Form SUPPL NEOVASC INC
Use these links to rapidly review the document
TABLE OF CONTENTS
TABLE OF CONTENTS
Filed
pursuant to General Instruction II.L. of Form F-10
File No. 333-211325
PROSPECTUS SUPPLEMENT
To Short Form Base Shelf Prospectus dated June 9, 2016
New Issue |
November 10, 2017 |

NEOVASC INC.
US$37,487,497
Series A Units Consisting of (I) One Common Share, (II) One Series A Warrant to Purchase One Common Share, (III) One Series B Warrant to Purchase One Common
Share and (IV) 0.40 Series C Warrants to Purchase a Unit Comprised of One Common Share, One Series A Warrant and One Series B Warrant
Series B Units Consisting of (I) either One Common Share or One Series D Warrant to Purchase One Common Share, (II) One Series A Warrant to Purchase One Common Share, (III) One Series B Warrant to Purchase One Common Share, (IV) 0.40 Series C Warrants to Purchase a Unit Comprised of One Common Share, One Series A Warrant and One Series B Warrant, and (V) 1.1765 Series F Warrants to Purchase One Common Share
US$1.46 per Unit
This prospectus supplement is hereby qualifying for distribution 6,609,588 Series A units (the "Series A Units") of Neovasc Inc. (the "Company", "Neovasc" or "We") and 19,066,780 Series B units (the "Series B Units" and together with the Series A Units, the "Units") of the Company, at a price of US$1.46 per Unit (the "Offering"), pursuant to the accompanying short form base shelf prospectus dated June 9, 2016. The Series B Units are being offered in lieu of Series A Units only to those purchasers who will be purchasing securities in the concurrent private placement more fully described elsewhere herein. See "Description of Securities Being Distributed Under the Concurrent Private Placement."
Each Series A Unit is comprised of (i) one common share of the Company (each, a "Unit Share"), (ii) one Series A common share purchase warrant of the Company (each, a "Series A Warrant"), (iii) one Series B common share purchase warrant of the Company (each, a "Series B Warrant") and (iv) 0.40 Series C warrants (each, a "Series C Warrant"), with each whole Series C Warrant exercisable to purchase a unit (each, a "Series C Unit") comprised of one common share of the Company, one Series A Warrant and one Series B Warrant. Each Series B Unit is comprised of (i) either one Unit Share or one pre-funded Series D common share purchase warrant of the Company (each, a "Series D Warrant"), (ii) one Series A Warrant, (iii) one Series B Warrant, (iv) 0.40 Series C Warrants, and (v) 1.1765 Series F common share purchase warrants of the Company (each, a "Series F Warrant"). The Series A Warrants, Series B Warrants, Series C Warrants, Series D Warrants and Series F Warrants are collectively referred to herein as, the "Warrants"). Each Series A Warrant will entitle the holder to purchase one common share of the Company (each, a "Series A Warrant Share") at an exercise price of US$1.61 per Series A Warrant Share for a period of five years following issuance. Each Series B Warrant will entitle the holder to purchase one common share of the Company (each, a "Series B Warrant Share") at an exercise price of US$1.61 per Series B Warrant Share for a period of two years following issuance. Each Series C Warrant will entitle the holder to purchase a Series C Unit comprised of a common share of the Company (each a "Series C Unit Share"), a Series A Warrant and a Series B Warrant, at an exercise price of US$1.46 per Series C Unit for a period of two years following issuance. Each Series D Warrant will entitle the holder to purchase one common share of the Company (each, a "Series D Warrant Share") at an exercise price of US$1.46 per Series D Warrant Share, all of which will be pre-funded except for a nominal exercise price of $0.01 per Series D Warrant Share for a period of five years following issuance. Each Series F Warrant will entitle the holder to purchase one common share of the Company (each, a "Series F Warrant Share" and together with the Series A Warrant Shares, Series B Warrant Shares, Series C Unit Shares, and Series D Warrant Shares, the "Warrant Shares") at an exercise price of US$1.61 per Series F Warrant Share for a period of two years following issuance. The Warrant Shares and Series C Units are subject to adjustment, at any time prior to their expiry. In certain circumstances each Series A Warrant, Series B Warrant, Series D and Series F Warrant may be exercised on a "net" or "cashless" basis. The Series A Units and Series B Units will separate into Unit Shares and Warrants immediately upon distribution. See "Description of Securities Being Distributed".
Canaccord Genuity Inc. ("Canaccord" or the "Underwriter") is acting as sole bookrunner in respect of the Offering pursuant to an underwriting agreement (the "Underwriting Agreement") dated as of November 9, 2017, between the Company and the Underwriter. See "Plan of Distribution". This prospectus supplement qualifies the distribution of the Units from Canada to the
United States. The Underwriter will only sell the Units in the United States either directly or through its duly registered U.S. broker dealer affiliates or agents. No Units will be sold to Canadian purchasers.
The outstanding common shares of the Company (the "Common Shares") are listed on the Nasdaq Capital Market (the "Nasdaq") and on the Toronto Stock Exchange (the "TSX") under the symbol "NVCN". On November 9, 2017, the last trading day before filing of this prospectus supplement, the closing price of the Common Shares was US$1.46 on the Nasdaq and C$1.86 on the TSX.
Investing in our securities involves a high degree of risk. You should carefully read the "Risk Factors" section in this prospectus supplement and the accompanying base shelf prospectus, as well as the information under the heading "Cautionary Note Regarding Forward-Looking Statements" in this prospectus supplement and consider such notes and information in connection with an investment in any securities.
Price: US$1.46 per Unit
| |
Public Offering Price |
Underwriting Commission |
Net Proceeds to the Company(1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Per Unit |
US$1.46 | US$0.0876 | US$1.3724 | |||||||
Total(2) |
US$37,487,497 | US$2,249,250 | US$35,238,247 | |||||||
Notes:
- (1)
- After
deducting the 6.0% commission to be paid to the Underwriter (the "Underwriting Commission"), but
before deducting the Company's expenses of the Offering, which are estimated at US$1.5 million and will be paid by the Company from the proceeds of the Offering. We refer you to the section
entitled "Plan of Distribution" for additional information regarding total Underwriting
Commission.
- (2)
- Total gross proceeds includes an aggregate of US$35,738 that will be funded upon the exercise of all of the Series D Warrants sold in the Offering.
The Company has not granted to the Underwriter any over-allotment option.
The Company has applied to list the Unit Shares and the Warrant Shares on the TSX and has submitted a notification of listing to list the Unit Shares and the Warrant Shares on the Nasdaq. Listing on the TSX and the Nasdaq will be subject to the Company fulfilling all of the listing requirements of the TSX and the Nasdaq. See "Plan of Distribution". There is no established public trading market for the Warrants, we do not expect a market to develop, and purchasers may not be able to resell Warrants purchased under this prospectus supplement and the accompanying prospectus. In addition, we do not intend to apply for listing of the Warrants on any securities exchange or other nationally recognized trading system. This may affect the pricing of the Warrants in the secondary market, the transparency and availability of trading prices, the liquidity of the Warrants, and the extent of issuer regulation. See "Risk Factors".
The Underwriter, as principal, conditionally offers the Units subject to prior sale, if as and when issued by us and accepted by the Underwriter, in accordance with the conditions contained in the Underwriting Agreement and subject to the approval of certain legal matters on behalf of Neovasc by Blake, Cassels & Graydon LLP, with respect to Canadian legal matters, and by Skadden, Arps, Slate, Meagher & Flom LLP, and Wilson Sonsini Goodrich & Rosati, with respect to U.S. legal matters, and on behalf of the Underwriter by Stikeman Elliott LLP, with respect to Canadian legal matters, and by Goodwin Procter LLP, with respect to U.S. legal matters. The Underwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part. Subject to the terms and conditions set forth in the Underwriting Agreement, the Underwriter has agreed to purchase all of the Units sold under the Underwriting Agreement if any of these Units are purchased. In connection with the Offering, the Underwriter may, subject to applicable laws, engage in transactions that stabilize the price of the Common Shares. See "Plan of Distribution".
The offering price of the Units was determined by arm's length negotiation between the Company and the Underwriter. If all of the Units are not sold at the public offering price, the Underwriter may change the offering price and the other selling terms. See "Plan of Distribution".
The Company will also be completing, concurrent with the Offering, a brokered private placement (the "Concurrent Private Placement"), through Canaccord Genuity Inc. acting as placement agent, of US$32,750,000 aggregate principal amount of senior secured convertible notes of the Company (the "Notes") and Series E warrants to purchase one common share of the Company (the "Series E Warrants"). The Notes will have an 18-month term, will be issued at an original issue price of US$850 per US$1,000 principal amount of Notes, and carry an interest rate of 0% per annum (increasing to 15% upon an event of default) from the closing date of the Concurrent Private Placement. Interest on the Notes will commence accruing on the date of issue, will be computed on the basis of a 360-day year and twelve 30-day months and will be payable in cash on January 1, 2018 and on the first day of each calendar quarter thereafter up to, and including, the maturity date. The Series E Warrants will have the same terms and conditions as the Series A Warrants, as more fully described under the heading "Description of Securities Being Distributed under the Offering". Completion of the Offering and the Concurrent Private Placement are each conditional upon completion of the other. See "Description of Securities Being Distributed under the Concurrent Private Placement."
It is expected that the Unit Shares will be delivered in book-entry form only through the facilities of the Depository Trust Company at the closing of the Offering, which is anticipated to be on or about November 17, 2017 or such other date as may be agreed upon between the Company and the Underwriter (the "Closing Date"). Certificates representing the Warrants will be in definitive form and available for delivery to purchasers at closing of the Offering. The Company expects that delivery of the Unit Shares and the
Warrants will be made against payment therefor on or about the Closing Date. Investors who wish to trade Unit Shares or Warrants prior to the Closing Date should consult their own advisors. See "Plan of Distribution."
The total gross proceeds from the Offering will be US$37,487,497. The Company estimates that the net proceeds from the Offering to the Company will be approximately US$33.74 million, after deducting the Underwriting Commission of US$2,249,250 and the Company's expenses of the Offering, which are estimated to be US$1.5 million and will be paid by the Company from the proceeds of the Offering. See "Plan of Distribution".
Certain of the Company's directors reside outside of Canada and have appointed an agent for service of process in Canada. See "Agent for Service of Process".
We are permitted under a multijurisdictional disclosure system adopted by the securities regulatory authorities in Canada and the United States to prepare this prospectus supplement and the accompanying base shelf prospectus in accordance with the disclosure requirements of Canada. Prospective investors in the United States should be aware that such requirements are different from those of the United States. The financial statements incorporated by reference in this prospectus supplement and the accompanying base shelf prospectus have been prepared in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board, and are subject to Canadian auditing and auditor independence standards. As a result, our financial statements may not be comparable to financial statements of United States companies.
Owning our securities may subject you to tax consequences both in Canada and the United States, including the Canadian federal income tax consequences applicable to a foreign controlled Canadian corporation that acquires Unit Shares, Warrants or the Warrant Shares. This prospectus supplement and the accompanying base shelf prospectus may not describe these tax consequences fully. You should read the tax discussion in this prospectus supplement and consult your own tax advisor with respect to your own particular circumstances.
Your ability to enforce civil liabilities under the United States federal securities laws may be affected adversely because we are incorporated in Canada, some of our officers and directors and the experts named in this prospectus supplement and the accompanying base shelf prospectus are Canadian residents, and a substantial portion of our assets and the assets of those officers, directors and experts are located outside of the United States.
NEITHER THE U.S. SECURITIES AND EXCHANGE COMMISSION ("SEC"), NOR ANY STATE SECURITIES REGULATOR HAS APPROVED OR DISAPPROVED THE SECURITIES OFFERED HEREBY OR DETERMINED IF THIS PROSPECTUS SUPPLEMENT OR THE ACCOMPANYING BASE SHELF PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENCE.
Our head office is located at Suite 5138 – 13562 Maycrest Way, Richmond, British Columbia, V6V 2J7, Canada, and our registered office is located at Suite 2600 – 595 Burrard Street, Vancouver, British Columbia, V7X 1L3, Canada.
Investors should rely only on current information contained in or incorporated by reference into this prospectus supplement and the accompanying base shelf prospectus as such information is accurate only as of the date of the applicable document. We have not authorized anyone to provide investors with different information. Information contained on our website shall not be deemed to be a part of this prospectus supplement or the accompanying base shelf prospectus or incorporated by reference herein and therein and should not be relied upon by prospective investors for the purpose of determining whether to invest in the securities. We will not make an offer of these securities in any jurisdiction where the offer or sale is not permitted. Investors should not assume that the information contained or incorporated by reference in this prospectus supplement and the accompanying base shelf prospectus is accurate as of any date other than the date on the face page of this prospectus supplement, the accompanying base shelf prospectus or the date of any documents incorporated by reference herein or therein, as applicable.
S-2
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first part is the prospectus supplement, which describes the terms of the Offering and adds to and updates information contained in the accompanying base shelf prospectus and the documents incorporated by reference therein. The second part is the accompanying base shelf prospectus, which gives more general information, some of which may not apply to the Unit Shares, Warrants or Warrant Shares. This prospectus supplement is deemed to be incorporated by reference into the accompanying base shelf prospectus solely for the purpose of the Offering. If information in this prospectus supplement is inconsistent with the accompanying base shelf prospectus or the information incorporated by reference, you should rely on this prospectus supplement. You should read both this prospectus supplement and the accompanying base shelf prospectus, together with the additional information about us to which we refer you in the section of this prospectus supplement entitled "Where You Can Find More Information."
You should rely only on the information contained in or incorporated by reference into this prospectus supplement and the accompanying base shelf prospectus. The Company has not authorized anyone to provide you with different information.
You should assume that the information contained in this prospectus supplement, the accompanying base shelf prospectus and the documents incorporated by reference herein and therein is accurate only as of their respective dates, regardless of the time of delivery of this prospectus supplement and the accompanying base shelf prospectus. Our business, financial condition, results of operations and prospects may have changed since those dates.
Market data and certain industry forecasts used in this prospectus supplement and the documents incorporated by reference in this prospectus supplement and the accompanying base shelf prospectus were obtained from market research, publicly available information and industry publications. We believe that these sources are generally reliable, but the accuracy and completeness of this information is not guaranteed. We have not independently verified such information, and we do not make any representation as to the accuracy of such information.
In this prospectus supplement and the accompanying base shelf prospectus, unless otherwise indicated, all dollar amounts and references to "US$" are to U.S. dollars and references to "C$" are to Canadian dollars. This prospectus supplement and the underlying base shelf prospectus and the documents incorporated by reference herein and therein contain translations of some Canadian dollar amounts into U.S. dollars solely for your convenience. See "Exchange Rate Information".
In this prospectus supplement and the accompanying base shelf prospectus, unless the context otherwise requires, references to "we", "us", "our" or similar terms, as well as references to "Neovasc" or the "Company", refer to Neovasc Inc., either alone or together with our subsidiaries.
The names Neovasc®, Tiara™ ("Tiara") and Neovasc Reducer™ ("Reducer") are our trademarks. Other trademarks, product names and company names appearing in this prospectus supplement and the accompanying base shelf prospectus and documents incorporated by reference in this prospectus supplement and the accompanying base shelf prospectus are the property of their respective owners.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement and the accompanying base shelf prospectus, including the documents incorporated by reference herein and therein, contain forward-looking statements within the meaning of applicable Canadian securities legislation and the U.S. Private Securities Litigation Reform Act of 1995 that may not be based on historical fact, including, without limitation, statements containing the words "believe", "may", "plan", "will", "estimate", "continue", "anticipate", "intend", "expect" and similar expressions. Forward-looking statements are necessarily based on estimates and assumptions made by us in light of our experience and perception of historical trends, current conditions and expected future developments, as well as the factors we believe are appropriate. Forward-looking statements in this prospectus supplement and the accompanying base
S-3
shelf prospectus and the documents incorporated by reference herein and therein include, but are not limited to, statements relating to:
- •
- our anticipated use of proceeds from the sale of the Units;
- •
- the conduct or possible outcomes of any actual or threatened legal proceedings, including the Company's ongoing litigation
with CardiAQ and the damages and interest awards affirmed by the U.S. Court of Appeals immediately due and payable on November 13, 2017 as described under the heading
"Legal Proceedings" in the AIF and "The Company — Recent
Developments" in this prospectus supplement;
- •
- our ability to continue as a going concern;
- •
- the amount of estimated additional litigation expenses required to defend the Company in lawsuits filed by CardiAQ;
- •
- our need for significant additional financing and our estimates regarding our capital requirements and future revenues,
expenses and profitability;
- •
- our intention to expand the indications for which we may market the Tiara (which does not have regulatory approval and is
not commercialized) and the Reducer (which has CE Mark approval for sale in the European Union), including our COSIRA-II trial in the United States relating to the Reducer;
- •
- clinical development of our products, including the results of current and future clinical trials and studies;
- •
- our intention to apply for CE Mark approval for the Tiara in the next one to two years;
- •
- the anticipated timing and locations of the first implantations in the TIARA-II trial and our intention to initiate
additional investigational sites in 2017 as required approvals are obtained;
- •
- our plans to develop and commercialize products, including the Tiara, and the timing and cost of these development
programs;
- •
- our strategy to refocus our business towards development and commercialization of the Reducer and the Tiara;
- •
- our ability to replace declining revenues from the tissue business with revenues from the Reducer and the Tiara in a
timely manner;
- •
- whether we will receive, and the timing and costs of obtaining, regulatory approvals for the Tiara and the Reducer;
- •
- the cost of post-market regulation if we receive necessary regulatory approvals;
- •
- our ability to enroll patients in our clinical trials, studies and compassionate use cases in Canada, the
United States and in Europe;
- •
- our intention to continue directing a significant portion of our resources into sales expansion;
- •
- the expected decline of consulting services revenue in the long-term as our consulting customers become contract
manufacturing customers or cease being customers;
- •
- our ability to get our products approved for use;
- •
- the benefits and risks of our products as compared to others;
- •
- our estimates of the size of the potential markets for our products including the anticipated market opportunity for
the Reducer;
- •
- our potential relationships with distributors and collaborators with acceptable development, regulatory and
commercialization expertise and the benefits to be derived from such collaborative efforts;
- •
- sources of revenues and anticipated revenues, including contributions from distributors and other third parties, product sales, license agreements and other collaborative efforts for the development and commercialization of products;
S-4
- •
- our creation of an effective direct sales and marketing infrastructure for approved products we elect to market and
sell directly;
- •
- the rate and degree of market acceptance of our products;
- •
- the timing and amount of reimbursement for our products; and
- •
- the impact of foreign currency exchange rates.
Forward-looking statements are based on estimates and assumptions made by the Company in light of its experience and its perception of historical trends, current conditions and expected future developments, as well as other factors that the Company believes are appropriate in the circumstances. Many factors could cause the Company's actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation:
- •
- risks relating to our litigation with CardiAQ, including the Company's ability to successfully satisfy the damages and
interest awards affirmed by the U.S. Court of Appeals due and payable on November 13, 2017, which create material uncertainty and which cast substantial doubt on our ability to continue
as a going concern;
- •
- the substantial doubt about our ability to continue as a going concern.
