Form 8-K Audentes Therapeutics, For: Jan 04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 4, 2018
AUDENTES THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
Delaware
(State or other jurisdiction of incorporation)
| 001-37833 | 46-1606174 | |
| (Commission File Number) |
(IRS Employer Identification No.) | |
| 600 California Street, 17th Floor San Francisco, California |
94108 | |
| (Address of principal executive offices) | (Zip Code) | |
(415) 818-1001
(Registrant’s telephone number, including area code)
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR 230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR 240.12b-2).
Emerging growth company ☒
If an emerging grown company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
| Item 7.01. | Regulation FD Disclosure. |
On January 4, 2018, Audentes Therapeutics, Inc. (the “Company”) issued a press release attached hereto as Exhibit 99.1 announcing the results of data from the first interim analysis of ASPIRO patients, and the investor presentation attached hereto as Exhibit 99.2. ASPIRO is a multicenter, multinational, open-label, ascending dose, delayed-treatment control study to evaluate the safety and preliminary efficacy of AT132 in approximately 12 XLMTM patients less than five years of age.
The information furnished with this report, including Exhibit 99.1 and Exhibit 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Exchange Act or the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits. |
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| AUDENTES THERAPEUTICS, INC. | ||
| By: | /s/ Thomas Soloway | |
| Thomas Soloway | ||
| Chief Financial Officer | ||
Date: January 4, 2018
Exhibit 99.1

Audentes Announces Positive Interim Data from First Dose Cohort of ASPIRO, a Phase 1/2 Clinical Trial of AT132 in Patients With X-Linked Myotubular Myopathy
- Significant improvements in neuromuscular function as assessed by the CHOP-INTEND scale
- Significant improvements in respiratory function as assessed by maximal inspiratory pressure (MIP)
- Multiple developmental milestones achieved at 12 weeks in first treated patient
Conference call and webcast January 4, 2018 at 8:00am EST
Webcast may be accessed via the Investor and Media page of the Audentes website
Call may be accessed by dialing 1-833-659-8620 and using conference ID#: 4957669
SAN FRANCISCO, January 4, 2018 / PRNewswire/ — Audentes Therapeutics, Inc. (Nasdaq: BOLD), a biotechnology company focused on developing and commercializing gene therapy products for patients living with serious, life-threatening rare diseases, today announced positive interim data from the first dose cohort of ASPIRO, a Phase 1/2 clinical trial of AT132 in patients with X-Linked Myotubular Myopathy (XLMTM). ASPIRO is a multicenter, multinational, open-label, ascending dose study to evaluate the safety and preliminary efficacy of AT132 in approximately 12 XLMTM patients less than five years of age.
“The early AT132 efficacy data observed in our first dose cohort of patients have exceeded our expectations,” stated Dr. Suyash Prasad, Senior Vice President and Chief Medical Officer of Audentes. “At the 12-week timepoint, Patient 1 has improved from a severely compromised baseline to achieve a CHOP-INTEND score and maximal inspiratory pressure that are approaching the ranges normally seen in healthy children. Importantly, Patient 1 has also attained several age-appropriate developmental milestones within this time period, including head-control, rolling over and sitting unassisted. While still early in the trial, we view these initial efficacy data as a promising indicator of the potential for AT132 to bring meaningful benefit to patients and families living with this devastating disease.”
ASPIRO Interim Data Summary
The interim data includes safety and efficacy assessments for the first dose cohort of ASPIRO, comprised of three AT132-treated patients dosed at 1x1014 vector genomes (vg) per kilogram (kg), and one delayed-treatment control patient. As of December 21, 2017, individual patient follow-up ranged from 4 to 12 weeks.
Safety Summary
There have been a total of six adverse events (AEs) reported in ASPIRO, two of which were determined to be serious adverse events (SAEs). Both SAEs occurred in Patient 3, the first of which was a hospitalization one week post-administration due to pneumonia and was deemed not treatment-related. Patient 3 was also hospitalized at week 7 post-administration due to a gastrointestinal infection and elevated troponin levels, the latter of which was deemed probably treatment-related and is responding to treatment with intravenous steroid administration and supportive care.
Of the four non-serious AEs, two have been determined to be probably or possibly treatment-related. Patient 1 experienced a mild, clinically asymptomatic exacerbation of a preexisting elevated bilirubin

