Form 8-K Protagonist Therapeutics For: Jan 08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 8, 2017
PROTAGONIST THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
001-37852 |
|
98-0505495 |
|
(State or other jurisdiction |
|
(Commission |
|
(IRS Employer |
Protagonist Therapeutics, Inc.
7707 Gateway Blvd., Suite 140
Newark, California 94560-1160
(Address of principal executive offices, including zip code)
(510) 474-0170
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Item 7.01 Regulation FD Disclosure.
Attached hereto as Exhibit 99.1 is a copy of a presentation that Protagonist Therapeutics, Inc. (“Protagonist”) intends to utilize in connection with various meetings with analysts, investors and others at the 36th Annual J.P. Morgan Healthcare Conference, commencing on January 8, 2017. Exhibit 99.1 is incorporated herein by reference.
The information in this Current Report on Form 8-K, including the attached Exhibit 99.1, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, except as expressly set forth by specific reference in such filing to this Current Report on Form 8-K.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
Protagonist Therapeutics, Inc. | |
|
|
|
|
|
Dated: January 8, 2018 |
|
|
|
|
|
|
|
|
By: |
/s/ Thomas P. O’Neil |
|
|
|
Thomas P. O’Neil |
|
|
|
Chief Financial Officer |
Forward Looking Statements This presentation contains forward-looking statements of Protagonist Therapeutics, Inc. (Protagonist). All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, our capital resources, business strategy, prospective products, potential market for our products, availability of funding, enrollment in our clinical trials, clinical trial results, product approvals and regulatory pathways, timing and likelihood of success, plans, objectives and opinions of management regarding future operations, future results of current and anticipated products and our potential receipt of milestone payments and royalties under our Exclusive License and Collaboration Agreement with Janssen Biotech, Inc., are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Protagonist discusses many of these risks and uncertainties in detail under the heading “Risk Factors” contained in our most recent periodic reports on Forms 10-K and 10-Q, which are filed with the Securities and Exchange Commission. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration (FDA). They are currently limited by Federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or Protagonist or any director, employee, agent or advisor of Protagonist. This presentation does not purport to be all inclusive or to contain all the information you may desire. 2

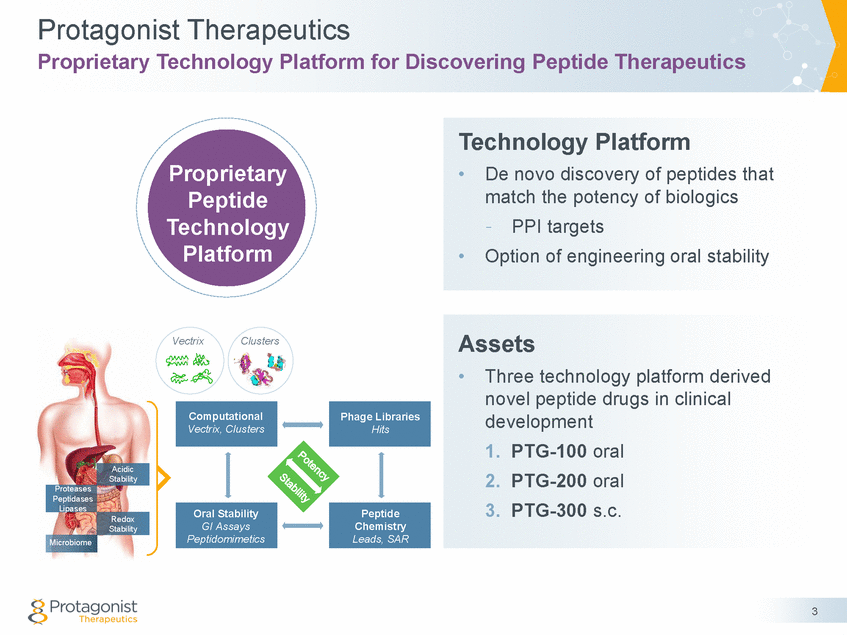
