Form 8-K Jounce Therapeutics, For: Jan 08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
________________________________
FORM 8-K
______________________________________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 8, 2018
________________________________________________________________________________________________________
JOUNCE THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
________________________________________________________________________________________________________
Delaware | 001-37998 | 45-4870634 | ||
(State or Other Jurisdiction of Incorporation) | (Commission File Number) | (IRS Employer Identification No.) | ||
780 Memorial Drive Cambridge, Massachusetts | 02139 | |
(Address of Principal Executive Offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (857) 259-3840
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
¨ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Item 2.02. Results of Operations and Financial Condition.
The information contained in Item 7.01 below and Exhibit 99.1 attached hereto, to the extent it relates to Jounce Therapeutics, Inc.’s (the “Company”) revenue and use of cash for the year ended December 31, 2017, is incorporated herein by reference.
Item 7.01. Regulation FD Disclosure.
The Company from time to time presents and/or distributes to the investment community at various conferences and meetings slide presentations to provide updates and summaries of its business. The Company is posting to the “Investors and Media” portion of its website at www.jouncetx.com a copy of its current corporate slide presentation. These slides are attached to this Current Report on Form 8-K as Exhibit 99.1. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
The information in Item 2.02 and Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
Exhibit No. | Description | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
JOUNCE THERAPEUTICS, INC. | ||
Date: January 8, 2018 | By: | /s/ Anna L. Barry |
Anna L. Barry | ||
Senior Vice President, Legal & Secretary | ||

Jounce Therapeutics
A Next Gen Immunotherapy Company

Legal Disclaimer
Various statements in this release concerning Jounce’s future expectations, plans and prospects, including without limitation, Jounce’s expectations
regarding operating expenses, collaboration revenue and other financial results, the timing, progress and results of research and development
programs, preclinical studies and clinical trials for Jounce’s product candidates and any future product candidates, the potential benefits of any of
these product candidates and the timing or likelihood of regulatory filings may constitute forward-looking statements for the purposes of the safe
harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws and are subject to substantial risks,
uncertainties and assumptions. You should not place reliance on these forward looking statements, which often include words such as “anticipate,”
“estimate,” “expect,” “intend,” “may,” “on track,” “plan,” “predict,” “target,” “potential” or similar terms, variations of such terms or the negative of
those terms. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company
cannot guarantee such outcomes. Actual results may differ materially from those indicated by these forward-looking statements as a result of
various important factors, including, without limitation, Jounce’s ability to successfully demonstrate the efficacy and safety of its product candidates
and future product candidates, the preclinical and clinical results for its product candidates, which may not support further development and
marketing approval, the potential advantages of Jounce’s product candidates, the development plans of its product candidates,actions of
regulatory agencies, which may affect the initiation, timing and progress of pre-clinical studies and clinical trials of its product candidates, Jounce’s
anticipated milestones, Jounce’s ability to obtain, maintain and protect its intellectual property, Jounce’s ability to enforce its patents against
infringers and defend its patent portfolio against challenges from third parties, the timing, cost or other aspects of a potential commercial launch of
Jounce’s product candidates and potential future sales of our current product candidates or any other potential products if any are approved for
marketing, competition from others developing products for similar uses, Jounce’s ability to manage operating expenses, Jounce’s ability to
maintain its collaboration with Celgene and establish or maintain future collaborations, Jounce’s dependence on third parties for development,
manufacture, marketing, sales and distribution of product candidates, the outcome of litigation, and unexpected expenditures, as well as those risks
more fully discussed in the section entitled “Risk Factors” in Jounce’s most recent Quarterly Report on Form 10-Q filed with the Securities and
Exchange Commission as well as discussions of potential risks, uncertainties, and other important factors in Jounce’s subsequent filings with the
Securities and Exchange Commission. All such statements speak only as of the date made, and the Company undertakes no obligation to update
or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
2 | Jounce Therapeutics © January, 2018

Jounce Therapeutics
Develop and
commercialize
Discover first-in-class
immunotherapies
Predictive
biomarkers
Tumor immune
composition
3 | Jounce Therapeutics © January, 2018
Developing first-in-class cancer immunotherapies
to match the right therapy to the right patients

