Form 8-K CTI BIOPHARMA CORP For: Jan 05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 5, 2018
CTI BIOPHARMA CORP.
(Exact name of registrant as specified in its charter)
Washington | 001-12465 | 91-1533912 | ||
(State or other jurisdiction of incorporation or organization) | (Commission File Number) | (I.R.S. Employer Identification Number) | ||
3101 Western Avenue, Suite 800
Seattle, Washington 98121
(Address of principal executive offices)
Registrant’s telephone number, including area code: (206) 282-7100
Not applicable
(Former name or former address, if changed since last report).
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01. Regulation FD Disclosure.
Commencing on or after January 8, 2018, members of management at CTI BioPharma Corp. (the “Company”) will be providing a corporate update to analysts and investors through a series of one-on-one meetings.
The information in this Current Report on Form 8-K, including the slides to be used in these presentations attached as Exhibit 99.1 hereto, are being furnished and not filed pursuant to Item 7.01 of Form 8-K. Such information shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference into any of the Company’s filings under the Securities Act of 1933, as amended (the "Securities Act") or the Exchange Act whether made before or after the date hereof and regardless of any general incorporation language in such filings, except to the extent expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Exhibit No. | Description | |||
99.1 | ||||
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
CTI BIOPHARMA CORP. | ||||
Date: January 5, 2018 | By: | /s/ David H. Kirske | ||
David H. Kirske | ||||
Chief Financial Officer | ||||
EXHIBIT INDEX
Exhibit No. | Description | |||||||
99.1 | ||||||||

© Copyright 2017 CTI BioPharma Corp. All rights reserved.
Corporate Presentation
January 2018

Forward Looking Statement
This presentation includes forward-looking statements within the meaning of the Safe Harbor provisions of the Private Securities Litigation Reform Act of
1995. Such statements are subject to a number of risks and uncertainties, the outcome of which could materially and/or adversely affect actual future
results and the trading price of CTI BioPharma's securities. Such statements include, but are not limited to, expectations with respect to the timing and
planned enrollment of and interim analysis for PAC203 and submission of responses to Day 120 list of questions, and our ability to interpret clinical trial
data and results for PERSIST-2 despite not satisfying the pre-specified minimum evaluable patient goal, expectations with respect to the potential
therapeutic utility of pacritinib, statements regarding CTI BioPharma’s expectations with respect to the potential of pacritinib to achieve treatment goals,
the development of CTI BioPharma and its product and product candidate portfolio, including the advancement of pacritinib and other pipeline programs,
CTI BioPharma’s ability to achieve its goals in 2018 and beyond, including achieving cost efficiency and year-on-year cost reduction goals, CTI
BioPharma’s intent to continue efforts to commercialize PIXUVRI in Europe in partnership with Servier and expand the market potential for PIXUVRI,