Protagonist Therapeutics Proprietary Technology Platform for Discovering Peptide Therapeutics Technology Platform Proprietary Peptide Technology Platform • De novo discovery of peptides that match the potency of biologics - PPI targets • Option of engineering oral stability Assets Vectrix Clusters • Three technology platform derived novel peptide drugs in clinical development 1. 2. 3. PTG-100 oral PTG-200 oral PTG-300 s.c. Acidic Stability Proteases Peptidases Lipases Redox Stability Microbiome 3 Peptide Chemistry Leads, SAR Oral Stability GI Assays Peptidomimetics Phage Libraries Hits Computational Vectrix, Clusters
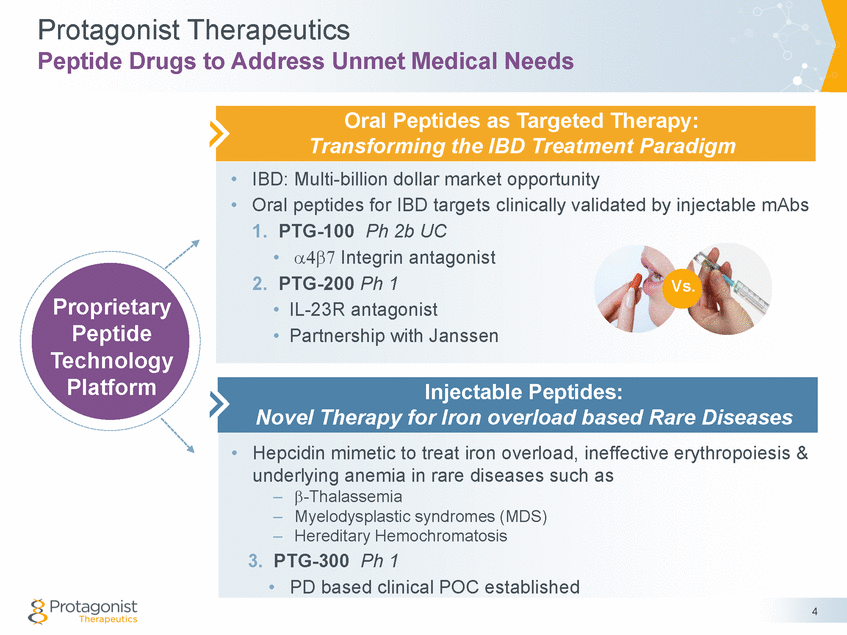
Protagonist Therapeutics Peptide Drugs to Address Unmet Medical Needs • • IBD: Multi-billion dollar market opportunity Oral peptides for IBD targets clinically validated by injectable mAbs 1. PTG-100 Ph 2b UC • Integrin antagonist 2. PTG-200 Ph 1 • IL-23R antagonist • Partnership with Janssen Vs. Proprietary Peptide Technology Platform • Hepcidin mimetic to treat iron overload, ineffective erythropoiesis & underlying anemia in rare diseases such as – – – -Thalassemia Myelodysplastic syndromes (MDS) Hereditary Hemochromatosis 3. PTG-300 Ph 1 • PD based clinical POC established 4 Injectable Peptides: Novel Therapy for Iron overload based Rare Diseases Oral Peptides as Targeted Therapy: Transforming the IBD Treatment Paradigm
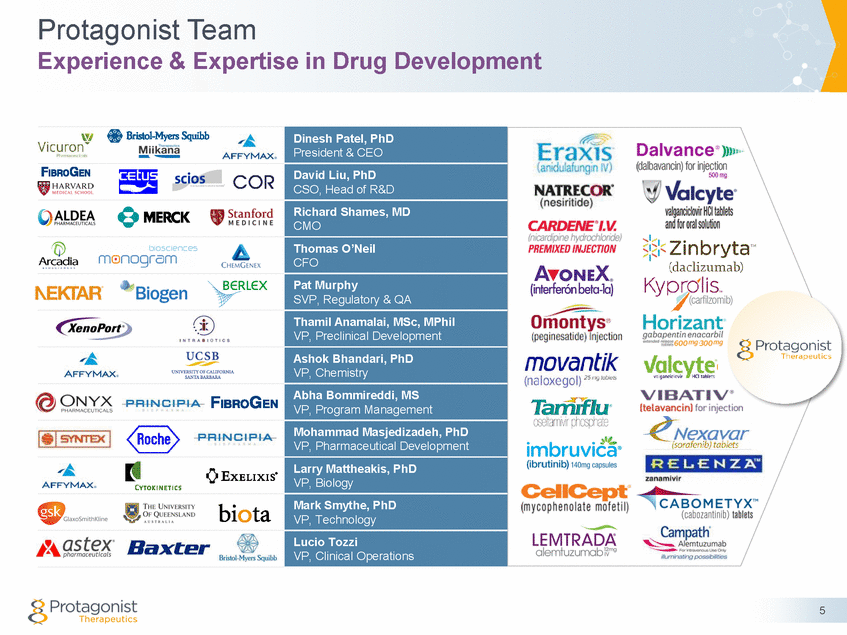
Protagonist Team Experience & Expertise in Drug Development 5 Dinesh Patel, PhD President & CEO David Liu, PhD CSO, Head of R&D Richard Shames, MD CMO Thomas O’Neil CFO Pat Murphy SVP, Regulatory & QA Thamil Anamalai, MSc, MPhil VP, Preclinical Development Ashok Bhandari, PhD VP, Chemistry Abha Bommireddi, MS VP, Program Management Mohammad Masjedizadeh, PhD VP, Pharmaceutical Development Larry Mattheakis, PhD VP, Biology Mark Smythe, PhD VP, Technology Lucio Tozzi VP, Clinical Operations
Peptide-based Therapeutics are on the Rise ‘Oral Peptides’ vs ‘Injectable Antibodies’ & side effects • Up to 80% new targets are ‘undruggable’ by small molecules –Protein-protein interaction (PPI) targets Antibodies vs peptides for ‘undruggable’ PPI targets –Oral peptides offer strong potential for differentiation vs. injectable antibodies • 6 Small Molecules Constrained Peptides Antibodies Molecular Size Route of Administration Features ~ 0.5 kDa Oral Do not bind to most PPI targets ~ 1 to 3 kDa Oral or Injectable High potency/selectivity against PPI targets ~ 150 kDa Injectable Expensive, inconvenient
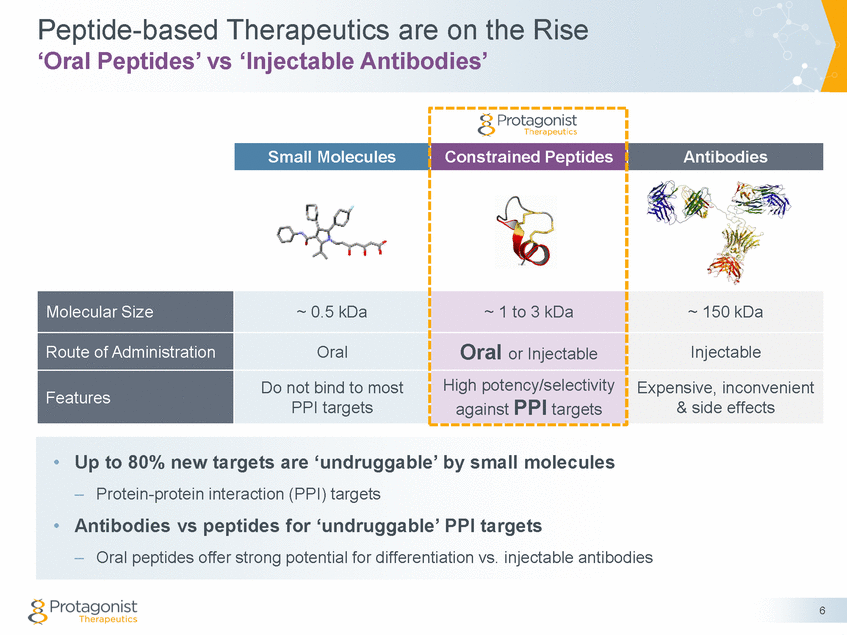