Jounce Investment Highlights
• Engine to drive sustained discovery
• New targets & biomarkers linked to tumor immune composition
Robust Translational Science Platform
Lead Candidate JTX-2011 Phase 2
Diverse, Broad Pipeline
Significant Strategic Collaboration
Experienced Founder & Management Team
• Phase 2 biomarker driven adaptive clinical trial
• Multiple solid tumor indications
• Targeting T cells and additional immune cell types
• Patients not well served by approved immunotherapies
• Strategic co-development and
co-commercialization collaboration
• Founders fundamental in the science of checkpoint therapy
• Management leadership roles in through approval
4 | Jounce Therapeutics © January, 2018

2017 Key Accomplishments
5 | Jounce Therapeutics © January, 2018
Upsized IPO, raising >$117m in gross proceeds
Established new corporate headquarters
Built discovery team to support Celgene collaboration and broader vision
Presentation and posters at AACR, ASCO and SITC
JTX-2011 ICONIC monotherapy and combination safety readout at ASCO,
RP2D established
Initiated ICONIC Phase 2 in both monotherapy and combination

2018 Value Drivers
JTX-2011 preliminary clinical
efficacy results in Q2
Tumor types that have completed enrollment
with biomarker and safety update
IND for JTX-4014 (anti-PD-1)
Initiate new JTX-2011 combination
Next development candidate into
IND enabling activities
Continued investment in our translational science platform and team
6 | Jounce Therapeutics © January, 2018

Immunotherapy Opportunities Beyond PD-1 Inhibitors
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
NSCLC,
1st line
Melanoma,
1st line
MSI-H,
2nd line+
Melanoma,
2nd line+
Bladder,
1st line
Renal,
2nd line+
Bladder,
2nd line+
NSCLC,
2nd line+
HNSCC,
1st line+
Liver,
2nd line+
Gastric,
3rd line+
ORR
for FD
A
A
ppro
v
e
d
A
n
ti
-P
D
-1
M
ono
th
e
ra
p
y
Therapeutic Opportunity
• Novel agents / combinations
• Indications with no PD-1 approval
• Indications with low PD-1 response rates
• PD-1 refractory patients
Strong biomarker selection
7 | Jounce Therapeutics © January, 2018

Immunotherapy opportunities beyond PD-1 inhibitors
T cells T cells +
macrophages
Macrophages No T cells or
macrophages
Hot / Cold Tumors
• PD-1 inhibitor amenable
• Opportunity to maximize
benefit
• High unmet medical need
• Opportunity to expand the
promise of immunotherapy
Translational Science Platform
Key to Expanding Immunotherapy Opportunities
Suite of integrated technologies
Proprietary
bioinformatics &
immune signatures
Multiplex IHC &
tumor histoculture
8 | Jounce Therapeutics © January, 2018
• Decode the TME
• Identify targets
• Inform biomarker
strategy
• Prioritize
indication
selection

JTX-2011 Shifts Balance of T Cells Towards Anti-Tumor Activity
APC
“Primed”
T effector cell
ICOS↑
TCR
1st signal
ICOS
JTX-2011
↑ IFN-γ↑
↑ IFN-γ↑
Activation of
T effector cells
T regulatory
cell
NK cell
X
Selective reduction of
intratumoral T
regulatory cells
“Non-Primed”
T effector cell
TCR
ICOS
9 | Jounce Therapeutics © January, 2018

