be subject also to a “branch profits tax.” The branch profits tax rate is 30%, although an applicable income tax treaty between the United States and the non-U.S. holder’s country of residence might provide for a lower rate.
U.S. Federal Estate Tax
The estates of nonresident alien individuals generally are subject to U.S. federal estate tax on property with a U.S. situs. Because we are a U.S. corporation, our common stock will be U.S. situs property and therefore will be included in the taxable estate of a nonresident alien decedent, unless an applicable estate tax treaty between the United States and the decedent’s country of residence provides otherwise.
Backup Withholding and Information Reporting
The Code and the Treasury regulations require those who make specified payments to report the payments to the IRS. Among the specified payments are dividends and proceeds paid by brokers to their customers. The required information returns enable the IRS to determine whether the recipient properly included the payments in income. This reporting regime is reinforced by “backup withholding” rules. These rules require the payors to withhold tax from payments subject to information reporting if the recipient fails to cooperate with the reporting regime by failing to provide his taxpayer identification number to the payor, furnishing an incorrect identification number, or failing to report interest or dividends on his returns. The backup withholding tax rate is currently 28%. The backup withholding rules do not apply to payments to corporations, whether domestic or foreign, provided they establish such exemption.
Payments to non-U.S. holders of dividends on common stock generally will not be subject to backup withholding, and payments of proceeds made to non-U.S. holders by a broker upon a sale of common stock will not be subject to information reporting or backup withholding, in each case so long as the non-U.S. holder certifies its nonresident status (and we or our paying agent do not have actual knowledge or reason to know the holder is a U.S. person or that the conditions of any other exemption are not, in fact, satisfied) or otherwise establishes an exemption. The certification procedures to claim treaty benefits described under “—Dividends” will generally satisfy the certification requirements necessary to avoid the backup withholding tax. We must report annually to the IRS any dividends paid to each non-U.S. holder and the tax withheld, if any, with respect to these dividends. Copies of these reports may be made available to tax authorities in the country where the non-U.S. holder resides.
Under the Treasury regulations, the payment of proceeds from the disposition of shares of our common stock by a non-U.S. holder made to or through a U.S. office of a broker generally will be subject to information reporting and backup withholding unless the beneficial owner certifies, under penalties of perjury, among other things, its status as a non-U.S. holder (and the broker does not have actual knowledge or reason to know the holder is a U.S. person) or otherwise establishes an exemption. The payment of proceeds from the disposition of shares of our common stock by a non-U.S. holder made to or through a non-U.S. office of a broker generally will not be subject to backup withholding and information reporting, except as noted below. Information reporting, but not backup withholding, will apply to a payment of proceeds, even if that payment is made outside of the United States, if you sell our common stock through a non-U.S. office of a broker that is:
| • | a U.S. person (including a foreign branch or office of such person); |
| • | a “controlled foreign corporation” for U.S. federal income tax purposes; |
S-25
Table of Contents
| • | a foreign person 50% or more of whose gross income from certain periods is effectively connected with a U.S. trade or business; or |
| • | a foreign partnership if at any time during its tax year (i) one or more of its partners are U.S. persons who, in the aggregate, hold more than 50% of the income or capital interests of the partnership or (ii) the foreign partnership is engaged in a U.S. trade or business; |
unless the broker has documentary evidence that the beneficial owner is a non-U.S. holder and certain other conditions are satisfied, or the beneficial owner otherwise establishes an exemption (and the broker has no actual knowledge or reason to know to the contrary).
Backup withholding is not an additional tax. Any amounts withheld from a payment to a holder of common stock under the backup withholding rules can be credited against any U.S. federal income tax liability of the holder and may entitle the holder to a refund, provided that the required information is furnished to the IRS in a timely manner.
Foreign Account Tax Compliance Act (FATCA)
A U.S. federal withholding tax of 30% may apply to dividends and the gross proceeds of a disposition of our common stock paid to a foreign financial institution (as specifically defined by the applicable rules) unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity holders of such institution, as well as certain account holders that are foreign entities with U.S. owners). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules. This U.S. federal withholding tax of 30% will also apply to dividends and the gross proceeds of a disposition of our common stock paid to a non-financial foreign entity unless such entity provides the withholding agent with either a certification that it does not have any substantial direct or indirect U.S. owners or provides information regarding direct and indirect U.S. owners of the entity. The 30% federal withholding tax described in this paragraph cannot be reduced under an income tax treaty with the United States or by providing an IRS Form W-8BEN or similar documentation. The withholding tax described above will not apply if the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from the rules. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. Holders should consult with their own tax advisors regarding the possible implications of the withholding described herein.
The withholding provisions described above generally will apply to proceeds from a sale or other disposition of common stock if such sale or other disposition occurs on or after January 1, 2019 and apply currently to payments of dividends on our common stock.
THE PRECEDING DISCUSSION OF U.S. FEDERAL TAX CONSIDERATIONS IS FOR GENERAL INFORMATION ONLY. IT IS NOT TAX ADVICE TO ANY NON-U.S. HOLDER IN ITS PARTICULAR CIRCUMSTANCES. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE PARTICULAR U.S. FEDERAL, STATE, LOCAL AND FOREIGN TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
S-26
Table of Contents
Merrill Lynch, Pierce, Fenner & Smith Incorporated, Cowen and Company, LLC and Leerink Partners LLC are acting as the representatives of each of the underwriters named below. Subject to the terms and conditions set forth in an underwriting agreement among us and the underwriters, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the number of shares of common stock set forth opposite its name below.
| Underwriter | Number of |
|||
| Merrill Lynch, Pierce, Fenner & Smith |
||||
| Cowen and Company, LLC |
||||
| Leerink Partners LLC |
||||
| Wedbush Securities Inc. |
||||
|
|
|
|||
| Total |
||||
|
|
|
|||
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the shares sold under the underwriting agreement if any of these shares are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
The representatives have advised us that the underwriters propose initially to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After the initial offering, the public offering price, concession or any other term of the offering may be changed.
The following table shows the public offering price, underwriting discount and proceeds before expenses to us. The information assumes either no exercise or full exercise by the underwriters of their option to purchase additional shares.
| Per Share |
Without Option |
With Option |
||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions |
$ | $ | $ | |||||||||
| Proceeds, before expenses, to us |
$ | $ | $ | |||||||||
The expenses of the offering, not including the underwriting discount, are estimated to be approximately $440,000. We have also agreed to reimburse the underwriters for up to $30,000 for their Financial Industry Regulatory Authority, Inc., or FINRA, counsel fee. In accordance with FINRA Rule 5110, this reimbursement is deemed underwriting compensation for this offering.
S-27
Table of Contents
Option to Purchase Additional Shares
We have granted an option to the underwriters, exercisable for 30 days after the date of this prospectus, to purchase up to additional shares at the public offering price, less the underwriting discount. If the underwriters exercise this option, each will be obligated, subject to conditions contained in the underwriting agreement, to purchase a number of additional shares proportionate to that underwriter’s initial amount reflected in the above table.
No Sales of Similar Securities
We, our executive officers and directors, and certain of our existing security holders have agreed not to sell or transfer any common stock or securities convertible into, exchangeable for, exercisable for, or repayable with common stock, for 60 days after the date of this prospectus without first obtaining the written consent of the representatives. Specifically, we and these other persons have agreed, with certain limited exceptions, not to directly or indirectly
| • | offer, pledge, sell or contract to sell any common stock, |
| • | sell any option or contract to purchase any common stock, |
| • | purchase any option or contract to sell any common stock, |
| • | grant any option, right or warrant for the sale of any common stock, |
| • | lend or otherwise dispose of or transfer any common stock, |
| • | request or demand that we file a registration statement related to the common stock, or |
| • | enter into any swap or other agreement that transfers, in whole or in part, the economic consequence of ownership of any common stock whether any such swap or transaction is to be settled by delivery of shares or other securities, in cash or otherwise. |
This lock-up provision applies to common stock and to securities convertible into or exchangeable or exercisable for or repayable with common stock. It also applies to common stock owned now or acquired later by the person executing the agreement or for which the person executing the agreement later acquires the power of disposition.
Nasdaq Global Market Listing
Our common stock is listed on The Nasdaq Global Market under the symbol “BOLD.”
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of the shares is completed, SEC rules may limit underwriters and selling group members from bidding for and purchasing our common stock. However, the representatives may engage in transactions that stabilize the price of the common stock, such as bids or purchases to peg, fix or maintain that price.
In connection with the offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not
S-28
Table of Contents
greater than the underwriters’ option to purchase additional shares described above. The underwriters may close out any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option granted to them. “Naked” short sales are sales in excess of such option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of common stock made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. The underwriters may conduct these transactions on The Nasdaq Global Market, in the over-the-counter market or otherwise.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor any of the underwriters make any representation that the representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Distribution
In connection with the offering, certain of the underwriters or securities dealers may distribute prospectuses by electronic means, such as e-mail.
Other Relationships
Some of the underwriters and their affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They have received, or may in the future receive, customary fees and commissions for these transactions. An affiliate of Cowen and Company, LLC served as the placement agent for our Series C convertible preferred stock financing in October 2015, and Merrill Lynch, Pierce, Fenner & Smith Incorporated, Cowen and Company, LLC and Wedbush Securities Inc. also served as underwriters in our initial public offering in July 2016 and follow-on equity offering in April 2017. In August 2017, we entered into an “at-the-market” program and sales agreement with Cowen and Company, LLC, under which the Company may, from time to time, offer and sell common stock having an aggregate offering value of up to $75.0 million.