Current IBD Targeted Therapy by Injectable Antibody A Growing Multi-Billion Dollar Market Drugs • Injectable Antibodies – Blockbuster drugs –Humira® & Remicade® for IBD; Global Humira ® 2016 sales ~ $ 16 B Low response rates –~30% non-responders; ~30% relapse rates (6-12 Mo’s) Safety concerns – black box warning –Serious infections & malignancy; Immunogenicity Lack of convenience & compliance mAbs with different MOA • TNF-α: Humira®, Remicade® • • • • α4β7 integrin: Entyvio® • IL12/IL-23: Stelara® Surgery Severe TNF mAbs 4th (Humira, Remicade) 3rd Oral Targeted Therapy Moderate AZA/6-MP, MTX • • • • α4β7 integrin: IL-23R: S1P1: JAK: PTG-100 PTG-200 Ozanimod Tofacitinib, Filgotinib 2nd Steroids: Prednisone Mild 1st 5-ASAs, Antibiotics Step-up Therapy 7 Current Treatment Paradigm – TNF mAbs dominate Targeted Therapy
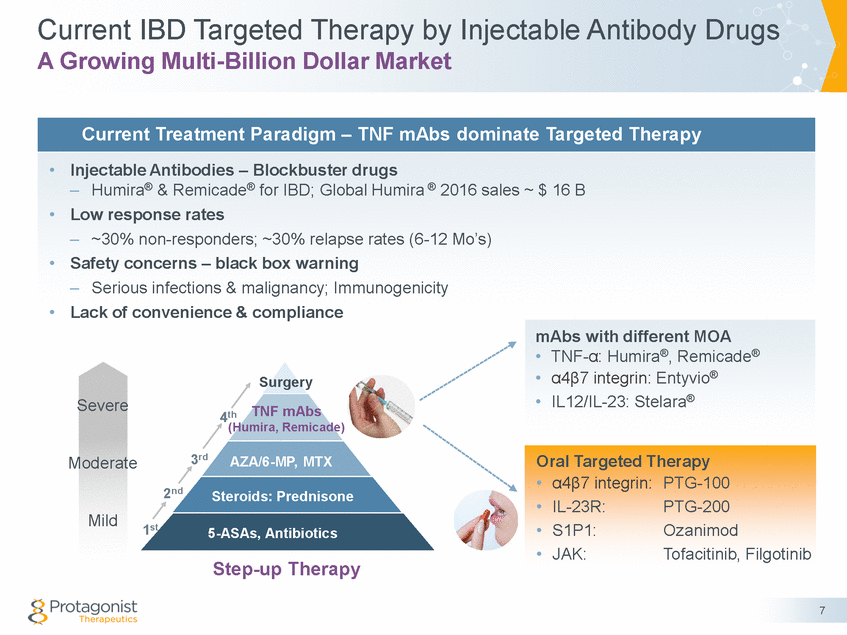
Oral Targeted Therapy for IBD Potential to Transform & Expand Current Treatment Paradigm • Potential advantages of oral, GI-restricted peptides PTG-100 & PTG-200 5-ASAs, Antibiotics Mild – – – – – Validated biological targets and/or pathways Reduced systemic infections Better safety & efficacy Better convenience & compliance New IP protection through mid 2030’s PTG-100 PTG-200 S1P1 Steroids 6-MP, MTX Biologics Moderate JAK Severe Surgery • Potential to transform IBD treatment paradigm Top-down Therapy – – Early introduction of targeted therapy Dose sparing, seamless combinations with other oral agents 8 Future Paradigm – Oral Targeted Therapy
PTG-100 α4β7 Antagonist for Inflammatory Bowel Disease 9

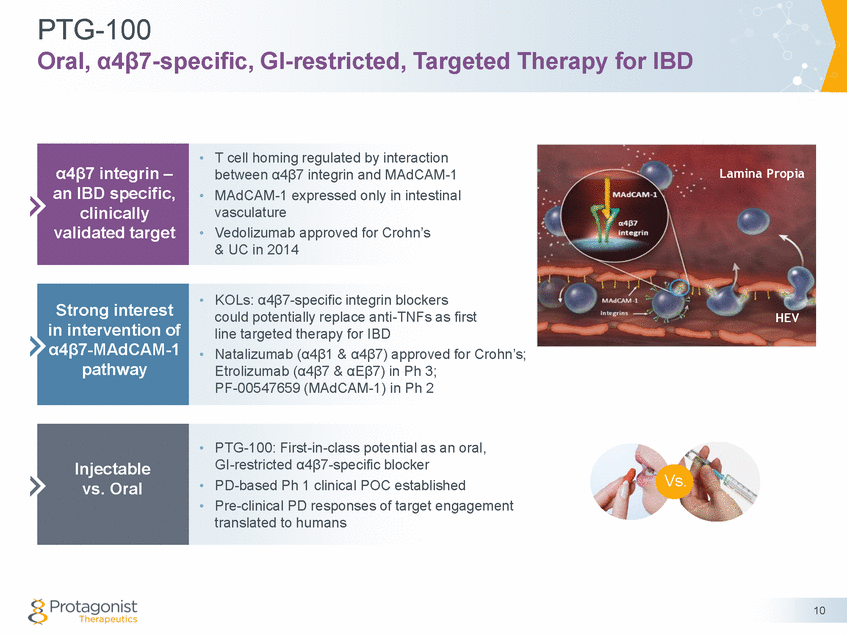
PTG-100 Oral, α4β7-specific, GI-restricted, Targeted Therapy for IBD • T cell homing regulated by interaction between α4β7 integrin and MAdCAM-1 MAdCAM-1 expressed only in intestinal vasculature Vedolizumab approved for Crohn’s & UC in 2014 • • • KOLs: α4β7-specific integrin blockers could potentially replace anti-TNFs as first line targeted therapy for IBD Natalizumab (α4β1 & α4β7) approved for Crohn’s; Etrolizumab (α4β7 & αEβ7) in Ph 3; PF-00547659 (MAdCAM-1) in Ph 2 • • PTG-100: First-in-class potential as an oral, GI-restricted α4β7-specific blocker PD-based Ph 1 clinical POC established Pre-clinical PD responses of target engagement translated to humans Vs. • • 10 Injectable vs. Oral Strong interest in intervention of α4β7-MAdCAM-1 pathway α4β7 integrin – an IBD specific, clinically validated target Lamina Propia HEV
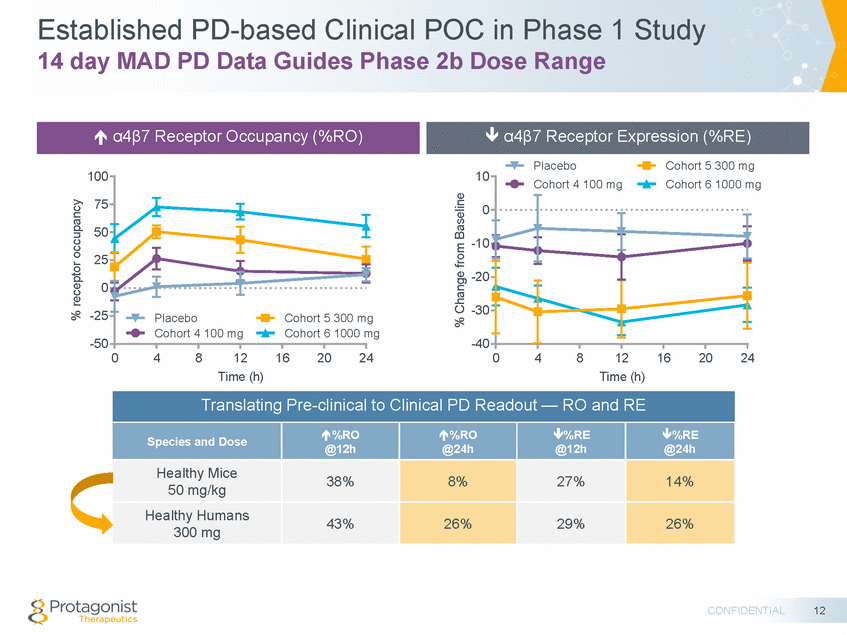
Translating Preclinical PD & Efficacy Results to Clinical Studies Target Engagement Blood Surrogate PD readouts α4β7 antagonists Effect of PTG-100 1. 2. PTG-100 (oral peptide) vs. DATK32 (i.p. mAb) 1. 2. % receptor occupancy (RO) % receptor expression (RE) Human Efficacy PD Translation Clinical POC PD based POC Clinical POC CONFIDENTIAL 11 Healthy humans Ph 1A/1B UC Subjects Ph 2b Mouse Effective Dose 70 kg Equivalent Dose 5-55 mg/kg ~ 30 to 300 mg Healthy Mice Colitis Mice