level, which was deemed possibly treatment-related and resolved with treatment. Patient 2 experienced a clinically asymptomatic elevation in liver enzyme levels toward the end of the protocol-specified prednisolone weaning period, which was deemed to be probably treatment-related and was controlled by extending the duration of steroid coverage.
Efficacy Summary
The key assessment of neuromuscular function in this first data set from Cohort 1 is the CHOP-INTEND scale, in which a maximal score of 64 reflects the level of neuromuscular function that a healthy baby is expected to approach by 3-6 months of age. Additional analyses to be reported based on longer term follow-up include the MFM-20 and Bayley-III™ scales of infant and toddler development (fine and gross motor function). Motor developmental milestones are captured within each of the neuromuscular assessments.
The key assessment of respiratory function in this first data set from Cohort 1 is a measurement of maximal inspiratory pressure (MIP), for which values ³ 80 cmH20 are considered normal in healthy children less than 5 years of age. Additional analyses to be reported based on longer term follow-up include measurement of maximal expiratory pressure (MEP), time per day on invasive ventilatory support (tracheostomy) or non-invasive respiratory support (BiPAP), and for those patients who are on 24-hour continuous ventilatory support, an assessment of ability to maintain adequate respiratory function while off a ventilator, termed “respiratory sprinting.”
Patient Interim Data Summaries:
| • | Patient 1: data set includes assessments through week 12 timepoint |
| • | Age 0.8 years (9 months) and on 12 hours of BiPAP per day at baseline |
| • | CHOP-INTEND increased from 29 at baseline to 56 at week 12 |
| • | MIP increased from 33 cmH20 at baseline to 80 cmH20 at week 12 |
| • | No age-appropriate first-year motor milestones were achieved at the baseline assessment; by week 12, Patient 1 had acquired several age appropriate skills, including the ability to control head movements, roll over by himself and sit unassisted for > 5 seconds |
| • | Patient 2: data set includes assessments through week 8 timepoint |
| • | Age 4.1 years and on 17 hours of invasive ventilation per day at baseline |
| • | CHOP-INTEND increased from 45 at baseline to 56 at week 8 |
| • | MIP increased from 44 cmH20 at baseline to 77 cmH20 at week 4 |
| • | Patient 3: data set includes assessments through week 4 timepoint |
| • | Age 2.6 years and on continuous (24-hour) invasive ventilation at baseline |
| • | CHOP-INTEND did not change meaningfully from 34 at baseline to 36 at week 4 |
| • | MIP increased from 26 cmH20 at baseline to 44 cmH20 at week 4 |
| • | Patient 4 (delayed-treatment control): data set includes assessments through week 4 timepoint |
| • | Age 4.0 years and on 12 hours of BiPAP per day at baseline |
| • | CHOP-INTEND did not change meaningfully from 49 at baseline to 46 at week 4 |
| • | MIP at baseline was 58 cmH20; MIP was not assessed at week 4 per protocol |
Physicians and caregivers have reported progressive qualitative improvements in disease severity in all treated patients. Ventilator settings (pressure, rate and volume of mechanical ventilation) have been reduced in Patients 1 and 2. All treated patients have demonstrated improvements in airway clearance

control, including swallowing and coughing, which is critical to preventing aspiration. By way of example, at baseline Patient 1 required suctioning of the oro-pharyngeal cavity several times per hour, and by week 12 he required no suctioning. In addition, investigators report anecdotally that all treated patients have increased limb and trunk strength, which is an early indicator of gross motor function improvement, and that the velocity and accuracy of their movements have increased. Caregivers also report that patients have increased vocalization, improving their ability to communicate.
“Physician and caregiver reports of patient progress are an important complement to the formal neuromuscular and respiratory data being collected,” stated Dr. Prasad. “These qualitative assessments of the child’s overall disease state and degree of change between visits provide further insight into the impact of AT132 on the XLMTM disease course.”
Audentes is currently reviewing the interim efficacy and safety data with the independent Data Monitoring Committee of ASPIRO prior to initiating enrollment and dosing of the next cohort. Audentes plans to provide the next update on interim data from ASPIRO in the second quarter of 2018.
“This is a promising start to ASPIRO,” stated Matthew R. Patterson, President and Chief Executive Officer of Audentes. “While the data are preliminary, we are very encouraged and will continue to advance this important work as rapidly as possible, keeping in mind our goal of bringing a potentially transformative treatment to patients living with this devastating disease.”
Conference Call
At 8:00 a.m. Eastern Time today, Audentes management will host a conference call and a simultaneous webcast to discuss the interim data from the first dose cohort of ASPIRO. To access a live webcast of the conference call, please visit the Investor and Media page of the Audentes website at www.audentestx.com. Alternatively, please call 1-833-659-8620 (U.S.) or 1-409-767-9247 (international) and dial the conference ID 4957669 to access the call. A replay of the webcast will be available on the Audentes website for approximately 30 days.
About ASPIRO, the Phase 1/2 Clinical Study of AT132
ASPIRO is a multicenter, multinational, open-label, ascending dose study to evaluate the safety and preliminary efficacy of AT132 in approximately 12 XLMTM patients less than five years of age. The study is designed to include nine AT132 treated subjects and three delayed-treatment concurrent control subjects, who will be treated after the optimal dose is selected. Primary endpoints include safety (adverse events and certain laboratory measures) and efficacy (assessments of neuromuscular and respiratory function). Secondary endpoints include the burden of disease and health related quality-of-life, and muscle tissue histology and biomarkers. The primary efficacy analysis is expected to be conducted at 12 months, with interim evaluations to be conducted at earlier time points. After the primary 12-month assessment, subjects are expected to be followed for another four years to assess long term safety, durability of effect and developmental progression.
About AT132 for X-Linked Myotubular Myopathy
AT132 is the Audentes product candidate being developed to treat XLMTM, a rare monogenic disease characterized by extreme muscle weakness, respiratory failure and early death, with an estimated 50% mortality rate by 18 months of age. XLMTM is caused by mutations in the MTM1 gene, which encodes the protein myotubularin. Myotubularin plays an important role in the development, maintenance and function of skeletal muscle cells. AT132 is comprised of an AAV8 vector containing a functional copy of the MTM1 gene. In January 2018, Audentes reported positive interim data from the first dose cohort of