Critical Pre-clinical Requirements for In Vivo Efficacy
Single Agent
TS
ICOS 3+
0 1 0 2 0 3 0 4 0
0
2 0 0
4 0 0
6 0 0
8 0 0
1 0 0 0
1 2 0 0
1 4 0 0
0 1 0 2 0 3 0 4 0
0
2 0 0
4 0 0
6 0 0
8 0 0
1 0 0
1 2 0 0
1 4 0 0
Days post-inoculation of SA1/N tumor cells
T
u
m
o
r
v
o
lu
m
e
(
m
m
3
) Control Anti-ICOS
1/10 7/10 0 1 0 2 0 3 0 4 0
0
5 0 0
1 0 0 0
1 5 0 0
A n im a ls c u re d o f tu m o rs a re
im m u n e to tu m o r re -c h a lle n g e
D a y s a fte r tu m o r r e -c h a lle n g e
T
u
m
o
r
v
o
lu
m
e
(
m
m
3
)
N o p r io r
tre a tm e n t
A n im a ls p re v io u s ly
c u re d b y a n ti-IC O S
ICOS 1+
Control antibody Anti-PD-1Anti-ICOS Anti-ICOS + Anti-PD-1
0 1 0 2 0 3 0 4 0
0
5 0 0
1 0 0 0
1 5 0 0
2 0 0 0
D a y s P o s t- In o c u la t io n
0 1 0 2 0 3 0 4 0
0
5 0 0
1 0 0 0
1 5 0 0
2 0 0 0
D a y s P o s t- In o c u la t io n
0 1 2 3 4
0
5 0 0
1 0 0 0
1 5 0 0
2 0 0 0
D a y s P o s t- In o c u la t io n
0 1 0 2 0 3 0 4 0
0
5 0 0
1 0 0 0
1 5 0 0
2 0 0 0
D a y s P o s t- I o c u la t io n
0/10 6/101/10 8/10
Combination with PD-1 Inhibitor
“ ”
”“
10 | Jounce Therapeutics © January, 2018

ICONIC: Adaptive, Biomarker-Driven Clinical Study
Phase 1 Dose Escalation, Safety, PK and PD
Single
Agent
Dose
Escalation
Safety, PK/PD read-out at ASCO 2017*
A
All-comers, no biomarker enrichment
Combo
with
nivolumab
Dose
EscalationB
PK/PD Expansions
PK/PD Expansions
Phase 1
Safety, PK and PD
Phase 2
Triggered Upon:
Identification of safe
dose where PK/PD
predicts anticipated
clinically effective
dose
* Phase 1 poster available on Jounce website
Any solid tumor type
HNSCC
NSCLC
NSCLC
HNSCC
TNBC
Melanoma
Gastric
Phase 2
Preliminary Efficacy
Q2 2018
11 | Jounce Therapeutics © January, 2018
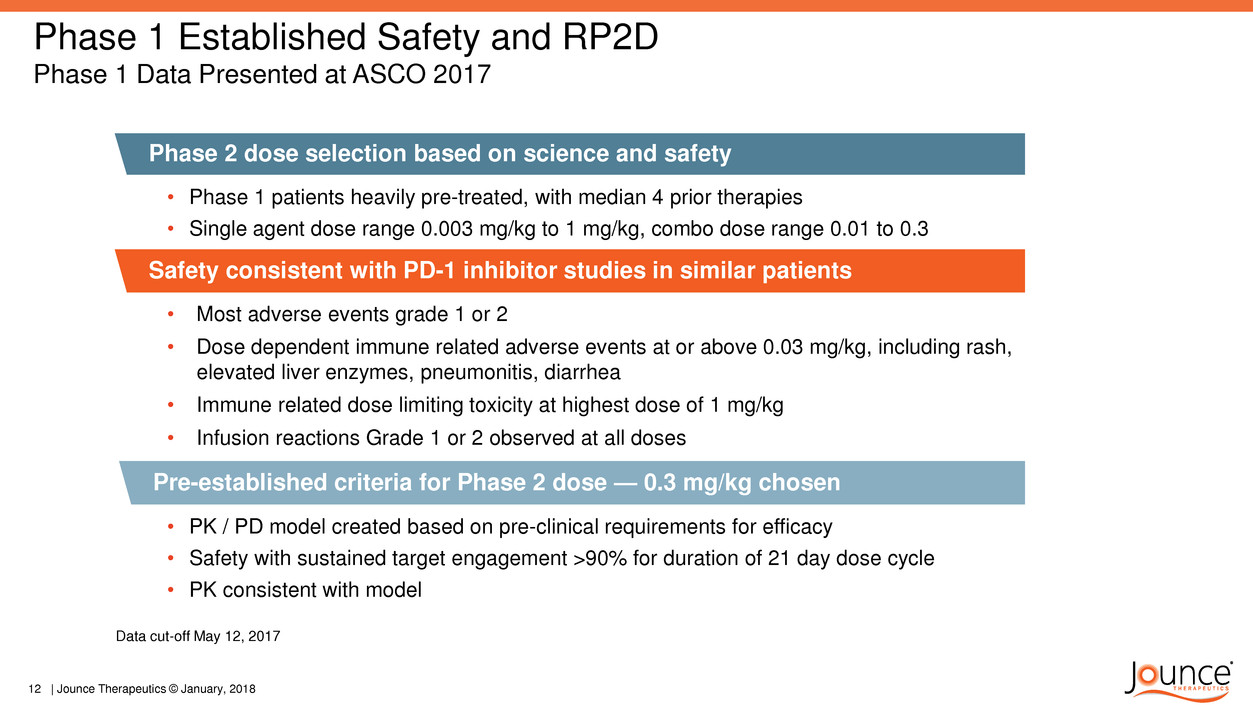
Phase 1 Established Safety and RP2D
Phase 1 Data Presented at ASCO 2017
Phase 2 dose selection based on science and safety
• Phase 1 patients heavily pre-treated, with median 4 prior therapies
• Single agent dose range 0.003 mg/kg to 1 mg/kg, combo dose range 0.01 to 0.3
• PK / PD model created based on pre-clinical requirements for efficacy
• Safety with sustained target engagement >90% for duration of 21 day dose cycle
• PK consistent with model
Pre-established criteria for Phase 2 dose — 0.3 mg/kg chosen
Safety consistent with PD-1 inhibitor studies in similar patients
• Most adverse events grade 1 or 2
• Dose dependent immune related adverse events at or above 0.03 mg/kg, including rash,
elevated liver enzymes, pneumonitis, diarrhea
• Immune related dose limiting toxicity at highest dose of 1 mg/kg
• Infusion reactions Grade 1 or 2 observed at all doses
12 | Jounce Therapeutics © January, 2018
Data cut-off May 12, 2017