Established PD-based Clinical POC in Phase 1 Study 14 day MAD PD Data Guides Phase 2b Dose Range Placebo Cohort 5 300 mg 100 10 mg 75 0 50 -10 25 -20 0 -30 -25 g mg -50 -40 0 4 8 12 Time (h) 16 20 24 0 4 8 12 Time (h) 16 20 24 @24h CONFIDENTIAL 12 % receptor occupancy % Change from Baseline Translating Pre-clinical to Clinical PD Readout — RO and RE Species and Dose %RO @12h %RO @24h %RE @12h %RE Healthy Mice 50 mg/kg 38% 8% 27% 14% Healthy Humans 300 mg 43% 26% 29% 26% Cohort 4 100 mg Cohort 6 1000 Placebo Cohort 5 300 m Cohort 4 100 mg Cohort 6 1000 α4β7 Receptor Expression (%RE) α4β7 Receptor Occupancy (%RO)
PTG-100: Clinical Development Plans Ph 2b Clinical POC/ analysis (Q1 2018) (Q4 2018) NHV (n=78) US Pre-AUS CTN US IND EFD = embryo-fetal development, PPN = pre-/post-natal development 13 201620172018 Q1Q2Q3Q4 Q1Q2Q3Q4 Q1Q2Q3Q4 CLINICAL DEVELOPMENT Ph 1-SAD/MAD/BAInterim futilityFinal analysis PD based Clinical POCDose finding in UCN = 60-80N = 240-260 REGULATORY IND FORMULATION Capsule dosage for Phase 2bTablet formulation for Phase 3 TOXICOLOGY 3-month tox6-month rat and 9-month monkey tox DRF ReprotoxEFD & PPN Reprotox
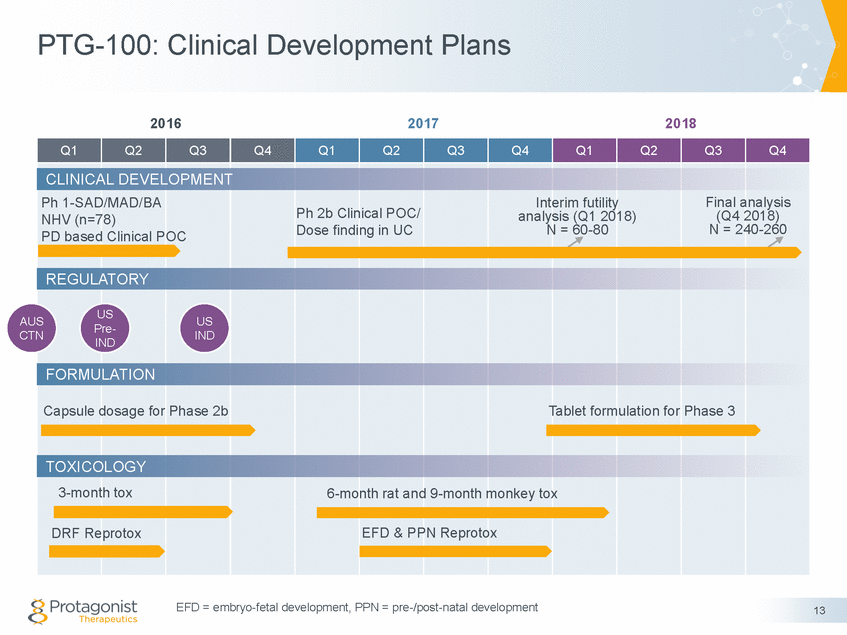
Phase 2b PROPEL Study in UC Patients Randomized, Double-blind, Placebo-controlled Adaptive Parallel Design Stage 1 Stage 2 Primary endpoints: • Clinical remission • Safety & tolerability Placebo Placebo 150 mg QD Dose A Secondary Endpoints: 300 mg QD Dose B • • • • Clinical response Endoscopic response Endoscopic remission Change in patient-reported outcomes PD (%RO & %RE) PK ADA 900 mg QD • • • Phase 2b study milestones • • • Study initiation (FPI) Dec 2016 Adaptive trial design; interim ‘futility’ analysis Q1 2018 Phase 2b completion (LPO) expected Q4 2018 14 Interim Analysis Interim Analysis • Futility analysis • Drop 1 (or 2) dose - hierarchical evaluation of clinical remission, response, endoscopy & PD (%RO & %RE) readouts Enrollment Criteria: • Biologics-naïve or TNFα-experienced (≤ 50%) • Stratification - Prior TNFα antagonist use 12 week induction study n ~60-80n ~160-180 Moderate to severe active UC n ~260, ~100 sites Final Analysis n ~240-260
PTG-100 Summary • Oral peptide PTG-100 ✔ approach with validated target • Proof of target engagement & efficacy in rodent models of colitis Side by side comparison of PTG-100 vs. DATK32 (mouse integrin mAb) Pre-Clinical ✔ • • • Blood receptor occupancy (%RO) Blood receptor expression (%RE) ✔ in Ph 1 NHV study • Interim futility analysis based go/no-go outcome in Q1 2018 Top line data expected in Q4 2018 In progress • 15 4 Ph 2B UC study PD based clinical 3 POC established 2 POC established Differentiated 1 a clinically •mAb Entyvio®