ASPIRO, a multicenter, ascending dose Phase 1/2 clinical study to evaluate the safety and preliminary efficacy of AT132 in approximately 12 XLMTM patients less than five years of age. The preclinical development of AT132 was conducted in collaboration with Genethon (www.genethon.fr).
About Audentes Therapeutics, Inc.
Audentes Therapeutics (Nasdaq: BOLD) is a clinical-stage biotechnology company focused on developing and commercializing gene therapy products for patients living with serious, life-threatening rare diseases. We are currently conducting a Phase 1/2 clinical study of our lead product candidate AT132 for the treatment of X-Linked Myotubular Myopathy (XLMTM) and have three additional product candidates in development, including AT342 for the treatment of Crigler-Najjar Syndrome, AT982 for the treatment of Pompe disease, and AT307 for the treatment of the CASQ2 subtype of Catecholaminergic Polymorphic Ventricular Tachycardia (CASQ2-CPVT). We are a focused, experienced and passionate team committed to forging strong, global relationships with the patient, research and medical communities.
For more information regarding Audentes, please visit www.audentestx.com.
About Genethon
Genethon, located in Evry, France, is a non-profit R&D organization dedicated to the development of biotherapies for orphan genetic diseases, from research to clinical validation. Genethon is specialized in the discovery and development of gene therapy drugs and has multiple ongoing programs at clinical, preclinical and research stage for neuromuscular, blood, immune system, liver and eye diseases.
Discover Genethon’s pipeline: http://www.genethon.fr/produits/
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: enrollment, dose and timing of the second cohort in ASPIRO and the clinical results from ASPIRO. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Although the company believes that the expectations reflected in such forward-looking statements are reasonable, the company cannot guarantee future events, results, actions, levels of activity, performance or achievements, and the timing and results of biotechnology development and potential regulatory approval is inherently uncertain. Forward-looking statements are subject to risks and uncertainties that may cause the company’s actual activities or results to differ significantly from those expressed in any forward-looking statement, including risks and uncertainties related to the company’s ability to advance its product candidates, obtain regulatory approval of and ultimately commercial its product candidates, the timing and results of preclinical and clinical trials, the company’s ability to fund development activities and achieve development goals, the company’s ability to protect intellectual property and other risks and uncertainties described under the heading “Risk Factors” in documents the company files from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this press release, and the company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof.

Audentes Contacts:
Investor Contact:
Andrew Chang, Investor Relations
415.818.1033
Media Contact:
Paul Laland
415.519.6610

ASPIRO Study Interim data as of December 21, 2017 Exhibit 99.2

Safe Harbor Except for statements of historical fact, any information contained in this presentation may be a forward-looking statement that reflects the Company’s current views about future events and are subject to risks, uncertainties, assumptions and changes in circumstances that may cause events or the Company’s actual activities or results to differ significantly from those expressed in any forward-looking statement. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “plan,” “expect,” “estimate,” “anticipate,” “intend,” “goal,” “strategy,” “believe” and similar expressions and variations thereof. Forward-looking statements include statements regarding the Company’s business strategy; potential growth opportunities; clinical development activities; the timing and results of Investigational New Drug application and Clinical Trial Authorisation submissions; the timing and results of preclinical studies and clinical trials; the nature and timing of potential regulatory approvals; and the likelihood of future commercialization of product candidates. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements, and the timing and results of biotechnology development and potential regulatory approval is inherently uncertain. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading “Risk Factors” in documents the Company has filed with the SEC. These forward-looking statements speak only as of the date of this presentation, and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. Certain information contained in this presentation may be derived from information provided by industry sources. We believe such information is accurate and that the sources from which it has been obtained are reliable. However, we cannot guarantee the accuracy of, and have not independently verified, such information. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products.