whether positive outcomes to PIX306 could lead to label expansion, and timing of PIX306 top-line results, CTI BioPharma’s plans to continue advancing
the development of its pipeline candidates through strategic product collaborations or cooperative group and investigator-sponsored trials, as well as the
identification and acquisition of additional pipeline opportunities. In particular, this presentation addresses top-line results regarding data from CTI
BioPharma’s Phase 3 trial of pacritinib for the treatment of patients with myelofibrosis whose platelet counts are less than or equal to 100,000 per
microliter. Meaningful interpretation of PERSIST-2 may not be possible because the pre-specified minimum evaluable patient goal was not met. Risks
that contribute to the uncertain nature of the forward-looking statements include, among others, risks associated with the biopharmaceutical industry in
general and with CTI BioPharma and its product and product candidate portfolio in particular including, among others, risks associated with the
following: that CTI BioPharma cannot predict or guarantee the outcome of preclinical and clinical studies, the potential failure of pacritinib to prove safe
and effective as determined by the FDA and/or the European Medicines Agency, changes to study protocol or design or sample size to address any
patient safety, efficacy or other issues raised by the FDA or otherwise, that top-line results observed to date may differ from future results or that
different conclusions or considerations may qualify such results once existing data has been more fully evaluated, that CTI BioPharma may not obtain
favorable determinations by other regulatory, patent and administrative governmental authorities, that CTI BioPharma may experience delays in the
commencement of preclinical and clinical studies, that the costs of developing pacritinib and CTI BioPharma’s other product candidates may rise; other
risks, including, without limitation, competitive factors, technological developments, that CTI BioPharma may not be able to sustain its current cost
controls or further reduce its operating expenses, that CTI BioPharma may not achieve previously announced goals, contractual milestones and
objectives as or when projected, that CTI BioPharma’s average net operating burn rate may increase, that CTI BioPharma will continue to need to raise
capital to fund its operating expenses, but may not be able to raise sufficient amounts to fund its continued operation as well as other risks listed or
described from time to time in CTI BioPharma’s most recent filings with the Securities Exchange Commission on Forms 10-K, 10-Q and 8-K. Except as
required by law, CTI BioPharma does not intend to update any of the statements in this presentation upon further developments.
2

CTI BioPharma
• Lead compound pacritinib (JAK2 inhibitor)
- Seeks to address a significant unmet medical need in myelofibrosis
- Focus on treating patients following ruxolitinib (Jakafi/Jakavi) therapy and/or with
thrombocytopenia
• Phase 2 dose-finding study (PAC203) on-going
- Interim analysis expected in 2Q 2018, expected to complete enrollment 2H 2018
• Pacritinib MAA under review in Europe for treatment of myelofibrosis patients with thrombocytopenia
- Day 120 list of questions received; expect to submit responses 1Q 2018