Phase 2 Indication Selection & Patient Enrichment via Biomarkers
ICOS Immunohistochemistry (IHC)
Re
na
l
Br
ea
st
Bl
ad
de
r
Ov
ar
ian
Pr
os
tat
e
St
om
ac
h
Co
lon
Me
lan
om
a
TN
BC
Lu
ng
HN
SC
C
0
50
100
%
T
um
or
s
3+
2+
1+
None
ICOS protein levels vary across indications
None (0) Low (1+) Medium (2+) High (3+)
Current Phase 2 patients represent high
areas of unmet need
All advanced, relapsed / refractory cancer patients
with no standard options
• NSCLC
• HNSCC
• Melanoma
• Gastric*
• TNBC
• New indications based on emerging science
PD-1 inhibitor failures
Generally IO naïve
* Recent PD-1 inhibitor approval
13 | Jounce Therapeutics © January, 2018

ICONIC: Adaptive, Biomarker-Driven Clinical Study
Phase 2 in Select Indications
Phase 1
Safety, PK and PD
Phase 2
Triggered Upon:
Identification of safe
dose where PK/PD
predicts anticipated
clinically effective
dose
Any solid tumor type
HNSCC
NSCLC
New indications based on
emerging science
NSCLC
HNSCC
TNBC
Melanoma
Gastric
New indications based
on emerging science
Phase 2
Preliminary Efficacy
Q2 2018
Enriched for patients with high ICOS expression
D
Combo
with
nivolumab
C Single
Agent
14 | Jounce Therapeutics © January, 2018

ICOS May Be an Ideal Target for Combination Therapy
APC
“Primed”
T effector cell
ICOS↑
TCR
1st signal
ICOS
Potential Combination Advantages of the ICOS Mechanism
CTLA-4 inhibitors
PD-1 inhibitors
Vaccines
Radiation
OX-40 agonists
ICOS
ICOS
ICOS
↑
↑
↑
• Variety of agents induce ICOS in T cells
• Induction may define combination opportunities
15 | Jounce Therapeutics © January, 2018