AT132 OveRview
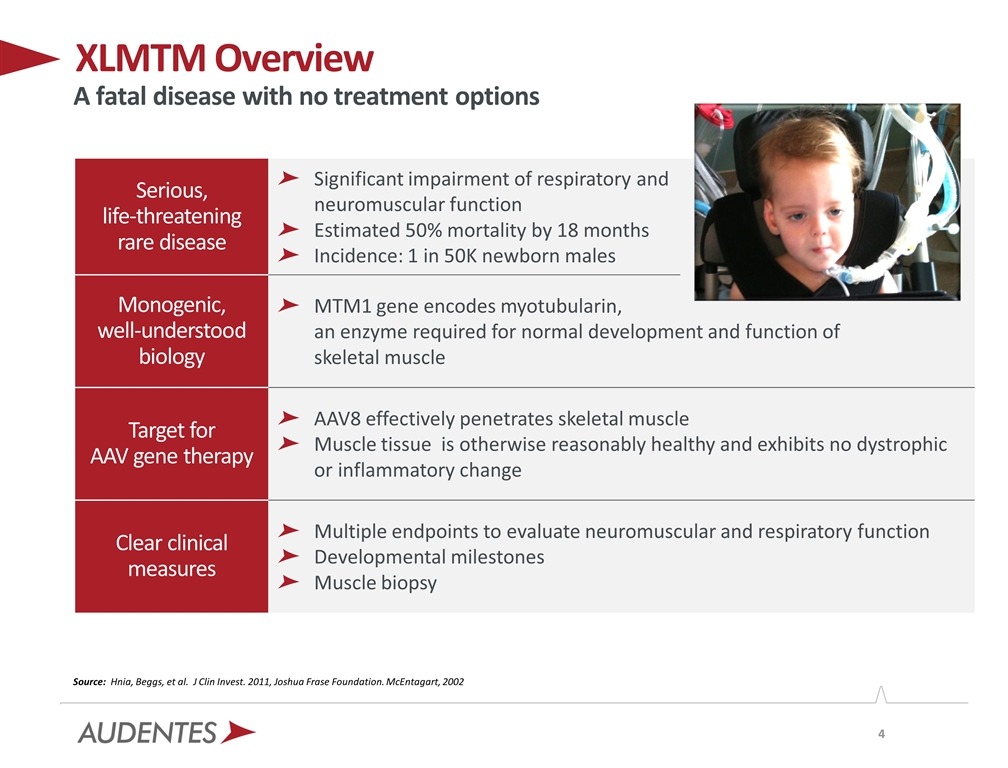
Serious, life-threatening rare disease Significant impairment of respiratory and neuromuscular function Estimated 50% mortality by 18 months Incidence: 1 in 50K newborn males Monogenic, well-understood biology MTM1 gene encodes myotubularin, an enzyme required for normal development and function of skeletal muscle Target for AAV gene therapy AAV8 effectively penetrates skeletal muscle Muscle tissue is otherwise reasonably healthy and exhibits no dystrophic or inflammatory change Clear clinical measures Multiple endpoints to evaluate neuromuscular and respiratory function Developmental milestones Muscle biopsy XLMTM Overview A fatal disease with no treatment options Source: Hnia, Beggs, et al. J Clin Invest. 2011, Joshua Frase Foundation. McEntagart, 2002
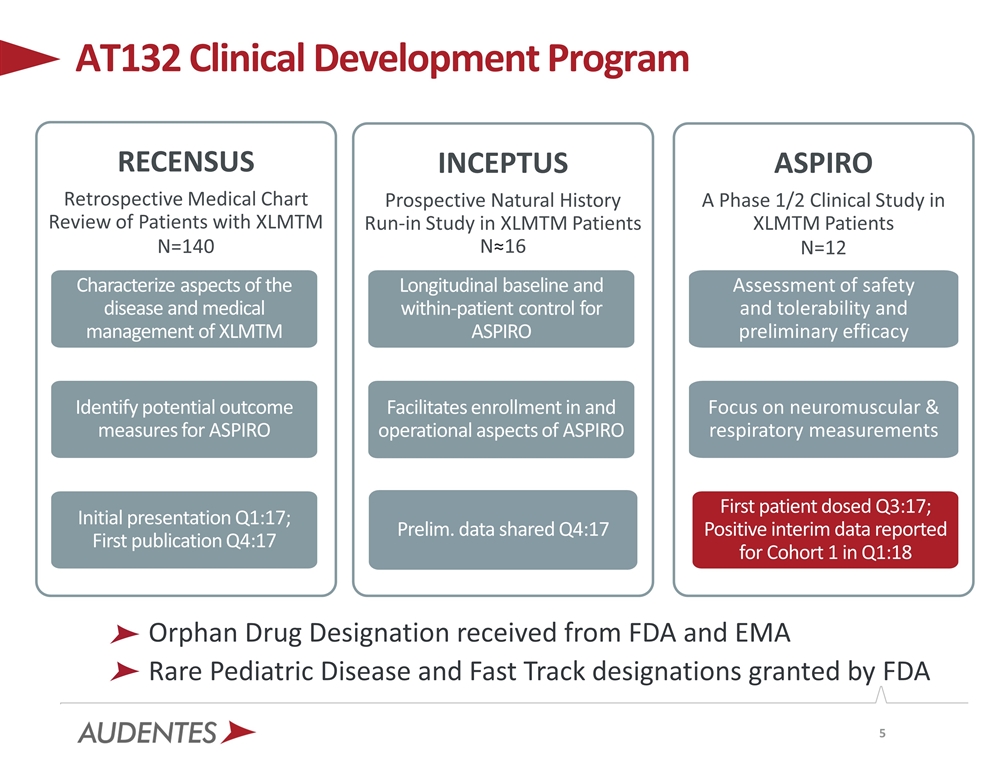
AT132 Clinical Development Program INCEPTUS Prospective Natural History Run-in Study in XLMTM Patients N≈16 Longitudinal baseline and within-patient control for ASPIRO Facilitates enrollment in and operational aspects of ASPIRO ASPIRO A Phase 1/2 Clinical Study in XLMTM Patients N=12 Assessment of safety and tolerability and preliminary efficacy Focus on neuromuscular & respiratory measurements RECENSUS Retrospective Medical Chart Review of Patients with XLMTM N=140 Characterize aspects of the disease and medical management of XLMTM Identify potential outcome measures for ASPIRO Initial presentation Q1:17; First publication Q4:17 Prelim. data shared Q4:17 First patient dosed Q3:17; Positive interim data reported for Cohort 1 in Q1:18 Orphan Drug Designation received from FDA and EMA Rare Pediatric Disease and Fast Track designations granted by FDA