- •
- risks relating to our need for significant additional future capital and our ability to raise additional funding on
satisfactory terms or at all;
- •
- risks relating to claims by third parties alleging infringement of their intellectual property rights;
- •
- our ability to establish, maintain and defend intellectual property rights in our products;
- •
- risks relating to results from clinical trials of our products, which may be unfavorable or perceived
as unfavorable;
- •
- our history of losses and significant accumulated deficit;
- •
- risks relating to immediate dilution of your securities;
- •
- risks relating to the Warrants and Notes resulting in additional and significant dilution to our shareholders;
- •
- risks relating to the possibility that our Common Shares may be delisted from the Nasdaq or the TSX, which could affect
their market price and liquidity;
- •
- risks associated with product liability claims, insurance and recalls;
- •
- risks relating to competition in the medical device industry, including the risk that one or more competitors may develop
more effective or more affordable products;
- •
- risks relating to our ability to achieve or maintain expected levels of market acceptance for our products, as well as our
ability to successfully build our in-house sales capabilities or secure third-party marketing or distribution partners;
- •
- our ability to convince public payors and hospitals to include our products on their approved products lists;
- •
- risks relating to new legislation, new regulatory requirements and the efforts of governmental and third party payors to
contain or reduce the costs of healthcare;
- •
- risks relating to increased regulation, enforcement and inspections of participants in the medical device industry,
including frequent government investigations into marketing and other business practices;
- •
- risks associated with the extensive regulation of our products and trials by governmental authorities, as well as the cost
and time delays associated therewith;
- •
- risks associated with post-market regulation of our products;
S-5
- •
- health and safety risks associated with our products and our industry;
- •
- risks associated with our manufacturing operations, including the regulation of our manufacturing processes by
governmental authorities and the availability of two critical components of the Reducer;
- •
- risk of animal disease associated with the use of our products;
- •
- risks relating to the manufacturing capacity of third-party manufacturers for our products, including risks of supply
interruptions impacting the Company's ability to manufacture its own products;
- •
- risks relating to breaches of anti-bribery laws by our employees or agents;
- •
- risks associated with future changes in financial accounting standards and new accounting pronouncements;
- •
- our dependence upon key personnel to achieve our business objectives;
- •
- our ability to maintain strong relationships with physicians;
- •
- risks relating to the sufficiency of our management systems and resources in periods of significant growth;
- •
- risks associated with consolidation in the health care industry, including the downward pressure on product pricing and
the growing need to be selected by larger customers in order to make sales to their members or participants;
- •
- our ability to successfully identify and complete corporate transactions on favorable terms or achieve anticipated
synergies relating to any acquisitions or alliances;
- •
- anti-takeover provisions in our constating documents which could discourage a third party from making a takeover bid
beneficial to our shareholders;
- •
- risks relating to conflicts of interests among the Company's officers and directors as a result of their involvement with
other issuers;
- •
- risks relating to the influence of significant shareholders of the Company over our business operations and
share price;
- •
- risks relating to adverse U.S. consequences if we are or become a "passive foreign investment company" under the
U.S. Internal Revenue Code;
- •
- risks relating to it being more expensive for us to raise capital in the future and dilution to investors;
- •
- our Common Share price being volatile;
- •
- difficulty enforcing actions against us, certain of our directors and officers or experts under U.S. federal
securities laws;
- •
- risks relating to the sale of a significant number of Common Shares;
- •
- risks relating to the restrictions on the Company entering into certain transactions;
- •
- risks relating to the exercise of Warrants, which may encourage short sales by third parties;
- •
- risks relating to the Warrants not being listed on any exchange;
- •
- risks relating to the holders of Warrants having no rights as shareholders;
- •
- risks relating to the Warrants having no value;
- •
- risks relating to our broad discretion in the use of proceeds; and
- •
- our intention not to pay dividends.
Forward-looking statements reflect our current views with respect to future events and are subject to risks and uncertainties and are necessarily based upon a number of estimates and assumptions that, while considered reasonable by us, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies, many of which, with respect to future events, are subject to change. The
S-6
material factors and assumptions used by us to develop such forward-looking statements include, but are not limited to:
- •
- the approval of this Offering and the Concurrent Private Placement by the United States District Court for the
District of Massachusetts;
- •
- our ability to continue as a going concern by using a portion of the net proceeds of this Offering and the Concurrent
Private Placement to satisfy the remaining balance of damages and interest accruals affirmed by the U.S. Court of Appeals;
- •
- our ability to comply with the listing requirements of the Nasdaq and the TSX;
- •
- the expected size and completion of the Concurrent Private Placement;
- •
- capital will be available on terms that are favorable to us;
- •
- our regulatory and clinical strategies will continue to be successful;
- •
- our current positive interactions with regulatory agencies will continue;
- •
- recruitment to clinical trials and studies will continue;
- •
- the time required to enroll, analyze and report the results of our clinical studies will be consistent with projected
timelines;
- •
- current and future clinical trials and studies will generate the supporting clinical data necessary to achieve approval of
marketing authorization applications;
- •
- the regulatory requirements for approval of marketing authorization applications will be maintained;
- •
- our current good relationships with our suppliers and service providers will be maintained;
- •
- our estimates of market size and reports reviewed by us are accurate;
- •
- our efforts to develop markets and generate revenue from the Reducer will be successful; and
- •
- genericisation of markets for the Tiara and the Reducer will develop.
By their very nature, forward-looking statements or information involve known and unknown risks, uncertainties and other factors that may cause our actual results, events or developments, or industry results, to be materially different from any future results, events or developments expressed or implied by such forward-looking statements or information. In evaluating these statements, prospective purchasers should specifically consider various factors, including the risks outlined herein and in the accompanying base shelf prospectus and in documents incorporated by reference herein and therein, under the heading "Risk Factors". Should one or more of these risks or uncertainties or a risk that is not currently known to us materialize, or should assumptions underlying the forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements are made as of the date of this prospectus supplement or, in the case of documents incorporated by reference in this prospectus supplement, as of the date of such documents, and we do not intend, and do not assume any obligation, to update these forward-looking statements, except as required by law. Investors are cautioned that forward- looking statements are not guarantees of future performance and investors are cautioned not to put undue reliance on forward-looking statements due to their inherent uncertainty.
DOCUMENTS INCORPORATED BY REFERENCE
This prospectus supplement is deemed to be incorporated by reference into the accompanying base shelf prospectus solely for the purposes of the Offering. Information has been incorporated by reference in this prospectus supplement from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated by reference in this prospectus supplement and not delivered with this prospectus supplement may be obtained on request without charge from Chris Clark, the Company Secretary of Neovasc, at Suite 5138 – 13562 Maycrest Way, Richmond, British Columbia, V6V 2J7, Canada, telephone: (604) 270-4344 or by accessing the disclosure documents through the Internet on the Canadian System for
S-7
Electronic Document Analysis and Retrieval ("SEDAR"), at www.sedar.com. Documents filed with, or furnished to, the SEC are available through the SEC's Electronic Data Gathering and Retrieval System ("EDGAR"), at www.sec.gov.
The following documents, filed with the securities commissions or similar regulatory authorities in certain provinces of Canada and filed with, or furnished to, the SEC are specifically incorporated by reference into, and form an integral part of, this prospectus supplement and the accompanying base shelf prospectus:
- •
- our annual information form for the fiscal year ended December 31, 2016, dated as of March 23, 2017
(the "AIF");
- •
- our audited annual consolidated financial statements for the fiscal years ended December 31, 2016 and 2015,
together with the notes thereto and the auditor's report thereon;
- •
- our management's discussion and analysis of financial condition and results of operations for the fiscal years ended
December 31, 2016 and 2015;
- •
- our unaudited consolidated condensed interim financial statements for the three and six month periods ended
June 30, 2017, as filed on SEDAR on August 10, 2017;
- •
- our management's discussion and analysis of our financial condition and results of operations for the three and six months
ended June 30, 2017, as filed on SEDAR on August 10, 2017; and
- •
- our management information circular dated May 8, 2017, distributed in connection with our annual general meeting of shareholders held on June 13, 2017.
Any documents of the type described in Section 11.1 of Form 44-101F1 Short Form Prospectuses filed by the Company with a securities commission or similar authority in any province of Canada subsequent to the date of this prospectus supplement and before withdrawal or completion of the Offering will be deemed to be incorporated by reference into this prospectus supplement.
In addition, to the extent that any document or information incorporated by reference into this prospectus supplement is filed with, or furnished to, the SEC pursuant to the Securities Exchange Act of 1934, as amended (the "Exchange Act"), after the date of this prospectus supplement, such document or information will be deemed to be incorporated by reference as an exhibit to the registration statement of which this prospectus supplement forms a part (in the case of a report on Form 6-K, if and to the extent expressly provided therein).
Any statement contained in this prospectus supplement or in the accompanying base shelf prospectus, or in a document incorporated or deemed to be incorporated by reference herein or therein will be deemed to be modified or superseded for purposes of this prospectus supplement to the extent that a statement contained herein or therein or in any other subsequently filed document that also is or is deemed to be incorporated by reference herein or therein modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement is not to be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of material fact or an omission to state a material fact that is required to be stated or is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement or the accompanying base shelf prospectus.
Upon our filing a new annual information form and the related annual financial statements and management's discussion and analysis with applicable securities regulatory authorities during the currency of this prospectus supplement and the accompanying base shelf prospectus, the previous annual information form, the previous annual financial statements and management's discussion and analysis and all quarterly financial statements and the related management's discussion and analysis, supplemental information, material change reports and information circulars filed prior to the commencement of our financial year in which the new annual information form is filed will be deemed no longer to be incorporated for purposes of future offers and sales of our securities under this prospectus supplement.
S-8
References to our website in any documents that are incorporated by reference into this prospectus supplement and the accompanying base shelf prospectus do not incorporate by reference the information on such website into this prospectus supplement or the accompanying base shelf prospectus, and we disclaim any such incorporation by reference.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
The following documents have been or will be filed with the SEC as part of the registration statement of which this prospectus supplement and the accompanying base shelf prospectus forms a part: (i) the documents listed under the heading "Documents Incorporated by Reference"; (ii) powers of attorney from our directors and officers; (iii) the consent of Grant Thornton LLP; and (iv) the Underwriting Agreement described under the heading "Plan of Distribution".
The following table sets forth for each period indicated: (i) the daily average exchange rate in effect at the end of the period; (ii) the high and low daily average exchange rate during such period; and (iii) the daily average exchange rate for such period, for one Canadian dollar, expressed in U.S. dollars, as quoted by the Bank of Canada.
| |
Six Months Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2017 | 2016 | 2015 | |||||||
| |
US$ |
US$ |
US$ |
|||||||
Closing |
0.7706 | 0.7705 | 0.8011 | |||||||
High |
0.7706 | 0.8025 | 0.8621 | |||||||
Low |
0.7276 | 0.6808 | 0.7793 | |||||||
Average |
0.7496 | 0.7527 | 0.8101 | |||||||
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||||||
| |
US$ |
US$ |
US$ |
|||||||
Closing |
0.7441 | 0.7232 | 0.8605 | |||||||
High |
0.8025 | 0.8621 | 0.9444 | |||||||
Low |
0.6808 | 0.7144 | 0.8569 | |||||||
Average |
0.7554 | 0.7830 | 0.9057 | |||||||
On November 9, 2017, the daily average exchange rate as quoted by the Bank of Canada was C$1.00 = US$0.7879.
S-9
Neovasc was incorporated on November 2, 2000 under the laws of the Province of British Columbia and was continued to federal jurisdiction under the Canada Business Corporations Act ("CBCA") on April 19, 2002. Neovasc has five wholly owned subsidiaries, three of which are material: (i) Neovasc Tiara Inc. ("NTI"), a corporation incorporated under the federal laws of Canada; (ii) Neovasc Medical Ltd. ("NML"), a corporation incorporated under the laws of Israel; and (iii) Neovasc Medical Inc. ("NMI"), a corporation incorporated under the laws of British Columbia.
Our registered office is located at Suite 2600 – 595 Burrard Street, Vancouver, British Columbia, V7X 1L3, Canada and our head office and principal place of business are located at Suite 5138 – 13562 Maycrest Way, Richmond, British Columbia, V6V 2J7, Canada.
Neovasc is a specialty medical device company that develops, manufactures and markets products for the rapidly growing cardiovascular marketplace. Its products include the Tiara technology in development for the transcatheter treatment of mitral valve disease and the Reducer for the treatment of refractory angina.
In 2009, Neovasc started initial activities to develop novel technologies for catheter-based treatment of mitral valve disease. Based on the early positive results of these activities, the Company formally launched a program to develop the Tiara. Neovasc established a separate entity, NTI, in March 2013 to develop and own the intellectual property related to the Tiara (Neovasc has transferred all intellectual property related to the Tiara to NTI). On February 3, 2014, Neovasc announced the first human implant of the Tiara under special access compassionate use exemptions. Subsequently 25 additional patients have been implanted with the Tiara bringing the total number of patients treated with the device to 26 as of this date. In December 2014, the Company announced that it had received approval from the U.S. Food and Drug Administration (the "FDA") to initiate the TIARA-I study in the United States. The TIARA-I study is a multinational, multicenter early feasibility study being conducted to assess the safety and performance of the Tiara and implantation procedure in high risk surgical patients suffering from severe Mitral Regurgitation. The study will include up to 15 patients enrolled at centers in the United States and up to 15 patients at centers in Canada and Europe. The first European patient was enrolled in the study in Antwerp, Belgium in late 2014 and the first patient in the United States was enrolled in mid-2015. The Tiara is currently available in two sizes (35mm and 40 mm); additional sizes are under development (45mm). Following completion of the TIARA-I study the Company intends to continue advancing the Tiara to commercialization and will be undertaking additional studies to support authorization to affix the CE Mark and FDA approval as appropriate. On November 28, 2016, the Company announced that it had received both regulatory and ethics committee approval to initiate the Tiara Transcatheter Mitral Valve Replacement Study (TIARA-II) in Italy. The TIARA-II study is a 115 patient, non-randomized, prospective clinical study evaluating the Tiara's safety and performance. It is expected that data from this study will be used to file for CE Mark approval. The first implantations in the TIARA-II trial were conducted by the medical team at San Raffaele Hospital in Milan, Italy in the first half of 2017. The Company is initiating additional investigational sites in Italy, Germany, the UK and other countries as required approvals are obtained.
In July 2008, Neovasc acquired NML, a pre-commercial vascular device company based in Israel. NML developed and owned intellectual property related to a novel catheter-based treatment for refractory angina, a debilitating condition resulting from inadequate blood flow to the heart muscle. The Company estimates that there are approximately 600,000 to 1.8 million Americans, with 50,000 to 100,000 new cases per year in the United States who are potential candidates for this treatment. The Company has completed development of the Reducer and obtained authorization to affix the CE Mark, which allows for marketing of the Reducer in the European marketplace. The Company initiated commercial sales of the Reducer in early 2015. In March 2014 the Company announced that results of its Coronary Sinus Reducer for Treatment of Refractory Angina ("COSIRA") trial had been presented at the ACC.14 medical conference. The COSIRA trial was a sham-controlled randomized, double-blinded study of the Reducer device in 104 patients with moderate to severe refractory angina. The results presented at ACC.14 confirmed that the COSIRA trial had met its primary endpoint demonstrating the efficacy of the Reducer device with statistical significance. The COSIRA trial results were published in the New England Journal of Medicine in February 2015. On November 6, 2017, Neovasc announced that it has received approval of the FDA to initiate the COSIRA-II IDE pivotal clinical trial. The
S-10
trial's purpose will be to demonstrate the safety and effectiveness of Neovasc's novel Reducer system for treatment of patients with refractory angina. Once completed, the trial data is intended to support an application to the FDA for approval to begin marketing Reducer in the United States. See "Use of Proceeds" for further information about the COSIRA-II trial.
Neovasc's business operations started in March 2002, with the acquisition of NMI. NMI manufactured a line of collagen-based surgical patch products made for use in cardiac reconstruction and vascular repair procedures as well as other surgeries. Neovasc, through NMI, also sells biological tissue to industry partners and other customers who incorporate this tissue into their own products such as transcatheter heart valves. Neovasc's biological products are made from chemically treated biocompatible pericardial tissue. In 2012, Neovasc sold the rights to manufacture a specific line of conventional surgical patch products to LeMaitre Vascular, Inc. for US$4.6 million, but retained rights to the underlying tissue technology for all other uses. Neovasc has refocused its use of this treated pericardial tissue to constitute key components in third-party medical products, such as transcatheter heart valves. The Company also provides customers with consulting services related to the development of these products with specific expertise related to the transcatheter heart valve field as well as contract manufacturing services for these valves at all stages of development through to commercial scale production.
The Company's core strategy is to focus on the continued development and commercialization of its products, the Tiara and the Reducer, providing minimally invasive medical devices for a cardiovascular market that the Company believes is both growing and under-served by current treatment solutions.
Recent Developments
On September 1, 2017, the United States Court of Appeals for the Federal Circuit issued an opinion and judgment affirming the judgment of the United States District Court for the District of Massachusetts in the ongoing lawsuit with CardiAQ. That judgment affirmed the money judgment against Neovasc and the decision on inventorship. It also affirmed the District Court's rejection of CardiAQ's claim for an injunction against Neovasc. On October 2, 2017, Neovasc filed a petition for rehearing at the Court of Appeals seeking to reverse the money judgment and inventorship decisions. On the same day, CardiAQ also filed a petition for rehearing on the denial of the injunction. On November 3, 2017, the Court of Appeals denied the petition for rehearing filed by CardiAQ and denied the petition for rehearing filed by Neovasc. The mandate of the court will issue on November 13, 2017 and the appeals process has been exhausted. The full judgment of approximately US$112 million, of which approximately US$70 million is already held in a trust account, will become enforceable on November 13, 2017. We must pay the damages and interest awards, including funding the remaining approximately US$42 million not held in trust, which exceeds our current cash resources. We intend to use a portion of the net proceeds of this Offering and the Concurrent Private Placement to satisfy the remaining balance of the awards not held in trust. Pursuant to the stay agreement the consummation of the Offering and the Concurrent Private Placement are subject to approval by the District Court.
On December 14, 2016, a hearing took place in Munich, Germany regarding the German lawsuit. Based on this hearing, and the underlying exchange of written submissions, the Munich court entered a judgment on June 16, 2017, granting co-ownership of the European patent application in question to CardiAQ. There are no monetary damages connected to this order, nor is there such a request from CardiAQ pending in the German litigation. Neovasc filed a notice of appeal against the decision with the Appeals Court of Munich on July 14, 2017. On July 20, 2017, CardiAQ filed a notice of appeal with the same court. Both parties substantiated their respective appeals, and by way of case management order of October 18, 2017, the Appeals Court of Munich has now provisionally set the hearing date in this matter for April 12, 2018. There is likely to be further exchanges of written submissions between the parties in the run-up to that hearing.
S-11
Investing in the Units is speculative and involves a high degree of risk. The following risk factors, as well as risks currently unknown to us, could materially adversely affect our future business, operations and financial condition and could cause them to differ materially from the estimates described in forward-looking information relating to the Company, or its business, property or financial results, each of which could cause purchasers of Units to lose part or all of their investment. In addition to the other information contained in this prospectus supplement, the accompanying base shelf prospectus and the documents incorporated by reference herein and therein, prospective investors should carefully consider the factors set out under "Risk Factors" in the accompanying base shelf prospectus, the AIF and the factors set out below in evaluating Neovasc and its business before making an investment in the Units.
Risks relating to the Company
The Company is subject to lawsuits that have diverted its resources and will result in the payment of significant damages and other remedies.
The Company is engaged in litigation with CardiAQ, as further described below. Litigation resulting from CardiAQ's claims has been, and is expected to continue to be, costly and time-consuming and have diverted the attention of management and key personnel from our business operations. On November 13, 2017, the approximately US$112 million owed in the U.S. Court of Appeals litigation with CardiAQ, including the approximately US$70 million already held in a trust account, will become due and payable. These monetary damages exceed our resources and could have a material adverse effect on the Company and its financial position. These circumstances create material uncertainty and cast substantial doubt about the Company's ability to continue as a going concern. This Offering and the Concurrent Private Placement are being undertaken to enable the Company to continue to operate its business and fund the remaining approximately US$42 million damages and interest awards not held in trust.
On June 6, 2014, Neovasc was named in a lawsuit filed by CardiAQ in the United States District Court for the District of Massachusetts concerning intellectual property rights ownership, unfair trade practices and a breach of contract relating to Neovasc's transcatheter mitral valve technology, including the Tiara. On May 19, 2016, a jury awarded US$70 million in favour of CardiAQ on certain trade secret claims. On October 31, 2016, a judge awarded an additional US$21 million in enhanced damages to the jury's award and ordered that certain CardiAQ employees be listed as inventors on a Neovasc patent related to the Tiara. On January 18, 2017, a judge granted CardiAQ's motion for pre- and post-judgment interest, all as more particularly described in the section titled "Legal Proceedings" in the AIF. The Company sought an expedited appeal of the judgment, including the underlying damages award upon which these figures were calculated and the decision on inventorship, before the United States Court of Appeals for the Federal Circuit. On September 1, 2017, the Court of Appeals entered an opinion and judgement affirming the District Court's decision on money judgement, inventorship and rejection of an injunction. Payment of the damages and interest awards is currently stayed pending completion of the appeal pursuant to a court order of December 23, 2016. Under the terms of the stay, Neovasc deposited US$70 million into a joint escrow account and entered into a general security agreement related to the remaining damages awarded by the court. The Court subsequently ordered that US$70 million to be transferred to a CardiAQ trust account. Neovasc will also require court approval for transactions outside the course of normal business, including this Offering and the Concurrent Private Placement, until such time that the Company posts the remaining amount of money judgment into the joint escrow account. On October 2, 2017, Neovasc filed a petition for rehearing at the Court of Appeals seeking to reverse the money judgment and inventorship decisions. On the same day, CardiAQ also filed a petition for rehearing on the denial of the injunction. On November 3, 2017, the Court of Appeals denied the petition for rehearing filed by CardiAQ and denied the petition for rehearing filed by the Company. The mandate of the court will issue on November 13, 2017 and the appeals process has been exhausted. The full judgment of approximately US$112 million, of which approximately US$70 million is already held in trust, will become enforceable on November 13, 2017. Neovasc must pay the damages and interest awards, including funding the remaining approximately US$42 million not held in trust, which exceeds the Company's current cash resources. See "The Company — Recent Developments".