JTX-4014: Anti-PD-1 Program, IND in 2018
JTX-4014 Product Profile
• Fully human IgG4 (hinge-stabilized) anti-PD-1 blocking antibody
• Selectively recognizes human, cynomolgus and mouse PD-1, and not closely related family members
• Blocks PD-1 binding to PD-L1 and PD-L2 across species
• JTX-4014 clone selected and undergoing GLP/GMP manufacture
• In-life portion of GLP safety studies in cynomolgus monkey and mouse completed
Preclinical Development
Clinical Development Plan
• JTX-4014 being developed for combination studies
• Safety and POC as a monotherapy to facilitate use as a combination agent
• IND filing in 2018
16 | Jounce Therapeutics © January, 2018

Translational Platform Expanding the Promise of Immunotherapy
Immunosuppressive
macrophage-rich
tumors
Immune cell-
lacking tumors Effector T cells
M2-like
suppressive
macrophages
Inflamed immune environment:
Susceptible to PD-1 inhibitors
Convert M2 mΦ to M1
Alter TME and recruit
immune cells
17 | Jounce Therapeutics © January, 2018

Proof of Biology of New Immunosuppressive Macrophage Targets
In Vivo (Using Genetically Engineered KO Mice)
MC38 tumor growth in normal mice MC38 tumor growth in mice lacking target
0 1 0 2 0 3 0
0
5 0 0
1 0 0 0
1 5 0 0
2 0 0 0
2 5 0 0
3 0 0 0
3 5 0 0
W T M C 3 8
D a y
T
u
m
o
r
V
o
lu
m
e
(
m
m
3
)
0 1 0 2 0 3 0
0
5 0 0
1 0 0 0
1 5 0 0
2 0 0 0
2 5 0 0
3 0 0 0
3 5 0 0
P ir B M C 3 8
D a y
T
u
m
o
r
V
o
lu
m
e
(m
m
3
)
• Genetically engineered KO mice of target releases immunosuppressive macrophage
effect, but does not deplete the macrophage cell type
Normal MC38 tumor
(macrophage staining)
18 | Jounce Therapeutics © January, 2018

Jounce Antibodies Convert Macrophages to Anti-Tumor Phenotype
• TNFa
• IL-1b
• CCL2
• IL-10
Pro-Inflammatory Anti-Inflammatory
Jounce antibodies promote M1-like activation Jounce antibodies attenuate M2-like activation
0 .0 0 0 1 0 .0 0 1 0 .0 1 0 .1 1 1 0
0
5
1 0
1 5
2 0
2 5
A n tib o d y c o n c e n tra t io n (n M )
T
N
F
a
p
ro
d
u
c
e
d
(
re
la
ti
v
e
t
o
L
P
S
a
lo
n
e
)
.0 0 0 1 0 .0 0 1 0 .0 1 0 .1 1 1 0
0 .6
0 .8
1 .0
1 .2
A n tib o d y c o n c e n tra t io n (n M )
IL
-1
0
p
ro
d
u
c
e
d
(
re
la
ti
v
e
t
o
L
P
S
a
lo
n
e
)
19 | Jounce Therapeutics © January, 2018

Jounce Immunotherapy Pipeline
Program Target ID Discovery Pre-clinical Clinical Development
JTX-2011 (ICOS)
Multiple Solid Tumor Indications
JTX-4014 (PD-1)
Lead Macrophage Program
Myeloid Enriched Solid Tumors
Macrophage Targeting
Multiple (Undisclosed)
T Reg
Multiple (Undisclosed)
B Cells
Multiple (Undisclosed)
Stromal Targeting
Multiple (Undisclosed)
Multiple Indications in Phase 2
Named Celgene Option Jounce ownedCelgene Target Pool
20 | Jounce Therapeutics © January, 2018