• PIXUVRI conditionally approved in EU for Relapsed Aggressive B-cell NHL
- Marketed by Servier
- Phase 3 PIX306 study has completed enrollment; top-line results expected 1H 2018
- Positive outcome has potential to lead to a label expansion
• New Management (CEO and CFO) and Board of Directors membership
Developing novel targeted therapies in blood-related
cancers
3

Focused and Well Financed
Key Milestones 2017
• Corporate
New management team members; 3 new independent Directors
Projected year-on-year cost reductions
Fundraising of $45mm in gross proceeds completed in July 2017
Major shareholders included BVF and OrbiMed
• Pacritinib
Removal of FDA clinical hold
First patients enrolled in PAC203 myelofibrosis study
MAA under review for myelofibrosis patients with platelet counts
≤100,000/µL
• Pixuvri
Expansion of Servier PIXUVRI collaboration with cost reductions
Completion of full enrollment of PIX306 label expansion trial
4
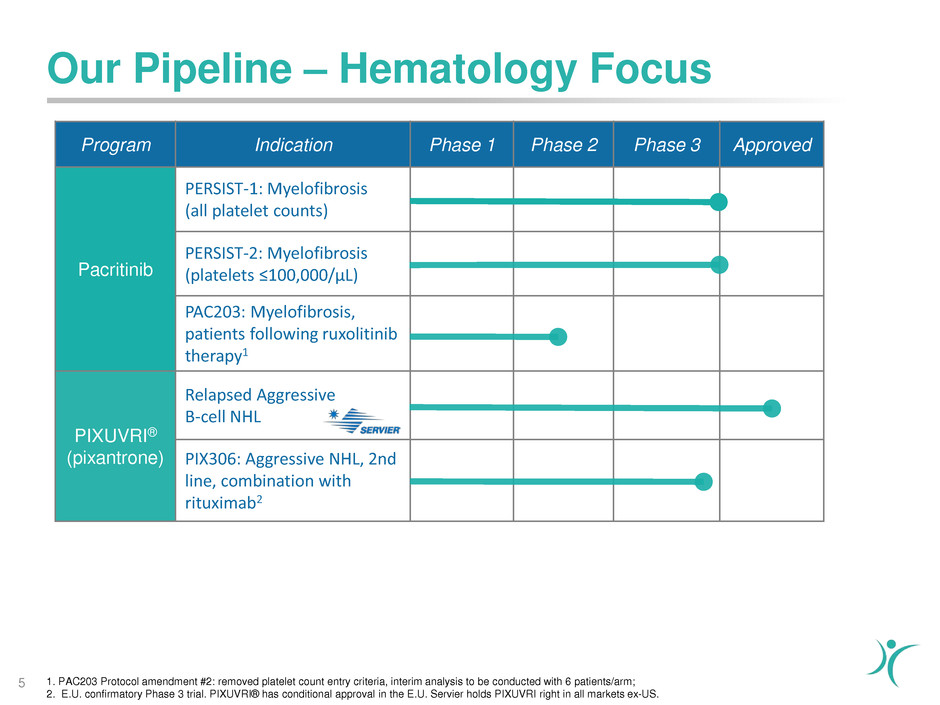
Our Pipeline – Hematology Focus
5
Program Indication Phase 1 Phase 2 Phase 3 Approved
Pacritinib
PERSIST-1: Myelofibrosis
(all platelet counts)
PERSIST-2: Myelofibrosis
(platelets ≤100,000/µL)
PAC203: Myelofibrosis,
patients following ruxolitinib
therapy1
PIXUVRI®
(pixantrone)
Relapsed Aggressive
B-cell NHL
PIX306: Aggressive NHL, 2nd
line, combination with
rituximab2
1. PAC203 Protocol amendment #2: removed platelet count entry criteria, interim analysis to be conducted with 6 patients/arm;
2. E.U. confirmatory Phase 3 trial. PIXUVRI® has conditional approval in the E.U. Servier holds PIXUVRI right in all markets ex-US.
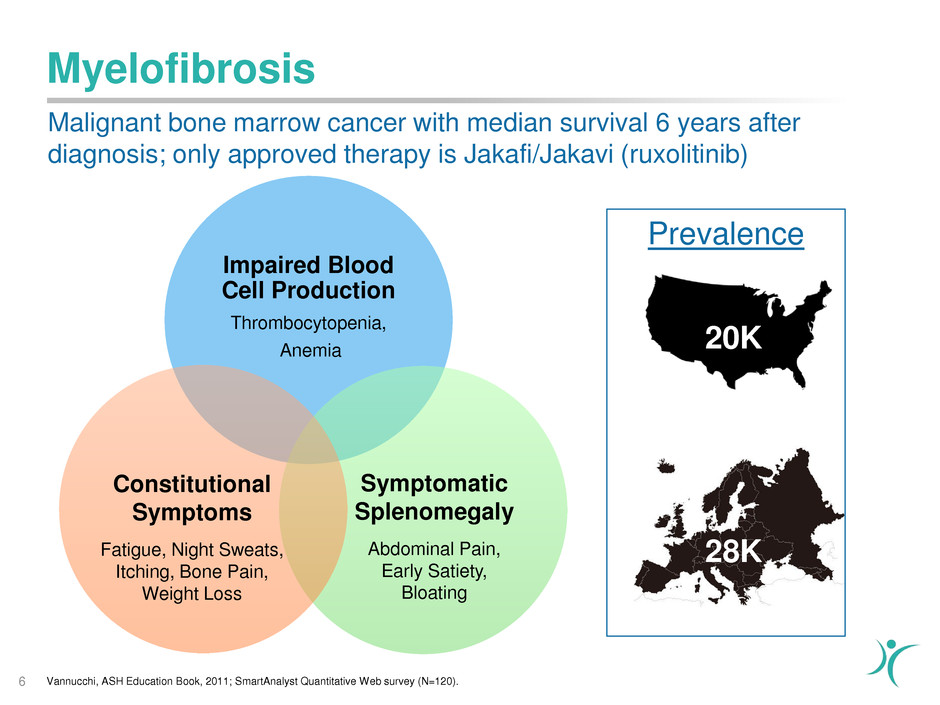
Myelofibrosis
6
Impaired Blood
Cell Production
Thrombocytopenia,
Anemia
Prevalence
20K
28K
Malignant bone marrow cancer with median survival 6 years after
diagnosis; only approved therapy is Jakafi/Jakavi (ruxolitinib)
Vannucchi, ASH Education Book, 2011; SmartAnalyst Quantitative Web survey (N=120).
Symptomatic
Splenomegaly
Abdominal Pain,
Early Satiety,
Bloating
Constitutional
Symptoms
Fatigue, Night Sweats,
Itching, Bone Pain,
Weight Loss
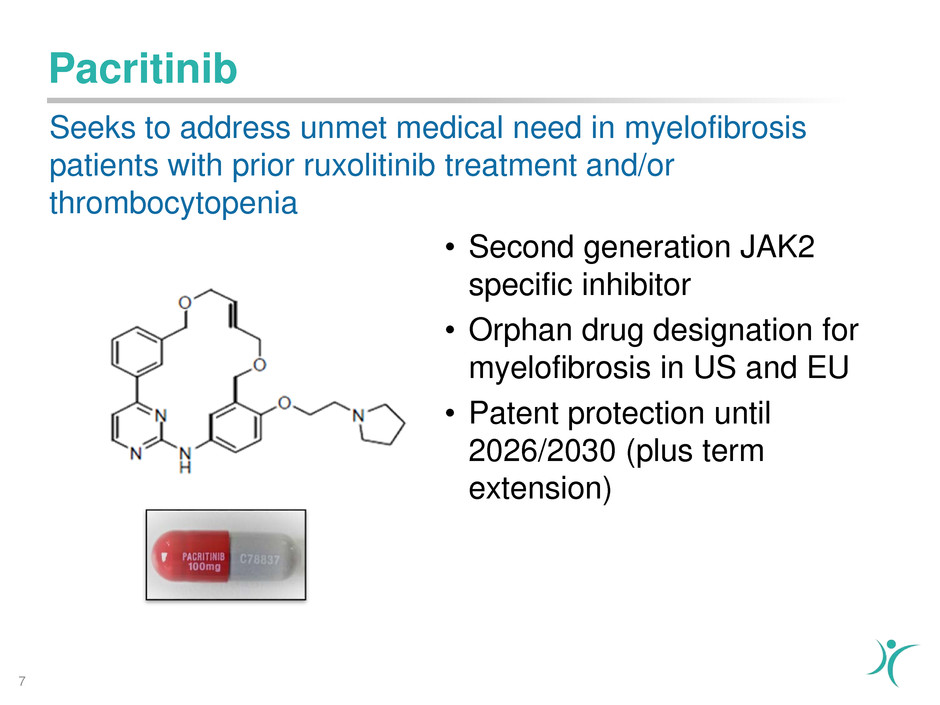
Pacritinib
• Second generation JAK2
specific inhibitor
• Orphan drug designation for
myelofibrosis in US and EU
• Patent protection until
2026/2030 (plus term
extension)
Seeks to address unmet medical need in myelofibrosis
patients with prior ruxolitinib treatment and/or
thrombocytopenia
7
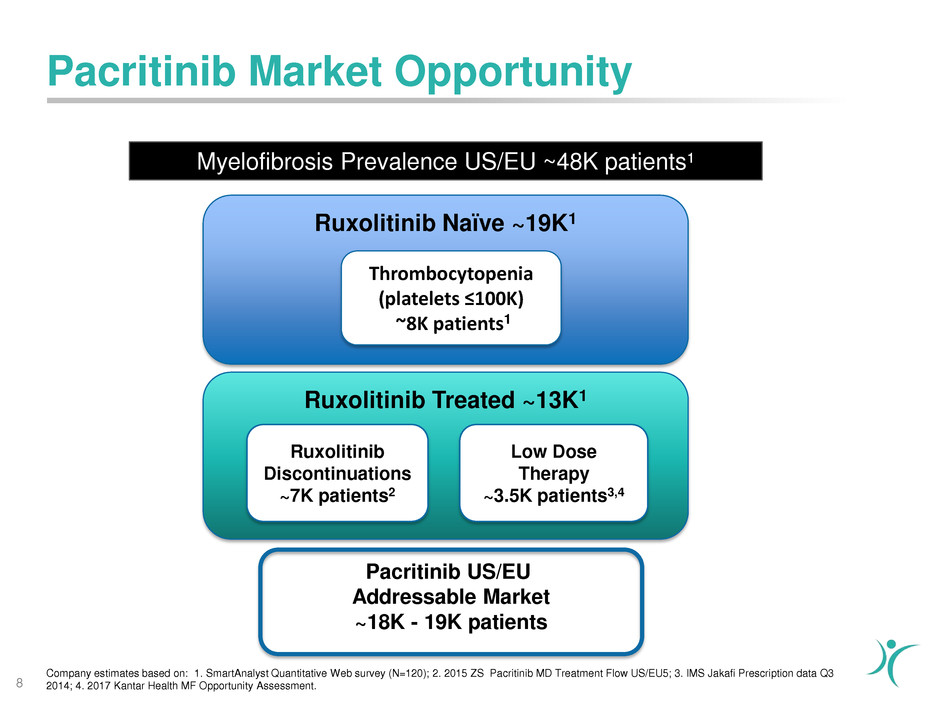
Pacritinib Market Opportunity
Myelofibrosis Prevalence US/EU ~48K patients¹
Company estimates based on: 1. SmartAnalyst Quantitative Web survey (N=120); 2. 2015 ZS Pacritinib MD Treatment Flow US/EU5; 3. IMS Jakafi Prescription data Q3
2014; 4. 2017 Kantar Health MF Opportunity Assessment.8
Ruxolitinib Naïve ~19K1
Ruxolitinib Treated ~13K1
Thrombocytopenia
(platelets ≤100K)
~8K patients1
Ruxolitinib
Discontinuations
~7K patients2
Low Dose
Therapy
~3.5K patients3,4
Pacritinib US/EU
Addressable Market
~18K - 19K patients
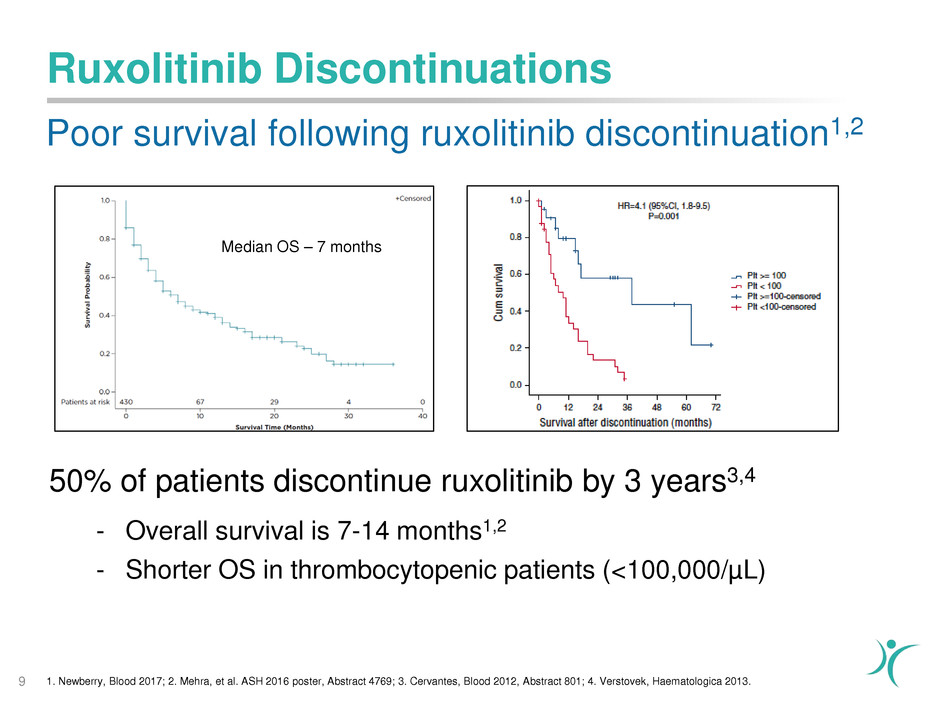
Ruxolitinib Discontinuations
Poor survival following ruxolitinib discontinuation1,2
9 1. Newberry, Blood 2017; 2. Mehra, et al. ASH 2016 poster, Abstract 4769; 3. Cervantes, Blood 2012, Abstract 801; 4. Verstovek, Haematologica 2013.