In addition, in the ordinary course of their business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
S-29
Table of Contents
Notice to Prospective Investors in the European Economic Area
In relation to each member state of the European Economic Area, no offer of ordinary shares which are the subject of the offering has been, or will be made to the public in that Member State, other than under the following exemptions under the Prospectus Directive:
| 1. | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| 2. | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the Representatives for any such offer; or |
| 3. | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of ordinary shares referred to in (a) to (c) above shall result in a requirement for the company or any representative to publish a prospectus pursuant to Article 3 of the Prospectus Directive, or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
Each person located in a Member State to whom any offer of ordinary shares is made or who receives any communication in respect of an offer of ordinary shares, or who initially acquires any ordinary shares will be deemed to have represented, warranted, acknowledged and agreed to and with each representative and the company that (i) it is a “qualified investor” within the meaning of the law in that Member State implementing Article 2(1)(e) of the Prospectus Directive; and (ii) in the case of any ordinary shares acquired by it as a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, the ordinary shares acquired by it in the offer have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Member State other than qualified investors, as that term is defined in the Prospectus Directive, or in circumstances in which the prior consent of the Representatives has been given to the offer or resale; or where ordinary shares have been acquired by it on behalf of persons in any Member State other than qualified investors, the offer of those ordinary shares to it is not treated under the Prospectus Directive as having been made to such persons.
The company, the representatives and their respective affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgments and agreements.
This prospectus has been prepared on the basis that any offer of shares in any Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of shares. Accordingly, any person making or intending to make an offer in that Member State of shares which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for the company or any of the representatives to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither the company nor the representatives have authorized, nor do they authorize, the making of any offer of shares in circumstances in which an obligation arises for the company or the representatives to publish a prospectus for such offer.
For the purposes of this provision, the expression an “offer of ordinary shares to the public” in relation to any ordinary shares in any Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the ordinary shares to be offered so as to enable an investor to decide to purchase or subscribe the ordinary shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (as amended) and includes any relevant implementing measure in each Member State.
The above selling restriction is in addition to any other selling restrictions set out below.
S-30
Table of Contents
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or SIX, or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the company, the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, or FINMA, and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in the Dubai International Financial Centre
This prospectus relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority, or DFSA. This prospectus is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth herein and has no responsibility for the prospectus. The shares to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the shares offered should conduct their own due diligence on the shares. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
Notice to Prospective Investors in Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission, or ASIC, in relation to the offering. This prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001, or Corporations Act, and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the shares may only be made to persons, or Exempt Investors, who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act.
S-31
Table of Contents
The shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring shares must observe such Australian on-sale restrictions.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice to Prospective Investors in Hong Kong
The shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to Prospective Investors in Japan
The shares have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) and, accordingly, will not be offered or sold, directly or indirectly, in Japan, or for the benefit of any Japanese Person or to others for re-offering or resale, directly or indirectly, in Japan or to any Japanese Person, except in compliance with all applicable laws, regulations and ministerial guidelines promulgated by relevant Japanese governmental or regulatory authorities in effect at the relevant time. For the purposes of this paragraph, “Japanese Person” shall mean any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or SFA, (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| (a) | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
S-32
Table of Contents
| (b) | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except:
| (a) | to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| (b) | where no consideration is or will be given for the transfer; |
| (c) | where the transfer is by operation of law; |
| (d) | as specified in Section 276(7) of the SFA; or |
| (e) | as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore. |
Notice to Prospective Investors in Canada
The shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
S-33
Table of Contents
The validity of the shares of common stock offered hereby will be passed upon for us by Fenwick & West LLP, San Francisco, California. Certain legal matters relating to this offering will be passed upon for the underwriters by Cooley LLP, San Francisco, California.
The consolidated financial statements of Audentes Therapeutics, Inc. as of December 31, 2016 and 2015, and for each of the years in the three year period ended December 31, 2016 have been incorporated by reference herein in reliance upon the report of KPMG LLP, an independent registered public accounting firm, incorporated by reference herein, and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the securities offered hereby. This prospectus supplement and the accompanying prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, the exhibits filed therewith or the documents incorporated by reference therein. For further information about us and the common stock offered hereby, reference is made to the registration statement, the exhibits filed therewith and the documents incorporated by reference therein. Statements contained in this prospectus supplement or the accompanying prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and in each instance we refer you to the copy of such contract or other document filed as an exhibit to the registration statement. A copy of the registration statement and the exhibits filed therewith may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street, NE, Washington, DC 20549, and copies of all or any part of the registration statement may be obtained from that office. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the website is www.sec.gov.
We are subject to the information and reporting requirements of the Exchange Act, and, in accordance with this law, file periodic reports and other information with the SEC. These periodic reports and other information are available for inspection and copying at the SEC’s public reference facilities and the website of the SEC referred to above. We also maintain a website at www.audentestx.com. You may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this prospectus supplement or the accompanying prospectus and the inclusion of our website address in this prospectus supplement or the accompanying prospectus is an inactive textual reference only. You may also inspect these documents at our corporate headquarters at 600 California Street, 17th Floor, San Francisco, California 94108, during normal business hours.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is an important part of this prospectus supplement and the accompanying prospectus, and information we file later with the SEC will automatically update and supersede this information.
We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (Commission File No. 001-37833):
| • | our Annual Report on Form 10-K for the year ended December 31, 2016, filed with the SEC on March 13, 2017 including certain information incorporated by reference therein from our Definitive Proxy Statement for our 2017 annual meeting of stockholders filed with the SEC on April 27, 2017; |
S-34
Table of Contents
| • | our Quarterly Reports on Forms 10-Q for the quarters ended March 31, 2017, June 30, 2017 and September 30, 2017, filed with the SEC on May 11, 2017, August 10, 2017 and November 14, 2017, respectively; |
| • | our Current Reports on Forms 8-K, filed with the SEC on May 5, 2017, June 7, 2017, June 13, 2017, July 14, 2017, September 27, 2017, November 17, 2017, December 13, 2017 and January 23, 2018 (in each case, except for information contained therein which is furnished rather than filed); and |
| • | the description of capital stock included in our registration statement on Form 8-A, filed with the SEC on July 13, 2016, and any amendments or reports filed for the purpose of updating such description. |
We will furnish without charge to you, on oral or written request, a copy of the documents incorporated by reference into this prospectus supplement or the accompanying prospectus but not delivered with the prospectus supplement, including any exhibits to the these documents. You should direct any requests for documents to 600 California Street, 17th Floor, San Francisco, California 94108, telephone (415) 818-1001. Copies of the above reports may also be accessed from our website at www.audentestx.com. Information contained on our website is not incorporated by reference into this prospectus supplement or the accompanying prospectus, and you should not consider any information on, or that can be accessed through, our website as part of this prospectus supplement or the accompanying prospectus (other than those filings with the SEC that we specifically incorporated by reference herein or therein).
This prospectus is part of a registration statement we filed with the SEC. We have incorporated exhibits into this registration statement. You should read the exhibits carefully for provisions that may be important to you.
You should rely only on the information incorporated by reference or provided in this prospectus supplement or the accompanying prospectus. We have not authorized anyone to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information in this prospectus supplement, the accompanying prospectus or in the documents incorporated by reference is accurate as of any date other than the date of those respective documents.
S-35
Table of Contents
PROSPECTUS
$250,000,000

Common Stock
Preferred Stock
Debt Securities
Warrants
Subscription Rights
Units
We may from time to time issue, in one or more series or classes, up to $250,000,000 in aggregate principal amount of our common stock, preferred stock, debt securities, warrants, subscription rights and/or units in one or more offerings. We may offer these securities separately or together in units, and we may offer securities as may be issuable upon conversion, redemption, repurchase, exchange or exercise of any of the securities registered hereunder, including any applicable antidilution provisions.
This prospectus provides a general description of the securities we may offer. Each time we offer securities, we will specify in the accompanying prospectus supplement the terms of the securities being offered. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement and any free writing prospectus may also add, update or change information contained in this prospectus. You should carefully read this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as any documents incorporated by reference, before you invest in our securities.
We may sell these securities to or through underwriters and also to other purchasers or through agents. We will set forth the names of any underwriters or agents, and any fees, conversions or discount arrangements, in the accompanying prospectus supplement. We may not sell any securities under this prospectus without delivery of the applicable prospectus supplement.
Our common stock is traded on The NASDAQ Global Market under the symbol “BOLD.” On August 7, 2017, the closing price for our common stock, as reported on The NASDAQ Global Market, was $19.83 per share. We are an “emerging growth company” as defined under the federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties referenced under the heading “Risk Factors” contained in this prospectus beginning on page 4 and any applicable prospectus supplement, and under similar headings in the other documents that are incorporated by reference into this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus is August 23, 2017.
Table of Contents
| Page | ||||
| ii | ||||
| 1 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| 12 | ||||
| 19 | ||||
| 21 | ||||
| 22 | ||||
| 23 | ||||
| 26 | ||||
| 26 | ||||
| 26 | ||||
| 26 | ||||
i
Table of Contents
This prospectus is part of a registration statement on Form S-3 that we filed with the United States Securities and Exchange Commission, or the SEC, using a “shelf” registration process. Under this shelf registration process, we may, from time to time, sell any combination of the securities described in this prospectus in one or more offerings up to a total amount of $250,000,000.