PTG-200 IL-23R Antagonist for Inflammatory Bowel Disease 16
PTG-200 Oral, IL-23 Receptor Specific, GI-restricted, Targeted Therapy for IBD Blocks IL-23 pathway p19 p35 p40 p40 Stelara® (IL-12/23, p40) approved for Psoriasis and CD, Phase 3 for UC Several IL-23 p19 antibodies in late-stage development for autoimmune indications (Risankizumab®, Brazikumab®) • • IL-23 IL-12 IL-17A, IL-17F, IL-22 TH17 stabilization IFN-γ TH1 development Inflammation Autoimmune diseases Cellular Immunity Bacterial & viral infections • • Localized & overexpressed in GI of IBD patients IL-23R gene mutations – possible IBD risk factor 17 -23R 12Rβ1 12Rβ2 12Rβ1 IL-23 receptor CELL MEMBRANE IL IL-IL-IL-Stelara® & several IL-23 specific antibodies in development PTG-200: Blocks IL-23 receptor & IL-23 pathway Potential first-in-class approach against clinically-validated IL-12/23 pathway p19 antibody: Stelara®: Blocks both IL-23 & IL-12 pathways
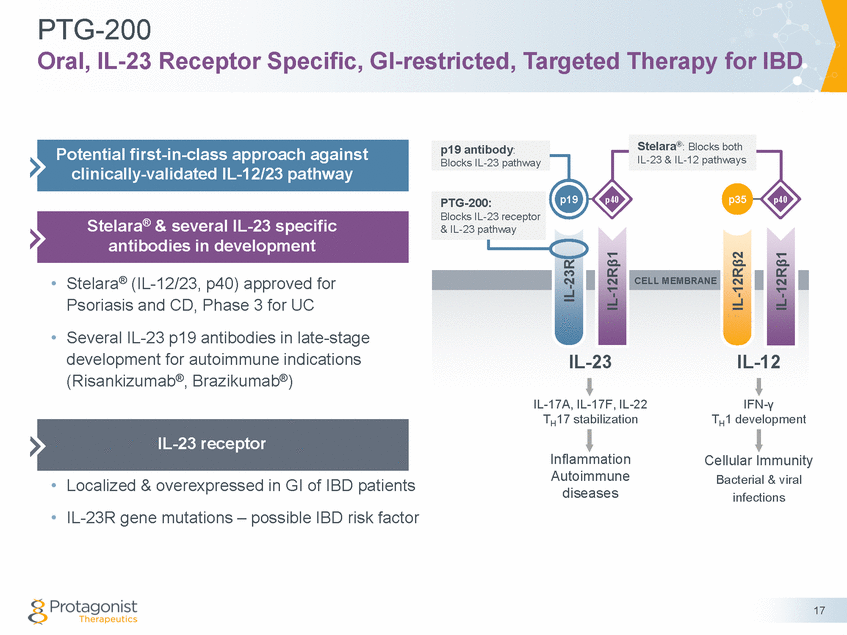
Discovery of Oral IL-23 Receptor Antagonist PTG-200 From Discovery to Pre-Clinical POC (1000+ peptides) to Ph1 Initiation Potency PK: Rats & Cynos ✓ Orally stable: fecal recovery in rats ✓ GI-restricted: <0.5% plasma exposure ✓ Principal exposure in various GI tissues PD & Efficacy • Efficacy in Colitis rats ✓ Colon macroscopic score ✓ Colon weight/length ratio ✓ Body weight loss ✓ histopathology • Reduced expression of IL-23 pathway cytokines Selectivity Development PTG-200 Oral Stability * Simulated Intestinal Fluid ** Simulated Gastric Fluid 18 Stability (t1/2, hr) SIF* SGF** >12 >10 ELISA, nM IL-23/IL-12 Rb1 IL-6/IL-6R >100,000 >100,000 ELISA (nM) Cell assay (nM) Human Human Primary 2.0 2.2
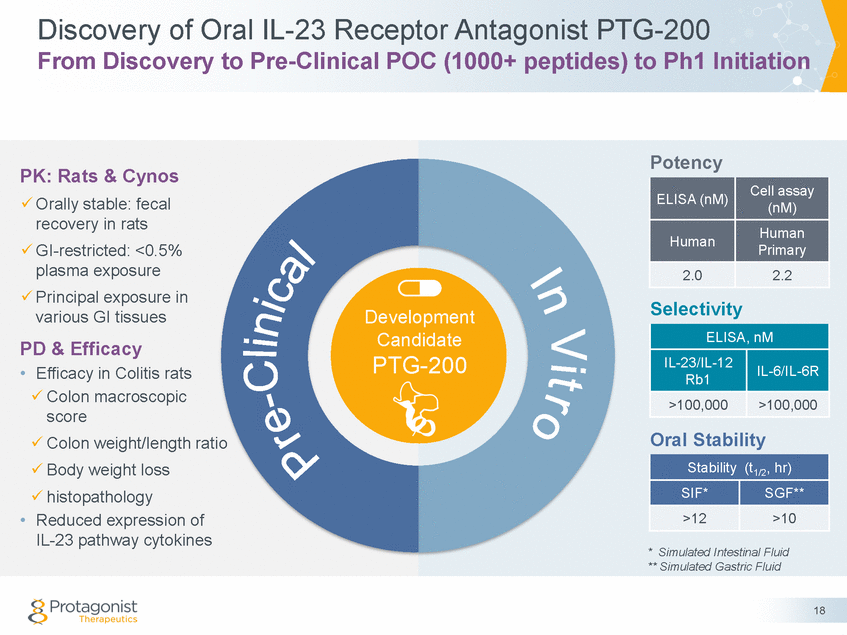
Protagonist & Janssen Collaboration Development and Commercialization of PTG-200 1. Janssen is a world leader in IBD –mAbs franchise: Remicade®, Simponi®, Stelara® 2. Mutual excitement around oral targeted therapy drugs for IBD 3. Further validation for the ‘oral’ hypothesis –Stelara® is an injectable IL-12/23 mAb antibody marketed by Janssen –PTG-200 is a first-in-class oral IL-23 receptor antagonist peptide 4. Protagonist & Janssen have a long standing relationship –Johnson & Johnson Innovation – JJDC is an early investor and shareholder in Protagonist 5. Competitive process –Janssen was the best choice for PTGX both financially & strategically 19 Protagonist’s Rationale
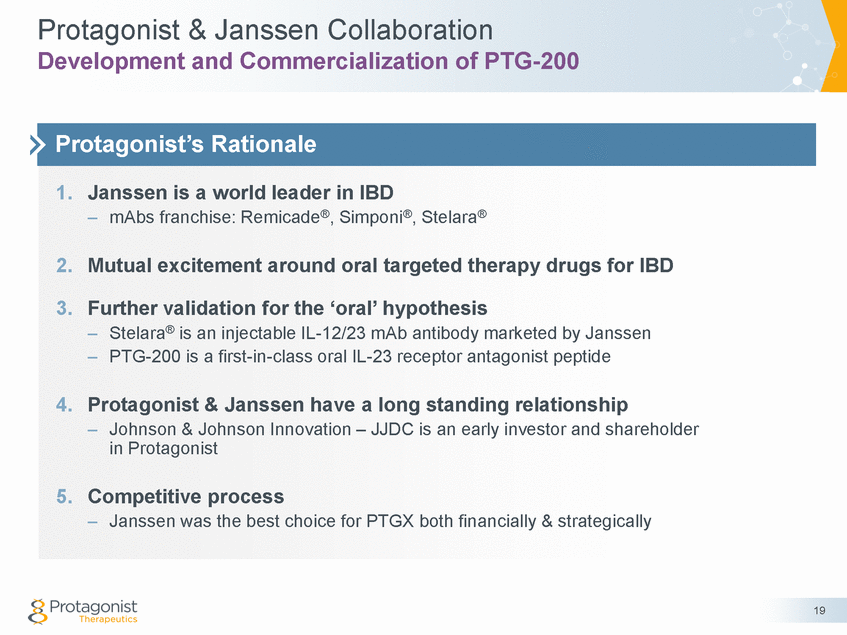