S-12
On June 23, 2014, CardiAQ also filed a complaint against Neovasc in Germany requesting that Neovasc assign its right to one of its European patent applications to CardiAQ. On July 7, 2014, the Company was made aware through a press release issued by CardiAQ of a stay in proceedings for Neovasc's European patent application that is the subject of the German lawsuit. This stay of proceedings was granted without an opportunity for Neovasc to respond to CardiAQ's allegations. The Company requested that the stay be lifted, but the request was denied by the European Patent office pending resolution of the German lawsuit. On December 14, 2016, a hearing took place in Munich, Germany regarding the German lawsuit. Based on this hearing, and the underlying exchange of written submissions, the Munich court entered a judgment on June 16, 2017, granting co-ownership of the European patent application in question to CardiAQ. There are no monetary damages connected to this order, nor is there such a request from CardiAQ pending in the German litigation. Neovasc filed a notice of appeal against the decision with the Appeals Court of Munich on July 14, 2017. On July 20, 2017, CardiAQ filed a notice of appeal with the same court. Both parties have in the meantime substantiated their respective appeals, and by way of case management order of October 18, 2017, the Appeals Court of Munich has now provisionally set the hearing date in this matter for April 12, 2018. There is likely to be further exchanges of written submissions between the parties in the run-up to that hearing.
On March 24, 2017, CardiAQ filed a complaint against Neovasc in the United States District Court for the District of Massachusetts. This lawsuit is related to the ongoing litigation with CardiAQ and concerns inventorship of two patents related to the Tiara. On October 4, 2017 CardiAQ amended the complaint to add an additional claim for inventorship of a third patent related to the Tiara. The complaint does not seek any money damages. The Company does not believe this additional lawsuit will have a material impact on the outcome of the Company's litigation with CardiAQ. The Company's deadline to submit a response to this complaint expires on November 16, 2017.
When the Company assesses that it is more likely that a present obligation exists at the end of the reporting period and that the possibility of an outflow of economic resources embodying economic benefits is probable, a provision is recognized and contingent liability disclosure is required. As at June 30, 2017, the Company has fully provided for the damages awards described above.
There is substantial doubt about our ability to continue as a going concern.
Our audited consolidated financial statements for the year ended December 31, 2016 were prepared under the assumption that we would continue our operations as a going concern. Our independent registered public accounting firm has included a "going concern" emphasis of matter paragraph in its report on our audited consolidated financial statements for the year ended December 31, 2016. This Offering and the Concurrent Private Placement are being undertaken to enable the Company to continue to operate its business after November 13, 2017, when the damages and interest awards totalling approximately US$112 million in connection with the CardiAQ U.S. Court of Appeals litigation become due and payable (see "Legal Proceedings" in the AIF and "The Company — Recent Developments" in this prospectus supplement for additional information). Litigation is inherently uncertain. The Company is faced with significant monetary damages that exceed its resources and will have a material adverse effect on the Company and its financial position. These circumstances create material uncertainty and cast substantial doubt about the Company's ability to continue as a going concern. Following payment of any such monetary awards from the proceeds of the Offering and the Concurrent Private Placement, the Company will require additional financing to continue to operate its business. There can be no assurance that such financing will be available on favorable terms, or at all, and the issuance of the Warrants and the Notes, which contain, among other things, provisions relating to future-priced conversion or exercise formula and full-ratchet anti-dilution, as well as every future exercise of the Class C Warrants, will make investment in our Common Shares less attractive to many investors, which may make it difficult to raise additional funding from equity issuances in the future while the Warrants and the Notes remain outstanding. The audited consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We have significant additional future capital needs and there are uncertainties as to our ability to raise additional funding.
We will require significant additional capital resources to expand our business, in particular the further development of our medical devices. Technical innovations often require substantial time and investment before
S-13
we can determine commercial viability. Advancing our products, market expansion of our currently marketed products or acquisition and development of any new products or medical devices will require considerable resources and additional access to capital markets. In addition, our future cash requirements may vary materially from those now expected. For example, our future capital requirements may increase if:
- •
- we experience more competition for our medical devices from other medical device companies or in more markets than
anticipated;
- •
- we experience delays or unexpected increases in costs in connection with obtaining regulatory approvals for our products
in the various markets where we hope to sell our products;
- •
- we experience unexpected or increased costs relating to preparing, filing, prosecuting, maintaining, defending and
enforcing patent claims, or other lawsuits, brought by either us or our competition;
- •
- we experience scientific progress sooner than expected in our discovery, research and development projects, if we expand
the magnitude and scope of these activities, or if we modify our focus as a result of our discoveries;
- •
- we experience setbacks in our progress with pre-clinical studies and clinical trials are delayed;
- •
- we are required to perform additional pre-clinical studies and clinical trials; or
- •
- we elect to develop, acquire or license new technologies, products or businesses.
We could potentially seek additional funding through corporate collaborations and licensing arrangements, through public or private equity or debt financing, or through other transactions. However, if sales are slow to increase or if capital market conditions in general, or with respect medical device companies such as ours, are unfavourable, our ability to obtain significant additional funding on acceptable terms, if at all, will be negatively affected. As described above, the Company will also require court approval for transactions outside the course of normal business, including this Offering and the Concurrent Private Placement, until the U.S. Court of Appeals litigation with CardiAQ is concluded or the Company posts the remaining amount of money judgment against the Company in that matter into the joint escrow account. The issuance of the Warrants and the Notes which contain, among other things, provisions relating to future-priced conversion or exercise formula and full-ratchet anti-dilution, as well as every future exercise of the Class C Warrants, will make investment in our Common Shares less attractive to many investors, which may make it difficult to raise additional funding from equity issuances in the future. Additional financing that we may pursue may involve the sale of our common shares or financial instruments that are exchangeable for, or convertible into, our common shares which could result in significant dilution to our shareholders.
If sufficient capital is not available, or if a transaction is not approved by the court in the U.S. Court of Appeals litigation with CardiAQ, we may be required to delay our business expansion or our research and development projects, either of which could have a material adverse effect on our business, financial condition, prospects or results of operations.
Third parties may claim we are infringing their intellectual property and we could suffer significant litigation or licensing expenses or be prevented from selling products.
We may be involved in substantial litigation regarding patent and other intellectual property rights in the medical device industry, other than the CardiAQ U.S. Court of Appeals litigation. We may be subject to challenges by third parties regarding our intellectual property, including, among others, claims regarding validity, enforceability, scope and effective term. From time to time, we have been and may in the future be forced to defend against claims and legal actions alleging infringement of the intellectual property rights of others, and such intellectual property litigation is typically costly and time-consuming. In particular, see "Legal Proceedings" in the AIF and "The Company — Recent Developments" for summaries of recent claims brought against us. Adverse determinations in any such litigation could result in significant liabilities to third parties or injunctions, or could require us to seek licenses from third parties and, if such licenses are not available on commercially reasonable terms, prevent us from manufacturing, selling or using certain products, any one of which could have a material adverse effect on us. In addition, some licenses may be non-exclusive, which could provide our competitors access to the same technologies.
S-14
Third parties could also obtain patents that may require us to either redesign products or, if possible, negotiate licenses from such third parties. Such licenses may materially increase our expenses. If we are unable to redesign products or obtain a license, we might have to exit a particular product offering.
The success of our business depends in part on our ability to obtain and maintain intellectual property protection for our technology and know-how, and operate without infringing the intellectual property rights of other companies. It is possible that as a result of future litigation our products currently marketed or under development may be found to infringe or otherwise violate third party intellectual property rights. Intellectual property litigation proceedings, if instituted against us, could result in substantial costs, inability to market our products including the Tiara, loss of our proprietary rights and diversion of our management's and technical team's attention and resources.
Our inability to protect our intellectual property could have a material adverse effect on our business.
Our success and competitive position are dependent in part upon our proprietary intellectual property. We rely on a combination of patents and trade secrets to protect our proprietary intellectual property, and we expect to continue to do so. Although we seek to protect our proprietary rights through a variety of means, we cannot guarantee that the protective steps we have taken are adequate to protect these rights. Patents issued to or licensed by us in the past or in the future may be challenged and held invalid. The scope of our patent claims also may vary between countries, as individual countries have distinctive patent laws. In addition, as our patents expire, we may be unsuccessful in extending their protection through patent term extensions. The expiration of, or the failure to maintain or extend our patents, could have a material adverse effect on us.
We also rely on confidentiality agreements with certain employees, consultants and other third parties to protect, in part, trade secrets and other proprietary information. These agreements could be breached and we may not have adequate remedies for such a breach. In addition, others could independently develop substantially equivalent proprietary information or gain access to our trade secrets or proprietary information.
We may spend significant resources to enforce our intellectual property rights and such enforcement could result in litigation. Intellectual property litigation is complex and can be expensive and time-consuming. However, our efforts in this regard may not be successful. We also may not be able to detect infringement. In addition, competitors may design around our technology or develop competing technologies. Patent litigation can result in substantial cost and diversion of effort. Intellectual property protection may also be unavailable or limited in some foreign countries, enabling our competitors to capture increased market position. The invalidation of key intellectual property rights or an unsuccessful outcome in lawsuits filed to protect our intellectual property could have a material adverse effect on our financial condition, results of operations or prospects.
Our products are continually the subject of clinical trials conducted by us, our competitors, or other third parties, the results of which may be unfavorable, or perceived as unfavorable, and could have a material adverse effect on our business, financial condition, and results of operations.
The regulatory approval process for new products and new indications for existing products requires extensive clinical trials and procedures, including early clinical experiences and regulatory studies. Unfavorable or inconsistent clinical data from current or future clinical trials or procedures conducted by us, our competitors, or third parties, or perceptions regarding this clinical data, could adversely affect our ability to obtain necessary approvals and the market's view of our future prospects. Such clinical trials and procedures are inherently uncertain and there can be no assurance that these trials or procedures will be completed in a timely or cost-effective manner or result in a commercially viable product. Failure to successfully complete these trials or procedures in a timely and cost-effective manner could have a material adverse effect on our prospects. Clinical trials or procedures may experience significant setbacks even after earlier trials have shown promising results. Further, preliminary results from clinical trials or procedures may be contradicted by subsequent clinical analysis. In addition, results from our clinical trials or procedures may not be supported by actual long-term studies or clinical experience. If preliminary clinical results are later contradicted, or if initial results cannot be supported by actual long-term studies or clinical experience, our business could be adversely affected. Clinical
S-15
trials or procedures may be suspended or terminated by us or regulatory authorities at any time if it is believed that the trial participants face unacceptable health risks.
A number of companies in the medical device industry have suffered significant setbacks in advanced clinical trials, even after positive results in earlier trials. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be delayed, repeated or terminated. In addition, failure to construct appropriate clinical trial protocols could result in the test or control group experiencing a disproportionate number of adverse events and could cause a clinical trial to be repeated or terminated.
Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, the Company may be required to report some of these relationships to the FDA. The FDA may conclude that a financial relationship between the company and a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. The FDA may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of regulatory approval of one or more of our product candidates.
We have a history of significant losses and a significant accumulated deficit.
We may incur losses in the future and our losses may increase. We have incurred net losses in each fiscal year since inception. For the six months ended June 30, 2017, we had a net loss of US$13,186,295 and at June 30, 2017, we had an accumulated deficit of US$214,969,901. We have increased our research and development expenses in recent periods and we plan further increases in the future as cash flows allow. The planned increases in research and development expenses may result in larger losses in future periods. As a result, we will need to generate significantly greater revenues than we have to date to achieve and maintain profitability. There can be no assurance that revenues will increase. Our business strategies may not be successful and we may not be profitable in any future period. Our operating results have varied in the past and they may continue to fluctuate in the future. In addition, our operating results may not follow any past trends.
You will experience immediate dilution.
Since the price per share of our Unit Shares being offered is higher than the net tangible book value per share of our Common Shares, you will suffer substantial dilution in the net tangible book value of the Common Shares you purchase in this Offering. Based on the combined public offering price of $1.46 per Unit, and after deducting the underwriting discount and estimated offering expenses payable by us, if you purchase Units in this offering, you will suffer immediate and substantial dilution.
The Warrants and Notes may result in significant dilution to our shareholders.
As part of this Offering we will issue the purchasers five-year Series A Warrants representing the right to acquire a Series A Warrant Share at an exercise price of $1.61 per share, two-year Series B Warrants representing the right to acquire a Series B Warrant Share at an exercise price of $1.61 per share, and two-year Series F Warrants representing the right to acquire a Series F Warrant Share at an exercise price of $1.61 per share. We will also issue the Notes and Series E Warrants pursuant to the Concurrent Private Placement. Each of the Series A Warrants, Series B Warrants, Series E Warrants, Series F Warrants and the Notes contain so-called full-ratchet anti-dilution provisions as well as other anti-dilution provisions that may be triggered upon any future issuance by us of Common Shares or Common Share equivalents at a price per share below the then-exercise price of the Warrants or conversion price of the Notes, subject to some exceptions, which could result in significant additional dilution to our shareholders. In addition, each of the Series A Warrants, Series B Warrants, Series E Warrants, Series F Warrants and the Notes contain future-priced conversion or exercise provisions and certain other provisions that could reset the conversion or exercise price of the securities based on the market price of the Common Shares at a future date. These provisions could result in the issuance of a large number of Common Shares if the market price for our Common Shares declines below the initial
S-16
conversion and exercise prices, thereby putting pressure on the market price of our Common Shares and increasing the risk of further dilution upon subsequent conversions or exercises of the securities. To the extent that purchasers of Units or Notes sell Common Shares issued upon the exercise of the Series A, Series B, Series C, Series D, Series E or Series F Warrants or the conversion of the Notes, or holders of the Class C Warrants exercise such securities, the market price of our Common Shares may decrease due to the additional selling pressure in the market. The risk of dilution from issuances of Common Shares underlying the Series A, Series B, Series D, Series E and Series F Warrants or pursuant to the conversion of the Notes may cause shareholders to sell their Common Shares, which could further contribute to any decline in the Common Share price.
Our Common Shares may be delisted from the Nasdaq or the TSX, which could affect their market price and liquidity. If our Common Shares were to be delisted, investors may have difficulty in disposing of their shares.
Our Common Shares are currently listed on the Nasdaq and on the TSX under the symbol "NVCN". We must meet continuing listing requirements to maintain the listing of our Common Shares on the Nasdaq and the TSX. For example, for continued listing, the Nasdaq requires, among other things, that listed securities maintain a minimum closing bid price of not less than US$1.00 per share. On July 1, 2016, we received a notice from The Nasdaq Listing Qualifications Department indicating that the minimum bid price for our Common Shares had fallen below US$1.00 for 30 consecutive business days, and that, therefore, we were no longer in compliance with Nasdaq Marketplace Rule 5550(a)(2) — bid price. We had 180 calendar days to regain compliance with the minimum bid price requirement. To regain compliance, the closing bid price of our Common Shares needed to be at least US$1.00 per share for a minimum of 10 consecutive business days. We regained compliance on December 16, 2016. On November 9, 2017, the last trading day before filing of this prospectus supplement the closing price of the common shares was US$1.46 on the Nasdaq. The dilution or perception of dilution from this Offering and the Concurrent Private Placement, pressure on the share price from the future-priced conversion and exercise features or exercises of the Class C Warrants of the securities, or from subsequent sales of Common Shares issued upon the exercise of the Warrants or the conversion of the Notes, may put downward pressure on the price of our Common Shares. If the price of our Common Shares falls below US$1.00 and we effect a reverse stock split to regain compliance with the Nasdaq minimum bid price requirement, this would trigger a repricing under the Warrants and Notes in accordance with the provisions therein. See "Description of the Securities Being Distributed under the Offering" and "Description of the Securities Being Distributed under the Concurrent Private Placement".
In addition to the specified criteria for continued listing, the Nasdaq also has broad discretionary public interest authority that it can exercise to apply additional or more stringent criteria for the continued listing of the Common Shares, or suspend or delist securities even though the securities meet all enumerated criteria for continued listing on the Nasdaq. We cannot assure you that the Nasdaq will not exercise such discretionary authority.
There can be no assurance that our Common Shares will remain listed on the Nasdaq or the TSX. If we fail to meet any of the Nasdaq's or the TSX's continued listing requirements, our Common Shares may be delisted. Any delisting of our Common Shares may adversely affect a shareholder's ability to dispose, or obtain quotations as to the market value, of such shares.
We are subject to the risks associated with product liability claims, insurance and recalls.
Prior to patient use, our products undergo extensive clinical testing and are approved by the applicable regulatory authorities. However, despite all reasonable efforts to ensure safety, it is possible that we or our partners may sell products which are defectively manufactured or labeled, contain defective components or are misused. Our products may also fail to meet patient expectations or produce harmful side effects. Such unexpected quality, safety or efficacy issues may be caused by a number of factors, including manufacturing defects, failure to adhere to good clinical practices, failure to adhere to good manufacturing practices, non-compliance with clinical protocols or the presence of other harmful conditions in a clinical trial inadequacies of product-related information conveyed to physicians or patients, or other factors or circumstances unique to the patient. Whether or not scientifically justified, such unexpected safety or efficacy concerns can arise and may lead to product recalls, loss of or delays in market acceptance, market withdrawals,
S-17
or declining sales, as well as product liability, consumer fraud and/or other claims. Additionally, we may be exposed to product liability claims from patients in clinical trials. Such liability might result from claims made directly by consumers or by medical device companies or others selling such products. It is impossible to predict the scope of injury or liability from such defects or unexpected reactions, or the impact on the market for such products of any allegations of these claims, even if unsupported, or the measure of damages which might be imposed as a result of any claims or the cost of defending such claims. Substantial damage awards and/or settlements have been handed down — notably in the United States and other common law jurisdictions — against medical device companies based on claims for injuries allegedly caused by the use of their products. Although our shareholders would not have personal liability for such damages, the expenses of litigation or settlements, or both, in connection with any such injuries or alleged injuries and the amount of any award imposed on us in excess of existing insurance coverage, if any, may have a material adverse impact on us and on the price of our common shares. In addition, we may not be able to avoid significant product liability exposure even if we take appropriate precautions, including maintaining product liability coverage (subject to deductibles and maximum payouts). Any liability that we may have as a result could have a material adverse effect on our business, financial condition and results of operations, to the extent insurance coverage for such liability is not available. Product liability claims in the future, regardless of their ultimate outcome, could have a material adverse effect on our reputation and on our ability to attract and retain customers for our products.
Use of our products in unapproved circumstances could expose us to liabilities.
The marketing approval from the FDA and other regulators of certain of our products are, or are expected to be, limited to specific indications. We are prohibited by law from marketing or promoting any unapproved use of our products. Physicians, however, in most jurisdictions, can use these products in ways or circumstances other than those strictly within the scope of the regulatory approval. Although the product training we provide to physicians and other health care professionals is limited to approved uses or for clinical trials, no assurance can be given that claims might not be asserted against us if our products are used in ways or for procedures that are not approved.
We have substantial competition in the medical device industry and with respect to our products.
The medical device industry is highly competitive and is characterized by extensive research and development and rapid technological change. Many companies, as well as research organizations, currently engage in, or have in the past engaged in, efforts related to the development of medical devices in the same therapeutic areas as we do. Due to the size of the cardiovascular market and the large unmet medical need for products that treat cardiovascular illnesses, a number of the world's largest medical device companies are developing, or could potentially develop, products that could compete with ours.
Many of the companies developing competing technologies and products may have significantly greater financial resources and expertise in discovery, research and development, manufacturing, pre-clinical studies and clinical testing, obtaining regulatory approvals and marketing than we do. Other smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Academic institutions, government agencies and other public and private research organizations may also conduct research, seek patent protection and establish collaborative arrangements for discovery, research, clinical development and marketing of products similar to ours. There is a risk that one or more of our competitors may develop more effective or more affordable products than us and that such competitors will commercialize products that will render our medical devices obsolete. We face competition with respect to product efficacy and safety, ease of use and adaptability to various modes of administration, acceptance by physicians, the timing and scope of regulatory approvals, availability of resources, reimbursement coverage, price and patent positions of others. In addition, these companies and institutions also compete with us in recruiting and retaining qualified personnel. If we fail to develop new products or enhance our existing products in the face of such strong competition, such competition could have a material adverse effect on our business, financial condition or results of operations.