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide one or more prospectus supplements that will contain specific information about the terms of the offering. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement and any free writing prospectus may also add, update or change information contained in this prospectus. You should read this prospectus, the accompanying prospectus supplement and any free writing prospectus together with the additional information described under the heading “Where You Can Find More Information.”
You should rely only on the information contained in or incorporated by reference in this prospectus, any accompanying prospectus supplement or in any related free writing prospectus filed by us with the SEC. We have not authorized anyone to provide you with different information. This prospectus and the accompanying prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the securities described in the accompanying prospectus supplement or an offer to sell or the solicitation of an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful. You should assume that the information appearing in this prospectus, any prospectus supplement, the documents incorporated by reference and any related free writing prospectus is accurate only as of their respective dates. Our business, financial condition, results of operations and prospects may have changed materially since those dates.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find More Information.”
Unless the context otherwise indicates, references in this prospectus to “Audentes Therapeutics”, “we”, “our”, “us” and “the Company” refer, collectively, to Audentes Therapeutics, Inc., a Delaware corporation.
We own various U.S. federal trademark registrations and applications and unregistered trademarks, including our corporate logo. This prospectus and the information incorporated herein by reference contains references to trademarks, service marks and trade names referred to in this prospectus and the information incorporated herein, including logos, artwork, and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks, service marks or trade names. We do not intend our use or display of other companies’ trade names, service marks or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus, any applicable prospectus supplement or any related free writing prospectus are the property of their respective owners.
ii
Table of Contents
This summary highlights information contained in other parts of this prospectus or incorporated by reference in this prospectus from our Annual Report on Form 10-K for the year ended December 31, 2016, and our other filings with the SEC listed below under the heading “Incorporation of Certain Information by Reference.” This summary does not contain all of the information you should consider in making your investment decision. Before deciding to invest in our securities, you should read the entire prospectus, the applicable prospectus supplement and any related free writing prospectus, and the information incorporated by reference herein in their entirety. You should carefully consider, among other things, the matters discussed under the heading “Risk Factors” contained in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus. Some of the statements in this prospectus constitute forward-looking statements that involve risks and uncertainties. See “Special Note Regarding Forward-Looking Statements.”
Our Company
We are a biotechnology company focused on developing and commercializing gene therapy products for patients suffering from serious, life-threatening rare diseases caused by single gene defects. We believe that gene therapy has powerful potential to treat these diseases through delivery of a functional copy of the affected gene to cells, resulting in production of the normal protein. We have built a compelling portfolio of product candidates, including AT132 for the treatment of X-Linked Myotubular Myopathy, or XLMTM, AT342 for the treatment of Crigler-Najjar Syndrome, or Crigler-Najjar, AT982 for the treatment of Pompe disease and AT307 for the treatment of the CASQ2 subtype of Catecholaminergic Polymorphic Ventricular Tachycardia, or CASQ2-CPVT. The Investigational New Drug applications, or INDs, for both AT132 and AT342 are active, and our collaborating institution, the University of Florida, has an active IND to conduct a proof-of-concept study of AT982 delivered via intra-muscular injection in adults with Pompe disease. We are conducting IND-enabling preclinical studies for the systemic administration of AT982 for the treatment of Pompe disease and planning to conduct exploratory preclinical studies evaluating intrathecal delivery of AT982. We maintain full global rights to all our product candidates.
We have developed a proprietary in-house cGMP manufacturing capability that we believe provides us with a core strategic advantage, enabling superior control over development timelines, costs and intellectual property. Our manufacturing facility is located in South San Francisco in a building that we have improved to support our research, process development and manufacturing capabilities in accordance with current Good Manufacturing Practices, or cGMP, requirements. We believe we have established a comprehensive platform for production of our adeno-associated virus vector, or AAV, product candidates and plan continued investment to further optimize our manufacturing capabilities to cost-effectively produce high-quality AAV vectors at both clinical and commercial scale.
The Securities We May Offer
With this prospectus, we may from time to time issue, in one or more series or classes, up to $250,000,000 in aggregate principal amount of our common stock, preferred stock, debt securities, warrants, subscription rights and/or units in one or more offerings. We may offer these securities separately or together in units, and we may offer securities as may be issuable upon conversion, redemption, repurchase, exchange or exercise of any of the securities registered hereunder, including any applicable antidilution provisions. Each time we offer securities, we will specify in the accompanying prospectus supplement the terms of the securities being offered. The following is a summary of the securities we may offer with this prospectus.
1
Table of Contents
Common Stock
We may offer shares of our common stock, par value $0.00001 per share.
Preferred Stock
We may offer shares of our preferred stock, par value $0.00001 per share, in one or more series. Our board of directors or a committee designated by the board will determine the dividend, voting, conversion and other rights of the series of shares of preferred stock being offered. Each series of preferred stock will be more fully described in the particular prospectus supplement that will accompany this prospectus, including redemption provisions, rights in the event of our liquidation, dissolution or the winding up, voting rights and rights to convert into common stock.
Debt Securities
We may offer general obligations, which may be secured or unsecured, senior or subordinated and convertible into shares of our common stock or preferred stock. In this prospectus, we refer to the senior debt securities and the subordinated debt securities together as the “debt securities.” Our board of directors will determine the terms of each series of debt securities being offered.
We will issue the debt securities under an indenture between us and a trustee. In this document, we have summarized general features of the debt securities from the indenture. We encourage you to read the indenture, which is an exhibit to the registration statement of which this prospectus is a part.
Warrants
We may offer warrants for the purchase of debt securities, shares of preferred stock or shares of common stock. We may issue warrants independently or together with other securities. Our board of directors will determine the terms of the warrants.
Subscription Rights
We may offer subscription rights for the purchase of common stock, preferred stock or debt securities. We may issue subscription rights independently or together with other securities. Our board of directors will determine the terms of the subscription rights.
Units
We may offer units consisting of some or all of the securities described above, in any combination, including common stock, preferred stock, warrants and/or debt securities. The terms of these units will be set forth in a prospectus supplement. The description of the terms of these units in the related prospectus supplement will not be complete. You should refer to the applicable form of unit and unit agreement for complete information with respect to these units.
Corporate Information
We were incorporated in Delaware in November 2012. Our principal executive offices are located at 600 California Street, 17th Floor, San Francisco, California 94108, and our telephone number is (415) 818-1001. Our website address is www.audentestx.com. The information contained on, or that can be accessed through, our website is not a part of this prospectus. Investors should not rely on any such information in deciding whether to purchase our securities.
2
Table of Contents
Ratio of Earnings to Fixed Charges
The following table sets forth our ratio of earnings to fixed charges for the periods shown. We had no shares of preferred stock outstanding as of March 31, 2017, and no paid dividends on shares of preferred stock during the periods indicated. Therefore, the ratios of earnings to combined fixed charges and preferred dividends are the same as the ratios of earnings to fixed charges presented below.
| Year Ended December 31, | Three Months Ended March 31, 2017 |
|||||||||||||||||||
| 2013 | 2014 | 2015 | 2016 | |||||||||||||||||
| Ratio of earnings to fixed charges(1) |
– | – | – | – | – | |||||||||||||||
| (1) | The ratio of earnings to fixed charges represents the number of times that fixed charges are covered by earnings. For the periods presented, “earnings” consists of our net losses and “fixed charges” consists of estimated interest expense within rental expense. In the years ended December 31, 2013, 2014, 2015 and 2016 and the three months ended March 31, 2017 earnings were insufficient to cover fixed charges by $3.1 million, $10.9 million, $26.9 million, $60.5 million and $18.3 million, respectively. |
3
Table of Contents
Investing in our securities involves a high degree of risk. You should carefully consider the risks and uncertainties referenced below and described in the documents incorporated by reference in this prospectus and any prospectus supplement, as well as other information we include or incorporate by reference into this prospectus and any applicable prospectus supplement, before making an investment decision. Our business, financial condition or results of operations could be materially adversely affected by the materialization of any of these risks. The trading price of our securities could decline due to the materialization of any of these risks, and you may lose all or part of your investment. This prospectus and the documents incorporated herein by reference also contain forward-looking statements that involve risks and uncertainties. Actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks described in the documents incorporated herein by reference, including (i) our Annual Report on Form 10-K for the fiscal year ended December 31, 2016 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2017, which are on file with the SEC and incorporated by reference into this prospectus, and (ii) other documents we file with the SEC that are deemed incorporated by reference into this prospectus.
4
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference into this prospectus contain forward-looking statements. Forward-looking statements include all statements that are not historical facts and can be identified by the words “believe,” “may,” “will,” “potentially,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “plan,” “expect,” and similar expressions that convey uncertainty of future events or outcomes.
These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those described in and incorporated by reference under the heading “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment, and new risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties, and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations, except as required by law.
You should read this prospectus, the documents incorporated by reference into this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration statement of which this prospectus is a part, with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
5
Table of Contents
We will retain broad discretion over the use of the net proceeds to us from the sale of our securities under this prospectus. Unless otherwise provided in the applicable prospectus supplement, we intend to use the net proceeds from the sale of securities under this prospectus for general corporate purposes, which may include funding research and development of our product candidates, expanding our manufacturing capabilities, increasing our working capital, acquisitions or investments in businesses, products or technologies that are complementary to our own and capital expenditures. We will set forth in the prospectus supplement our intended use for the net proceeds received from the sale of any securities. Pending the application of the net proceeds, we intend to invest the net proceeds in short-term or long-term, investment-grade, interest-bearing securities.