Janssen Collaboration for Executed May 26, 2017 PTG-200 • Janssen receives exclusive WW license to PTG-200 • • • Phase 1: 100% Phase 2: 20% Phase 3 & beyond: 0% • • Janssen to lead WW commercialization PTGX has the right to co-detail up to 30% the US calls; gets reimbursed PTGX economics remains the same regardless co-detail option exercise • • Tiered royalties on worldwide net sales -Ten to mid-teens percent 20 Royalty Commercialization & Co-detailing Financial Summary Upfront $50M Phase 2 Interim Analysis $125M Phase 2 Completion $200M All Other Milestone Payments $615M Potential Total $990M Protagonist share of development costs License
Financials Funding R&D efforts through 2019 21 •$104.9 M in cash, cash equivalents and investments at 3Q 2017 end – Includes $50 M up-front payment received in August 2017 from Janssen as part of PTG-200 licensing and collaboration agreement •$60 M follow-on offering in October 2017 – With the additional funding from Janssen collaboration and proceeds from follow-on offering, sufficient financial resources to fund operations through 2019 •$90 M raise through an upsized IPO in August 2016 •$67 M in Series A through C financing - strategics, VCs, cross-overs

Protagonist Platform PTG-300 and Other Potential Future Applications 22

Peptide Technology Platform Potential for Broad Utility vs. Diverse Undruggable Targets Oral IL-23R antagonist Oral agonist GI non-IBD Oral α4β7 antagonist IL-6 antagonist Hepcidin mimetic Nav 1.7 blocker 23 Peptide Technology Platform
PTG-300: Hepcidin Mimetic Protagonist’s 3rd Asset in Clinical Development Hepcidin • • A natural hormone and master regulator of iron homeostasis Absence or deficiency of hepcidin associated with ineffective erythropoiesis and chronic anemia in iron-overload related rare diseases – β-thalassemia – Myelodysplastic syndromes (MDS) Hereditary Hemochromatosis • PTG-300 • • A hepcidin mimetic discovered through Protagonist’s peptide technology platform Potential advantages of a mimetic: Engineer drug-like properties – Potency & efficacy, PK, Stability, ease of synthesis (COGS) Current status: • Preliminary PD based clinical POC established in Phase 1 study in NHVs 24 PTG-300: Hepcidin Mimetic for Treating Iron Overload related Rare Diseases
Hepcidin: Regulator of Iron Homeostasis PTG-300 Hepcidin Mimetic Iron recycled by macrophages gets picked up by ferroportin; PTG-300 inhibits iron export to circulation Macrophage Hepcidin mimetic PTG-300 binds and degrades ferroportin; inhibiting iron export to circulation mimicking natural hepcidin’ regulation of iron Ferroportin PT Hepcidin Fe DMT1 Enterocyte Ferroportin TF-Fe Fe PTG RBC Intestine Bone Marrow 25 D’Angelo G. Role of hepcidin in the pathophysiology and diagnosis of anemia. Blood Res. 2013;48:10-15. Transferin-iron Delivery to red blood cells Dietary Iron Stored Iron

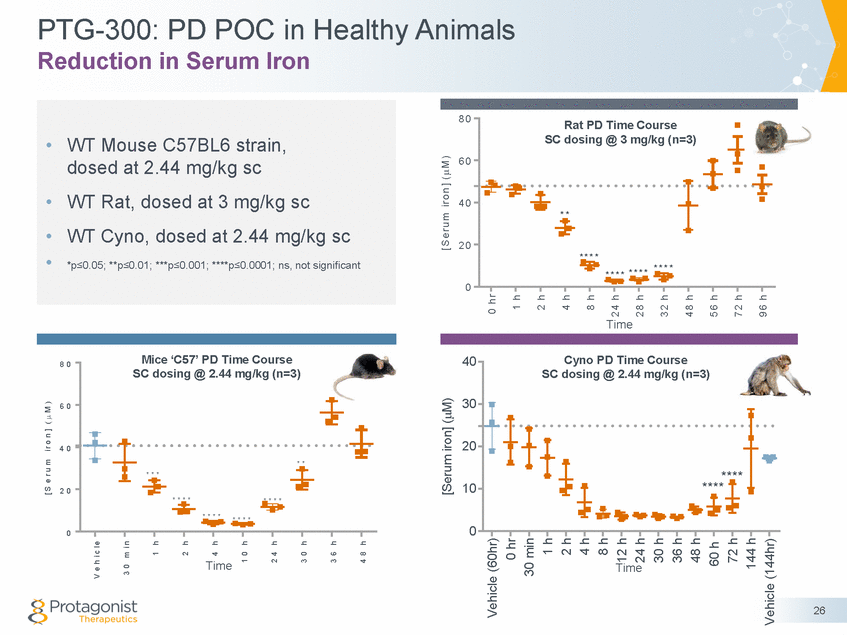