50% of patients discontinue ruxolitinib by 3 years3,4
- Overall survival is 7-14 months1,2
- Shorter OS in thrombocytopenic patients (<100,000/µL)
Median OS – 7 months
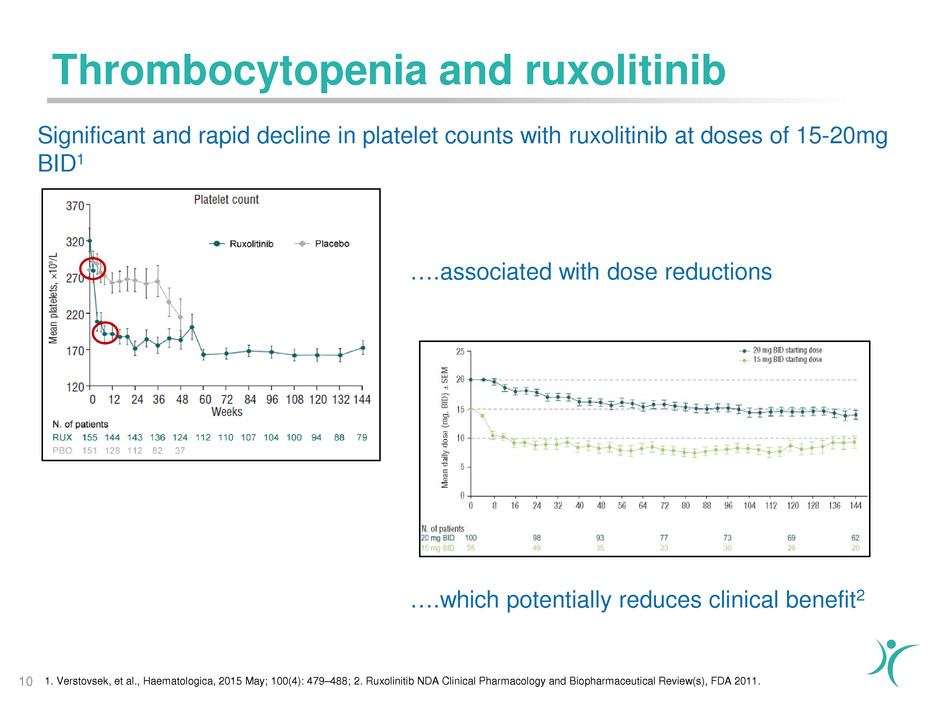
Significant and rapid decline in platelet counts with ruxolitinib at doses of 15-20mg
BID1
1. Verstovsek, et al., Haematologica, 2015 May; 100(4): 479–488; 2. Ruxolinitib NDA Clinical Pharmacology and Biopharmaceutical Review(s), FDA 2011.10
….associated with dose reductions
….which potentially reduces clinical benefit2
Thrombocytopenia and ruxolitinib
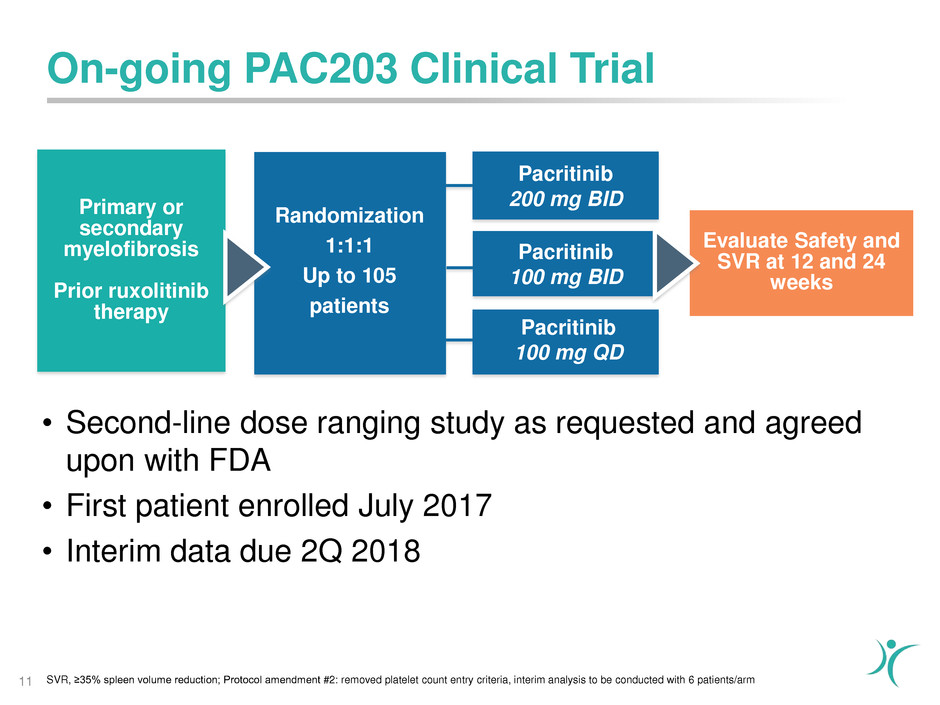
On-going PAC203 Clinical Trial
11
Pacritinib
100 mg BID
Primary or
secondary
myelofibrosis
Prior ruxolitinib
therapy
Randomization
1:1:1
Up to 105
patients
Evaluate Safety and
SVR at 12 and 24
weeks
Pacritinib
100 mg QD
Pacritinib
200 mg BID
• Second-line dose ranging study as requested and agreed
upon with FDA
• First patient enrolled July 2017
• Interim data due 2Q 2018
SVR, ≥35% spleen volume reduction; Protocol amendment #2: removed platelet count entry criteria, interim analysis to be conducted with 6 patients/arm

Rationale for PAC203 Clinical Trial
• Identify the lowest effective dose of pacritinib
• Evaluate safety and SVR at 12 and 24 weeks
• Evaluate long-term exposure and safety data
• Evaluate dosing flexibility
12
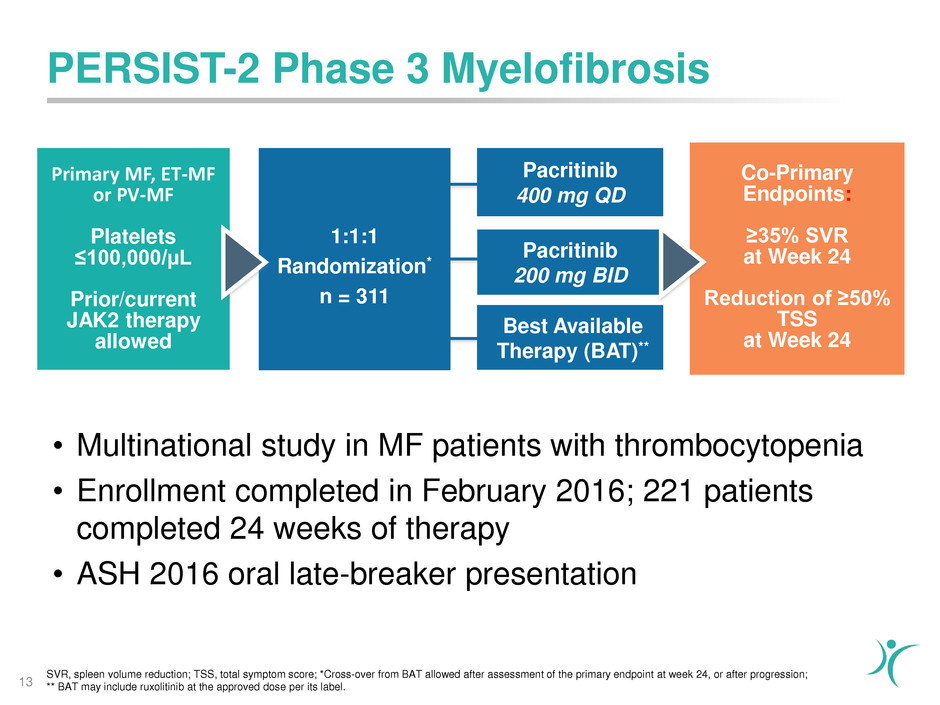
SVR, spleen volume reduction; TSS, total symptom score; *Cross-over from BAT allowed after assessment of the primary endpoint at week 24, or after progression;