6
Table of Contents
General
Our authorized capital stock consists of 300,000,000 shares of common stock, $0.00001 par value per share, and 10,000,000 shares of undesignated preferred stock, $0.00001 par value per share. As of March 31, 2017, there were 21,767,984 shares of our common stock outstanding, and no shares of preferred stock outstanding.
Common Stock
Dividend rights
Subject to preferences that may apply to any shares of preferred stock outstanding at the time, the holders of our common stock are entitled to receive dividends out of funds legally available if our board of directors, in its discretion, determines to issue dividends and then only at the times and in the amounts that our board of directors may determine.
Voting rights
Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. We have not provided for cumulative voting for the election of directors in our restated certificate of incorporation. Accordingly, holders of a majority of the shares of our common stock are able to elect all of our directors. Our restated certificate of incorporation establishes a classified board of directors, divided into three classes with staggered three-year terms. Only one class of directors is elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms.
No preemptive or similar rights
Our common stock is not entitled to preemptive rights, and is not subject to conversion, redemption or sinking fund provisions.
Right to receive liquidation distributions
Upon our liquidation, dissolution or winding-up, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our common stock and any participating preferred stock outstanding at that time, subject to prior satisfaction of all outstanding debt and liabilities and the preferential rights of and the payment of liquidation preferences, if any, on any outstanding shares of preferred stock..
Preferred Stock
Our board of directors is authorized, subject to limitations prescribed by Delaware law, to issue from time to time up to 10,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each series and to fix the designation, powers, preferences and rights of the shares of each series and any of their qualifications, limitations or restrictions, in each case without further vote or action by our stockholders. Our board of directors is also be able to increase or decrease the number of shares of any series of preferred stock, but not below the number of shares of that series then outstanding, without any further vote or action by our stockholders. Our board of directors may be able to authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of our company and might adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock. We have no current plan to issue any shares of preferred stock.
7
Table of Contents
Registration Rights
The holders of certain outstanding shares of our common stock, or their permitted transferees, are entitled to rights with respect to the registration of these shares under the Securities Act. These shares are referred to as registrable securities. These rights are provided under the terms of an amended and restated investors’ rights agreement between us and the holders of these shares, and include demand registration rights, short-form registration rights and piggyback registration rights. In any registration made pursuant to such amended and restated investors’ rights agreement, all fees, costs and expenses of underwritten registrations, including fees and disbursements of one counsel to the selling stockholders not to exceed $30,000, will be borne by us and all selling expenses, including estimated underwriting discounts and selling commissions, will be borne by the holders of the shares being registered.
The registration rights terminate five years following the completion of our initial public offering or, with respect to any particular stockholder, at such time as we have completed this offering and that stockholder can sell all of its shares during any three-month period pursuant to Rule 144 of the Securities Act.
Demand Registration Rights
Under the terms of the amended and restated investors’ rights agreement, if we receive a written request from the holders of at least 66.67% of the common stock (i) issued or issuable upon conversion of then-outstanding shares of preferred stock held by preferred stockholders under the amended and restated investors’ rights agreement and (ii) then held by Genethon, voting together as a single class on an as-converted to common stock basis, that we file a registration statement under the Securities Act covering the registration of outstanding registrable securities, then we will be required to use commercially reasonable efforts to register, as soon as practicable, and in any event within 90 days of such written request, all of the shares requested to be registered for public resale, if the amount of registrable securities to be registered will have anticipated aggregate gross proceeds (net of underwriting discounts, commissions, taxes and certain fees and expenses of counsel for selling stockholders) of at least $25.0 million. We are required to effect only two registrations pursuant to this provision of the amended and restated investors’ rights agreement. We may postpone the filing of a registration statement no more than once during any 12-month period for up to 90 days if our board of directors determines that the filing would be materially detrimental to us and our stockholders, but we shall not register any securities for our own account or that of any other stockholder during such 90-day period, subject to certain exceptions. We are not required to effect a demand registration under certain additional circumstances specified in the amended and restated investors’ rights agreement.
Form S-3 Registration Rights
Any holder of registrable securities then outstanding can request that we register all or part of their shares on Form S-3 if we are eligible to file a registration statement on Form S-3 and if the aggregate price to the public of the shares offered is at least $5.0 million (net of underwriting discounts, commissions, taxes and certain fees and expenses of counsel for selling stockholders). We shall not be obligated to effect a registration if we have effected two registrations within the 12-month period immediately preceding the date of the request. We may postpone the filing of a registration statement no more than once during any 12-month period for up to 90 days if our board of directors determines that the filing would be materially detrimental to us and our stockholders, but we shall not register any securities for our own account or that of any other stockholder during such 90-day period, subject to certain exceptions. We are not required to effect a registration on Form S-3 under certain additional circumstances specified in the amended and restated investors’ rights agreement.
Piggyback Registration Rights
In connection with this offering, holders of our registrable securities were entitled to, and the necessary percentage of holders waived, their rights to notice of this offering and to include their
8
Table of Contents
registrable securities in this offering. If we register any of our securities for public sale in another offering, holders of registrable securities will have the right to include their shares in the registration statement. However, this right does not apply to a registration relating to employee benefit plans, a registration relating to a corporate reorganization, a registration on any registration form that does not include substantially the same information as would be required to be included in a registration statement covering the sale of registrable securities or a registration in which the only common stock being registered is common stock issuable upon conversion of debt securities that are also being registered. If the total number of securities requested by stockholders to be included in such offering exceeds the number of securities to be sold (other than by us) that the underwriters in their reasonable discretion determine is compatible with the success of the offering, then we will be required to include in the offering only that number of securities that we and the underwriters determine will not jeopardize the success of the offering. In this case, the number of shares held by the selling stockholders to be registered will be allocated among the selling stockholders in proportion the number of registrable securities owned or held by each selling stockholders or in such other proportions as mutually agreed to by all such selling stockholders. However, the number of shares to be registered by these holders cannot be reduced below 30% of the total shares covered by the registration statement.
Anti-Takeover Provisions
The provisions of Delaware law, our restated certificate of incorporation and our restated bylaws could have the effect of delaying, deferring or discouraging another person from acquiring control of our company. These provisions, which are summarized below, may have the effect of discouraging takeover bids. They are also designed, in part, to encourage persons seeking to acquire control of us to negotiate first with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms.
Delaware Law
We are subject to the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, regulating corporate takeovers. In general, DGCL Section 203 prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder for a period of three years following the date on which the person became an interested stockholder unless:
| ∎ | prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| ∎ | the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, but not the outstanding voting stock owned by the interested stockholder, (i) shares owned by persons who are directors and also officers and (ii) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| ∎ | at or subsequent to the date of the transaction, the business combination is approved by the board of directors of the corporation and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66.67% of the outstanding voting stock that is not owned by the interested stockholder. |
Generally, a business combination includes a merger, asset or stock sale, or other transaction or series of transactions together resulting in a financial benefit to the interested stockholder. An interested stockholder is a person who, together with affiliates and associates, owns or, within three
9
Table of Contents
years prior to the determination of interested stockholder status, did own 15% or more of a corporation’s outstanding voting stock. We expect the existence of this provision to have an anti-takeover effect with respect to transactions our board of directors does not approve in advance. We also anticipate that DGCL Section 203 may also discourage attempts that might result in a premium over the market price for the shares of common stock held by stockholders.
Restated Certificate of Incorporation and Restated Bylaws Provisions
Our restated certificate of incorporation and our restated bylaws include a number of provisions that could deter hostile takeovers or delay or prevent changes in control of our company, including the following:
| ∎ | Board of directors vacancies. Our restated certificate of incorporation and restated bylaws authorize only our board of directors to fill vacant directorships, including newly created seats. In addition, the number of directors constituting our board of directors is permitted to be set only by a resolution adopted by a majority vote of our entire board of directors. These provisions would prevent a stockholder from increasing the size of our board of directors and then gaining control of our board of directors by filling the resulting vacancies with its own nominees. This makes it more difficult to change the composition of our board of directors but promotes continuity of management. |
| ∎ | Classified board. Our restated certificate of incorporation and restated bylaws provide that our board of directors will be classified into three classes of directors, each with staggered three-year terms. A third party may be discouraged from making a tender offer or otherwise attempting to obtain control of us as it is more difficult and time consuming for stockholders to replace a majority of the directors on a classified board of directors. See “Management—Board of Directors.” |
| ∎ | Stockholder action; special meetings of stockholders. Our restated certificate of incorporation provides that our stockholders may not take action by written consent, but may only take action at annual or special meetings of our stockholders. As a result, a holder controlling a majority of our capital stock would not be able to amend our restated bylaws or remove directors without holding a meeting of our stockholders called in accordance with our restated bylaws. Further, our restated bylaws and restated certificate of incorporation provide that special meetings of our stockholders may be called only by a majority of our board of directors, the chairman of our board of directors, our Chief Executive Officer or our President, thus prohibiting a stockholder from calling a special meeting. These provisions might delay the ability of our stockholders to force consideration of a proposal or for stockholders controlling a majority of our capital stock to take any action, including the removal of directors. |
| ∎ | Advance notice requirements for stockholder proposals and director nominations. Our restated bylaws provide advance notice procedures for stockholders seeking to bring business before our annual meeting of stockholders or to nominate candidates for election as directors at our annual meeting of stockholders. Our restated bylaws also specify certain requirements regarding the form and content of a stockholder’s notice. These provisions might preclude our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders if the proper procedures are not followed. We expect that these provisions might also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company. |
| ∎ | No cumulative voting. The Delaware General Corporation Law provides that stockholders are not entitled to the right to cumulate votes in the election of directors unless a corporation’s certificate of incorporation provides otherwise. Our restated certificate of incorporation does not provide for cumulative voting. |
10
Table of Contents
| ∎ | Directors removed only for cause. Our restated certificate of incorporation provides that stockholders may remove directors only for cause and only by the affirmative vote of the holders of at least two-thirds of our outstanding common stock. |
| ∎ | Amendment of charter provisions. Any amendment of the above provisions in our restated certificate of incorporation would require approval by holders of at least two-thirds of our outstanding common stock. |
| ∎ | Issuance of undesignated preferred stock. Our board of directors has the authority, without further action by the stockholders, to issue up to 10,000,000 shares of undesignated preferred stock with rights and preferences, including voting rights, designated from time to time by our board of directors. The existence of authorized but unissued shares of preferred stock would enable our board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or other means. |
| ∎ | Choice of forum. Our restated certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for: any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our restated certificate of incorporation or our restated bylaws; any action to interpret, apply, enforce or determine the validity of our restated certificate of incorporation or our restated bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that a court could find these types of provisions to be inapplicable or unenforceable. |
Exchange Listing
Our common stock is listed on The NASDAQ Global Market under the symbol “BOLD.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC. The transfer agent’s address is 6201 15th Avenue, Brooklyn, New York 11219, and its telephone number is (800) 937-5449.