ASPIRO: to aspire…
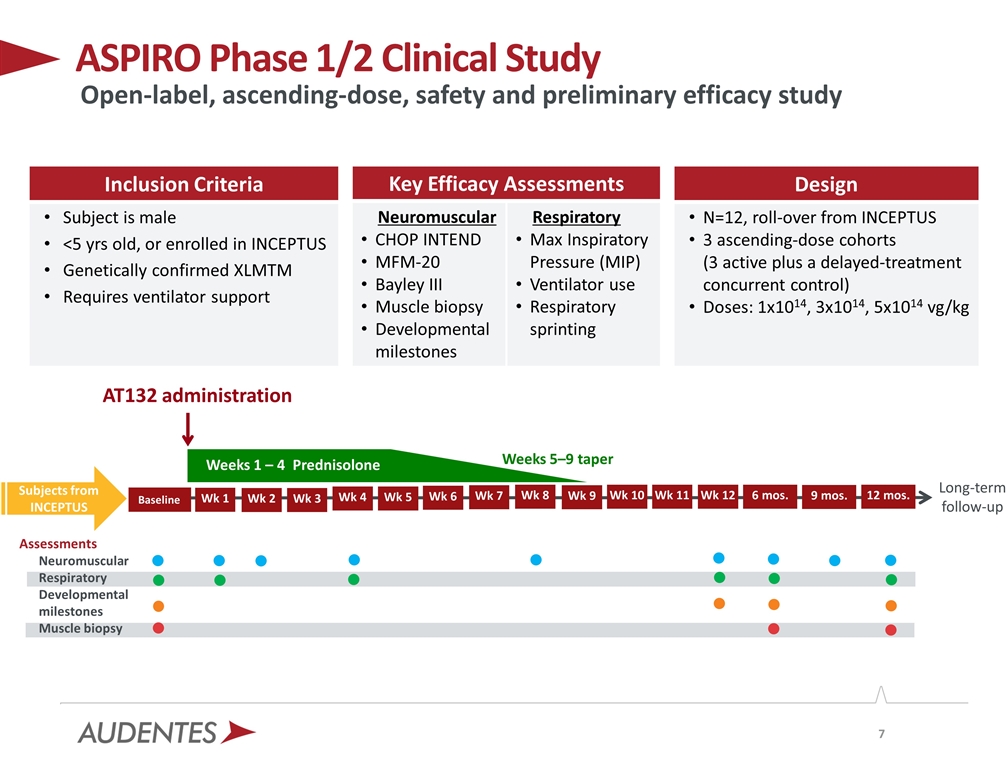
ASPIRO Phase 1/2 Clinical Study Baseline AT132 administration Weeks 5–9 taper Long-term follow-up 6 mos. Inclusion Criteria Subject is male <5 yrs old, or enrolled in INCEPTUS Genetically confirmed XLMTM Requires ventilator support Key Efficacy Assessments Neuromuscular CHOP INTEND MFM-20 Bayley III Muscle biopsy Developmental milestones Respiratory Max Inspiratory Pressure (MIP) Ventilator use Respiratory sprinting Design N=12, roll-over from INCEPTUS 3 ascending-dose cohorts (3 active plus a delayed-treatment concurrent control) Doses: 1x1014, 3x1014, 5x1014 vg/kg Open-label, ascending-dose, safety and preliminary efficacy study Wk 12 Wk 11 Wk 10 Weeks 1 – 4 Prednisolone 9 mos. 12 mos. Baseline Wk 1 Wk 2 Wk 3 Wk 4 Wk 5 Wk 6 Wk 8 Wk 9 Wk 7 Assessments Neuromuscular Respiratory Developmental milestones Muscle biopsy Subjects from INCEPTUS
ASPIRO Clinical Study Sites Same study sites as INCEPTUS natural history run-in study
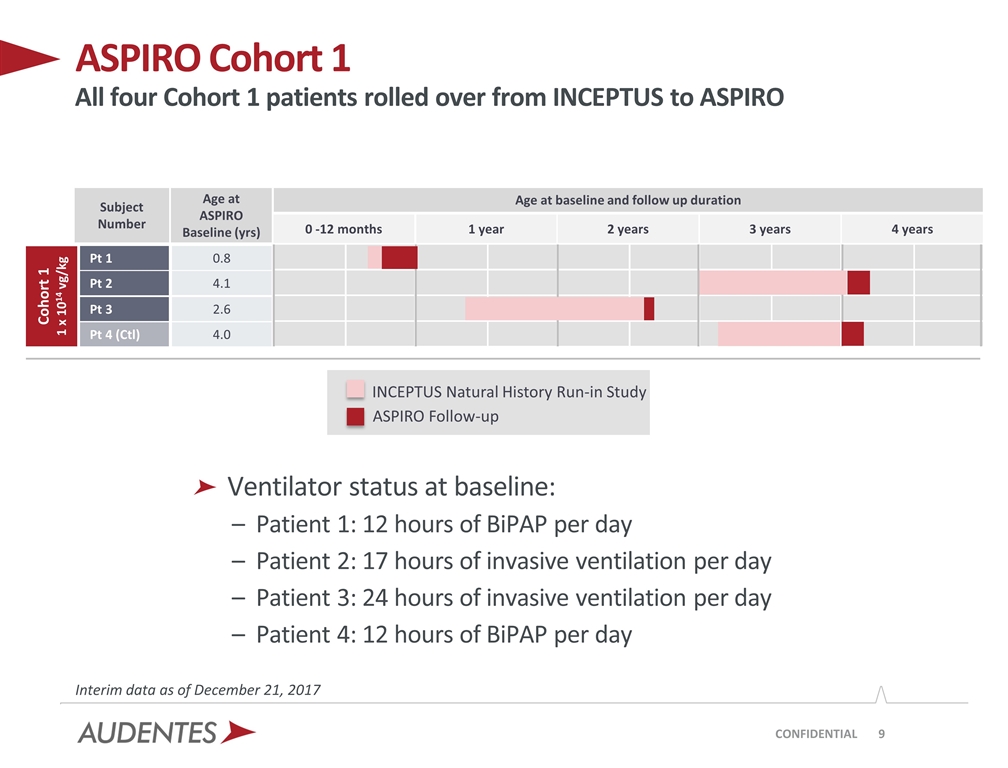
ASPIRO Cohort 1 All four Cohort 1 patients rolled over from INCEPTUS to ASPIRO CONFIDENTIAL ASPIRO Follow-up Interim data as of December 21, 2017 Age at baseline and follow up duration Age at ASPIRO Baseline (yrs) 0.8 2.6 4.1 4.0 Subject Number Pt 1 Pt 3 Pt 2 Pt 4 (Ctl) 0 -12 months 1 year 2 years 4 years 3 years Cohort 1 1 x 1014 vg/kg Ventilator status at baseline: Patient 1: 12 hours of BiPAP per day Patient 2: 17 hours of invasive ventilation per day Patient 3: 24 hours of invasive ventilation per day Patient 4: 12 hours of BiPAP per day INCEPTUS Natural History Run-in Study