** BAT may include ruxolitinib at the approved dose per its label.
Pacritinib
200 mg BID
Primary MF, ET-MF
or PV-MF
Platelets
≤100,000/µL
Prior/current
JAK2 therapy
allowed
1:1:1
Randomization*
n = 311
Co-Primary
Endpoints:
≥35% SVR
at Week 24
Reduction of ≥50%
TSS
at Week 24
Best Available
Therapy (BAT)**
Pacritinib
400 mg QD
PERSIST-2 Phase 3 Myelofibrosis
13
• Multinational study in MF patients with thrombocytopenia
• Enrollment completed in February 2016; 221 patients
completed 24 weeks of therapy
• ASH 2016 oral late-breaker presentation
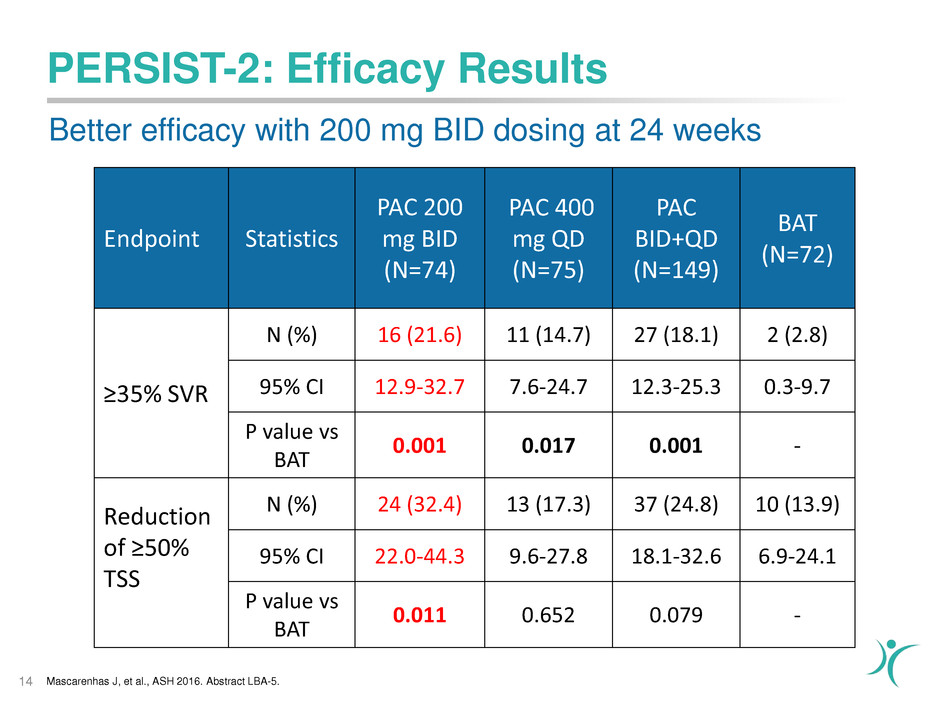
PERSIST-2: Efficacy Results
14
Endpoint Statistics
PAC 200
mg BID
(N=74)
PAC 400
mg QD
(N=75)
PAC
BID+QD
(N=149)
BAT
(N=72)
≥35% SVR
N (%) 16 (21.6) 11 (14.7) 27 (18.1) 2 (2.8)
95% CI 12.9-32.7 7.6-24.7 12.3-25.3 0.3-9.7
P value vs
BAT
0.001 0.017 0.001 -
Reduction
of ≥50%
TSS
N (%) 24 (32.4) 13 (17.3) 37 (24.8) 10 (13.9)
95% CI 22.0-44.3 9.6-27.8 18.1-32.6 6.9-24.1
P value vs
BAT
0.011 0.652 0.079 -
Mascarenhas J, et al., ASH 2016. Abstract LBA-5.
Better efficacy with 200 mg BID dosing at 24 weeks
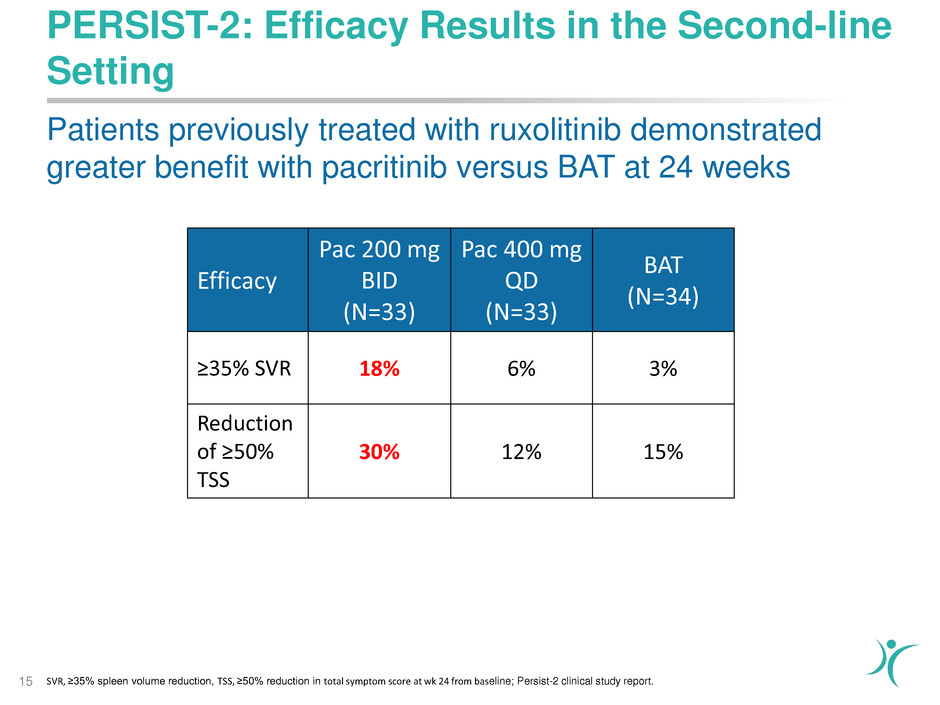
PERSIST-2: Efficacy Results in the Second-line
Setting
15
Efficacy
Pac 200 mg
BID
(N=33)
Pac 400 mg
QD
(N=33)
BAT
(N=34)
≥35% SVR 18% 6% 3%
Reduction
of ≥50%
TSS
30% 12% 15%
Patients previously treated with ruxolitinib demonstrated
greater benefit with pacritinib versus BAT at 24 weeks
SVR, ≥35% spleen volume reduction, TSS, ≥50% reduction in total symptom score at wk 24 from baseline; Persist-2 clinical study report.