11
Table of Contents
DESCRIPTION OF DEBT SECURITIES
General
We will issue the debt securities offered by this prospectus and any accompanying prospectus supplement under an indenture to be entered into between us and the trustee identified in the applicable prospectus supplement. The terms of the debt securities will include those stated in the indenture and those made part of the indenture by reference to the Trust Indenture Act of 1939, as in effect on the date of the indenture. We have filed a copy of the form of indenture as an exhibit to the registration statement in which this prospectus is included. The indenture will be subject to and governed by the terms of the Trust Indenture Act of 1939.
We may offer under this prospectus up to an aggregate principal amount of $250,000,000 in debt securities, or if debt securities are issued at a discount, or in a foreign currency, foreign currency units or composite currency, the principal amount as may be sold for an aggregate public offering price of up to $250,000,000. Unless otherwise specified in the applicable prospectus supplement, the debt securities will represent our direct, unsecured obligations and will rank equally with all of our other unsecured indebtedness.
We may issue the debt securities in one or more series with the same or various maturities, at par, at a premium, or at a discount. We will describe the particular terms of each series of debt securities in a prospectus supplement relating to that series, which we will file with the SEC. The prospectus supplement relating to the particular series of debt securities being offered will specify the particular amounts, prices and terms of those debt securities. These terms may include:
| ∎ | the title of the series; |
| ∎ | the aggregate principal amount, and, if a series, the total amount authorized and the total amount outstanding; |
| ∎ | the issue price or prices, expressed as a percentage of the aggregate principal amount of the debt securities; |
| ∎ | any limit on the aggregate principal amount; |
| ∎ | the date or dates on which principal is payable; |
| ∎ | the interest rate or rates (which may be fixed or variable) or, if applicable, the method used to determine such rate or rates; |
| ∎ | the date or dates from which interest, if any, will be payable and any regular record date for the interest payable; |
| ∎ | the place or places where principal and, if applicable, premium and interest, is payable; |
| ∎ | the terms and conditions upon which we may, or the holders may require us to, redeem or repurchase the debt securities; |
| ∎ | the denominations in which such debt securities may be issuable, if other than denominations of $1,000 or any integral multiple of that number; |
| ∎ | whether the debt securities are to be issuable in the form of certificated securities (as described below) or global securities (as described below); |
| ∎ | the portion of principal amount that will be payable upon declaration of acceleration of the maturity date if other than the principal amount of the debt securities; |
| ∎ | the currency of denomination; |
| ∎ | the designation of the currency, currencies or currency units in which payment of principal and, if applicable, premium and interest, will be made; |
| ∎ | if payments of principal and, if applicable, premium or interest, on the debt securities are to be made in one or more currencies or currency units other than the currency of denomination, the manner in which the exchange rate with respect to such payments will be determined; |
12
Table of Contents
| ∎ | if amounts of principal and, if applicable, premium and interest may be determined by reference to an index based on a currency or currencies or by reference to a commodity, commodity index, stock exchange index or financial index, then the manner in which such amounts will be determined; |
| ∎ | the provisions, if any, relating to any collateral provided for such debt securities; |
| ∎ | any addition to or change in the covenants and/or the acceleration provisions described in this prospectus or in the indenture; |
| ∎ | any events of default, if not otherwise described below under “Events of Default”; |
| ∎ | the terms and conditions, if any, for conversion into or exchange for shares of our common stock or preferred stock; |
| ∎ | any depositaries, interest rate calculation agents, exchange rate calculation agents or other agents; and |
| ∎ | the terms and conditions, if any, upon which the debt securities shall be subordinated in right of payment to our other indebtedness. |
We may issue discount debt securities that provide for an amount less than the stated principal amount to be due and payable upon acceleration of the maturity of such debt securities in accordance with the terms of the indenture. We may also issue debt securities in bearer form, with or without coupons. If we issue discount debt securities or debt securities in bearer form, we will describe material U.S. federal income tax considerations and other material special considerations which apply to these debt securities in the applicable prospectus supplement.
We may issue debt securities denominated in or payable in a foreign currency or currencies or a foreign currency unit or units. If we do, we will describe the restrictions, elections, and general tax considerations relating to the debt securities and the foreign currency or currencies or foreign currency unit or units in the applicable prospectus supplement.
Debt securities offered under this prospectus and any prospectus supplement will be subordinated in right of payment to certain of our outstanding senior indebtedness. In addition, we will seek the consent of the holders of any such senior indebtedness prior to issuing any debt securities under this prospectus to the extent required by the agreements evidencing such senior indebtedness.
Registrar and Paying Agent
The debt securities may be presented for registration of transfer or for exchange at the corporate trust office of the security registrar or at any other office or agency that we maintain for those purposes. In addition, the debt securities may be presented for payment of principal, interest and any premium at the office of the paying agent or at any office or agency that we maintain for those purposes.
Conversion or Exchange Rights
Debt securities may be convertible into or exchangeable for shares of our common stock. The terms and conditions of conversion or exchange will be stated in the applicable prospectus supplement. The terms will include, among others, the following:
| ∎ | the conversion or exchange price; |
| ∎ | the conversion or exchange period; |
| ∎ | provisions regarding the convertibility or exchangeability of the debt securities, including who may convert or exchange; |
| ∎ | events requiring adjustment to the conversion or exchange price; |
| ∎ | provisions affecting conversion or exchange in the event of our redemption of the debt securities; and |
| ∎ | any anti-dilution provisions, if applicable. |
13
Table of Contents
Registered Global Securities
If we decide to issue debt securities in the form of one or more global securities, then we will register the global securities in the name of the depositary for the global securities or the nominee of the depositary, and the global securities will be delivered by the trustee to the depositary for credit to the accounts of the holders of beneficial interests in the debt securities.
The prospectus supplement will describe the specific terms of the depositary arrangement for debt securities of a series that are issued in global form. None of us, the trustee, any payment agent or the security registrar will have any responsibility or liability for any aspect of the records relating to or payments made on account of beneficial ownership interests in a global debt security or for maintaining, supervising or reviewing any records relating to these beneficial ownership interests.
No Protection in the Event of Change of Control
The indenture does not have any covenants or other provisions providing for a put or increased interest or otherwise that would afford holders of our debt securities additional protection in the event of a recapitalization transaction, a change of control or a highly leveraged transaction. If we offer any covenants or provisions of this type with respect to any debt securities covered by this prospectus, we will describe them in the applicable prospectus supplement.
Covenants
Unless otherwise indicated in this prospectus or the applicable prospectus supplement, our debt securities will not have the benefit of any covenants that limit or restrict our business or operations, the pledging of our assets or the incurrence by us of indebtedness. We will describe in the applicable prospectus supplement any material covenants in respect of a series of debt securities.
Merger, Consolidation or Sale of Assets
The form of indenture provides that we will not consolidate with or merge into any other person or convey, transfer, sell or lease our properties and assets substantially as an entirety to any person, unless:
| ∎ | we are the surviving person of such merger or consolidation, or if we are not the surviving person, the person formed by the consolidation or into or with which we are merged or the person to which our properties and assets are conveyed, transferred, sold or leased, is a corporation organized and existing under the laws of the U.S., any state or the District of Columbia or a corporation or comparable legal entity organized under the laws of a foreign jurisdiction and has expressly assumed all of our obligations, including the payment of the principal of and, premium, if any, and interest on the debt securities and the performance of the other covenants under the indenture; and |
| ∎ | immediately before and immediately after giving effect to the transaction on a pro forma basis, no event of default, and no event which, after notice or lapse of time or both, would become an event of default, has occurred and is continuing under the indenture. |
Events of Default
Unless otherwise specified in the applicable prospectus supplement, the following events will be events of default under the indenture with respect to debt securities of any series:
| ∎ | we fail to pay any principal or premium, if any, when it becomes due; |
| ∎ | we fail to pay any interest within 30 days after it becomes due; |
| ∎ | we fail to observe or perform any other covenant in the debt securities or the indenture for 60 days after written notice specifying the failure from the trustee or the holders of not less than 25% in aggregate principal amount of the outstanding debt securities of that series; and |
14
Table of Contents
| ∎ | certain events involving bankruptcy, insolvency or reorganization of us or any of our significant subsidiaries. |
The trustee may withhold notice to the holders of the debt securities of any series of any default, except in payment of principal of or premium, if any, or interest on the debt securities of a series, if the trustee considers it to be in the best interest of the holders of the debt securities of that series to do so.