Safety and Tolerability
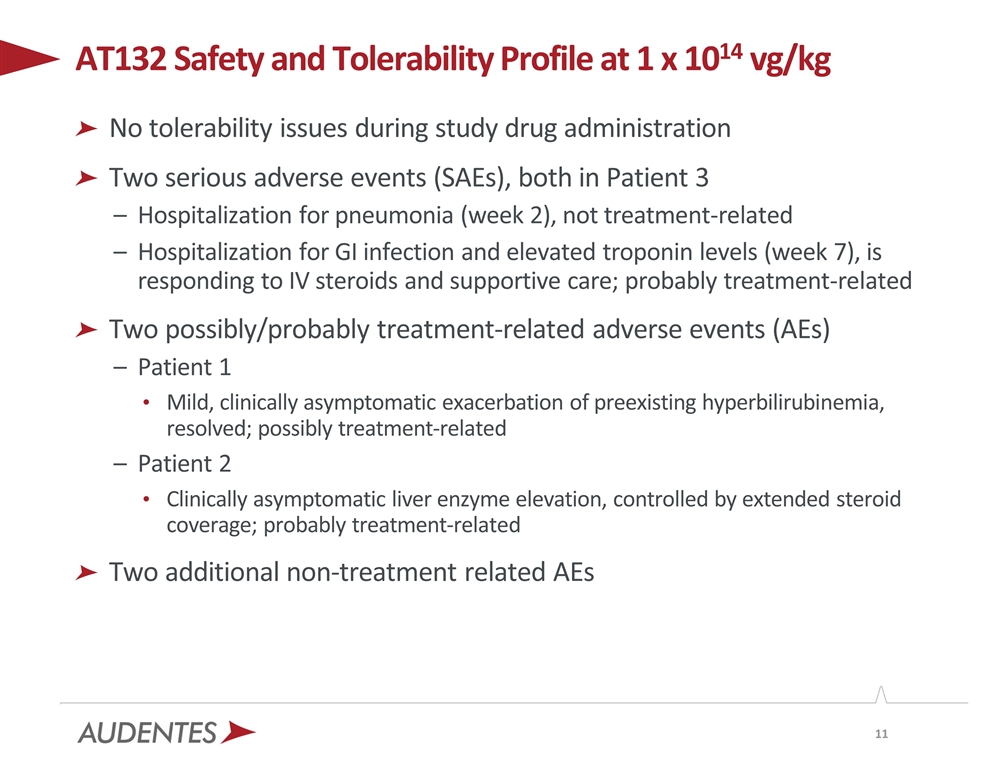
No tolerability issues during study drug administration Two serious adverse events (SAEs), both in Patient 3 Hospitalization for pneumonia (week 2), not treatment-related Hospitalization for GI infection and elevated troponin levels (week 7), is responding to IV steroids and supportive care; probably treatment-related Two possibly/probably treatment-related adverse events (AEs) Patient 1 Mild, clinically asymptomatic exacerbation of preexisting hyperbilirubinemia, resolved; possibly treatment-related Patient 2 Clinically asymptomatic liver enzyme elevation, controlled by extended steroid coverage; probably treatment-related Two additional non-treatment related AEs AT132 Safety and Tolerability Profile at 1 x 1014 vg/kg