Jounce and Celgene Strategic Collaboration
Co-develop and Co-commercialize Innovative Immunotherapies
Collaboration Highlights
• Global strategic collaboration, enabling comprehensive clinical strategy
• Option based deal, preset opt-in fees and profit share, cost sharing after opt-in
• Jounce retains significant development, commercial and economic rights
− Upon opt-in, Jounce leads JTX-2011 US co-development/co-commercialization
• $225M upfront, $36M equity investment, and up to $2.3B in potential milestones
Program US Jounce US Celgene Ex-US Jounce
JTX-2011 Lead 60% 40% Royalty
1st Option post-lead 25% 75% Royalty
Options 2-4 50% 50% Royalty
JTX-4014 Shared Globally for Combinations with Collaboration Products
Profit Sharing
Committed to
working together
to change the
course of
cancer
21 | Jounce Therapeutics © January, 2018
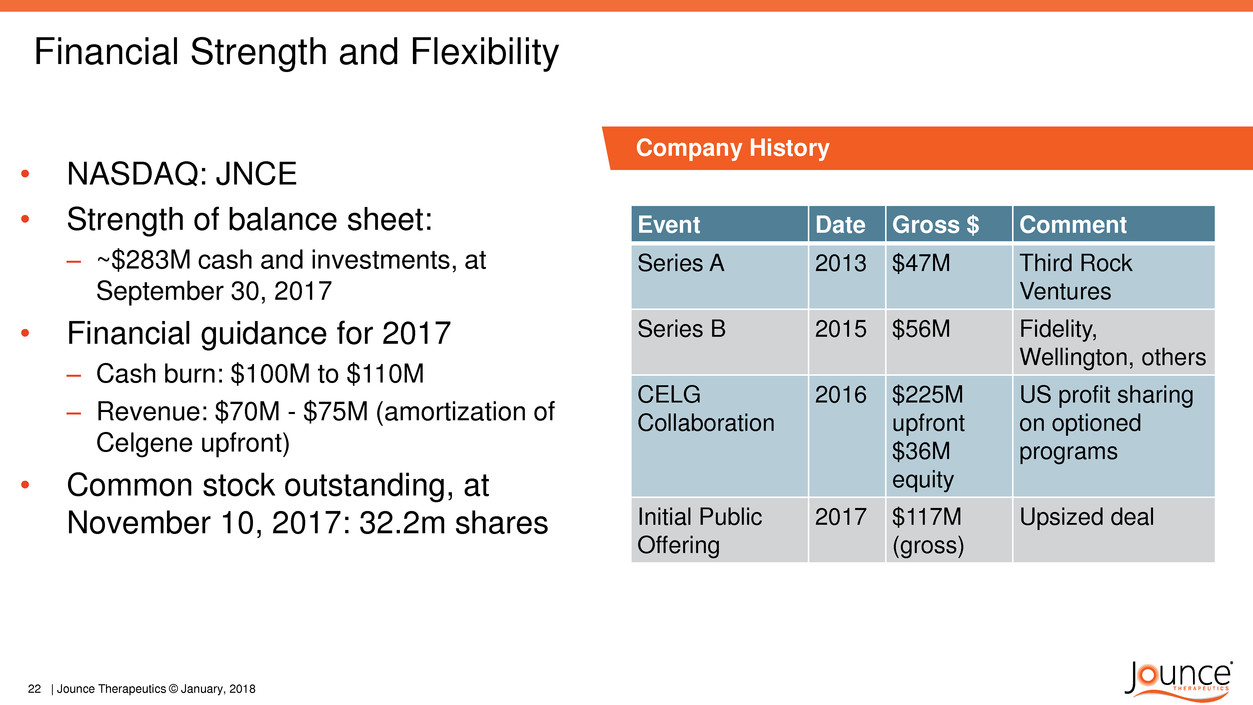
Financial Strength and Flexibility
Event Date Gross $ Comment
Series A 2013 $47M Third Rock
Ventures
Series B 2015 $56M Fidelity,
Wellington, others
CELG
Collaboration
2016 $225M
upfront
$36M
equity
US profit sharing
on optioned
programs
Initial Public
Offering
2017 $117M
(gross)
Upsized deal
• NASDAQ: JNCE
• Strength of balance sheet:
– ~$283M cash and investments, at
September 30, 2017
• Financial guidance for 2017
– Cash burn: $100M to $110M
– Revenue: $70M - $75M (amortization of
Celgene upfront)
• Common stock outstanding, at
November 10, 2017: 32.2m shares
Company History
22 | Jounce Therapeutics © January, 2018