If an event of default (other than an event of default resulting from certain events of bankruptcy, insolvency or reorganization) occurs, and is continuing, then the trustee or the holders of not less than 25% in aggregate principal amount of the outstanding debt securities of any series may accelerate the maturity of the debt securities. If this happens, the entire principal amount, plus the premium, if any, of all the outstanding debt securities of the affected series plus accrued interest to the date of acceleration will be immediately due and payable. At any time after the acceleration, but before a judgment or decree based on such acceleration is obtained by the trustee, the holders of a majority in aggregate principal amount of outstanding debt securities of such series may rescind and annul such acceleration if:
| ∎ | all events of default (other than nonpayment of accelerated principal, premium or interest) have been cured or waived; |
| ∎ | all lawful interest on overdue interest and overdue principal has been paid; and |
| ∎ | the rescission would not conflict with any judgment or decree. |
In addition, if the acceleration occurs at any time when we have outstanding indebtedness that is senior to the debt securities, the payment of the principal amount of outstanding debt securities may be subordinated in right of payment to the prior payment of any amounts due under the senior indebtedness, in which case the holders of debt securities will be entitled to payment under the terms prescribed in the instruments evidencing the senior indebtedness and the indenture.
If an event of default resulting from certain events of bankruptcy, insolvency or reorganization occurs, the principal, premium and interest amount with respect to all of the debt securities of any series will be due and payable immediately without any declaration or other act on the part of the trustee or the holders of the debt securities of that series.
The holders of a majority in principal amount of the outstanding debt securities of a series will have the right to waive any existing default or compliance with any provision of the indenture or the debt securities of that series and to direct the time, method and place of conducting any proceeding for any remedy available to the trustee, subject to certain limitations specified in the indenture.
No holder of any debt security of a series will have any right to institute any proceeding with respect to the indenture or for any remedy under the indenture, unless:
| ∎ | the holder gives to the trustee written notice of a continuing event of default; |
| ∎ | the holders of at least 25% in aggregate principal amount of the outstanding debt securities of the affected series make a written request and offer reasonable indemnity to the trustee to institute a proceeding as trustee; |
| ∎ | the trustee fails to institute a proceeding within 60 days after such request; and |
| ∎ | the holders of a majority in aggregate principal amount of the outstanding debt securities of the affected series do not give the trustee a direction inconsistent with such request during such 60-day period. |
These limitations do not, however, apply to a suit instituted for payment on debt securities of any series on or after the due dates expressed in the debt securities.
15
Table of Contents
We will periodically deliver certificates to the trustee regarding our compliance with our obligations under the indenture.
Modification and Waiver
From time to time, we and the trustee may, without the consent of holders of the debt securities of one or more series, amend the indenture or the debt securities of one or more series, or supplement the indenture, for certain specified purposes, including:
| ∎ | to provide that the surviving entity following a change of control permitted under the indenture will assume all of our obligations under the indenture and debt securities; |
| ∎ | to provide for certificated debt securities in addition to uncertificated debt securities; |
| ∎ | to comply with any requirements of the SEC under the Trust Indenture Act of 1939; |
| ∎ | to provide for the issuance of and establish the form and terms and conditions of debt securities of any series as permitted by the indenture; |
| ∎ | to cure any ambiguity, defect or inconsistency, or make any other change that does not materially and adversely affect the rights of any holder; and |
| ∎ | to appoint a successor trustee under the indenture with respect to one or more series. |
From time to time we and the trustee may, with the consent of holders of at least a majority in principal amount of an outstanding series of debt securities, amend or supplement the indenture or the debt securities series, or waive compliance in a particular instance by us with any provision of the indenture or the debt securities. We may not, however, without the consent of each holder affected by such action, modify or supplement the indenture or the debt securities or waive compliance with any provision of the indenture or the debt securities in order to:
| ∎ | reduce the amount of debt securities whose holders must consent to an amendment, supplement, or waiver to the indenture or such debt security; |
| ∎ | reduce the rate of or change the time for payment of interest or reduce the amount of or postpone the date for payment of sinking fund or analogous obligations; |
| ∎ | reduce the principal of or change the stated maturity of the debt securities; |
| ∎ | make any debt security payable in money other than that stated in the debt security; |
| ∎ | change the amount or time of any payment required or reduce the premium payable upon any redemption, or change the time before which no such redemption may be made; |
| ∎ | waive a default in the payment of the principal of, premium, if any, or interest on the debt securities or a redemption payment; |
| ∎ | waive a redemption payment with respect to any debt securities or change any provision with respect to redemption of debt securities; or |
| ∎ | take any other action otherwise prohibited by the indenture to be taken without the consent of each holder affected by the action. |
Defeasance of Debt Securities and Certain Covenants in Certain Circumstances
The indenture permits us, at any time, to elect to discharge our obligations with respect to one or more series of debt securities by following certain procedures described in the indenture. These procedures will allow us either:
| ∎ | to defease and be discharged from any and all of our obligations with respect to any debt securities except for the following obligations (which discharge is referred to as “legal defeasance”): |
1. to register the transfer or exchange of such debt securities;
16
Table of Contents
2. to replace temporary or mutilated, destroyed, lost or stolen debt securities;
3. to compensate and indemnify the trustee; or
4. to maintain an office or agency in respect of the debt securities and to hold monies for payment in trust; or
| ∎ | to be released from our obligations with respect to the debt securities under certain covenants contained in the indenture, as well as any additional covenants which may be contained in the applicable supplemental indenture (which release is referred to as “covenant defeasance”). |
In order to exercise either defeasance option, we must irrevocably deposit with the trustee or other qualifying trustee, in trust for that purpose:
| ∎ | money; |
| ∎ | U.S. Government Obligations (as described below) or Foreign Government Obligations (as described below) that through the scheduled payment of principal and interest in accordance with their terms will provide money; or |
| ∎ | a combination of money and/or U.S. Government Obligations and/or Foreign Government Obligations sufficient in the written opinion of a nationally-recognized firm of independent accountants to provide money; |
that, in each case specified above, provides a sufficient amount to pay the principal of, premium, if any, and interest, if any, on the debt securities of the series, on the scheduled due dates or on a selected date of redemption in accordance with the terms of the indenture.
In addition, defeasance may be effected only if, among other things:
| ∎ | in the case of either legal or covenant defeasance, we deliver to the trustee an opinion of counsel, as specified in the indenture, stating that as a result of the defeasance neither the trust nor the trustee will be required to register as an investment company under the Investment Company Act of 1940; |
| ∎ | in the case of legal defeasance, we deliver to the trustee an opinion of counsel stating that we have received from, or there has been published by, the Internal Revenue Service a ruling to the effect that, or there has been a change in any applicable federal income tax law with the effect that (and the opinion shall confirm that), the holders of outstanding debt securities will not recognize income, gain or loss for U.S. federal income tax purposes solely as a result of such legal defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner, including as a result of prepayment, and at the same times as would have been the case if legal defeasance had not occurred; |
| ∎ | in the case of covenant defeasance, we deliver to the trustee an opinion of counsel to the effect that the holders of the outstanding debt securities will not recognize income, gain or loss for U.S. federal income tax purposes as a result of covenant defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if covenant defeasance had not occurred; and |
| ∎ | certain other conditions described in the indenture are satisfied. |
If we fail to comply with our remaining obligations under the indenture and applicable supplemental indenture after a covenant defeasance of the indenture and applicable supplemental indenture, and the debt securities are declared due and payable because of the occurrence of any undefeased event of default, the amount of money and/or U.S. Government Obligations and/or Foreign Government Obligations on deposit with the trustee could be insufficient to pay amounts due under the debt securities of the affected series at the time of acceleration. We will, however, remain liable in respect of these payments.
17
Table of Contents
The term “U.S. Government Obligations” as used in the above discussion means securities that are direct obligations of or non-callable obligations guaranteed by the United States of America for the payment of which obligation or guarantee the full faith and credit of the United States of America is pledged.
The term “Foreign Government Obligations” as used in the above discussion means, with respect to debt securities of any series that are denominated in a currency other than U.S. dollars, (1) direct obligations of the government that issued or caused to be issued such currency for the payment of which obligations its full faith and credit is pledged or (2) obligations of a person controlled or supervised by or acting as an agent or instrumentality of such government the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by that government, which in either case under clauses (1) or (2), are not callable or redeemable at the option of the issuer.