Neuromuscular function
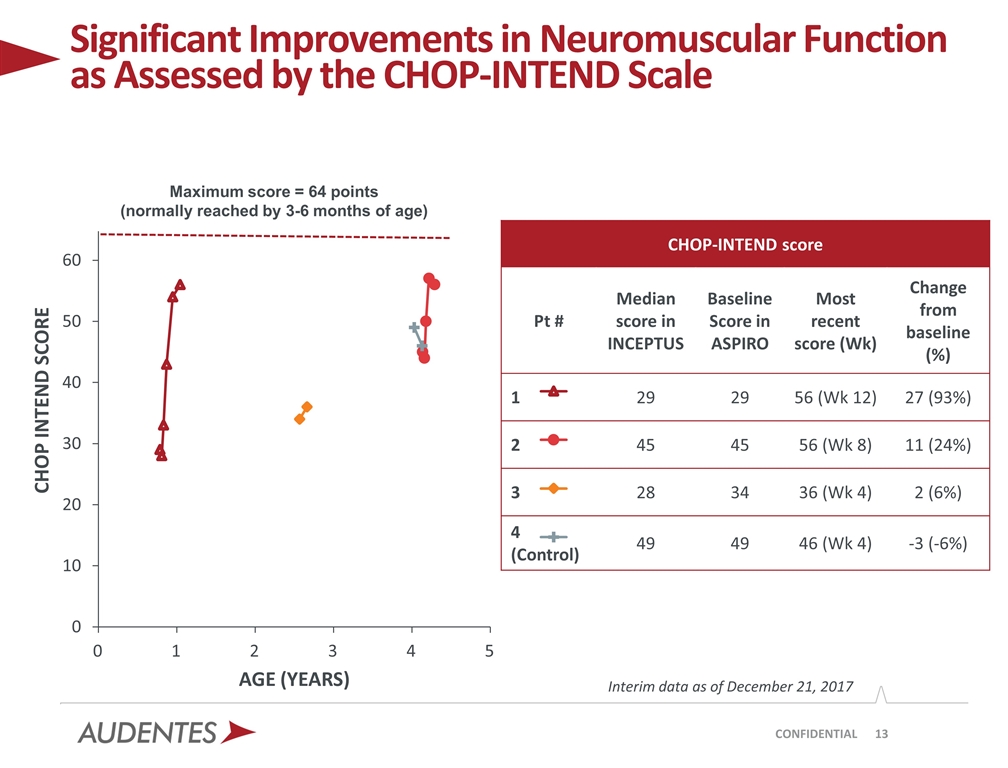
Significant Improvements in Neuromuscular Function as Assessed by the CHOP-INTEND Scale CONFIDENTIAL Maximum score = 64 points (normally reached by 3-6 months of age) Interim data as of December 21, 2017 CHOP-INTEND score Pt # Median score in INCEPTUS Baseline Score in ASPIRO Most recent score (Wk) Change from baseline (%) 1 29 29 56 (Wk 12) 27 (93%) 2 45 45 56 (Wk 8) 11 (24%) 3 28 34 36 (Wk 4) 2 (6%) 4 (Control) 49 49 46 (Wk 4) -3 (-6%)
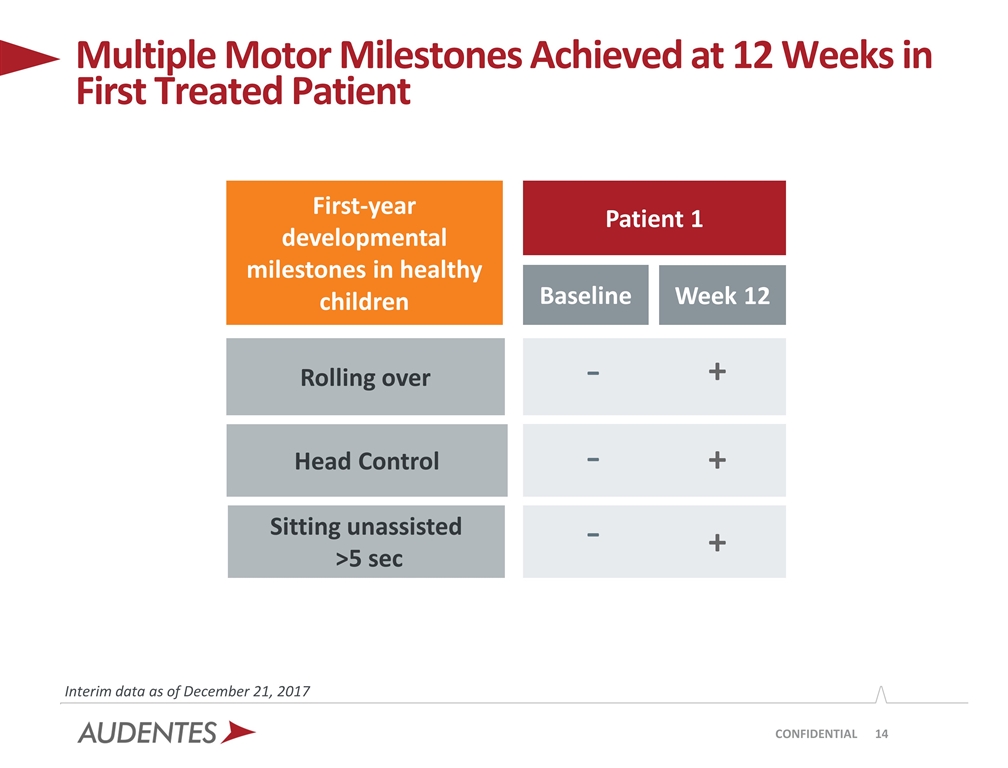
CONFIDENTIAL Multiple Motor Milestones Achieved at 12 Weeks in First Treated Patient Interim data as of December 21, 2017 First-year developmental milestones in healthy children Patient 1 Baseline Week 12 Rolling over Head Control Sitting unassisted >5 sec - - - + + +

Respiratory function
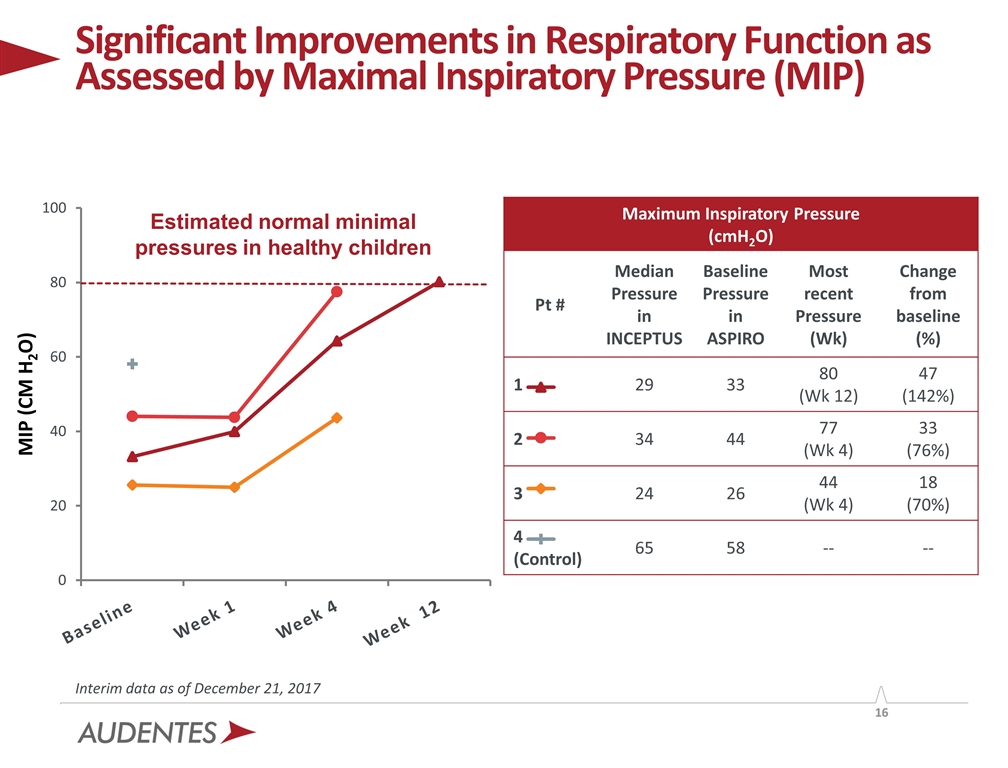
Significant Improvements in Respiratory Function as Assessed by Maximal Inspiratory Pressure (MIP) Estimated normal minimal pressures in healthy children Interim data as of December 21, 2017 Maximum Inspiratory Pressure (cmH2O) Pt # Median Pressure in INCEPTUS Baseline Pressure in ASPIRO Most recent Pressure (Wk) Change from baseline (%) 1 29 33 80 (Wk 12) 47 (142%) 2 34 44 77 (Wk 4) 33 (76%) 3 24 26 44 (Wk 4) 18 (70%) 4 (Control) 65 58 -- --