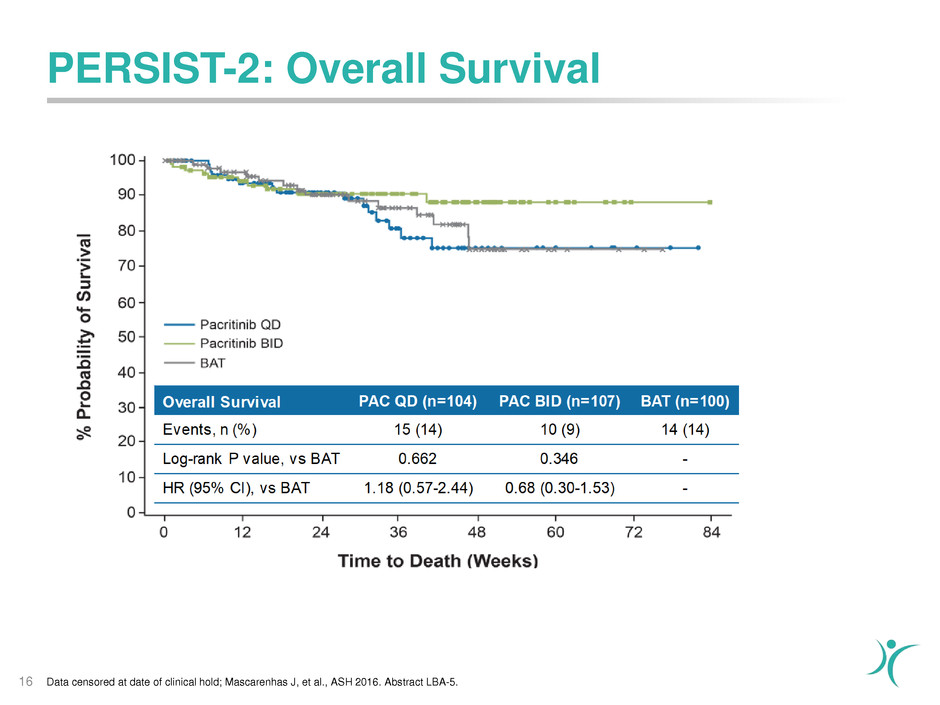
PERSIST-2: Overall Survival
16 Data censored at date of clinical hold; Mascarenhas J, et al., ASH 2016. Abstract LBA-5.
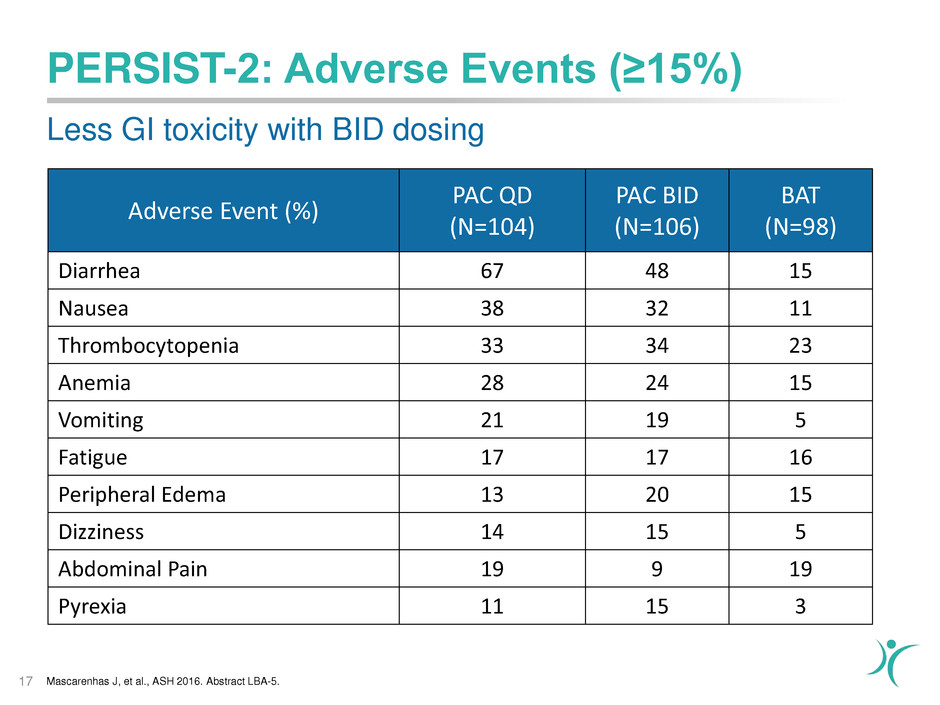
PERSIST-2: Adverse Events (≥15%)
17
Adverse Event (%)
PAC QD
(N=104)
PAC BID
(N=106)
BAT
(N=98)
Diarrhea 67 48 15
Nausea 38 32 11
Thrombocytopenia 33 34 23
Anemia 28 24 15
Vomiting 21 19 5
Fatigue 17 17 16
Peripheral Edema 13 20 15
Dizziness 14 15 5
Abdominal Pain 19 9 19
Pyrexia 11 15 3
Less GI toxicity with BID dosing
Mascarenhas J, et al., ASH 2016. Abstract LBA-5.
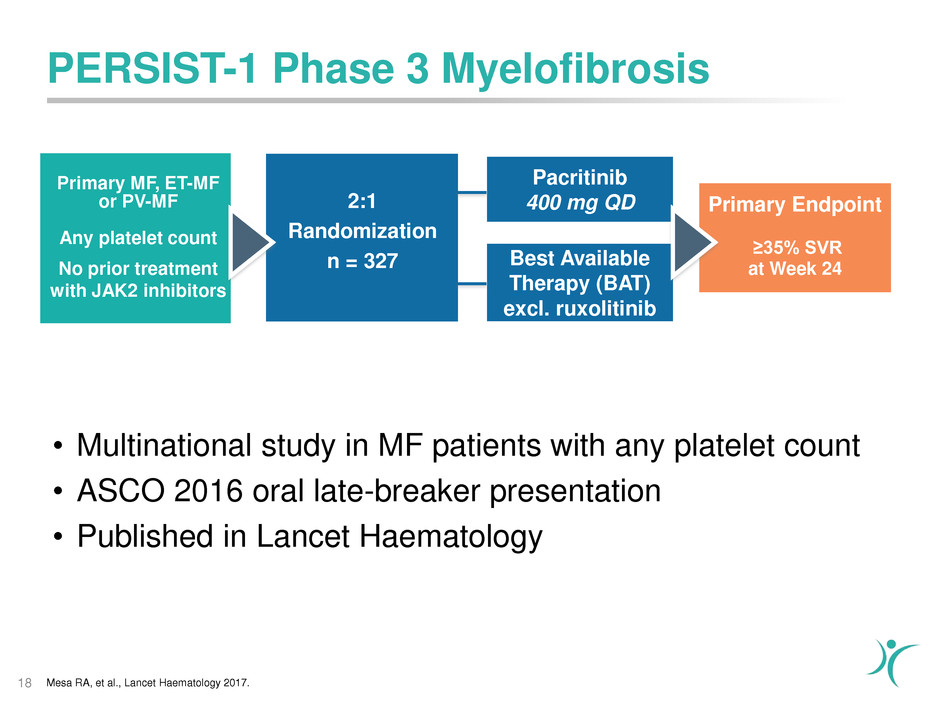
Primary MF, ET-MF
or PV-MF
Any platelet count
No prior treatment
with JAK2 inhibitors
2:1
Randomization
n = 327
Primary Endpoint
≥35% SVR
at Week 24Best Available
Therapy (BAT)
excl. ruxolitinib
Pacritinib
400 mg QD
PERSIST-1 Phase 3 Myelofibrosis
18
• Multinational study in MF patients with any platelet count
• ASCO 2016 oral late-breaker presentation
• Published in Lancet Haematology
Mesa RA, et al., Lancet Haematology 2017.