S-18
Our approved products may not achieve or maintain expected levels of market acceptance, which could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our securities to decline.
Even if we are able to obtain regulatory approvals for our products, the success of those products is dependent upon achieving and maintaining market acceptance. New medical devices that appear promising in development may fail to reach the market or may have only limited or no commercial success. Levels of market acceptance for our products could be impacted by several factors, many of which are not within our control, including but not limited to:
- •
- safety, efficacy, convenience and cost-effectiveness of our products compared to products of our competitors;
- •
- scope of approved uses and marketing approval;
- •
- timing of market approvals and market entry;
- •
- difficulty in, or excessive costs to, manufacture;
- •
- infringement or alleged infringement of the patents or intellectual property rights of others;
- •
- availability of alternative products from our competitors;
- •
- acceptance of the price of our products; and
- •
- ability to market our products effectively at the retail level.
In addition, the success of any new product will depend on our ability to either successfully build our in-house sales capabilities or to secure new, or to realize the benefits of existing arrangements with, third-party marketing or distribution partners. Seeking out, evaluating and negotiating marketing or distribution agreements may involve the commitment of substantial time and effort and may not ultimately result in an agreement. In addition, the third-party marketing or distribution partners may not be as successful in promoting our products as we had anticipated. If we are unable to commercialize new products successfully, whether through a failure to achieve market acceptance, a failure to build our own in-house sales capabilities, a failure to secure new marketing partners or to realize the benefits of our arrangements with existing marketing partners, there may be a material adverse effect on our business, financial condition and results of operations and it could cause the market value of our securities to decline.
In addition, by the time any products are ready to be commercialized, the proposed market for these products may have changed. Our estimates of the number of patients who have received or might have been candidates to use a specific product may not accurately reflect the true market or market prices for such products or the extent to which such products, if successfully developed, will actually be used by patients. Our failure to successfully introduce and market our products that are under development would have a material adverse effect on our business, financial condition, and results of operations.
If we are not able to convince public payors and hospitals to include our products on their approved product lists, our revenues may not meet expectations and our business, results of operations and financial condition may be adversely affected.
The direct cost of implanting or using our medical devices is seldom paid by individual patients. Successful commercialization of such devices will depend largely upon the availability of reimbursement for the surgery and medical costs associated with the product from third-party payors. We expect that our products will be purchased by health-care providers, clinics, and hospitals that will subsequently bill various third-party payors such as government programs and private insurance plans. These expectant payors carefully review and increasingly challenge the prices charged for medical devices and services. Provincial government sponsored health programs in Canada and similar programs in the United States reimburse hospitals a pre-determined fixed amount for the costs associated with a particular procedure based on the patient's discharge diagnosis and similarly reimburse the surgeon or physician based on the procedure performed, without taking into consideration the actual costs incurred by either party or the actual cost of the device. New products are being scrutinized increasingly with respect to whether or not they will be covered by the various health plans and at what level of reimbursement.
S-19
Third-party payors may determine that our products are unnecessary, not cost-effective, too experimental, or are primarily intended for non-approved indications.
Our business may be materially adversely affected by new legislation, new regulatory requirements, and the continuing efforts of governmental and third party payors to contain or reduce the costs of healthcare through various means, including the U.S. healthcare reform legislation signed in 2010.
The government and regulatory authorities in Canada, the United States, Europe and other markets in which we sell our products may propose and adopt new legislation and regulatory requirements relating to medical product approval criteria and manufacturing requirements. Such legislation or regulatory requirements, or the failure to comply with such, could adversely impact our operations and could have a material adverse effect on our business, financial condition and results of operations.
The growth of overall healthcare costs as a percentage of gross domestic product in many countries means that governments and payors are under intense pressure to control healthcare spending even more tightly. These pressures are particularly strong given the ongoing effects of the global economic and financial crisis, including the continuing debt crisis in certain countries in Europe, and the risk of a similar crisis in the United States. As a result, our businesses and the healthcare industry in general are operating in an ever more challenging environment with very significant pricing pressures. In recent years, national, federal, provincial, state, and local officials and legislators have proposed, or are reportedly considering proposing, a variety of price based reforms to the healthcare systems in the European Union, the United States and other countries. Some proposals include measures that would limit or eliminate payments for certain medical procedures and treatments or subject pricing to government control. Furthermore, in certain foreign markets, the pricing or profitability of healthcare products is subject to government controls and other measures that have been prepared by legislators and government officials. While we cannot predict whether any such legislative or regulatory proposals or reforms will be adopted, the adoption of any such proposals or reforms could adversely affect the commercial viability of our existing and potential products.
In March 2010, then U.S. President Obama signed into law the Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act of 2010, or the ACA. The ACA imposed new taxes on medical device makers in the form of a 2.3% excise tax on all U.S. medical device sales. In 2015, Congress imposed a 2-year moratorium on this medical device tax, so that medical device sales during the period between January 1, 2016 and December 31, 2017 are exempt from the tax. Absent further legislative action, the tax will be automatically reinstated for medical device sales starting on January 1, 2018. The device tax, if reinstated, could materially and adversely affect our business, cash flows and results of operations. The law also focuses on a number of Medicare provisions aimed at improving quality and decreasing costs. It is uncertain at this point what negative unintended consequences these provisions will have on patient access to new technologies. The Medicare provisions include value-based payment programs, increased funding of comparative effectiveness research, reduced hospital payments for avoidable readmissions and hospital acquired conditions, and pilot programs to evaluate alternative payment methodologies that promote care coordination (such as bundled physician and hospital payments). Additionally, the ACA includes a reduction in the annual rate of inflation for Medicare payments to hospitals and the establishment of an independent payment advisory board to recommend ways of reducing the rate of growth in Medicare spending beginning. Some of the provisions of the ACA have yet to be fully implemented, while certain provisions have been subject to judicial and Congressional challenges. In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, that while not a law, is widely viewed as the first step toward the passage of legislation that would repeal certain aspects of the ACA. Further, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal burden on states or a cost, fee, tax, penalty or regulatory burden on individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Legislation, including the American Health Care Reform Act of 2017, has been drafted to repeal and replace parts of the ACA, but it is uncertain when a bill would be passed and what any replacement law would encompass. Thus, the full impact of the ACA, or any law replacing elements of it, on our business remains unclear. We cannot predict what health care programs and regulations will be ultimately implemented at the federal or state level, or the effect of any future legislation or regulation. However, any
S-20
changes that lower reimbursement for our products or reduce medical procedure volumes could adversely affect our business and results of operations.
Our industry is the subject of numerous governmental investigations into marketing and other business practices. These investigations could result in the commencement of civil and/or criminal proceedings, substantial fines, penalties, and/or administrative remedies, divert the attention of our management, and have an adverse effect on our financial condition and results of operations.
Our industry is the subject of numerous governmental investigations into marketing and other business practices. This has included increased regulation, enforcement, inspections, and governmental investigations of the medical device industry and disclosure of financial relationships with health care professionals. In the United States, the laws in which we are subject to include:
- •
- the federal Anti-Kickback Statute, which prohibits, among other things,
soliciting, receiving, offering or providing remuneration intended to induce the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare
or Medicaid programs. This statute has been applied to medical device manufacturer marketing practices, educational programs, pricing policies and relationships with healthcare providers. A person or
entity does not need to have actual knowledge of this statute or specific intent to violate it to have committed a violation;
- •
- federal civil and criminal false claims laws and civil monetary penalty laws, including civil whistleblower or qui tam
actions that prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment or approval to the federal government that are false or fraudulent, knowingly making a
false statement material to an obligation to pay or transmit money or property to the federal government or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay
or transmit money or property to the federal government. The government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a
false or fraudulent claim for purposes of the false claims statutes;
- •
- the federal Health Insurance Portability and Accountability Act of 1996,
("HIPAA"), and its implementing regulations, which created federal criminal laws that prohibit, among other things, executing a scheme to defraud any
healthcare benefit program or making false statements relating to healthcare matters;
- •
- HIPAA, as amended by the Health Information Technology for Economic and Clinical Health
Act, also imposes certain regulatory and contractual requirements regarding the privacy, security and transmission of individually identifiable health information;
- •
- federal "sunshine" requirements imposed by the Patient Protection and Affordable Care
Act, as amended by the Health Care and Education Reconciliation Act, on device manufacturers regarding any "transfer of value" made or distributed to physicians and teaching
hospitals; and
- •
- state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws that require device companies to comply with the industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of certain health information, many of which differ from each other in significant ways and often are not pre-empted by HIPAA.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities, including certain sales and marketing practices and financial arrangements with physicians, could be subject to challenge under one or more of such laws. Any action against us, even if we successfully defend against it, could result in the commencement of civil and/or criminal proceedings, exclusion from governmental health care programs, substantial fines, penalties, and/or administrative remedies, divert the attention of our management, and have an adverse effect on our financial condition and results of operations. We anticipate that the government will continue to scrutinize our
S-21
industry closely, and that additional regulation by governmental authorities, both foreign and domestic, may increase compliance costs, exposure to litigation and other adverse effects to our operations.
Our products are subject to extensive regulation, which can be costly and time consuming, cause unanticipated delays, or prevent the receipt of the required approvals to commercialize products.
The pre-clinical and clinical trials of any products developed by us and the manufacturing, labeling, sale, distribution, export or import, marketing, advertising and promotion of any of those products are subject to rigorous regulation by federal, provincial, state and local governmental authorities. Our medical devices are principally regulated in the United States by the FDA, in Canada by the Health Canada (particularly, the Therapeutic Products Directorate), in the European Union by the European Medicines Agency ("EMA"), and by other similar regulatory authorities in other jurisdictions. Government regulation substantially increases the cost and risk of researching, developing, manufacturing and selling products. Following several widely-publicized issues in recent years, the FDA and similar regulatory authorities in other jurisdictions have become increasingly focused on product safety. This development has led to requests for more clinical trial data, for the inclusion of a significantly higher number of patients in clinical trials and for more detailed analysis of trial results. Consequently, the process of obtaining regulatory approvals, particularly from the FDA, has become more costly, time consuming and challenging than in the past. Any product developed by us or our future collaborative partners, if any, must receive all relevant regulatory approvals or clearances from the applicable regulatory authorities before it may be marketed and sold in a particular country.
Any of our products that receive regulatory approval could be subject to extensive post-market regulation that can affect sales, marketing and profitability.
With respect to any products for which we obtain regulatory approval, we will be subject to post-marketing regulatory obligations, including the requirements by the FDA, EMA and similar agencies in other jurisdictions to maintain records regarding product safety and to report to regulatory authorities serious or unexpected adverse events. The occurrence of unanticipated serious adverse events or other safety problems could cause the governing agencies to impose significant restrictions on the indicated uses for which the product may be marketed, impose other restrictions on the distribution or sale of the product or require potentially costly post-approval studies. In addition, post-market discovery of previously unknown safety problems or increased severity or significance of a pre-existing safety signal could result in withdrawal of the product from the market and product recalls. Compliance with extensive post-marketing record keeping and reporting requirements requires a significant commitment of time and funds, which may limit our ability to successfully commercialize approved products.
Our industry is subject to health and safety risks.
We produce products for human implantation and use. While we take substantial precautions such as laboratory and clinical testing, clinical studies, quality control and assurance testing and controlled production methods, the associated health and safety risks cannot be eliminated. Our products may be found to be, or to contain substances that are harmful to the health of our patients and customers and which, in extreme cases, may cause serious health conditions or death. This sort of finding may expose us to substantial risk of litigation and liability.
Further, we could be forced to discontinue production of certain products, which would harm our profitability. Neovasc maintains product liability insurance coverage; however, there is no guarantee that our current coverage will be sufficient or that we can secure insurance coverage in the future at commercially viable rates or with the appropriate limits.
We may face risks associated with our manufacturing operations.
Manufacturing operations are subject to numerous unanticipated technological problems and delays. Our manufacturing processes, products and their various components are, and will be, subject to regulations specified by the various regulatory bodies such as Health Canada and the FDA. There can be no assurance that we will be able to comply with all stated manufacturing regulations. Failure or delay by the Company to comply with such regulations or to satisfy regulatory inspections could have an adverse effect on the Company's business and operations.
S-22
Additionally, two critical components of the Reducer are not readily available. The balloon portion of the delivery system is technically challenging to manufacture and the Reducer device, while a basic technology, must be manufactured in Israel due to restrictions on the transfer of intellectual property and manufacturing out of Israel stemming from certain research grants received by NML prior to the acquisition in July 2008.
Use of our products may increase the risk of animal disease.
Our critical raw material used in most of our customers' devices is animal derived pericardial tissue. As this raw material is derived from an animal, it is subject to many inconsistencies and potential risks. The most notable risk is the disease Bovine Spongiform Encephalopathy ("BSE"), also known as mad cow disease which can arise from bovine tissue. Although the tissue originates from the United States where strict controls are in place to prevent diseased animals from being processed, it cannot be assured that the livestock in the United States will remain free from BSE. There is also no assurance that our supplier will regularly deliver tissue with the specifications required to manufacture its products.
The manufacture of our products is highly regulated and complex and we may experience supply interruptions that could harm our ability to manufacture products.
We use a broad range of raw and organic materials and other items in the design and manufacture of our products. Our products are manufactured from treated natural animal tissue and man-made materials. Our non-implantable products are manufactured from man-made raw materials including resins, chemicals, electronics and metals. We purchase certain of the materials and components used in the manufacture of our products from external suppliers, and we purchase certain supplies from single sources for reasons of quality assurance, cost-effectiveness, availability or constraints resulting from regulatory requirements. General economic conditions could adversely affect the financial viability of our suppliers, resulting in their inability to provide materials and components used in the manufacture of our products. While we work closely with suppliers to monitor their financial viability and to assure continuity of supply and maintain high quality and reliability, these efforts may not be successful. In addition, due to the rigorous regulations and requirements of regulatory authorities regarding the manufacture of our products (including the need for approval of any change in supply arrangements), we may have difficulty establishing additional or replacement sources on a timely basis or at all if the need arises. Although alternative supplier options are considered and identified, we typically do not pursue regulatory qualification of alternative sources due to the strength of our existing supplier relationships and the time and expense associated with the regulatory validation process. A change in suppliers could require significant effort or investment in circumstances where the items supplied are integral to product performance or incorporate unique technology, and the loss of any existing supply contract could have a material adverse effect on us.
In particular, the Tiara valve is made up of two major components: the leaflets and skirt, which are made from the Peripatch and the nitinol frame, which is manufactured by a well-established specialty manufacturer in the medical device industry. However, if this supplier were unable to provide the nitinol frame in the future, it would seriously impact the further development of the Tiara.
Regulatory agencies from time to time have limited or banned the use of certain materials used in the manufacture of medical device products. In these circumstances, transition periods typically provide time to arrange for alternative materials.
We are dependent on limited products for substantially all of our current revenues. If the volume or price of these products decline or the costs of related manufacturing, distribution or marketing increase, it could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our securities to decline.
Sales of a limited number of our products represent substantially all of our current revenues. If the volume or pricing of our existing significant products decline in the future, or our cost to manufacture, distribute our existing significant products increase in the future, our market our business, financial condition and results of operations could be materially adversely affected and this could cause the market value of our securities to decline. In addition, if these products were to become subject to any other issues, such as material adverse changes in prescription growth rates, unexpected side effects, regulatory proceedings, material product liability
S-23
litigation, publicity affecting doctor or patient confidence or pressure from competitive products, the adverse impact on our business, financial condition, results of operations and the market value of our securities could be significant.
We may face exposure to adverse movements in foreign currency exchange rates.
Our business has expanded internationally and as a result, a significant portion of our revenues, expenses, current assets and current liabilities are preliminary denominated in U.S. dollars, Euros and other foreign currencies. The functional currency of Neovasc and its subsidiaries is the Canadian dollar and the presentation currency of our financial statements is U.S. dollars. A decrease in the value of such foreign currencies relative to the Canadian dollar could result in losses in revenues from currency exchange rate fluctuations. To date, we have not hedged against risks associated with foreign exchange rate exposure.
If we were to lose our foreign private issuer status under U.S. federal securities laws, we would likely incur additional expenses associated with compliance with the U.S. securities laws applicable to U.S. domestic issuers.
As a foreign private issuer, as defined in Rule 3b-4 under the Exchange Act we are exempt from certain of the provisions of the U.S. federal securities laws. For example, the U.S. proxy rules and the Section 16 reporting and "short swing" profit rules do not apply to foreign private issuers. However, if we were to lose our status as a foreign private issuer, these regulations would immediately apply and we would also be required to commence reporting on forms required of U.S. companies, such as Forms 10-K, 10-Q and 8-K, rather than the forms currently available to us, such as Forms 40-F and 6-K. Compliance with these additional disclosure and timing requirements under these securities laws would likely result in increased expenses and would require our management to devote substantial time and resources to comply with new regulatory requirements. Further, to the extent that we were to offer or sell our securities outside of the United States, we would have to comply with the more restrictive Regulation S requirements that apply to U.S. companies, and we would no longer be able to utilize the multijurisdictional disclosure system forms for registered offerings by Canadian companies in the United States, which could limit our ability to access the capital markets in the future.
Failure to comply with the U.S. Foreign Corrupt Practices Act ("FCPA"), as well as the anti-bribery laws of the nations in which we conduct business (such as the UK's Bribery Act or the Corruption of Foreign Public Officials Act of Canada ("CFPOA"), could subject us to penalties and other adverse consequences.
Our business is subject to the FCPA which generally prohibits companies and company employees from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. The FCPA also requires companies to maintain accurate books and records and internal controls, including at foreign-controlled subsidiaries. In addition, we are subject to other anti-bribery laws of the nations in which we conduct business that apply similar prohibitions as the FCPA (e.g., the UK's Bribery Act, the CFPOA and the OECD Anti-Bribery Convention). Our employees or other agents may, without our knowledge and despite our efforts, engage in prohibited conduct under our policies and procedures and the FCPA or other anti-bribery laws that we may be subject to for which we may be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
Legislative actions, potential new accounting pronouncements, and higher insurance costs are likely to impact our future financial position or results of operations.
Future changes in financial accounting standards may cause adverse, unexpected revenue fluctuations and affect our financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with greater frequency and are expected to occur in the future. Compliance with changing regulations of corporate governance and public disclosure may result in additional expenses. All of these uncertainties are leading generally toward increasing insurance costs, which may adversely affect our business, results of operations and our ability to purchase any such insurance, at acceptable rates or at all, in the future.
S-24
We are dependent upon our key personnel to achieve our business objectives.
As a technology-driven company, intellectual input from key management and personnel is critical to achieve our business objectives. Consequently, our ability to retain these individuals and attract other qualified individuals is critical to our success. The loss of the services of key individuals might significantly delay or prevent achievement of our business objectives. In addition, because of a relative scarcity of individuals with the high degree of education and scientific achievement required for our business, competition among medical device companies for qualified employees is intense and, as a result, we may not be able to attract and retain such individuals on acceptable terms, or at all. We do not maintain "key person" life insurance on any of our officers, employees, or consultants, and so any delay in replacing such persons, or an inability to replace them with persons of similar expertise, would have a material adverse effect on our business, financial condition and results of operations.
We also have relationships with scientific collaborators at academic and other institutions, some of whom conduct research at our request or assist us in formulating our research and development strategies. These scientific collaborators are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, even though our collaborators are required to sign confidentiality agreements prior to working with us, they may have arrangements with other companies to assist such other companies in developing technologies that may prove competitive to us.
Incentive provisions for our key executives include the granting of stock options that vest over time, designed to encourage such individuals to stay with us. However, a low share price, whether as a result of disappointing progress in our sales or development programs or as a result of market conditions generally, could render such agreements of little value to our key executives. In such event, our key executives could be susceptible to being hired away by our competitors who could offer a better compensation package. If we are unable to attract and retain key personnel our business, financial conditions and results of operations may be adversely affected.
The continuing development of many of our products depends upon us maintaining strong relationships with physicians.