Qualitative ASSESSMENTS
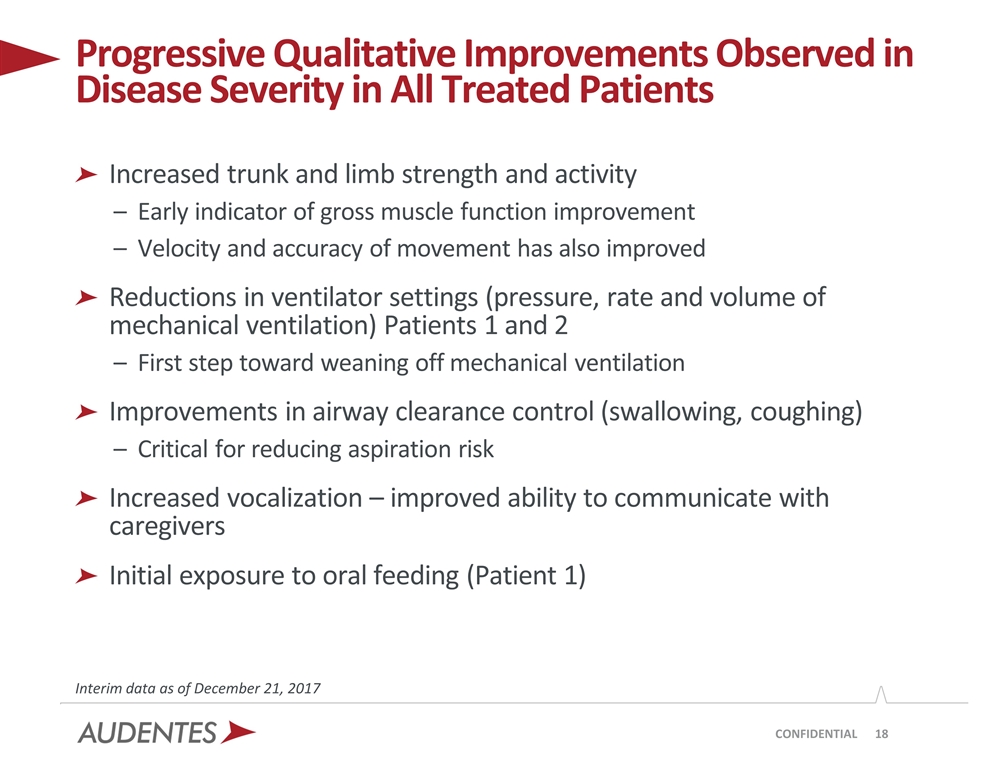
CONFIDENTIAL Increased trunk and limb strength and activity Early indicator of gross muscle function improvement Velocity and accuracy of movement has also improved Reductions in ventilator settings (pressure, rate and volume of mechanical ventilation) Patients 1 and 2 First step toward weaning off mechanical ventilation Improvements in airway clearance control (swallowing, coughing) Critical for reducing aspiration risk Increased vocalization – improved ability to communicate with caregivers Initial exposure to oral feeding (Patient 1) Progressive Qualitative Improvements Observed in Disease Severity in All Treated Patients Interim data as of December 21, 2017
Patient 1 chop intend video assessments baseline vs Week 12

Six adverse events to date (3 treatment-related); all resolved or responding to treatment Significant improvements in neuromuscular function as assessed by the CHOP-INTEND scale Multiple developmental milestones achieved at 12 weeks in Patient 1 Significant improvements in respiratory function as assessed by Maximal Inspiratory Pressure (MIP) Progressive qualitative improvements observed in disease severity in all treated patients Interim efficacy and safety data review underway with independent Data Monitoring Committee prior to initiating enrollment of next cohort Next study update is scheduled for Q2:18 ASPIRO Key Findings and Program Next Steps

ASPIRO Study Interim data as of December 21, 2017
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Leerink Partners Starts Boundless Bio Inc. (BOLD) at Outperform
- LA Wine Country Stylish Wedding Transportation, Luxury Limousines Available
- Municipality Finance issues a GBP 50 million tap under its MTN programme
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share