Regarding the Trustee
We will identify the trustee with respect to any series of debt securities in the prospectus supplement relating to the applicable debt securities. You should note that if the trustee becomes a creditor of ours, the indenture and the Trust Indenture Act of 1939 limit the rights of the trustee to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim, as security or otherwise. The trustee and its affiliates may engage in, and will be permitted to continue to engage in, other transactions with us and our affiliates. If, however, the trustee acquires any “conflicting interest” within the meaning of the Trust Indenture Act of 1939, it must eliminate such conflict or resign.
The holders of a majority in principal amount of the then outstanding debt securities of any series may direct the time, method and place of conducting any proceeding for exercising any remedy available to the trustee. If an event of default occurs and is continuing, the trustee, in the exercise of its rights and powers, must use the degree of care and skill of a prudent person in the conduct of his or her own affairs. Subject to that provision, the trustee will be under no obligation to exercise any of its rights or powers under the indenture at the request of any of the holders of the debt securities, unless they have offered to the trustee reasonable indemnity or security.
No Individual Liability of Incorporators, Stockholders, Officers or Directors
Each indenture provides that no incorporator and no past, present or future stockholder, officer or director of our company or any successor corporation in those capacities will have any individual liability for any of our obligations, covenants or agreements under the debt securities or such indenture.
Governing Law
The indentures and the debt securities will be governed by, and construed in accordance with, the laws of the State of New York.
18
Table of Contents
General
We may issue warrants for the purchase of our debt securities, preferred stock, common stock, or any combination thereof. Warrants may be issued independently or together with our debt securities, preferred stock or common stock and may be attached to or separate from any offered securities. Each series of warrants will be issued under a separate warrant agreement to be entered into between us and a bank or trust company, as warrant agent. The warrant agent will act solely as our agent in connection with the warrants. The warrant agent will not have any obligation or relationship of agency or trust for or with any holders or beneficial owners of warrants. This summary of certain provisions of the warrants is not complete. For the terms of a particular series of warrants, you should refer to the prospectus supplement for that series of warrants and the warrant agreement for that particular series.
Debt Warrants
The prospectus supplement relating to a particular issue of warrants to purchase debt securities will describe the terms of the debt warrants, including the following:
| ∎ | the title of the debt warrants; |
| ∎ | the offering price for the debt warrants, if any; |
| ∎ | the aggregate number of the debt warrants; |
| ∎ | the designation and terms of the debt securities, including any conversion rights, purchasable upon exercise of the debt warrants; |
| ∎ | if applicable, the date from and after which the debt warrants and any debt securities issued with them will be separately transferable; |
| ∎ | the principal amount of debt securities that may be purchased upon exercise of a debt warrant and the exercise price for the warrants, which may be payable in cash, securities or other property; |
| ∎ | the dates on which the right to exercise the debt warrants will commence and expire; |
| ∎ | if applicable, the minimum or maximum amount of the debt warrants that may be exercised at any one time; |
| ∎ | whether the debt warrants represented by the debt warrant certificates or debt securities that may be issued upon exercise of the debt warrants will be issued in registered or bearer form; |
| ∎ | information with respect to book-entry procedures, if any; |
| ∎ | the currency or currency units in which the offering price, if any, and the exercise price are payable; |
| ∎ | if applicable, a discussion of material U.S. federal income tax considerations; |
| ∎ | the antidilution provisions of the debt warrants, if any; |
| ∎ | the redemption or call provisions, if any, applicable to the debt warrants; |
| ∎ | any provisions with respect to the holder’s right to require us to repurchase the debt warrants upon a change in control or similar event; and |
| ∎ | any additional terms of the debt warrants, including procedures and limitations relating to the exchange, exercise, and settlement of the debt warrants. |
Debt warrant certificates will be exchangeable for new debt warrant certificates of different denominations. Debt warrants may be exercised at the corporate trust office of the warrant agent or any other office indicated in the prospectus supplement. Prior to the exercise of their debt warrants, holders of debt warrants will not have any of the rights of holders of the debt securities purchasable upon exercise and will not be entitled to payment of principal or any premium, if any, or interest on the debt securities purchasable upon exercise.
19
Table of Contents
Equity Warrants
The prospectus supplement relating to a particular series of warrants to purchase our common stock or preferred stock will describe the terms of the warrants, including the following:
| ∎ | the title of the warrants; |
| ∎ | the offering price for the warrants, if any; |
| ∎ | the aggregate number of warrants; |
| ∎ | the designation and terms of the common stock or preferred stock that may be purchased upon exercise of the warrants; |
| ∎ | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each security; |
| ∎ | if applicable, the date from and after which the warrants and any securities issued with the warrants will be separately transferable; |
| ∎ | the number of shares of common stock or preferred stock that may be purchased upon exercise of a warrant and the exercise price for the warrants; |
| ∎ | the dates on which the right to exercise the warrants shall commence and expire; |
| ∎ | if applicable, the minimum or maximum amount of the warrants that may be exercised at any one time; |
| ∎ | the currency or currency units in which the offering price, if any, and the exercise price are payable; |
| ∎ | if applicable, a discussion of material U.S. federal income tax considerations; |
| ∎ | the antidilution provisions of the warrants, if any; |
| ∎ | the redemption or call provisions, if any, applicable to the warrants; |
| ∎ | any provisions with respect to a holder’s right to require us to repurchase the warrants upon a change in control or similar event; and |
| ∎ | any additional terms of the warrants, including procedures and limitations relating to the exchange, exercise and settlement of the warrants. |
Holders of equity warrants will not be entitled:
| ∎ | to vote, consent, or receive dividends; |
| ∎ | receive notice as stockholders with respect to any meeting of stockholders for the election of our directors or any other matter; or |
| ∎ | exercise any rights as stockholders. |
20
Table of Contents
DESCRIPTION OF SUBSCRIPTION RIGHTS
We may issue subscription rights to purchase our common stock, preferred stock or debt securities. These subscription rights may be offered independently or together with any other security offered hereby and may or may not be transferable by the stockholder receiving the subscription rights in such offering. In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for after such offering.
The prospectus supplement relating to any subscription rights we offer, if any, will, to the extent applicable, include specific terms relating to the offering, including some or all of the following:
| ∎ | the price, if any, for the subscription rights; |
| ∎ | the exercise price payable for our common stock, preferred stock or debt securities upon the exercise of the subscription rights; |
| ∎ | the number of subscription rights to be issued to each stockholder; |
| ∎ | the number and terms of our common stock, preferred stock or debt securities which may be purchased per each subscription right; |
| ∎ | the extent to which the subscription rights are transferable; |
| ∎ | any other terms of the subscription rights, including the terms, procedures and limitations relating to the exchange and exercise of the subscription rights; |
| ∎ | the date on which the right to exercise the subscription rights shall commence, and the date on which the subscription rights shall expire; |
| ∎ | the extent to which the subscription rights may include an over-subscription privilege with respect to unsubscribed securities or an over-allotment privilege to the extent the securities are fully subscribed; and |
| ∎ | if applicable, the material terms of any standby underwriting or purchase arrangement which may be entered into by us in connection with the offering of subscription rights. |
The description in the applicable prospectus supplement of any subscription rights we offer will not necessarily be complete and will be qualified in its entirety by reference to the applicable subscription rights certificate, which will be filed with the SEC if we offer subscription rights. We urge you to read the applicable subscription rights certificate and any applicable prospectus supplement in their entirety.
21
Table of Contents
We may issue units consisting of some or all of the securities described above, in any combination, including common stock, preferred stock, warrants and/or debt securities. The terms of these units will be set forth in a prospectus supplement. The description of the terms of these units in the related prospectus supplement will not be complete. You should refer to the applicable form of unit and unit agreement for complete information with respect to these units.
22
Table of Contents
We may sell securities:
| ∎ | through underwriters; |
| ∎ | through dealers; |
| ∎ | through agents; |
| ∎ | directly to purchasers; or |
| ∎ | through a combination of any of these methods or any other method permitted by law. |
In addition, we may issue the securities as a dividend or distribution or in a subscription rights offering to our existing security holders.
We may directly solicit offers to purchase securities, or agents may be designated to solicit such offers. In the prospectus supplement relating to such offering, we will name any agent that could be viewed as an underwriter under the Securities Act and describe any commissions that we must pay to any such agent. Any such agent will be acting on a best efforts basis for the period of its appointment or, if indicated in the applicable prospectus supplement, on a firm commitment basis. This prospectus may be used in connection with any offering of our securities through any of these methods or other methods described in the applicable prospectus supplement.
The distribution of the securities may be effected from time to time in one or more transactions:
| ∎ | at a fixed price, or prices, which may be changed from time to time; |
| ∎ | at market prices prevailing at the time of sale; |
| ∎ | at prices related to such prevailing market prices; or |
| ∎ | at negotiated prices. |
Each prospectus supplement will describe the method of distribution of the securities and any applicable restrictions.
The prospectus supplement with respect to the securities of a particular series will describe the terms of the offering of the securities, including the following:
| ∎ | the name of the agent or any underwriters; |
| ∎ | the public offering or purchase price; |
| ∎ | any discounts and commissions to be allowed or paid to the agent or underwriters; |
| ∎ | all other items constituting underwriting compensation; |
| ∎ | any discounts and commissions to be allowed or paid to dealers; and |
| ∎ | any exchanges on which the securities will be listed. |
If any underwriters or agents are used in the sale of the securities in respect of which this prospectus is delivered, we will enter into an underwriting agreement, sales agreement or other agreement with them at the time of sale to them, and we will set forth in the prospectus supplement relating to such offering the names of the underwriters or agents and the terms of the related agreement with them.