If we fail to maintain our working relationships with physicians, many of our products may not be developed and marketed in line with the needs and expectations of the professionals who use and support our products, which could cause a decline in our earnings and profitability. The research, development, marketing, and sales of our new and improved products is dependent upon our maintaining working relationships with physicians. We rely on these professionals to provide us with considerable knowledge and experience regarding the development, marketing, and sale of our products. Physicians assist us as researchers, marketing and product consultants, inventors, and public speakers. If we are unable to maintain our strong relationships with these professionals and continue to receive their advice and input, the development and marketing of our products could suffer, which could have a material adverse effect on our consolidated earnings, financial condition, and/or cash flows.
A period of significant growth can place a strain on management systems.
If we experience a period of significant growth in the number of personnel, this could place a strain upon its management systems and resources. Our future will depend in part on the ability of its officers and other key employees to implement and improve its financial and management controls, reporting systems and procedures on a timely basis and to expand, train and manage its employee workforce. There can be no assurance that we will be able to effectively manage such growth. Our failure to do so could have a material adverse effect upon our business, prospects, results of operation and financial condition.
Consolidation in the health care industry could have an adverse effect on our revenues and results of operations.
Many health care industry companies, including health care systems, are consolidating to create new companies with greater market power. Organizations such as group purchase organizations, independent delivery networks, and large single accounts such as the U.S. Veterans Administration, continue to consolidate purchasing decisions for many of our health care provider customers. As a result, transactions with customers
S-25
are larger, more complex, and tend to involve more long-term contracts. The purchasing power of these larger customers has increased, and may continue to increase, causing downward pressure on product pricing. If we are not one of the providers selected by one of these organizations, we may be precluded from making sales to its members or participants. Even if we are one of the selected providers, we may be at a disadvantage relative to other selected providers that are able to offer volume discounts based on purchases of a broader range of medical equipment and supplies. Further, we may be required to commit to pricing that has a material adverse effect on our revenues and profit margins, business, financial condition and results of operations. We expect that market demand, governmental regulation, third-party reimbursement policies and societal pressures will continue to change the worldwide health care industry, resulting in further business consolidations and alliances, which may exert further downward pressure on the prices of our products and could adversely impact our business, financial condition, and results of operations.
We may or may not successfully identify and complete corporate transactions on favorable terms or achieve anticipated synergies relating to any acquisitions or alliances, and such acquisitions could result in unforeseen operating difficulties and expenditures, require significant management resources and require significant charges.
As a part of our growth strategy, we regularly explore potential acquisitions of complementary businesses, technologies, services or products as well as potential strategic alliances or divestitures of assets or a sale of the Company. We may be unable to find suitable acquisition candidates or appropriate partners with which to form alliances. Even if we identify appropriate acquisition or alliance candidates, we may be unable to complete the acquisitions or alliances on favorable terms, if at all. Acquisition activities can be thwarted by overtures from competitors for the targeted candidates, government regulation and replacement product developments in our industry. In addition, the process of integrating an acquired business, technology, service or product into our existing operations could result in unforeseen difficulties and expenditures. Integration of an acquired company often requires significant expenditures as well as significant management resources that otherwise would be available for ongoing development of our other businesses. Moreover, we may not realize the anticipated financial or other benefits of an acquisition or alliance.
We may be required to take charges or write-downs in connection with acquisitions. In particular, acquisitions of businesses engaged in the development of new products may give rise to in-process research and development assets. To the extent that the value of these assets declines, we may be required to write down the value of the assets. Also, in connection with certain asset acquisitions, we may be required to take an immediate charge related to acquired in-process research and development. Either of these situations could result in substantial charges, which could adversely affect our results of operations.
Future acquisitions could also involve the issuance of equity securities, the incurrence of debt, contingent liabilities or amortization of expenses related to other intangible assets, any of which could adversely impact our financial condition or results of operations. In addition, equity or debt financing required for such acquisitions may not be available.
Any corporate transaction will be accompanied by certain risks including but not limited to:
- •
- exposure to unknown liabilities of acquired companies and the unknown issues with any associated technologies
or research;
- •
- higher than anticipated acquisition costs and expenses;
- •
- exposure to other companies' shares that shareholders could receive as consideration for our shares in a corporate
transaction;
- •
- the difficulty and expense of integrating operations, systems, and personnel of acquired companies;
- •
- disruption of our ongoing business;
- •
- inability to retain key customers, distributors, vendors and other business partners of the acquired company; and
- •
- diversion of management's time and attention.
S-26
We may not be able to successfully overcome these risks and other problems associated with acquisitions and this may adversely affect our business, financial condition or results of operations.
Anti-takeover provisions could discourage a third party from making a takeover offer that could be beneficial to our shareholders.
Some of the provisions in our articles of incorporation and by-laws could delay or prevent a third party from acquiring us or replacing members of our board of directors, even if the acquisition or the replacements would be beneficial to our shareholders. Such provisions include the following:
- •
- shareholders cannot amend our articles of incorporation unless at least two-thirds of the shares entitled to vote approve
the amendment; and
- •
- our board of directors can, without shareholder approval, issue preferred shares having any terms, conditions, rights and preferences that the board determines.
These provisions could also reduce the price that certain investors might be willing to pay for our securities and result in the market price for our securities, including the market price for our common shares, being lower than it would be without these provisions.
Conflicts of interest may arise among the Company's officers and directors as a result of their involvement with other issuers.
Certain of our directors and officers serve as directors, officers, promoters and members of management of other companies and, therefore, it is possible that a conflict may arise between their duties as a director or officer, and their duties as a director or officer of such other companies. There can be no assurance that in the carrying out of their duties with respect to us, these persons will not find themselves in situations which could give rise to conflicts of interest. There can be no assurance that if conflicts do arise, they will be resolved in a manner favourable to us. There can be no assurance that future transactions or arrangements between the companies and any of such entities will be advantageous to us.
Significant shareholders of the Company could influence our business operations and sales of our shares by such significant shareholders could influence our share price.
Frost Gamma Investments Trust (the "Frost Group") and Boston Scientific Corporation ("Boston Scientific") are each significant shareholders of the Company, holding approximately 19% and 15% of our outstanding common shares, respectively, prior to the completion of this Offering and the Concurrent Private Placement. The exercise of voting rights associated with shares held by the Frost Group and Boston Scientific at meetings of shareholders may have significant influences on our business operations. Also, the Frost Group and Boston Scientific each own shares for the purpose of investment, and therefore, if either the Frost Group or Boston Scientific sells those shares in the market in the future, it could have significant influences on our share price, depending on the market environment at the time of such sale.
There may be adverse U.S. tax consequences for investors in the United States if we are or become a "passive foreign investment company" under the U.S. Internal Revenue Code.
Potential investors that are U.S. Holders (as defined under the heading "Certain Income Tax Considerations — Certain U.S. Federal Income Tax Considerations") should be aware that although we do not currently anticipate that we will be treated as a "passive foreign investment company" ("PFIC") in the current taxable year or in the foreseeable future, the determination as to whether we are a PFIC for any taxable year is based on the application of complex U.S. federal income tax rules, which are subject to differing interpretations, and is not determinable until after the end of such taxable year. Further, the determination is based in part on the mix, use and value of our assets, which values may be treated as changing for U.S. federal income tax purposes as our market capitalization changes. Because of the above described uncertainties, there can be no assurance that the U.S. Internal Revenue Service ("IRS") will not challenge the determination made by us concerning our PFIC status or that we will not be a PFIC for any taxable year. U.S. Holders should read "Certain
S-27
Income Tax Considerations — Certain U.S. Federal Income Tax Considerations" for more information, and consult their own tax advisors regarding the application of the PFIC rules to their investments in the Units.
Risks relating to the Unit Shares, Warrants and Warrant Shares.
Cashless exercise and adjustment provisions in the Series A Warrants, Series B Warrants, Series D Warrants, Series E Warrants, Series F Warrants and Notes may make it more difficult and expensive for us to raise additional capital in the future and may result in further dilution to investors in this offering of Units.
The Series A Warrants, Series B Warrants, Series D Warrants, Series E Warrants and Series F Warrants include, among other things, provisions relating to future-priced conversion or exercise formulae and full-ratchet anti-dilution provisions and may be exercised on a "net" or "cashless" basis if there is no effective registration statement covering the issuance of the Warrant Shares. Under such circumstances, Holders of such Warrants may, in lieu of making a cash payment when exercising a Series A Warrant, Series B Warrant, Series D Warrant, Series E Warrant or Series F Warrant elect instead to receive the "net" number of Series A Warrant Shares, Series B Warrant Shares, Series D Warrant Shares, Series E Warrant Shares or Series F Warrant Shares determined in accordance with a formula referred to in the respective Warrant as the "Alternate Cashless Exercise" and pursuant to other terms and conditions. See "Description of Securities Being Distributed — Warrants". If we are unable to raise additional capital at an effective price per Common Share that is higher than the exercise price of these Series A Warrants, Series B Warrants, Series D Warrants, Series E Warrants or Series F Warrants or the conversion price of the Notes, the anti-dilution provisions contained in these securities may make it more difficult and more expensive to raise capital in the future. Any reduction in the exercise prices of our Series A Warrants, Series B Warrants, Series D Warrants, Series E Warrants and Series F Warrants or the conversion price of the Notes, or any increase in the number of Series A, Series B, Series D, Series E and Series F Warrant Shares or Common Shares issuable upon the exercise of the Series A, Series B, Series D, Series E and Series F Warrants or the conversion of the Notes, or the exercise of the Class C Warrants, may also result in additional dilution in the per share net tangible book value of our Common Shares, including any Unit Shares you purchase in this Offering.
Our Common Share price has experienced volatility and may be subject to fluctuation in the future based on market conditions.
The market prices for the securities of medical companies, including our own, have historically been highly volatile. The market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of any particular company. In addition, because of the nature of our business, certain factors such as our announcements and the public's reaction, our operating performance and the performance of competitors and other similar companies, government regulations, changes in earnings estimates or recommendations by research analysts who track our securities or securities of other companies in the medical sector, general market conditions, announcements relating to litigation, the arrival or departure of key personnel and the factors listed under the heading "Cautionary Note Regarding Forward-Looking Statements" and "Risk Factors" can have an adverse impact on the market price of the Common Shares. For example, from January 1, 2016 to November 9, 2017, the closing price of the Common Shares on the TSX has ranged from a low of C$0.51 to a high of C$6.86 and from January 1, 2016 to November 9, 2017 the closing price of the Common Shares on the Nasdaq has ranged from a low of US$0.39 to a high of US$4.67.
Any negative change in the public's perception of our prospects could cause the price of our securities, including the price of the Unit Shares or the Warrant Shares, to decrease dramatically. Furthermore, any selling pressure caused by the Offering or the Concurrent Private Placement, the conversion of the Notes or the exercise of the Warrants, adjustments to the exercise prices of the Warrants or the conversion price of the Notes as a result of anti-dilution or future-priced conversion or exercise provisions or otherwise, or negative change in the public's perception of the prospects of medical companies in general, could depress the price of our securities, including the price of the Unit Shares or the Warrant Shares, regardless of our results. See the risk factors entitled "The sale of Common Shares issued upon exercise of the Warrants or conversion of the Notes could encourage short sales by third parties which could further depress the price of the Common Shares" and "The Warrants and Notes may result in significant dilution to our shareholders". Following declines in the market price of
S-28
a company's securities, securities class-action litigation is often instituted. Litigation of this type, if instituted, could result in substantial costs and a diversion of our management's attention and resources.
The Series C Warrants contain provisions that restrict the Company's ability to enter into Fundamental Transactions.
The Series C Warrants contain provisions that restrict the Company's ability to enter into a transaction whereby (i) the Company or any of its subsidiaries, (1) consolidate or merge with any other person, (2) sell, lease, license, assign, transfer, convey or otherwise dispose of all or substantially all of its respective properties or assets to any other person, (3) allow any other person to make a purchase, tender or exchange offer that is accepted by the holders of more than 50% of the outstanding Common Shares of the Company, (4) consummate share purchase agreement or other business combination with any other person whereby such other person acquires more than 50% of the outstanding Common Shares of the Company, (5) reorganize, recapitalize or reclassify the Common Shares of the Company, (ii) any "person" or "group" is or shall become the "beneficial owner" of 50% of the aggregate ordinary voting power represented by issued and outstanding Common Shares of the Company, or (iii) any transaction or series of related transactions which, directly or indirectly, could result in the issuance of Common Shares of the Company or convertible securities (each a "Fundamental Transaction"), unless (i) the successor entity assumes in writing all of the obligations of the Company under the Series C Warrant and other transaction documents, including entering into agreements to deliver to the holder in exchange for the Series C Warrant a security of the successor entity evidenced by a written instrument substantially similar in form and substance to the Series C Warrant; and (ii) the successor entity is a publicly traded corporation listed on The New York Stock Exchange, the NYSE American, the Nasdaq Global Select Market, the Nasdaq Global Market, the OTCQB or the Nasdaq (the "Eligible Markets"). These provisions may impact the Company's ability to effect a transaction that it believes is in the best interest of the stakeholders, including a transaction with a foreign acquirer that is not listed on an Eligible Market.
You may be unable to enforce actions against us, certain of our directors and officers or the experts named in this prospectus supplement under U.S. federal securities laws.
We are a company continued under the federal laws of Canada. Most of our directors and officers as well as the experts named in this prospectus supplement and the accompanying base shelf prospectus, reside principally in Canada. Because all or a substantial portion of our assets and the assets of these persons are located outside of the United States, it may not be possible for you to effect service of process within the United States upon us or those persons. Furthermore, it may not be possible for you to enforce against us or those persons in the United States, judgments obtained in U.S. courts based upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States. There is doubt as to the enforceability, in original actions in Canadian courts, of liabilities based upon U.S. federal securities laws and as to the enforceability in Canadian courts of judgments of U.S. courts obtained in actions based upon the civil liability provisions of the U.S. federal securities laws. Therefore, it may not be possible to enforce those actions against us, certain of our directors and officers or the experts named in this prospectus supplement.
Sales of a significant number of Common Shares in the public markets, or the perception of such sales, could depress the market price of the Unit Shares and the Warrant Shares.
Sales of a substantial number of Unit Shares and Warrant Shares or other equity-related securities in the public markets by the Company or its shareholders could depress the market price of the Unit Shares and Warrant Shares and impair our ability to raise capital through the sale of additional equity securities. We cannot predict the effect that future sales of Unit Shares and Warrant Shares or other equity-related securities would have on the market price of the Unit Shares and Warrant Shares. The price of the Common Shares could be affected by possible sales of Unit Shares and Warrant Shares or by hedging or arbitrage trading activity which we expect to occur involving the Unit Shares and Warrant Shares.
S-29
The sale of Common Shares issued upon exercise of the Warrants or conversion of the Notes could encourage short sales by third parties which could further depress the price of the Common Shares.
Any downward pressure on the price of Common Shares caused by the sale of the Warrant Shares issued upon the exercise of the Warrants or the Common Shares issued upon conversion of the Notes could encourage short sales by third parties. In a short sale, a prospective seller borrows Common Shares from a shareholder or broker and sells the borrowed Common Shares. The prospective seller hopes that the Common Share price will decline, at which time the seller can purchase Common Shares at a lower price for delivery back to the lender. The seller profits when the Common Share price declines because it is purchasing Common Shares at a price lower than the sale price of the borrowed Common Shares. Such sales could place downward pressure on the price of our Common Shares by increasing the number of Common Shares being sold, which could further contribute to any decline in the market price of our Common Shares.
The Warrants will not be listed on any exchange.
The Warrants will not be listed on any exchange and the Company does not intend to list the Warrants on any exchange in the future. Investors may be unable to sell the Warrants at the prices desired or at all. There is no existing trading market for the Warrants and there can be no assurance that a liquid market will develop or be maintained for the Warrants, or that an investor will be able to sell any of the Warrants at a particular time (if at all). The liquidity of the trading market in the Warrants and the sale price, if any, for the Warrants, may be adversely affected by, among other things:
- •
- changes in the overall market for the Warrants;
- •
- changes in the Company's financial performance or prospects;
- •
- changes or perceived changes in the Company's creditworthiness;
- •
- the prospects for companies in the medical device industry generally;
- •
- the number of holders of the Warrants; and
- •
- the interest of securities dealers in making a market for the Warrants.
Holders of our Warrants will have no rights as a shareholder until such holders exercise their Warrants and acquire our Common Shares.
Until holders of Warrants acquire Warrant Shares upon exercise of the Warrants, holders of Warrants will have no rights with respect to the Warrant Shares underlying such Warrants. Upon exercise of the Warrants, the holders thereof will be entitled to exercise the rights of a shareholder only as to matters for which the record date occurs after the exercise date.
The Series A, Series B, Series C and Series F Warrants may not have any value.
The Series A Warrants will have an exercise price of US$1.61 per Series A Warrant Share, subject to adjustment. They will be exercisable immediately and will expire five years after their date of issuance. The Series B Warrants will have an exercise price of US$1.61 per Series B Warrant Share, subject to adjustment. They will be exercisable two months after the Closing Date and will expire two years after their date of issuance. The Series C Warrants will have an exercise price of $1.46 per Series C Unit, subject to adjustment. They will be exercisable immediately and will expire two years after the date of issuance. The Series F Warrants will have an exercise price of US$1.61 per Series F Warrant Share, subject to adjustment. They will be exercisable two months after their date of issuance and will expire five years after their date of issuance. In the event our Common Share price does not exceed the exercise prices of either the Series A, Series B and Series F Warrants during the period when they are exercisable, the Series A, Series B and Series F Warrants may not have any value. As a result, the Series C Warrant may also not have any value, given that it is composed of one Series C Unit Share, one Series A Warrant and one Series B Warrant.
S-30
We will have broad discretion in the use of the net proceeds of the Offering and may not use them to effectively manage our business.
We will have broad discretion over the use of the net proceeds from the Offering. Because of the number and variability of factors that will determine our use of such proceeds, our ultimate use might vary substantially from our planned use. You may not agree with how we allocate or spend the proceeds from the Offering. We may pursue acquisitions, collaborations or other opportunities that do not result in an increase in the market value of our securities, including the market value of the Unit Shares, Warrants or Warrant Shares, and that may increase our losses.
We do not intend to pay dividends in the foreseeable future.
We have never declared or paid any dividends on the Common Shares. We intend, for the foreseeable future, to retain our future earnings, if any, to finance our further development and the expansion of our business. The payment of future dividends, if any, will be reviewed periodically by our board of directors and will depend upon, among other things, conditions then existing factors, including earnings, financial conditions, cash on hand, financial requirements to fund our research and development activities, development and growth, and other factors that our board of directors may consider appropriate in the circumstances.
Significant holders or beneficial holders of our Common Shares may not be permitted to exercise the Warrants that they hold.
The terms of the Warrants prohibit a holder from exercising its Warrants if doing so would result in such holder (together with such holder's affiliates) beneficially owning more than 9.99% of the number of Common Share outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Warrants. As a result, you may not be able to exercise your Warrants for Common Shares at a time when it would be financially beneficial for you to do so. In such circumstance you could seek to sell your Warrants to realize value, but you may be unable to do so.
The Company estimates that its net proceeds from the Offering and the Concurrent Private Placement will be approximately US$59.90 million, after deducting the Underwriting Commission of US$2,249,250, placement agent fees in the amount of US$1,670,250 payable in connection with the Concurrent Private Placement and our expenses of the Offering and the Concurrent Private Placement, which are estimated to be US$1,500,000.
The Company intends to use the net proceeds from the Offering and the Concurrent Private Placement to fund clinical trial costs, product development and sustaining engineering for the Tiara and the Reducer and for general corporate purposes, including funding cash flows from operations. The Company has negative operating cash flow and it is expected that a portion of the proceeds from the Offering and the Concurrent Private Placement will be used to fund operating cash flow.
Principal Purpose
|
Estimated Amount to be Expended (US$000s) |
|||
|---|---|---|---|---|
Payment of the balance of the damages and interest award to CardiAQ (after subtracting the US$70 million that Neovasc has paid into escrow) |
42,206 | |||
Clinical trial costs and product development and sustaining engineering for the Tiara |
11,500 | |||
General corporate purposes(1) |
6,199 | |||
Total(2) |
59,905 | |||
Notes:
- (1)
- Funds included in general corporate purposes include approximately US$4,250,000 for construction of a clean room facility and may also be allocated to corporate expenses, business development, potential future acquisitions, litigation defence costs, severance and reorganization expenses and to other purposes.
S-31
- (2)
- After deduction of the Underwriting Commission, placement agent fees payable in connection with the Concurrent Private Placement and the estimated expenses of the Offering and Concurrent Private Placement. Total net proceeds assumes receipt of an aggregate of US$35,738 upon the exercise of all of the Series D Warrants sold in the Offering.