PTG-300: PD POC in Healthy Animals Reduction in Serum Iron 8 0 Rat PD Time Course SC dosing @ 3 mg/kg (n=3) 6 0 4 0 * * 2 0 * * * * * * * * * * * * * * * * 0 Time Mice ‘C57’ PD Time Course SC dosing @ 2.44 mg/kg (n=3) Cyno PD Time Course 40 8 0 30 6 0 20 4 0 * * * * * 10 2 0 * * * * * * * * * * * * * * * * 0 0 Time Time 26 [ S e r u m i r o n ] ( M ) V e h i c l e 3 0 m i n 1 h 2 h 4 h 1 0 h 2 4 h 3 0 h 3 6 h 4 8 h [Serum iron] (M) [ S e r u m i r o n ] ( M ) Vehicle (60hr) 0 hr 30 min 1 h 2 h 4 h 8 h 12 h 24 h 30 h 36 h 48 h 60 h 72 h 144 h Vehicle (144hr) 0 h r 1 h 2 h 4 h 8 h 2 4 h 2 8 h 3 2 h 4 8 h 5 6 h 7 2 h 9 6 h SC dosing @ 2.44 mg/kg (n=3) **** **** •WT Mouse C57BL6 strain, dosed at 2.44 mg/kg sc •WT Rat, dosed at 3 mg/kg sc •WT Cyno, dosed at 2.44 mg/kg sc • *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001; ns, not significant
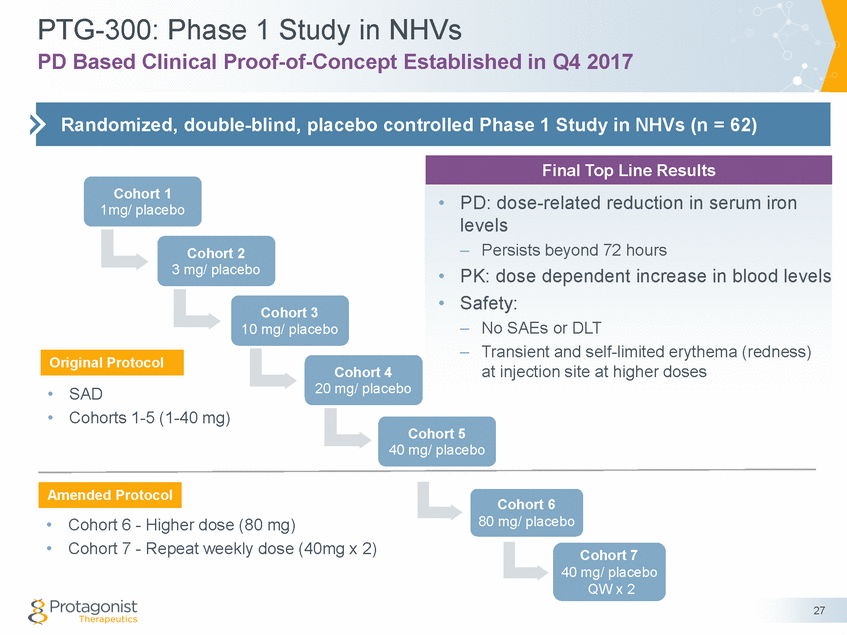
PTG-300: Phase 1 Study in NHVs PD Based Clinical Proof-of-Concept Established in Q4 2017 Cohort 1 1mg/ placebo Cohort 2 3 mg/ placebo Cohort 3 10 mg/ placebo Cohort 4 20 mg/ placebo • • SAD Cohorts 1-5 (1-40 mg) Cohort 5 40 mg/ placebo Cohort 6 80 mg/ placebo • • Cohort 6 - Higher dose (80 mg) Cohort 7 - Repeat weekly dose (40mg x 2) Cohort 7 40 mg/ placebo QW x 2 27 Amended Protocol Original Protocol Final Top Line Results •PD: dose-related reduction in serum iron levels –Persists beyond 72 hours •PK: dose dependent increase in blood levels •Safety: –No SAEs or DLT –Transient and self-limited erythema (redness) at injection site at higher doses Randomized, double-blind, placebo controlled Phase 1 Study in NHVs (n = 62)
PTG-300 Efficacy based POC in -thalassemia Mouse Model -thalassemic mouse model (Hbbth3+ Mice); 6 week treatment • • Evaluate effects on – Ineffective erythropoiesis – Iron overload • • PTG-300 1 mg/kg Q2D saline DFP (deferiprone, oral iron chelator) 1.25mg/mL water ad libitum Combination of PTG-300 Q2D and DFP water ad libitum • Comparison to iron chelator • 28 Objectives Study design
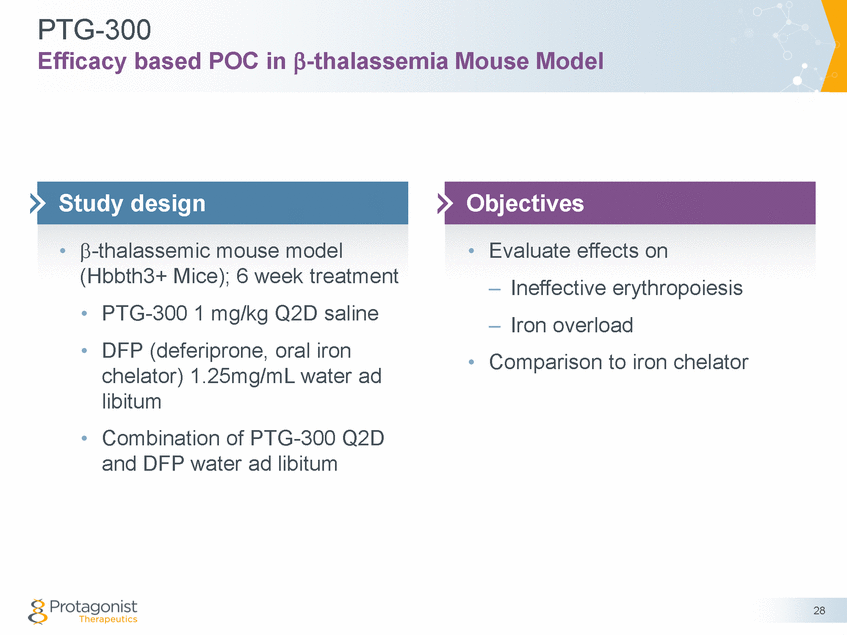
PTG-300: Efficacy based POC in β-thalassemia Mouse Model Improved Erythropoiesis 12 11 10 9 8 7 6 5 17 16 15 14 13 12 11 10 9 8 *** **** ** **** ns * ** ns 50 40 30 20 10 0 60 ns 40 ns ** *** 20 0 29 Reticulocyte Percentage (%) RBC (x106 cells/L) Vehicle DFP PTG300 DFP + PTG300 HCT (%) HGB (g/dL) • PTG-300 1 mg/kg dosed Q2D for 6 weeks (Hbbth3+ Mice) • DFP (deferiprone, oral iron chelator) dosed at 1.25mg/mL • *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001; ns, not significant WTth3 + Vehicleth3 + DFPth3 + PTG300th3 + DFP + PTG300 **** ns **** **** Reticulocyte (%) Hematocrit * WT Hemoglobin Red Blood Cells
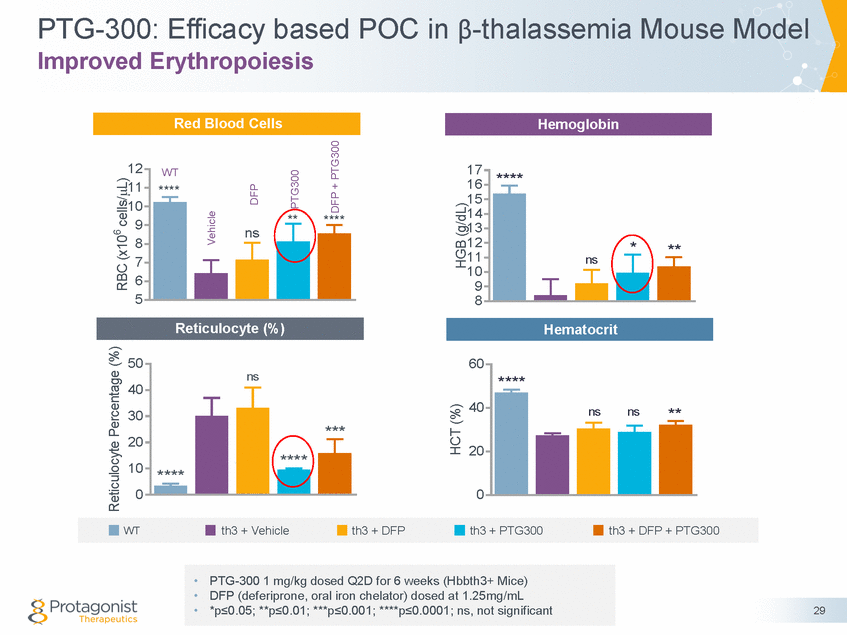