In connection with the offering of securities, we may grant to the underwriters an option to purchase additional securities with an additional underwriting commission, as may be set forth in the accompanying prospectus supplement. If we grant any such option, the terms of such option will be set forth in the prospectus supplement for such securities.
23
Table of Contents
If a dealer is used in the sale of the securities in respect of which the prospectus is delivered, we will sell such securities to the dealer, as principal. The dealer, who may be deemed to be an “underwriter” as that term is defined in the Securities Act, may then resell such securities to the public at varying prices to be determined by such dealer at the time of resale.
If we offer securities in a subscription rights offering to our existing security holders, we may enter into a standby underwriting agreement with dealers, acting as standby underwriters. We may pay the standby underwriters a commitment fee for the securities they commit to purchase on a standby basis. If we do not enter into a standby underwriting arrangement, we may retain a dealer-manager to manage a subscription rights offering for us.
Agents, underwriters, dealers and other persons may be entitled under agreements which they may enter into with us to indemnification by us against certain civil liabilities, including liabilities under the Securities Act, and may be customers of, engage in transactions with or perform services for us in the ordinary course of business.
If so indicated in the applicable prospectus supplement, we will authorize underwriters or other persons acting as our agents to solicit offers by certain institutions to purchase securities from us pursuant to delayed delivery contracts providing for payment and delivery on the date stated in the prospectus supplement. Each contract will be for an amount not less than, and the aggregate amount of securities sold pursuant to such contracts shall not be less nor more than, the respective amounts stated in the prospectus supplement. Institutions with whom the contracts, when authorized, may be made include commercial and savings banks, insurance companies, pension funds, investment companies, educational and charitable institutions and other institutions, but shall in all cases be subject to our approval. Delayed delivery contracts will not be subject to any conditions except that:
| ∎ | the purchase by an institution of the securities covered under that contract shall not at the time of delivery be prohibited under the laws of the jurisdiction to which that institution is subject; and |
| ∎ | if the securities are also being sold to underwriters acting as principals for their own account, the underwriters shall have purchased such securities not sold for delayed delivery. The underwriters and other persons acting as our agents will not have any responsibility in respect of the validity or performance of delayed delivery contracts. |
Offered securities may also be offered and sold, if so indicated in the prospectus supplement, in connection with a remarketing upon their purchase, in accordance with a redemption or repayment pursuant to their terms, or otherwise, by one or more remarketing firms, acting as principals for their own accounts or as agents for us. Any remarketing firm will be identified and the terms of its agreement, if any, with us and its compensation will be described in the applicable prospectus supplement. Remarketing firms may be deemed to be underwriters in connection with their remarketing of offered securities.
Certain agents, underwriters and dealers, and their associates and affiliates, may be customers of, have borrowing relationships with, engage in other transactions with, or perform services, including investment banking services, for us or one or more of our respective affiliates in the ordinary course of business.
In order to facilitate the offering of the securities, any underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the securities or any other securities the prices of which may be used to determine payments on such securities. Specifically, any underwriters may overallot in connection with the offering, creating a short position for their own accounts. In addition, to cover overallotments or to stabilize the price of the securities or of any such other securities, the
24
Table of Contents
underwriters may bid for, and purchase, the securities or any such other securities in the open market. Finally, in any offering of the securities through a syndicate of underwriters, the underwriting syndicate may reclaim selling concessions allowed to an underwriter or a dealer for distributing the securities in the offering if the syndicate repurchases previously distributed securities in transactions to cover syndicate short positions, in stabilization transactions or otherwise. Any of these activities may stabilize or maintain the market price of the securities above independent market levels. Any such underwriters are not required to engage in these activities and may end any of these activities at any time.
We may engage in at the market offerings into an existing trading market in accordance with Rule 415(a)(4) under the Securities Act. In addition, we may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable prospectus supplement so indicates, in connection with those derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of stock. The third party in such sale transactions will be an underwriter and, if not identified in this prospectus, will be named in the applicable prospectus supplement (or a post-effective amendment). In addition, we may otherwise loan or pledge securities to a financial institution or other third party that in turn may sell the securities short using this prospectus and an applicable prospectus supplement. Such financial institution or other third party may transfer its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
Under Rule 15c6-1 of the Exchange Act, trades in the secondary market generally are required to settle in two business days, unless the parties to any such trade expressly agree otherwise or the securities are sold by us to an underwriter in a firm commitment underwritten offering. The applicable prospectus supplement may provide that the original issue date for your securities may be more than two scheduled business days after the trade date for your securities. Accordingly, in such a case, if you wish to trade securities on any date prior to the second business day before the original issue date for your securities, you will be required, by virtue of the fact that your securities initially are expected to settle in more than two scheduled business days after the trade date for your securities, to make alternative settlement arrangements to prevent a failed settlement.
The securities may be new issues of securities and may have no established trading market. The securities may or may not be listed on a national securities exchange. We can make no assurance as to the liquidity of or the existence of trading markets for any of the securities.
The specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
The underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business for which they receive compensation.
The anticipated date of delivery of offered securities will be set forth in the applicable prospectus supplement relating to each offer.
25
Table of Contents
Certain legal matters in connection with this offering will be passed upon for us by Fenwick & West LLP, San Francisco, California. Any underwriters will also be advised about the validity of the securities and other legal matters by their own counsel, which will be named in the applicable prospectus supplement.
The consolidated financial statements of Audentes Therapeutics, Inc. as of December 31, 2016 and 2015, and for each of the years in the three-year period ended December 31, 2016, have been incorporated by reference herein in reliance upon the report of KPMG LLP, independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the securities offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, the exhibits filed therewith or the documents incorporated by reference therein. For further information about us and the securities offered hereby, reference is made to the registration statement, the exhibits filed therewith and the documents incorporated by reference therein. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and in each instance we refer you to the copy of such contract or other document filed as an exhibit to the registration statement. A copy of the registration statement and the exhibits filed therewith may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street, NE, Washington, DC 20549, and copies of all or any part of the registration statement may be obtained from that office. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the website is www.sec.gov.
We are subject to the information and reporting requirements of the Exchange Act, and, in accordance with this law, file periodic reports and other information with the SEC. These periodic reports and other information are available for inspection and copying at the SEC’s public reference facilities and the website of the SEC referred to above. We also maintain a website at www.audentestx.com. You may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this prospectus and the inclusion of our website address in this prospectus is an inactive textual reference only. You may also inspect these documents at our corporate headquarters at 600 California Street, 17th Floor, San Francisco, California 94108, during normal business hours.
The SEC allows us to incorporate by reference the information and reports we file with it, which means that we can disclose important information to you by referring you to these documents. The information incorporated by reference is an important part of this prospectus, and information that we file later with the SEC will automatically update and supersede the information already incorporated by
26
Table of Contents
reference. We are incorporating by reference the documents listed below, which we have already filed with the SEC, and any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including all filings made after the date of the filing of this registration statement and prior to the effectiveness of this registration statement, except as to any portion of any future report or document that is not deemed filed under such provisions, after the date of this prospectus and prior to the termination of this offering:
| ∎ | our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, filed with the SEC on March 13, 2017; |
| ∎ | the information specifically incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2016 from our definitive proxy statement on Schedule 14A, which was filed with the SEC on April 27, 2017; |
| ∎ | our Quarterly Report on Form 10-Q for the quarter ended March 31, 2017, filed with the SEC on May 11, 2017; |
| ∎ | our Current Reports on Form 8-K filed with the SEC on May 5, 2017, June 7, 2017, June 13, 2017 and July 14, 2017 (in each case, except for information contained therein which is furnished rather than filed); and |
| ∎ | the description of capital stock included in our registration statement on Form 8-A, filed with the SEC on July 13, 2016, and any amendments or reports filed for the purpose of updating such description. |
Upon request, we will provide, without charge, to each person, including any beneficial owner, to whom a copy of this prospectus is delivered, a copy of the documents incorporated by reference into this prospectus but not delivered with the prospectus. You may request a copy of these filings, and any exhibits we have specifically incorporated by reference as an exhibit in this prospectus, at no cost, by writing or telephoning us at 600 California Street, 17th Floor, San Francisco, California 94108; (415) 818-1001.
You may also access these documents, free of charge on the SEC’s website at www.sec.gov or on our website at www.audentestx.com. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information on, or that can be accessed from, our website as part of this prospectus or any accompanying prospectus supplement.
This prospectus is part of a registration statement we filed with the SEC. We have incorporated exhibits into this registration statement. You should read the exhibits carefully for provisions that may be important to you.
You should rely only on the information incorporated by reference or provided in this prospectus or any prospectus supplement. We have not authorized anyone to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information in this prospectus or in the documents incorporated by reference is accurate as of any date other than the date on the front of this prospectus or those documents.
27
Table of Contents
$150,000,000

Common Stock
PROSPECTUS SUPPLEMENT
BofA Merrill Lynch
Cowen
Leerink Partners
Wedbush PacGrow
, 2018
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Piper Sandler Starts Boundless Bio Inc. (BOLD) at Overweight
- GOODRX ALERT: Bragar Eagel & Squire, P.C. Announces that a Class Action Lawsuit Has Been Filed Against GoodRx Holdings, Inc. and Encourages Investors to Contact the Firm
- Mueller Water Products Announces Quarterly Dividend
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share