The net proceeds of the Offering and the Concurrent Private Placement will be used primarily to fund the approximately US$42 million balance of the awards granted in the U.S. Court of Appeals litigation with CardiAQ (after subtracting the US$70 million that Neovasc has paid into escrow) as described under the caption "The Company — Recent Developments", with remaining funds being used to partially fund the Tiara program and in particular to support completion of the TIARA-II study and the Reducer program, in particular funding the REDUCE-I Registry and COSIRA-II trial, each as described below. The TIARA-II study is a 115 patient, non-randomized, prospective clinical study intended to provide the clinical data required to support obtaining CE Mark approval for the Tiara which would enable Neovasc to market the device in Europe. The key business objective of this activity is to enable sales of the product into the European marketplace. The TIARA-II study is estimated to cost approximately US$18-20 million. The exact timing for completion of enrollment in the study is unknown at this time and is dependent on a number of factors including screening rates and local regulatory approvals. Neovasc is targeting to complete enrollment and receive CE Mark approval and begin Tiara sales in Europe in approximately 2019. However, due to the inherent uncertainty around gaining regulatory approval to market an implantable heart valve product, this timeline is subject to extension. Neovasc is managing and conducting the TIARA-II trial itself in conjunction with certain service providers who undertake certain portions of data collection, data management, data analysis, safety and event monitoring and similar functions. The Tiara is currently manufactured for use in these trials by Neovasc at its own facilities following required medical device quality requirements. In the event of a positive outcome from the TIARA-II trial enabling Neovasc to obtain CE Mark approval, the Tiara would be commercially manufactured in the same manner at Neovasc's facility.
The Reducer has received CE Mark approval and is currently commercially available in Europe. It is not currently approved for sale in the United States. Proceeds of the Offering and the Concurrent Private Placement allocated to this program will be primarily targeted to support the ongoing REDUCE-I Registry which is collecting post-market data from European centers on the device. Proceeds will also go towards initial work to plan initiation of an FDA investigational device exemption trial (COSIRA-II) intended to support FDA approval to begin marketing the product in the United States. Neovasc recently obtained approval for the COSIRA-II trial, and is presently evaluating options for initiating this trial. The Company does not presently have the funds available to conduct the COSIRA-II trial, which is expected to cost approximately US$20-25 million, so it is not possible to accurately forecast when the trial will be completed or a detailed budget. The Company will be responsible for collecting data for the REDUCE-I Registry and conducting the COSIRA-II trial, other than certain required activities that are subcontracted out. The key business goals of these activities will be to (1) provide post-market data to support commercial efforts to sell the Reducer in Europe; and (2) obtain approval to begin selling the Reducer in the United States. Following the COSIRA-II trial, the FDA will need to review and approve the Reducer, the cost and timing of which is unknown at this time.
The development of the Tiara and Reducer will require additional capital exceeding the Company's cash on hand resources even after giving effect to the Offering and the Concurrent Private Placement. In addition, actual costs and development time may exceed management's current expectations. It is unlikely that the Company will generate sufficient operating cash flow to meet its total capital obligations in the proposed development time frame. Accordingly, the Company will need to raise additional capital in the future over and above the current Offering and the Concurrent Private Placement.
In addition to the foregoing, we are engaged in other litigation resulting from claims made by CardiAQ, which has been, and is expected to continue to be, costly. A majority of the net proceeds from the Offering and Concurrent Private Placement will be used to satisfy Neovasc's significant monetary damages, which will require us to raise additional capital for future operations. See "Risk Factors".
While the Company intends to spend the net proceeds of the Offering and the Concurrent Private Placement as stated above, there may be circumstances where, for sound business reasons, a re-allocation of funds may be necessary or advisable. The actual amount that the Company spends in connection with each of the intended uses of proceeds may vary significantly from the amounts specified above, and will depend on a number of factors, including those listed under the heading "Risk Factors" in this prospectus supplement and the accompanying base shelf prospectus and the documents incorporated by reference herein and therein.
S-32
The following table sets forth information in respect of the Common Shares that we issued upon the exercise of options granted under our incentive stock option plan during the twelve month period preceding the date of this prospectus supplement.
Exercise Date
|
Number of Common Shares | Exercise Price | |||||
|---|---|---|---|---|---|---|---|
February 9, 2017 |
16,000 | C$ | 1.45 | ||||
April 17, 2017 |
39,100 | C$ | 1.45 | ||||
April 19, 2017 |
32,000 | C$ | 1.45 | ||||
April 20, 2017 |
2,000 | C$ | 1.45 | ||||
April 21, 2017 |
800 | C$ | 1.45 | ||||
April 24, 2017 |
77,000 | C$ | 1.45 | ||||
April 25, 2017 |
22,300 | C$ | 1.45 | ||||
April 26, 2017 |
20,800 | C$ | 1.45 | ||||
April 27, 2017 |
2,000 | C$ | 1.45 | ||||
April 28, 2017 |
2,000 | C$ | 1.45 | ||||
July 31, 2017 |
13,343 | C$ | 0.01 | ||||
September 19, 2017 |
10,000 | C$ | 1.30 | ||||
Total |
237,343 | ||||||
The following table sets forth information in respect of options to acquire Common Shares that we granted under our incentive stock option plan during the twelve month period preceding the date of this prospectus supplement.
Grant Date
|
Number of Options | Grant Price | |||||
|---|---|---|---|---|---|---|---|
March 31, 2017 |
1,744,500 | C$ | 1.90 | ||||
June 28, 2017 |
100,000 | C$ | 1.83 | ||||
Total |
1,844,500 | ||||||
The following table sets forth information in respect of Common Shares that we issued, other than on exercise of stock options as set out above, during the twelve month period preceding the date of this prospectus supplement.
Issuance Date
|
Number of Common Shares | Issue Price | |||||
|---|---|---|---|---|---|---|---|
December 13, 2016 |
11,817,000 | US$ | 0.60 | ||||
Total |
11,817,000 | ||||||
No other Common Shares, preferred shares, debt securities or warrants, or securities exchangeable or convertible into Common Shares, preferred shares, debt securities or warrants have been issued during the twelve month period preceding the date of this prospectus supplement.
S-33
Our Common Shares are listed under the trading symbol NVCN on the TSX in Canada and on the Nasdaq in the United States. Our Common Shares began trading on the TSX on June 23, 2014. The following table sets forth, for the periods indicated, the reported high, low and closing prices (in Canadian dollars) and volume traded on the TSX.
Month
|
High | Low | Close | Volume | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
November 2016 |
1.10 | 0.61 | 0.70 | 3,680,595 | |||||||||
December 2016 |
4.44 | 0.67 | 2.32 | 27,358,444 | |||||||||
January 2017 |
2.82 | 1.48 | 1.48 | 3,296,051 | |||||||||
February 2017 |
1.90 | 1.40 | 1.79 | 1,652,292 | |||||||||
March 2017 |
2.82 | 1.74 | 2.05 | 2,568,297 | |||||||||
April 2017 |
2.48 | 2.00 | 2.11 | 946,063 | |||||||||
May 2017 |
2.39 | 1.87 | 1.95 | 615,021 | |||||||||
June 2017 |
2.3 | 1.63 | 1.80 | 454,579 | |||||||||
July 2017 |
1.88 | 1.19 | 1.34 | 481,018 | |||||||||
August 2017 |
1.50 | 0.87 | 0.98 | 481,018 | |||||||||
September 2017 |
2.32 | 0.91 | 2.11 | 1,858,258 | |||||||||
October 2017 |
2.17 | 1.82 | 1.89 | 445,000 | |||||||||
November 1 – 9, 2017 |
1.99 | 1.68 | 1.86 | 371,000 | |||||||||
Our Common Shares began trading on the Nasdaq on May 21, 2014. The following table sets forth, for the periods indicated, the reported high, low and closing prices (in U.S. dollars) and volume traded on the Nasdaq.
Month
|
High | Low | Close | Volume | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
November 2016 |
1.10 | 0.61 | 0.70 | 3,680,595 | |||||||||
December 2016 |
4.44 | 0.67 | 2.32 | 27,358,444 | |||||||||
January 2017 |
2.82 | 1.48 | 1.48 | 3,296,051 | |||||||||
February 2017 |
1.90 | 1.40 | 1.79 | 1,652,292 | |||||||||
March 2017 |
2.82 | 1.74 | 2.05 | 2,568,297 | |||||||||
April 2017 |
1.85 | 1.49 | 1.55 | 12,854,296 | |||||||||
May 2017 |
1.74 | 1.35 | 1.45 | 5,855,270 | |||||||||
June 2017 |
1.71 | 1.17 | 1.38 | 3,874,425 | |||||||||
July 2017 |
1.43 | 0.96 | 1.10 | 3,159,485 | |||||||||
August 2017 |
1.19 | 0.70 | 0.79 | 3,628,884 | |||||||||
September 2017 |
1.89 | 0.72 | 1.70 | 15,026,709 | |||||||||
October 2017 |
1.74 | 1.31 | 1.47 | 2,743,795 | |||||||||
November 1 – 9, 2017 |
1.54 | 1.31 | 1.46 | 2,222,600 | |||||||||
DESCRIPTION OF SECURITIES BEING DISTRIBUTED UNDER THE OFFERING
The Offering consists of 6,609,588 Series A Units and 19,066,780 Series B Units at a price of US$1.46 per Unit. Each Series A Unit is comprised of one Unit Share, one Series A Warrant to purchase one Series A Warrant Share, one Series B Warrant to purchase one Series B Warrant Share and 0.40 Series C Warrants, with each whole Series C Warrant exercisable to purchase one Series C Unit, comprised of one Series C Unit Share, one Series A Warrant and one Series B Warrant. Each Series B Unit is comprised of either one Unit Share or one pre-funded Series D Warrant to purchase one Series D Warrant Share, one Series A Warrant to purchase one Series A Warrant Share, one Series B Warrant to purchase one Series B Warrant Share, 0.40 Series C Warrants, with each whole Series C Warrant exercisable to purchase one Series C Unit, comprised of one Series C Unit Share, one Series A Warrant and one Series B Warrant, and 1.1765 Series F Warrants, with each whole Series F Warrant exercisable to purchase one Series F Warrant Share.
Our authorized share capital consists of an unlimited number of Common Shares and an unlimited number of preferred shares. As of the date of this prospectus supplement, we had 78,920,688 Common Shares and no
S-34
preferred shares of any series issued and outstanding. In addition, as of the date of this prospectus supplement, there were 9,227,366 Common Shares issuable upon the exercise of outstanding stock options at a weighted average exercise price of US$3.55 and no outstanding Common Share purchase warrants.
Common Shares
All of our Common Shares are of the same class and, once issued, rank equally as to entitlement to dividends, voting powers (one vote per Common Share) and participation in assets upon dissolution, liquidation or winding-up. No Common Shares have been issued subject to call or assessment. Our Common Shares contain no pre-emptive or conversion or exchange rights and have no provisions for redemption, retraction or purchase for cancellation or surrender or sinking or purchase funds. In addition, there are no provisions restricting the issuance of additional Common Shares or requiring a holder of Common Shares to contribute additional capital. Provisions as to the modification, amendment or variation of such rights or provisions are contained in our articles and by-laws and in the CBCA.
Warrants
The material terms and provisions of the Warrants being offered under this prospectus supplement and the accompanying base shelf prospectus are summarized below. Certain capitalized terms used in this section titled "Description of the Securities Being Distributed under the Offering" are defined in the form of Series A Warrant, the form of Series B Warrant, the form of Series C Warrant, the form of Series D Warrant and the form of Series F Warrant. The following summary is subject to, and is qualified in its entirety by reference to, the form of Series A Warrant, the form of Series B Warrant, the form of Series C Warrant, the form of Series D Warrant and the form of Series F Warrant, each of which will be issued under the Offering and will be filed on SEDAR under our profile at www.sedar.com and furnished to the SEC as an exhibit to a Report on Form 6-K following closing of the Offering. You should review a copy of the form of Series A Warrant, the form of Series B Warrant, the form of Series C Warrant, the form of Series D Warrant and the form of Series F Warrant for a complete description of the terms and conditions applicable to the Warrants.
There will be no market through which the Warrants may be sold and purchasers may not be able to resell the Warrants purchased in the Offering. This may affect the pricing of the Warrants in the secondary market, the transparency and availability of trading prices and the liquidity of the Warrants. See "Risk Factors". Except by virtue of such holder's ownership of our Common Shares, the holder of a Warrant does not have the rights or privileges of a holder of our Common Shares, including any voting rights, until the holder exercises the Warrant.
Series A Warrants
The Series A Warrants are exercisable beginning on the date of issuance, and at any time prior to 11:59 p.m. (New York time) on the date that is five years after the date of issuance. The Series A Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice, thereby canceling all or a portion of the Series A Warrant. No fractional Warrant Shares will be issued in connection with the exercise of a Series A Warrant. Any entitlement to Series A Warrant Shares shall be rounded to the nearest whole Series A Warrant Share. The holder will not have the right to exercise any portion of the Series A Warrant if the holder (together with its affiliates and any other persons acting as a group together with the holder or any of the holder's affiliates) would beneficially own in excess of 9.99% of the number of our Common Shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Series A Warrants.
Each Series A Warrant represents the right to purchase one Series A Warrant Share at a notional exercise price equal to US$1.61 per Series A Warrant Share (the "Series A Exercise Price"), subject to adjustment. The notional exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Shares. The Series A Warrants are subject to full ratchet anti-dilution provisions in certain circumstances. Pursuant to these provisions, if the Company issues or sells, or is deemed to have issued or sold by issuance of options or convertible securities in accordance with the Series A Warrant, any Common Shares for a consideration per share (the "New Issuance Price") less than the Series A Exercise Price, then immediately after such dilutive
S-35
issuance, the Series A Exercise Price then in effect shall be reduced to the New Issuance Price. If the Company issues options or convertible securities, the holder of a Series A Warrant may elect to replace the Series A Exercise Price with the variable price of such option or convertible security in accordance with the terms of the Series A Warrant. If there is a stock split, stock dividend, stock combination or similar transaction and the market price at the time of the event is lower than the Series A Exercise Price, the Series A Exercise Price will be adjusted accordingly. Simultaneously with any adjustment to the Series A Exercise Price as described above, the number of Series A Warrant Shares that may be purchased upon exercise of the Series A Warrants shall be increased or decreased proportionately so that after such adjustment the Series A Exercise Price payable under the adjusted number of Series A Warrant Shares is equal to the Series A Exercise Price in effect immediately prior to the Series A Exercise Price adjustment.
If there is any share split, share dividend, share combination, recapitalization or other similar transaction involving the Common Shares (each, a "Share Combination Event") at any time after the issuance of a Series A Warrant and the Event Market Price (as defined in the Series A Warrant) is less than the Series A Exercise Price then, on the sixteenth trading day following such Share Combination Event, the Series A Exercise Price then in effect will be reduced to the Event Market Price.
If, at the time a holder exercises its Series A Warrant, there is no effective registration statement covering the issuance of the shares underlying the Series A Warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of Common Shares determined according to a formula set forth in the Series A Warrant.
The Series A Warrant holders are entitled to participate in any dividends or other distributions by the Company and the sale, by the Company, of any options, convertible securities or rights to purchase stock, warrants, securities or other property pro rata to the shareholders of the Common Shares as if they had exercised their Series A Warrant and were holders of the Series A Warrant Shares.
In the event of a Fundamental Transaction (as described under the risk factor entitled "The Series C Warrants contain provisions that restrict the Company's ability to enter into Fundamental Transactions") and at the request of the holder of the Series A Warrant, the Company must purchase the Series A Warrant from such holder on the date of such request by paying to the holder cash in an amount equal to the value of the unexercised Series A Warrants according to the Black Scholes Option Pricing Model. In the event of a default and if the Series A Warrant holder currently holds Notes, the Company must, at the Series A Warrant holder's request, purchase the Series A Warrant from the holder in cash according to the Black Scholes Option Pricing Model.
Subject to applicable laws, the Series A Warrants may be offered for sale, sold, transferred or assigned without our consent.
Series B Warrants
The Series B Warrants are exercisable beginning on the date of issuance, and at any time prior to 11:59 p.m. (New York time) on the date that is two years after the date of issuance. The Series B Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice, thereby canceling all or a portion of the Series B Warrant. No fractional Warrant Shares will be issued in connection with the exercise of a Series B Warrant. Any entitlement to Series B Warrant Shares shall be rounded to the nearest whole Series B Warrant Share. The holder will not have the right to exercise any portion of the Series B Warrant if the holder (together with its affiliates and any other persons acting as a group together with the holder or any of the holder's affiliates) would beneficially own in excess of 9.99% of the number of our Common Shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Series B Warrants.
Each Series B Warrant represents the right to purchase one Series B Warrant Share at a notional exercise price equal to US$1.61 per Series B Warrant Share (the "Series B Exercise Price"), subject to adjustment. The notional exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Shares.
S-36
The Series B Warrants are subject to full ratchet anti-dilution provisions in certain circumstances. Pursuant to these provisions, if the Company issues or sells, or is deemed to have issued or sold by issuance of options or convertible securities in accordance with the Series B Warrant, any Common Shares for the New Issuance Price less than the Series B Exercise Price, then immediately after such dilutive issuance, the Series B Exercise Price then in effect shall be reduced to the New Issuance Price. If the Company issues options or convertible securities, the holder of a Series B Warrant may elect to replace the Series B Exercise Price with the variable price of such option or convertible security in accordance with the terms of the Series B Warrant. If there is a stock split, stock dividend, stock combination or similar transaction and the market price at the time of the event is lower than the Series B Exercise Price, the Series B Exercise Price will be adjusted accordingly. Simultaneously with any adjustment to the Series B Exercise Price as described above, the number of Series B Warrant Shares that may be purchased upon exercise of the Series B Warrants shall be increased or decreased proportionately so that after such adjustment the Series B Exercise Price payable under the adjusted number of Series B Warrant Shares is equal to the Series B Exercise Price in affect immediately prior to the Series B Exercise Price adjustment.
If there is a Share Combination Event at any time after the issuance of a Series B Warrant and the Event Market Price (as defined in the Series B Warrant) is less than the Series B Exercise Price then, on the sixteenth trading day following such Share Combination Event, the Series B Exercise Price then in effect will be reduced to the Event Market Price.
If, at the time a holder exercises its Series B Warrant, there is no effective registration statement covering the issuance of the shares underlying the Series B Warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of Common Shares determined according to a formula set forth in the Series B Warrant.
The Series B Warrant holders are entitled to participate in any dividends or other distributions by the Company and the sale, by the Company, of any options, convertible securities or rights to purchase stock, warrants, securities or other property pro rata to the shareholders of the Common Shares as if they had exercised their Series B Warrant and were holders of the Series B Warrant Shares.
In the event of a Fundamental Transaction and at the request of the holder of the Series B Warrant, the Company must purchase the Series B Warrant from such holder on the date of such request by paying to the holder cash in an amount equal to the value of the unexercised Series B Warrants according to the Black Scholes Option Pricing Model. In the event of a default (as defined in the Notes) and if the Series B Warrant holder currently holds Notes, the Company must, at the Series B Warrant holder's request, purchase the Series B Warrant from the holder in cash according to the Black Scholes Option Pricing Model.
Subject to applicable laws, the Series B Warrants may be offered for sale, sold, transferred or assigned without our consent.
At any time two months after the issuance of the Series B Warrants and prior to their expiration, the holder of the Series B Warrant may, in its sole discretion, exercise the Series B Warrant in whole or in part and, in lieu of making any cash payment otherwise contemplated to be made to the Company upon such exercise in payment of the exercise price, elect instead to receive upon such exercise a number of Series B Warrant Shares equal to the Alternate Net Number. The "Alternate Net Number" is equal to the product of (i) the quotient obtained by dividing (x) the total number of Series B Warrant Shares with respect to which the Series B Warrant is being exercised and (y) the maximum number of Series B Warrant Shares (as adjusted for share splits, share dividends, share combinations, recapitalizations or other similar events) initially issuable upon a cash exercise of the Series B Warrant on the date of issuance and (ii) the quotient obtained by dividing (A) the difference obtained by subtracting (x) the the lowest daily volume weighted average price during the ten trading days period ending on and including such exercise date (the "Market Price") from (y) the exercise price as of the subscription date (as adjusted for share splits, share dividends, share combinations, recapitalizations or other similar events) by (B) 85% of the Market Price.