Perry Karsen
Chairman
Cary Pfeffer, M.D.
Director
Robert Tepper, M.D.
Director
Robert Kamen, Ph.D.
Director
Duncan Higgons
Director
Barbara Duncan
Director
Luis A. Diaz, Jr., M.D.
Director
Richard Murray, Ph.D.
Director
Leadership Team
Highly Experienced in Oncology and Immunotherapy
Executive Team
Richard Murray, Ph.D.
CEO and President
Elizabeth Trehu, M.D.
Chief Medical Officer
Kim Drapkin, CPA
Chief Financial Officer
Deborah Law, D.Phil.
Chief Scientific Officer
Stephen Farrand, Ph.D.
Chief Technical Officer
Hugh Cole
Chief Business Officer & Head
of Corporate Development
Jim Allison, Ph.D.
Chair Immunology
Founders / Advisory Board
Padmanee Sharma, M.D., Ph.D.
Professor
Board of Directors
Thomas Gajewski, M.D., Ph.D.
Professor
Robert Schreiber, Ph.D.
Professor, Director
Louis Weiner, M.D.
Professor, Chair
23 | Jounce Therapeutics © January, 2018
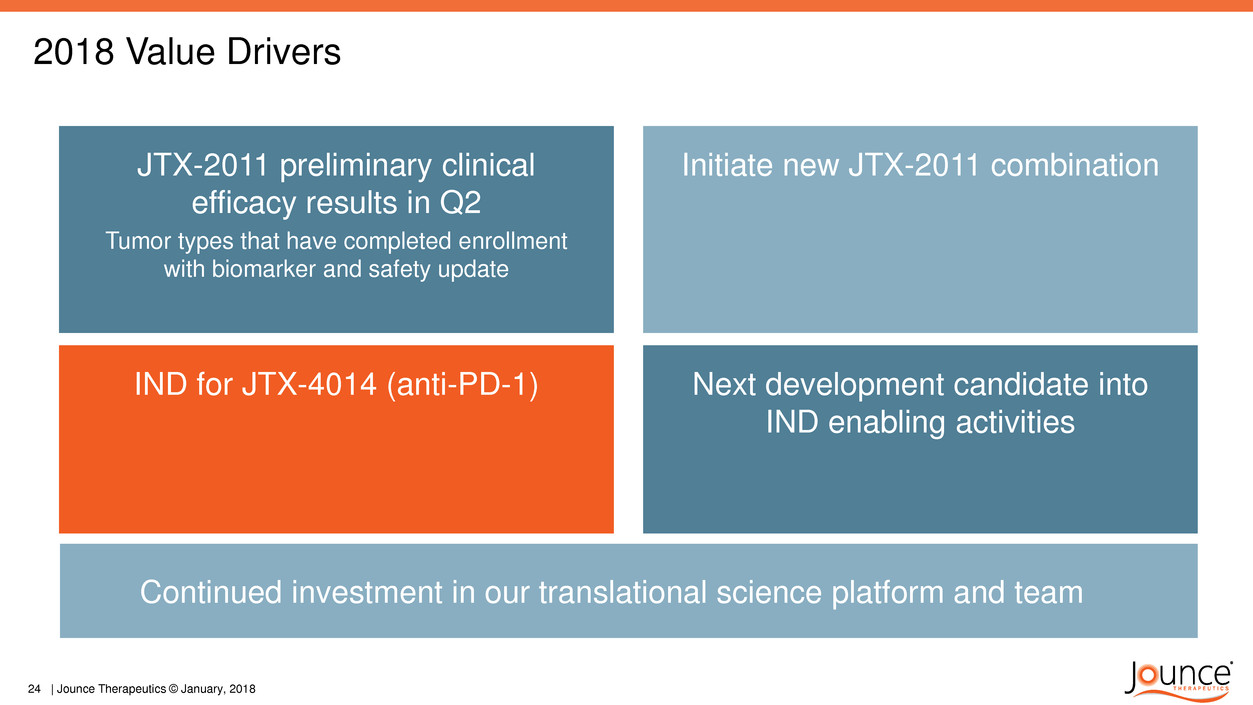
2018 Value Drivers
JTX-2011 preliminary clinical
efficacy results in Q2
Tumor types that have completed enrollment
with biomarker and safety update
IND for JTX-4014 (anti-PD-1)
Initiate new JTX-2011 combination
Next development candidate into
IND enabling activities
Continued investment in our translational science platform and team
24 | Jounce Therapeutics © January, 2018

Jounce Therapeutics
A Next Gen Immunotherapy Company