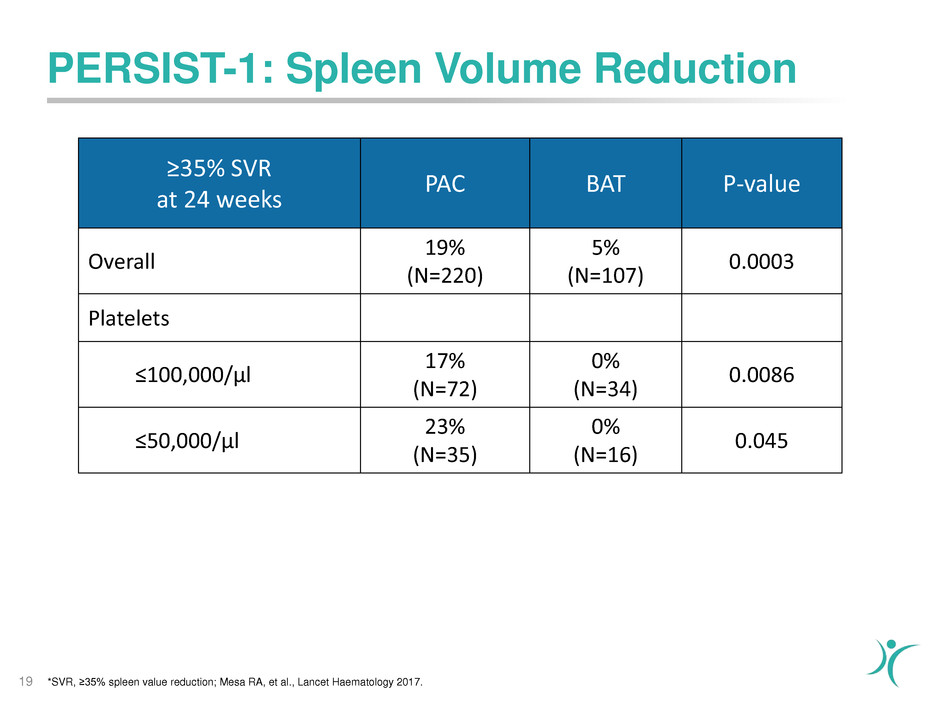
PERSIST-1: Spleen Volume Reduction
19
≥35% SVR
at 24 weeks
PAC BAT P-value
Overall
19%
(N=220)
5%
(N=107)
0.0003
Platelets
≤100,000/µl
17%
(N=72)
0%
(N=34)
0.0086
≤50,000/µl
23%
(N=35)
0%
(N=16)
0.045
*SVR, ≥35% spleen value reduction; Mesa RA, et al., Lancet Haematology 2017.
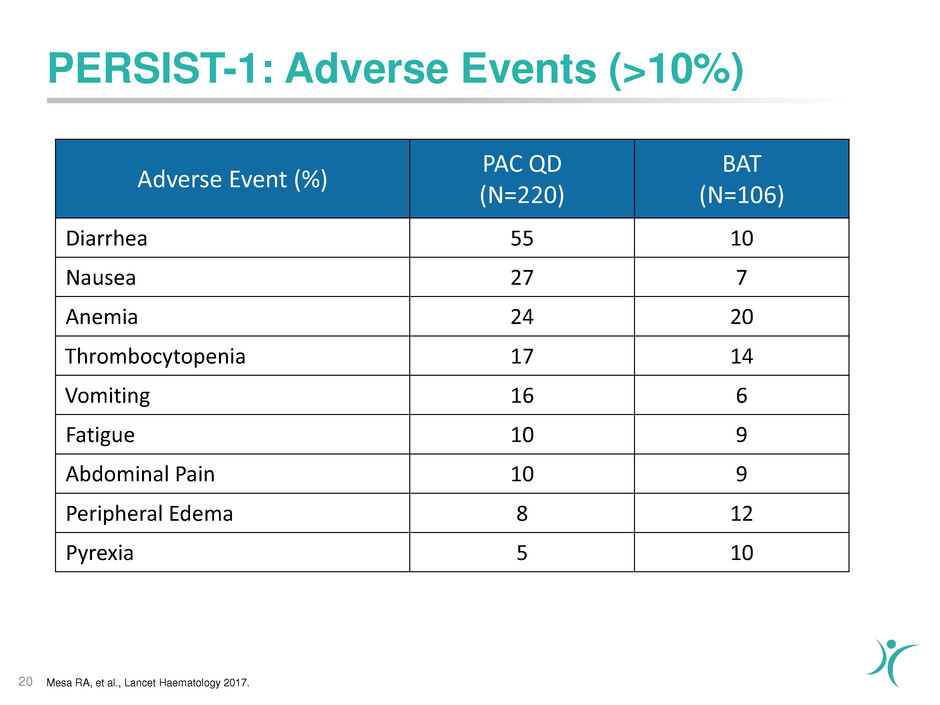
PERSIST-1: Adverse Events (>10%)
20
Adverse Event (%)
PAC QD
(N=220)
BAT
(N=106)
Diarrhea 55 10
Nausea 27 7
Anemia 24 20
Thrombocytopenia 17 14
Vomiting 16 6
Fatigue 10 9
Abdominal Pain 10 9
Peripheral Edema 8 12
Pyrexia 5 10
Mesa RA, et al., Lancet Haematology 2017.
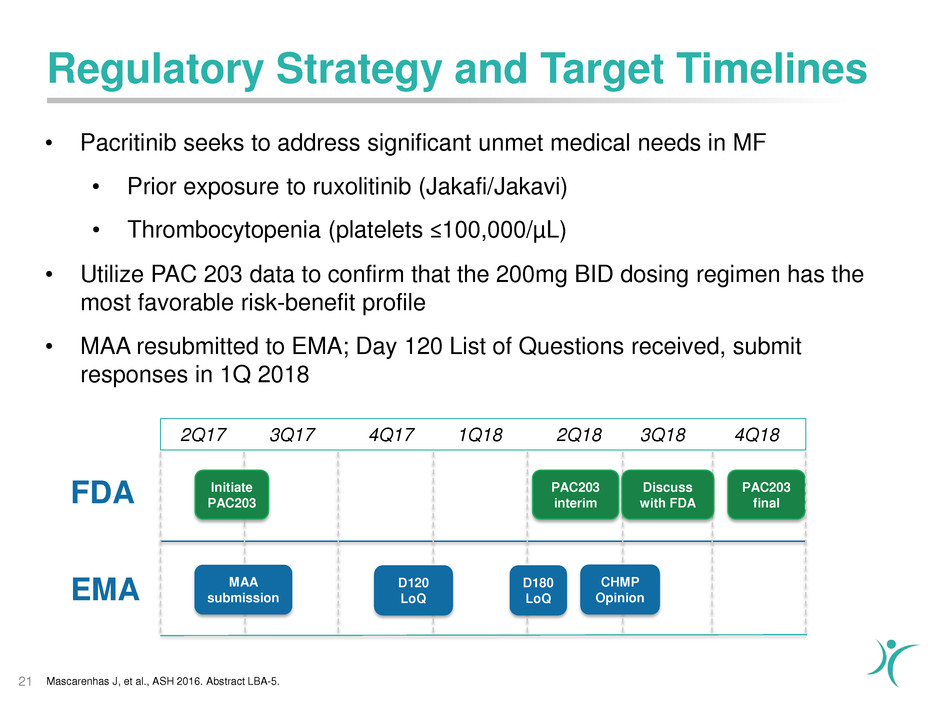
Regulatory Strategy and Target Timelines
FDA
21
2Q17 3Q17 4Q17 1Q18 2Q18 3Q18 4Q18
EMA MAA submission D180 LoQD120LoQ CHMPOpinion
PAC203
interim
PAC203
final
• Pacritinib seeks to address significant unmet medical needs in MF
• Prior exposure to ruxolitinib (Jakafi/Jakavi)
• Thrombocytopenia (platelets ≤100,000/µL)
• Utilize PAC 203 data to confirm that the 200mg BID dosing regimen has the
most favorable risk-benefit profile
• MAA resubmitted to EMA; Day 120 List of Questions received, submit
responses in 1Q 2018
Initiate
PAC203
Discuss
with FDA
Mascarenhas J, et al., ASH 2016. Abstract LBA-5.