The effect of the Alternate Net Number mechanism is that the number of Series B Warrant Shares issuable increases as the Market Price falls. As an example, if the Market Price at the time of exercise is 95% of the
S-37
Series B Exercise Price, as of the subscription date, then, if the holders exercise all of the Series B Warrants for the Alternate Net Number a total of 1.590 million Series B Warrant Shares will be issued. Further, if the Market Price at the time of exercise is 90% of the Series B Exercise Price, as of the subscription date, then, if the holders exercise all of the Series B Warrants for the Alternate Net Number a total of 3.356 million Series B Warrant Shares will be issued.
Series C Warrants
The Series C Warrants are exercisable beginning on the date of issuance, and at any time prior to 11:59 p.m. (New York time) on the date that is two years after the date of issuance. Each Series C Warrant may be exercised for a Series C Unit, with each Series C Unit being comprised of a Series C Warrant Share, a Series A Warrant and a Series B Warrant. The Series C Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice, thereby canceling all or a portion of the Warrant. No fractional Series C Unit Shares will be issued in connection with the exercise of a Series C Warrant. Any entitlement to Series C Unit Shares be rounded to the nearest whole Series C Unit Share. The holder will not have the right to exercise any portion of the Series C Warrant if the holder (together with its affiliates and any other persons acting as a group together with the holder or any of the holder's affiliates) would beneficially own in excess of 9.99% of the number of our Common Shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Series C Warrants.
Each Series C Warrant represents the right to purchase one Series C Unit at a notional exercise price equal to US$1.46 per Series C Unit, subject to adjustment. The notional exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Shares. In addition, any Series A Warrant or Series B Warrant issued will be adjusted for any events which occur between the Closing Date and the exercise of the Series C Warrants so that any newly issued Series A Warrant and Series B Warrant will be adjusted to reflect such event as if such Series A Warrant and Series B Warrant were issued on the date of such event.
The Company is restricted from entering into or being party to a Fundamental Transaction (as described under the risk factor entitled "The Series C Warrants contain provisions that restrict the Company's ability to enter into Fundamental Transactions") unless (i) the successor entity assumes in writing all of the obligations of the Company under the Series C Warrant and other transaction documents, including entering into agreements to deliver to the holder in exchange for the Series C Warrant a security of the successor entity evidenced by a written instrument substantially similar in form and substance to the Series C Warrant; and (ii) the successor entity is a publicly traded corporation listed on an Eligible Market. In addition, prior to a Fundamental Transaction pursuant to which holders of Common Shares are entitled to receive securities or other assets with respect to or in exchange for Common Shares, the Company shall make appropriate provision to ensure that the holder of a Series C Warrant will have the right to receive, upon an exercise of the Series C Warrants any time after the consummation of the Fundamental Transaction and prior to the expiration of their Series C Warrants, and in lieu of the Series C Warrant Shares, such securities, cash or any other property which the holder would have been entitled to receive under the Fundamental Transaction had the Series C Warrant been exercised immediately prior to the Fundamental Transaction.
Subject to applicable laws, the Series C Warrants may be offered for sale, sold, transferred or assigned without our consent.
The effect of the Alternate Net Number mechanism in the Series B Warrants underlying the Series C Units is that the number of Series B Warrant Shares issuable increases as the Market Price falls. As an example, if the Market Price at the time of exercise is 95% of the Series B Exercise Price, as of the subscription date, then, if the holders exercise all of the Series B Warrants underlying the Series C Units for the Alternate Net Number a total of 0.636 million Series B Warrant Shares will be issued. Further, if the Market Price at the time of exercise is 90% of the Series B Exercise Price, as of the subscription date, then, if the holders exercise all of the Series B Warrants underlying the Series C Units for the Alternate Net Number a total of 1.343 million Series B Warrant Shares will be issued.
S-38
Series D Warrants
We are also offering to those purchasers, whose purchase of Units in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than the initial beneficial ownership limitation following the consummation of this offering, the opportunity to purchase, in lieu of Common Shares forming part of the Units that would result in ownership in excess of the initial beneficial ownership limitation, one Series D Warrant to purchase one Series D Warrant Share. The pre-funded Series D Warrants will be exercisable immediately and at any time prior to 11:59 p.m. (New York time) on the date that is five years following the date of issuance. The exercise price will be pre-funded except for a nominal exercise price of $0.01 per Series D Warrant. The Series D Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice, thereby canceling all or a portion of the Series D Warrant. No fractional Warrant Shares will be issued in connection with the exercise of a Series D Warrant. Any entitlement to Series D Warrant Shares shall be rounded to the next whole Series D Warrant Share. The holder will not have the right to exercise any portion of the Series D Warrant if the holder (together with its affiliates and any other persons acting as a group together with the holder or any of the holder's affiliates) would beneficially own in excess of 9.99% of the number of our Common Shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Series D Warrants.
Each Series D Warrant represents the right to purchase one Series D Warrant Share at a notional exercise price equal to US$1.46 per Series D Warrant Share (the "Series D Exercise Price"), subject to adjustment, all of which will be pre-funded except for a nominal exercise price of US$0.01 per Series D Warrant. The notional exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Shares.
If, at the time a holder exercises its Series D Warrant, there is no effective registration statement covering the issuance of the shares underlying the Series D Warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of common shares determined according to a formula set forth in the Series D Warrant.
The Series D Warrant holders are entitled to participate in any dividends or other distributions by the Company and the sale, by the Company, of any options, convertible securities or rights to purchase stock, warrants, securities or other property pro rata to the shareholders of the Common Shares as if they had exercised their Series B Warrant and were holders of the Series D Warrant Shares.
Subject to applicable laws, the Series D Warrants may be offered for sale, sold, transferred or assigned without our consent.
Series F Warrants
The Series F Warrants are exercisable beginning on the date of issuance, and at any time prior to 11:59 p.m. (New York time) on the date that is two years after the date of issuance. The Series F Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice, thereby canceling all or a portion of the Series F Warrant. No fractional Warrant Shares will be issued in connection with the exercise of a Series F Warrant. Any entitlement to Series F Warrant Shares shall be rounded to the nearest whole Series F Warrant Share. The holder will not have the right to exercise any portion of the Series F Warrant if the holder (together with its affiliates and any other persons acting as a group together with the holder or any of the holder's affiliates) would beneficially own in excess of 9.99% of the number of our Common Shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Series F Warrants.
Each Series F Warrant represents the right to purchase one Series F Warrant Share at a notional exercise price equal to US$1.61 per Series F Warrant Share (the "Series F Exercise Price"), subject to adjustment. The notional exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Shares. The Series F Warrants are subject to full ratchet anti-dilution provisions in certain circumstances. Pursuant to
S-39
these provisions, if the Company issues or sells, or is deemed to have issued or sold by issuance of options or convertible securities in accordance with the Series F Warrant, any Common Shares for the New Issuance Price less than the Series F Exercise Price, then immediately after such dilutive issuance, the Series F Exercise Price then in effect shall be reduced to the New Issuance Price. If the Company issues options or convertible securities, the holder of a Series F Warrant may elect to replace the Series F Exercise Price with the variable price of such option or convertible security in accordance with the terms of the Series F Warrant. If there is a stock split, stock dividend, stock combination or similar transaction and the market price at the time of the event is lower than the Series F Exercise Price, the Series F Exercise Price will be adjusted accordingly. Simultaneously with any adjustment to the Series F Exercise Price as described above, the number of Series F Warrant Shares that may be purchased upon exercise of the Series F Warrants shall be increased or decreased proportionately so that after such adjustment the Series F Exercise Price payable under the adjusted number of Series F Warrant Shares is equal to the Series F Exercise Price in affect immediately prior to the Series F Exercise Price adjustment.
If there is a Share Combination Event at any time after the issuance of a Series F Warrant and the Event Market Price (as defined in the Series F Warrant) is less than the Series F Exercise Price then, on the sixteenth trading day following such Share Combination Event, the Series A Exercise Price then in effect will be reduced to the Event Market Price.
If, at the time a holder exercises its Series F Warrant, there is no effective registration statement covering the issuance of the shares underlying the Series F Warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of Common Shares determined according to a formula set forth in the Series F Warrant.
The Series F Warrant holders are entitled to participate in any dividends or other distributions by the Company and the sale, by the Company, of any options, convertible securities or rights to purchase stock, warrants, securities or other property pro rata to the shareholders of the Common Shares as if they had exercised their Series F Warrant and were holders of the Series F Warrant Shares.
In the event of a Fundamental Transaction (as described under the risk factor entitled "The Series C Warrants contain provisions that restrict the Company's ability to enter into Fundamental Transactions") and at the request of the holder of the Series F Warrant, the Company must purchase the Series F Warrant from such holder on the date of such request by paying to the holder cash in an amount equal to the value of the unexercised Series F Warrants according to the Black Scholes Option Pricing Model. In the event of a default (as defined in the Notes) and if the Series F Warrant holder currently holds Notes, the Company must, at the Series F Warrant holder's request, purchase the Series F Warrant from the holder in cash according to the Black Scholes Option Pricing Model.
Subject to applicable laws, the Series F Warrants may be offered for sale, sold, transferred or assigned without our consent.
At any time two months after the issuance of the Series F Warrants and prior to their expiration, the holder of the Series F Warrant may, in its sole discretion, exercise the Series F Warrant in whole or in part and, in lieu of making any cash payment otherwise contemplated to be made to the Company upon such exercise in payment of the exercise price, elect instead to receive upon such exercise a number of Series F Warrant Shares equal to the Alternate Net Number. The "Alternate Net Number" is equal to the product of (i) the quotient obtained by dividing (x) the total number of Series F Warrant Shares with respect to which the Series F Warrant is being exercised and (y) the maximum number of Series F Warrant Shares (as adjusted for share splits, share dividends, share combinations, recapitalizations or other similar events) initially issuable upon a cash exercise of the Series F Warrant on the date of issuance and (ii) the quotient obtained by dividing (A) the difference obtained by subtracting (x) the the lowest daily volume weighted average price during the ten trading days period ending on and including such exercise date (the "Market Price") from (y) the exercise price as of the subscription date (as adjusted for share splits, share dividends, share combinations, recapitalizations or other similar events) by (B) 85% of the Market Price.
S-40
The effect of the Alternate Net Number mechanism is that the number of Series F Warrant Shares issuable increases as the Market Price falls. As an example, if the Market Price at the time of exercise is 95% of the Series F Exercise Price, as of the subscription date, then, if the holders exercise all of the Series F Warrants for the Alternate Net Number a total of 1.389 million Series F Warrant Shares will be issued. Further, if the Market Price at the time of exercise is 90% of the Series F Exercise Price, as of the subscription date, then, if the holders exercise all of the Series F Warrants for the Alternate Net Number a total of 2.932 million Series F Warrant Shares will be issued.
The effect of the Alternate Net Number mechanisms described above is that the maximum number of Common Shares issuable by the Company pursuant to the Offering is based on the future market price of the Common Shares. For illustration purposes, taking into account the total number of Warrant Shares issuable under the Offering and assuming full conversion of the Notes and exercise of the Warrants and Series E Warrants into Common Shares, if the Market Price of the Common Shares remained at US$1.46 per Common Share (being the closing price of the Common Shares on November 9, 2017) on the date of exercise, the maximum number of Common Shares issuable pursuant to the Transaction would be 175.1 million, representing approximately 222% of Neovasc's current issued and outstanding number of Common Shares. If the Market Price of the Common Shares on the date of exercise reduces to a point lower than approximately 60% of the closing price of the Common Shares on November 9, 2017, the Alternate Net Number mechanism would result in an increase in the number of shares issuable and that number materially increases more as the price reduces.
Dividend Policy
We have not paid any dividends to date on our Common Shares. We do not currently expect to pay any dividends on our Common Shares for the foreseeable future.
Preferred Shares
We may issue our preferred shares from time to time in one or more series. The terms of each series of preferred shares, including the number of shares, the designation, rights, preferences, privileges, priorities, restrictions, conditions and limitations, will be determined at the time of creation of each such series by our board of directors, without shareholder approval, provided that all preferred shares will rank equally within their class as to dividends and distributions in the event of our dissolution, liquidation or winding-up.
DESCRIPTION OF SECURITIES BEING DISTRIBUTED UNDER THE
CONCURRENT PRIVATE PLACEMENT
Senior Secured Convertible Note
The material terms and provisions of the Senior Secured Convertible Notes (the "Notes") being offered under the Concurrent Private Placement are summarized below. Certain capitalized terms used in this section titled "Description of the Securities Being Distributed under the Concurrent Private Placement" are defined in the form of Note. The following summary is subject to, and is qualified in its entirety by reference to, the form of Note, which will be issued under the Concurrent Private Placement and will be filed on SEDAR under our profile at www.sedar.com and furnished to the SEC as an exhibit to a Report on Form 6-K following the closing of the Offering and the Concurrent Private Placement. You should review a copy of the form of Note for a complete description of the terms and conditions applicable to the Notes.
The Notes will be issued as an original issue price of US$850 per US$1,000 principal amount of notes. Interest on the Notes will commence accruing on the date of issuance at the 0% interest rate, computed on the basis of a 360-day year and twelve 30-day months and payable in cash on January 1, 2018 and on the first day of each calendar quarter thereafter up to, and including the date of maturity on which the principal of the Notes is repayable. Upon an event of default, the interest rate shall automatically be increased to fifteen percent per annum. The Notes will be senior to all other indebtedness and secured by all assets.
At any time after the issuance, any portion of the outstanding and unpaid amount remaining under the Note will be convertible into Common Shares (the "Note Conversion Shares"). The conversion rate will be the number of Common Shares issuable upon conversion of any conversion amount determined by dividing (x) the
S-41
sum of the portion of the principal to be converted, redeemed or otherwise with respect to which this determination is being made and all accrued and unpaid Interest with respect to such portion of the principal amount and accrued and unpaid late charges with respect to such portion of such principal and any such interest by (y) 1.46 (the "Note Conversion Price"), subject to certain adjustments.
The Notes contain a future-priced conversion mechanism upon the earlier of (x) the later of (i) upon the occurrence of an event of default, the twentieth day following the cure of such event of default and (ii) the twentieth day following the holder of the Note having received notice of such event of default and (y) the fourth month anniversary of the closing of the sale of the Notes. The Notes are subject to full ratchet anti-dilution provisions in certain circumstances. Pursuant to these provisions, if the Company issues or sells any Common Shares for a consideration per share (the "Note Issuance Price") less than the Note Conversion Price then immediately after such dilutive issuance, the Note Conversion Price then in effect shall be reduced to the Note Issuance Price. If the Company issues options or convertible securities, the holder of a Note may elect to replace the Note Conversion Price with the variable price of such option or convertible security in accordance with the terms of the Note. If there is a stock split, stock dividend, stock combination or similar transaction and the market price of the Common Shares at the time of the event is lower than the Note Conversion Price, then on the sixteenth trading date following such event, the Note Conversion Price will be reduced to the Event Market Price (as defined in the form of Note). Simultaneously with any adjustment to the Note Conversion Price as described above, the Note Conversion Shares issuable upon conversion of the Notes shall be increased or decreased proportionately so that after such adjustment the Note Conversion Price payable upon such conversion is equal to the Note Conversion Price in effect immediately prior to the Note Conversion Price.
With effect from and after 5:00 p.m. New York City time on the date (the "Conversion Price Reset Date") nine months immediately following the Issuance Date, the Conversion Price (as defined in the Notes) will be adjusted to be the lower of (x) the then-current Conversion Price and (y) the greater of (i) the amount in USD equal to the VWAP (as defined in the Notes) for the Common Stock on the Conversion Price Reset Date (or, if the Conversion Price Reset Date is not a Trading Day (as defined in the Notes), the immediately following Trading Day) and (ii) US$0.50.
The terms of the Notes prohibit a holder from converting its Notes if doing so would result in such holder (together with such holder's affiliates) beneficially owning more than 9.99% of the number of Common Share outstanding immediately after giving effect to the conversion, as such percentage ownership is determined in accordance with the terms of the Notes.
The Company shall not issue any fraction of a share issued upon any conversion. If the issuance would result in the issuance of a fraction of a share of Common Shares, the Company shall round such fraction of a share of Common Shares up to the nearest whole share.
Upon a change of control of the Company, the portion of the Note subject to redemption shall be redeemed by the Company in cash at the premium price equal to the Change of Control Redemption Price (which is 125%).
The Note holders are entitled to participate in any dividends or other distributions by the Company and the sale, by the Company, of any options, convertible securities or rights to purchase stock, warrants, securities or other property pro rata to the holders of Common Shares as if they had converted their Notes and were holders of the Note Conversion Shares.
The Company shall not enter into or be party to a Fundamental Transaction (as described under the risk factor entitled "The Series C Warrants contain provisions that restrict the Company's ability to enter into Fundamental Transactions") unless the Successor Entity assumes in writing all of the obligations of the Company under the Note and it delivers to each holder of Notes in exchange for such Notes a security of the successor entity evidenced by a written instrument substantially similar in form and substance to the Notes.
Under the Notes, an event of default triggers a redemption right with a redemption premium regardless of whether the event of default is cured. Under the Notes, a change of control triggers a redemption right with a redemption premium.
S-42
The Notes contain certain covenants, which include: restricted payments upon an event of default, restrictions on distributions, and restrictions on asset transfers (other than ordinary course of business).
This Note and any Common Shares issued upon conversion of this Note may be offered, sold, assigned or transferred by the holder without the consent of the Company.
General Security Agreement
The Note is secured by a general security agreement to be dated as of November 17, 2017 granted by the Company to and in the favour of Bio IP Ventures II LLC, as collateral agent for the benefit of the Noteholders over all of our present and after-acquired personal property, which includes all of our assets in the U.S., Canada and Israel related to Tiara and Reducer.
Series E Warrants
The Series E Warrants are exercisable beginning on the date of issuance, and at any time prior to 11:59 p.m. (New York time) on the date that is five years after the date of issuance. The Series E Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice, thereby canceling all or a portion of the Series E Warrant. No fractional Warrant Shares will be issued in connection with the exercise of a Series E Warrant. Any entitlement to Series E Warrant Shares shall be rounded to the nearest whole Series E Warrant Share. The holder will not have the right to exercise any portion of the Series E Warrant if the holder (together with its affiliates and any other persons acting as a group together with the holder or any of the holder's affiliates) would beneficially own in excess of 9.99% of the number of our Common Shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Series E Warrants.
Each Series E Warrant represents the right to purchase one Series E Warrant Share at a notional exercise price equal to US$1.61 per Series E Warrant Share (the "Series E Exercise Price"), subject to adjustment. The notional exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Shares. The Series E Warrants are subject to full ratchet anti-dilution provisions in certain circumstances. Pursuant to these provisions, if the Company issues or sells, or is deemed to have issued or sold by issuance of options or convertible securities in accordance with the Series E Warrant, any Common Shares for a consideration per share (the "New Issuance Price") less than the Series E Exercise Price, then immediately after such dilutive issuance, the Series E Exercise Price then in effect shall be reduced to the New Issuance Price. If the Company issues options or convertible securities, the holder of a Series E Warrant may elect to replace the Series E Exercise Price with the variable price of such option or convertible security in accordance with the terms of the Series E Warrant. If there is a stock split, stock dividend, stock combination or similar transaction and the market price at the time of the event is lower than the Series E Exercise Price, the Series E Exercise Price will be adjusted accordingly. Simultaneously with any adjustment to the Series E Exercise Price as described above, the number of Series E Warrant Shares that may be purchased upon exercise of the Series E Warrants shall be increased or decreased proportionately so that after such adjustment the Series E Exercise Price payable under the adjusted number of Series E Warrant Shares is equal to the Series E Exercise Price in effect immediately prior to the Series E Exercise Price adjustment.
If there is a Share Combination Event at any time after the issuance of a Series E Warrant and the Event Market Price (as defined in the Series E Warrant) is less than the Series E Exercise Price then, on the sixteenth trading day following such Share Combination Event, the Series E Exercise Price then in effect will be reduced to the Event Market Price.
If, at the time a holder exercises its Series E Warrant, there is no effective registration statement covering the issuance of the shares underlying the Series E Warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of Common Shares determined according to a formula set forth in the Series E Warrant.
S-43
The Series E Warrant holders are entitled to participate in any dividends or other distributions by the Company and the sale, by the Company, of any options, convertible securities or rights to purchase stock, warrants, securities or other property pro rata to the shareholders of the Common Shares as if they had exercised their Series E Warrant and were holders of the Series E Warrant Shares.