PTG-300: Efficacy based POC in β-thalassemia Mouse Model Decreased Splenomegaly and Liver Iron Overload 800 0.03 600 ns 0.02 400 * 0.01 ** 200 *** **** **** 0 0.00 30 Spleen/Body Weight (g/g) Total Liver Fe (ng /mg) wet tissue • PTG-300 1 mg/kg dosed Q2D for 6 weeks (Hbbth3+ Mice) • DFP (deferiprone, oral iron chelator) dosed at 1.25mg/mL • *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001; ns, not significant WTth3 + Vehicleth3 + DFPth3 + PTG300th3 + DFP + PTG300 **** * *** Non-heme Iron in Liver (ng/mg) Spleen/Body Weight (g/g)
PTG-300: Efficacy based POC in β-thalassemia Mouse Model Improved Erythropoiesis, Decreased Splenomegaly and Liver Iron • PTG-300 statistically improved erythropoiesis, decreased splenomegaly and liver iron overload DFP only decreased liver iron overload and did not statistically improve erythropoiesis or decrease splenomegaly • • PTG-300 may potentially address pre-existing anemia and liver iron overload through iron restriction to restore iron homeostasis Potential to address sequelae in β-thalassemia –Reduce transfusions and subsequent iron overload –Prevent splenomegaly and need for splenectomy with a reduction of thrombosis risk Potential to treat other diseases characterized by ineffective erythropoiesis and iron overload, e.g. low risk MDS • • 31 Conclusions Results
PTG-300: Proposed Clinical Development Plan • Single global phase 2/3 clinical trial in -thalassemia, MDS patients (basket protocol) Phase 2 dose-range finding – Open label single-arm study to identify safe and effective starting dose Phase 3 placebo-controlled pivotal trial – Randomized, double-blind, placebo-controlled 2-arm study • • • Phase 2 and 3 patients can roll over to open label extension 32

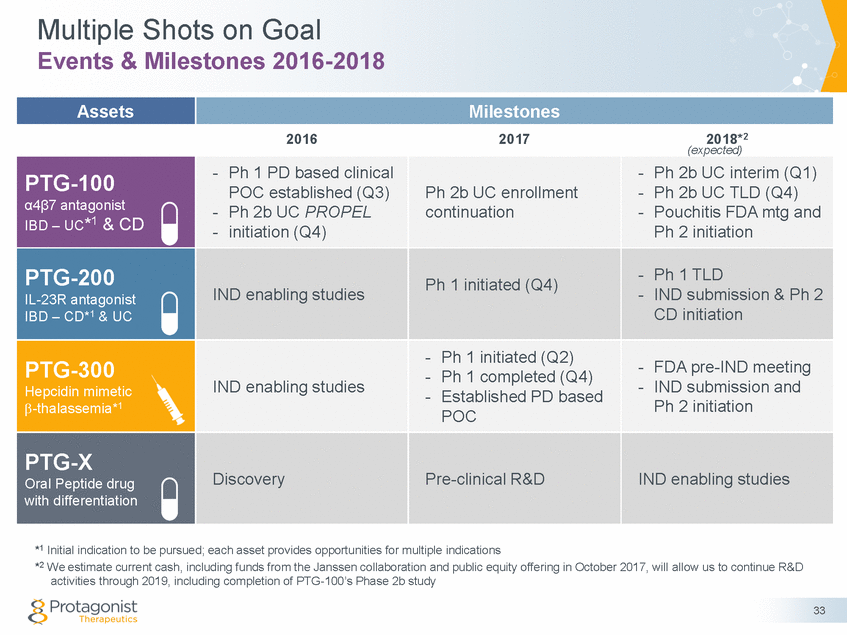
Multiple Shots on Goal Events & Milestones 2016-2018 (expected) - Ph 2b UC TLD (Q4) Ph 2 initiation - IND submission & Ph 2 - IND submission and *1 Initial indication to be pursued; each asset provides opportunities for multiple indications *2 We estimate current cash, including funds from the Janssen collaboration and public equity offering in October 2017, will allow us to continue R&D activities through 2019, including completion of PTG-100’s Phase 2b study 33 Assets Milestones 2016 2017 2018*2 PTG-100 α4β7 antagonist IBD – UC*1 & CD - Ph 1 PD based clinical POC established (Q3) - Ph 2b UC PROPEL - initiation (Q4) Ph 2b UC enrollment continuation - Ph 2b UC interim (Q1) - Pouchitis FDA mtg and PTG-200 IL-23R antagonist IBD – CD*1 & UC IND enabling studies Ph 1 initiated (Q4) - Ph 1 TLD CD initiation PTG-300 Hepcidin mimetic -thalassemia*1 IND enabling studies - Ph 1 initiated (Q2) - Ph 1 completed (Q4) - Established PD based POC - FDA pre-IND meeting Ph 2 initiation PTG-X Oral Peptide drug with differentiation Discovery Pre-clinical R&D IND enabling studies
Thank You