PIXUVRI: Approved in Europe
• Conditional approval in 2012
• 3rd - 4th line aggressive B-cell NHL
• Partnered with Servier (ex-US)
22
®
( P I X A N T R O N E )
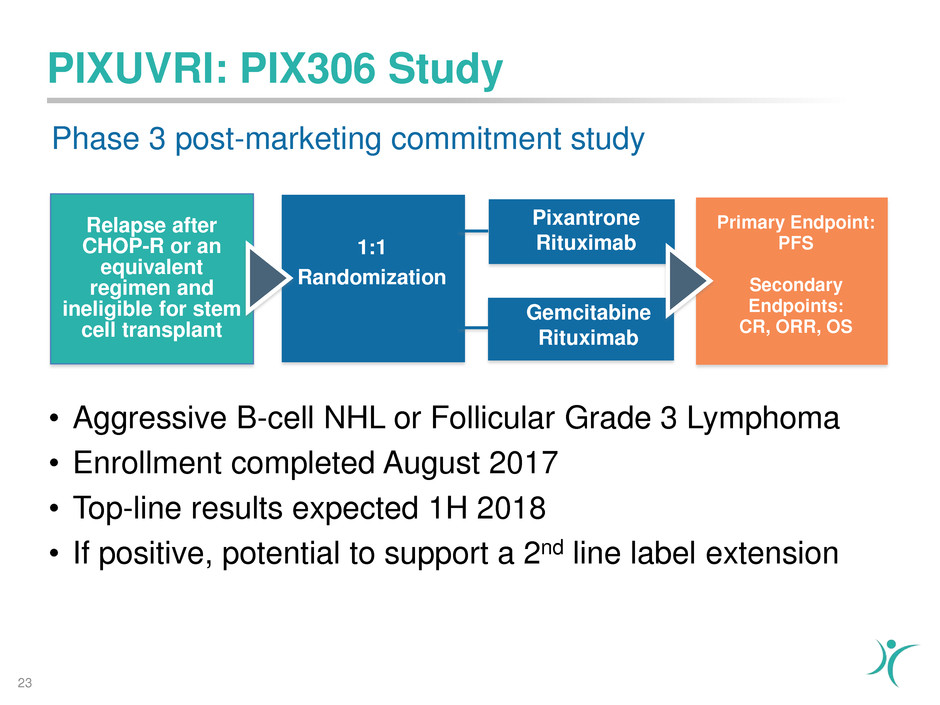
PIXUVRI: PIX306 Study
Relapse after
CHOP-R or an
equivalent
regimen and
ineligible for stem
cell transplant
1:1
Randomization
Primary Endpoint:
PFS
Secondary
Endpoints:
CR, ORR, OS
Gemcitabine
Rituximab
Pixantrone
Rituximab
Phase 3 post-marketing commitment study
• Aggressive B-cell NHL or Follicular Grade 3 Lymphoma
• Enrollment completed August 2017
• Top-line results expected 1H 2018
• If positive, potential to support a 2nd line label extension
23

Financial and Corporate
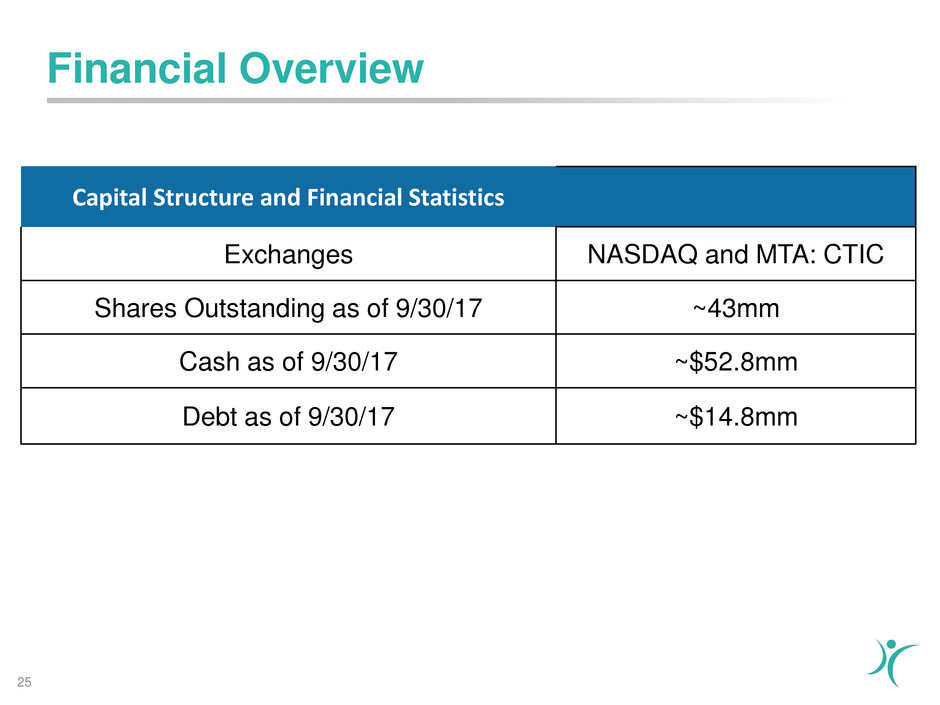
Financial Overview
Capital Structure and Financial Statistics
Exchanges NASDAQ and MTA: CTIC
Shares Outstanding as of 9/30/17 ~43mm
Cash as of 9/30/17 ~$52.8mm
Debt as of 9/30/17 ~$14.8mm
25

Upcoming Milestones and Objectives
• Pacritinib
- Submit responses to D120 LoQ (1Q 2018)
- Interim analysis of PAC203 study (2Q 2018)
- Complete enrollment of PAC203 study (2H 2018)
- CHMP opinion (mid-2018)
• Pixuvri
- Top-line data on PIX306 (1H 2018)
26