In the event of a Fundamental Transaction (as described under the risk factor entitled "The Series C Warrants contain provisions that restrict the Company's ability to enter into Fundamental Transactions") and at the request of the holder of the Series E Warrant, the Company must purchase the Series E Warrant from such holder on the date of such request by paying to the holder cash in an amount equal to the value of the unexercised Series E Warrants according to the Black Scholes Option Pricing Model. In the event of a default (as defined in the Notes) and if the Series E Warrant holder currently holds Notes, the Company must, at the Series E Warrant holder's request, purchase the Series E Warrant from the holder in cash according to the Black Scholes Option Pricing Model.
Subject to applicable laws, the Series E Warrants may be offered for sale, sold, transferred or assigned without our consent.
Since June 30, 2017, the date of our financial statements for the most recently completed financial period, there have been no material changes in our consolidated share and loan capital other than as outlined under "Prior Sales". For information on the issuance of Common Shares pursuant to the exercise of options pursuant to our incentive stock option plan, see "Prior Sales".
The following table sets forth our cash and cash equivalents, cash held in escrow, long-term financial liabilities and capitalization as of June 30, 2017 on an actual basis and as adjusted to give effect to the Offering and the Concurrent Private Placement as though it had occurred on such date. This table should be read in conjunction with the Company's audited annual financial statements for the fiscal years ended December 31, 2016 and 2015.
| |
As at June 30, 2017 (in thousands of US$ except Common Shares) |
As at June 30, 2017 after giving effect to the Offering and the Concurrent Private Placement (in thousands of US$ except Common Shares)(2) |
As at June 30, 2017 after giving effect to the Offering and the Concurrent Private Placement and settlement of the damages and interest award to CardiAQ (in thousands of US$ except Common Shares)(2) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Cash and cash equivalents |
$ | 11,581 | $ | 71,486 | (1) | $ | 29,280 | (1) | ||
Cash held in escrow |
$ | 70,190 | $ | 70,190 | $ | nil | ||||
Long-term financial liabilities |
$ | 112,207 | $ | 112,207 | $ | nil | ||||
Outstanding Common Shares (unlimited authorized) |
78,910,688 | 101,013,226 | 101,013,226 | |||||||
Notes:
- (1)
- After
deduction of the Underwriting Commission, placement agent fees payable in connection with the Concurrent Private Placement and the estimated expenses
of the Offering and Concurrent Private Placement. Total net proceeds assumes receipt of an aggregate of US$35,738 upon the exercise of all of the Series D Warrants sold in the Offering.
- (2)
- Figures, including estimated expenses of the Offering, converted to U.S. dollars using the Bank of Canada daily average rate of exchange on November 9, 2017 of C$1.2692 per US$1.00. See "Exchange Rate Information".
S-44
The Offering
The Company is offering the Units through the Underwriter pursuant to the Underwriting Agreement. Pursuant to the Underwriting Agreement, the Company has agreed to sell and the Underwriter has agreed to purchase on November 17, 2017, the Units at a price of US$37,487,497, less the US$2,249,250 underwriting commission, payable in cash to the Company against delivery. The obligations of the Underwriter under the Underwriting Agreement may be terminated at its discretion on the basis of any domestic or international event or act or occurrence that has materially disrupted, or in the opinion of the Underwriter will in the immediate future materially disrupt, the market for the Company's securities or securities in general and may also be terminated upon the occurrence of certain stated events. The Underwriter is, however, obligated to take up and pay for all of the Units if any of the Units are purchased under the Underwriting Agreement.
The Offering is being made only in the United States pursuant to the multijurisdictional disclosure system implemented by the SEC and the securities regulatory authorities in Canada. The Units will be offered in the United States by the Underwriter either directly or through its duly registered U.S. broker dealer affiliates or agents. The Units are offered subject to a number of conditions, including:
- •
- receipt and acceptance of the Units by the Underwriter;
- •
- approval of legal matters by the Underwriter's counsel, including the validity of the Unit Shares, Warrants and
Warrant Shares;
- •
- the Underwriter's right to withdraw, cancel or modify offers to the public and to reject orders in whole or in
part; and
- •
- other conditions contained in the Underwriting Agreement, such as the receipt by the Underwriter of officers' certificates and legal opinions.
The Company has not granted to the Underwriter any over-allotment option.
The Company has applied to the TSX for an exemption from the requirement to obtain shareholder approval in connection with the Offering and Concurrent Private Placement on the basis of financial hardship, given that the immediacy of our need to address our financial difficulties would not afford us sufficient time to hold a meeting of our shareholders. The approval of the financial hardship exemption from the TSX forms the basis for the Company's request to be exempt from the Nasdaq's similar shareholder approval requirement as a foreign private issuer. Listing of the Unit Shares and the Warrant Shares on the TSX and the Nasdaq will be subject to the Company fulfilling all the requirements of the TSX and the Nasdaq.
The offering price of the Units was determined by arm's length negotiation between the Company and the Underwriter. The Units will not be certificated and the Unit Shares, and Warrants will be issued separately but will be purchased together in the Offering. See "Description of Securities Being Distributed under the Offering".
The Company has applied to list the Unit Shares and the Warrant Shares on the TSX. Listing on the TSX will be subject to the Company fulfilling all of the listing requirements of the TSX. The Company has submitted a notification of listing to list the Unit Shares and the Warrant Shares on the Nasdaq.
Commissions
Units sold by the Underwriter to the public will initially be offered at the offering price set forth on the cover of this prospectus supplement. If all of the Units are not sold at the public offering price, the Underwriter may change the offering price and the other selling terms. Upon execution of the Underwriting Agreement, the Underwriter will be obligated to purchase the Units at the prices and upon the terms stated therein and, as a result, will thereafter bear any risk associated with changing the offering price to the public or other selling terms.
S-45
The following table shows the per Unit and total Underwriting Commission the Company will pay to the Underwriter:
Per Unit |
US$0.0876 | |||
Total |
US$2,249,250 |
We estimate that the total expenses of the Offering payable by us, not including the Underwriting Commission, will be approximately US$1,500,000. We have agreed to reimburse the Underwriter for certain of its fees and expenses, including the expenses of counsel to the Underwriter, in an amount not to exceed US$350,000 in the aggregate.
Covenants
We and our officers and directors and certain of our shareholders have agreed that, subject to certain exceptions, for a period of 180 days from the date of the Underwriting Agreement, we and they will not, without the prior written consent of the Underwriter, directly or indirectly, issue, offer, pledge, sell, agree to issue, offer pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lead or otherwise transfer or dispose of any of our Common Shares or any securities convertible into or exchangeable for our Common Shares, or make any public announcement of any of the foregoing, or otherwise enter into any swap, derivative or other transaction or arrangement that transfers to another, in whole or in part, any economic consequence of ownership of any of our Common Shares or any securities convertible into or exchangeable for our Common Shares. The Frost Group will not be entering into such lock-up agreements on 2,300,000 of its Common Shares of the Company or any securities it purchases pursuant to the Offering.
The Company has agreed in the Underwriting Agreement to indemnify the Underwriter against certain liabilities, including liabilities under the U.S. Securities Act of 1933, as amended, and, where such indemnification is unavailable, to contribute to payments that the Underwriter may be required to make in respect of such liabilities.
In connection with the Offering, the Underwriter may engage in passive market making transactions in our Common Shares on the Nasdaq prior to the pricing and completion of the Offering. Passive market making consists of displaying bids on the Nasdaq no higher than the bid prices of independent market makers and making purchases at prices no higher than these independent bids and effected in response to order flow. Net purchases by a passive market maker on each day are generally limited to a specified percentage of the passive market maker's average daily trading volume in the common stock during a specified period and must be discontinued when such limit is reached. Passive market making may cause the price of our Common Shares to be higher than the price that otherwise would exist in the open market in the absence of these transactions. If passive market making is commenced, it may be discontinued at any time.
In order to facilitate the Offering, the Underwriter may engage in transactions that stabilize, maintain or otherwise affect the market price of our Common Shares in accordance with Regulation M under the Exchange Act.
The Underwriter may over-allot Common Shares in connection with the Offering, thus creating a short position for its own account. Short sales involve the sale by the Underwriter of a greater number of shares than it is committed to purchase in the Offering. To cover these short sales positions or to stabilize the market price of our Common Shares, the Underwriter may bid for, and purchase, Common Shares in the open market. These transactions may be effected on the TSX, the Nasdaq or otherwise. Additionally, the Underwriter may also reclaim selling concessions allowed to a dealer. Similar to other purchase transactions, the Underwriter's purchases to cover the syndicate short sales or to stabilize the market price of our Common Shares may have the effect of raising or maintaining the market price of our Common Shares or preventing or mitigating a decline in the market price of our Common Shares. As a result, the price of our Common Shares may be higher than the price that might otherwise exist in the open market. No representation is made as to the magnitude or effect of any such stabilization or other activities. The Underwriter is not required to engage in these activities and, if commenced, may discontinue any of these activities at any time.
S-46
Pursuant to rules and policy statements of certain Canadian securities regulators, the Underwriter may not, at any time during the period ending on the date the selling process for the Units ends and all stabilization arrangements relating to the Units are terminated, bid for or purchase Common Shares. The foregoing restrictions are subject to certain exceptions including (a) a bid for or purchase of Common Shares if the bid or purchase is made through the facilities of the TSX, in accordance with the Universal Market Integrity Rules of Market Regulation Services Inc., (b) a bid or purchase on behalf of a client, other than certain prescribed clients, provided that the client's order was not solicited by the Underwriter, or if the client's order was solicited, the solicitation occurred before the commencement of a prescribed restricted period, and (c) a bid or purchase to cover a short position entered into prior to the commencement of a prescribed restricted period. The Underwriter may engage in market stabilization or market balancing activities on the TSX where the bid for or purchase of the Common Shares is for the purpose of maintaining a fair and orderly market in the Common Shares, subject to price limitations applicable to such bids or purchases. Such transactions, if commenced, may be discontinued at any time.
Canaccord Genuity Inc. is acting as placement agent in connection with the Concurrent Private Placement, in which it will receive a placement agent fee in the amount of US$1,670,250.
From time to time, the Underwriter and/or its affiliates may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us for which they would expect to receive customary fees and commissions.
In addition, in the ordinary course of its business activities, the Underwriter and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The Underwriter and its affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Copies of this prospectus supplement and the accompanying base shelf prospectus in electronic format may be made available on the websites maintained by the Underwriter. In addition, Common Shares may be sold by the Underwriter to securities dealers who resell shares to online brokerage account holders.
Neovasc expects to deliver the Units on or about the fifth business day following the time of sale ("T + 5"), as agreed to by Neovasc and the Underwriter. Pursuant to Rule 15c6-1(a) under the Exchange Act, trades in the secondary market generally are required to settle in two business days, unless the parties to any such trade expressly agree otherwise. Accordingly, investors who wish to trade the Unit Shares or the Warrants prior to the delivery date may be required to specify an alternate settlement cycle at the time of trade to prevent a failed settlement. Investors who wish to trade the Unit Shares or the Warrant Shares prior to the delivery date should consult their own advisors.
CERTAIN INCOME TAX CONSIDERATIONS
Certain U.S. Federal Income Tax Considerations
The following is a summary of certain U.S. federal income tax considerations generally applicable to a "U.S. Holder" of the ownership and disposition of the Unit Shares, Warrant Shares and Warrants acquired pursuant to this Offering. This summary addresses only holders who acquire and hold the Units Shares, Warrant Shares and Warrants as capital assets (generally, property held for investment purposes). This summary does not address all potentially relevant U.S. federal income tax matters, and unless otherwise specifically provided, it does not address any state, local, foreign, alternative minimum, unearned income "Medicare" contribution, estate or gift tax consequences of holding or disposing of the Unit Shares, Warrant Shares and Warrants acquired pursuant to this Offering.
As used herein, the term "U.S. Holder" means any beneficial owner of Units Shares, Warrant Shares or Warrants, who, for U.S. federal income tax purposes, is: (i) a citizen or individual resident of the United States; (ii) a corporation (or other entity classified as a corporation for U.S. federal tax purposes) organized under the laws of the United States or of any state thereof or the District of Columbia, (iii) an estate whose income is
S-47
subject to U.S. federal income taxation regardless of its source, and (iv) a trust (A) if a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust, or (B) that has elected to be treated as a U.S. person under applicable U.S. Treasury Regulations, and, in each case, (a) who is a resident of the United States for purposes of the Canada-United States Income Tax Convention (1980), as amended (the "Canada-U.S. Tax Convention") or, (b) whose Unit Shares, Warrant Shares or Warrants would not, for purposes of the Canada-U.S. Tax Convention, be attributable to a permanent establishment in Canada and (c) who otherwise would qualify for the full benefits of the Canada-U.S. Tax Convention.
If a partnership (or other entity or arrangement treated as a partnership for U.S. federal tax purposes) holds our Unit Shares, Warrant Shares or Warrants, the tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. Partnerships (or other entities or arrangements classified as a partnership for U.S. federal tax purposes) holding our Unit Shares, Warrant Shares or Warrants, and their partners and other owners, should consult their own tax advisors to determine the U.S. federal, state, local and other tax consequences that may be relevant to them.
This summary is based on the Canada-U.S. Tax Convention, the U.S. Internal Revenue Code of 1986, as amended (the "Code"), administrative pronouncements and rulings of the U.S. Internal Revenue Service (the "IRS"), judicial decisions and existing and proposed U.S. Treasury Regulations, changes to any of which subsequent to the date of this prospectus supplement may affect the tax consequences described herein, possibly on a retroactive basis. This summary is for general guidance only and does not address the consequences applicable to certain categories of shareholders subject to special treatment under the Code, including tax-exempt organizations, pass-through entities, certain financial institutions, insurance companies, qualified retirement plans, individual retirement accounts or other tax-deferred accounts, persons that hold our Unit Shares, Warrant Shares or Warrants as part of a straddle, hedging transaction, conversion transaction, constructive sale or other arrangement involving more than one position, persons that acquired our Unit Shares, Warrant Shares or Warrants in connection with the exercise of employee stock options or otherwise as compensation for services, dealers in securities or foreign currencies, traders in securities that elect to use a mark-to-market method of accounting, U.S. persons whose functional currency (as defined in the Code) is not the U.S. dollar, former citizens or permanent residents of the United States, or persons that own directly, indirectly or by application of the constructive ownership rules of the Code 10% or more of our shares by voting power or by value. Persons considering the purchase of the Units offered hereunder should consult their own tax advisors with regard to the application of the income tax laws of the United States and any other taxing jurisdiction to their particular circumstances.
Prospective investors should consult their own tax advisors with respect to the income tax considerations relevant to them, having regard to their particular circumstances.
Treatment of Units
Each Series A Unit is comprised of one Unit Share, one Series A Warrant, one Series B Warrant and 0.40 Series C Warrants, each of which will be treated as a separate instrument for U.S. federal income tax purposes. The amount paid for a Series A Unit must be allocated between the Unit Share, the Series A Warrant, the Series B Warrant, and the Series C Warrant based on their respective fair market values at the time of the Offering, and the initial tax basis of each will equal the amount so allocated. For this purpose, the Company intends to allocate approximately US$1.46 of the issue price of each Series A Unit as consideration for the issue of each Unit Share, US$nil of the issue price as consideration for the issue of the Series A Warrant, US$nil of the issue price as consideration for the issue of the Series B Warrant, and US$nil of the issue price as consideration for the issue of the Series C Warrant.
Each Series B Unit is comprised of one Unit Share or one pre-funded Series D Warrant, one Series A Warrant, one Series B Warrant, 0.40 Series C Warrants and 1.1765 Series F Warrants, each of which will be treated as a separate instrument for U.S. federal income tax purposes. The amount paid for a Series B Unit must be allocated between the Unit Share or Series D Warrant, the Series A Warrant, the Series B Warrant, the Series C Warrant and the Series F Warrant based on their respective fair market values at the time of the Offering, and the initial tax basis of each will equal the amount so allocated. For this purpose, the Company intends to allocate approximately US$nil of the issue price of each Series B Unit as consideration for the issue of
S-48
each Unit Share or Series D Warrant, US$nil of the issue price as consideration for the issue of the Series A Warrant, US$nil of the issue price as consideration for the issue of the Series B Warrant, US$nil of the issue price as consideration for the issue of the Series C Warrant and US$nil of the issue price as consideration for the issue of the Series F Warrant.
Although the Company believes that its allocation is reasonable, the IRS is not bound by the Company's allocation of the purchase price for the Units, and the IRS or a U.S. court may disagree with the allocation set forth herein. Each U.S. Holder should consult its own tax advisor regarding the allocation of the purchase price for the Units purchased in the Offering. The holding period for the Unit Share and Warrants will begin on the day after the date of acquisition.
Treatment of the Series D Warrants
The U.S. federal income tax characterization of pre-funded warrants is uncertain. Although the Series D Warrants are issued in the form of warrants, because the Series D Warrants are pre-funded except for a nominal exercise price of $0.01 per Series D Warrant Share, holders of the Series D Warrants may be treated for U.S. federal income tax purposes as holding the underlying Series D Warrant Shares. Because of this uncertainty, this summary does not address the U.S. federal income tax consequences of the ownership or disposition of Series D Warrants or Series D Warrant Shares. Prospective purchasers of Series B Units containing Series D Warrants should consult their own tax advisors regarding the U.S. federal income tax consequences applicable to the ownership and disposition of the Series D Warrants and Series D Warrant Shares, including the effect of the passive foreign investment company ("PFIC") rules.
The Shares
Distributions
Subject to the PFIC rules discussed below, a U.S. Holder will generally recognize, to the extent out of our current and accumulated earnings and profits (determined in accordance with U.S. federal income tax principles), dividend income on the receipt of distributions on our Unit Shares, Series A, Series B and Series F Warrant Shares and Series C Unit Shares (collectively, the "Shares") (including amounts withheld to pay Canadian withholding taxes). We do not intend to calculate our earnings and profits under U.S. federal income tax rules. Accordingly, U.S. Holders should expect that a distribution will generally be treated as a dividend for U.S. federal income tax purposes.
The amount of any dividend paid to a U.S. Holder in Canadian dollars (including amounts withheld to pay Canadian withholding taxes) will be includible in income in a U.S. dollar value amount by reference to the exchange rate between the U.S. dollar and the Canadian dollar in effect on the date of receipt of such dividend by the U.S. Holder, regardless of whether the Canadian dollars so received are in fact converted into U.S. dollars. A U.S. Holder will have a tax basis in the Canadian dollars equal to their U.S. dollar value on the date of receipt. If the Canadian dollars received are converted into U.S. dollars on the date of receipt, the U.S. Holder should generally not be required to recognize foreign currency gain or loss in respect of the dividend. If the Canadian dollars received are not converted into U.S. dollars on the date of receipt, a U.S. Holder may recognize foreign currency gain or loss on a subsequent conversion or other disposition of the Canadian dollars. Such gain or loss will generally be treated as U.S. source ordinary income or loss.
We believe that we are a "qualified foreign corporation" and therefore, distributions treated as dividends and received by certain non-corporate U.S. Holders will be taxed at preferential rates, provided applicable holding period and certain other requirements are satisfied. Any amount of such distributions treated as dividends will generally not be eligible for the "dividends received" deduction ordinarily available to certain U.S. corporate shareholders.
Distributions on our Shares that are treated as dividends will generally constitute income from sources outside the United States and will generally be categorized for U.S. foreign tax credit purposes as "passive category income." A U.S. Holder may be eligible to elect to claim a U.S. foreign tax credit against its U.S. federal income tax liability, subject to applicable limitations and holding period requirements, for Canadian tax withheld, if any, from distributions received in respect of the Shares. A U.S. Holder that does not elect to claim a U.S. foreign tax credit may instead claim a deduction for Canadian tax withheld, but only for a taxable
S-49
year in which the U.S. Holder elects to do so with respect to all non-U.S. income taxes paid or accrued in such taxable year. The rules relating to U.S. foreign tax credits are complex, and each U.S. Holder should consult its own tax adviser regarding the application of such rules.
Sale, Exchange or Other Taxable Disposition
Subject to the PFIC rules discussed below, upon a sale, exchange or other taxable disposition of a Share, a U.S. Holder will generally recognize a capital gain or loss equal to the difference between the amount realized on such sale, exchange or other taxable disposition (or, if the amount realized is denominated in Canadian dollars, its U.S. dollar equivalent, generally, for U.S. Holders that use the cash method and for electing U.S. Holders that use accrual method, determined by reference to the spot rate of exchange on the date of settlement) and the holder's tax basis of such Share. Such gain or loss will be a long-term capital gain or loss if the Share has been held for more than one year and will be short-term capital gain or loss if the holding period is equal to or less than one year. Such gain or loss will generally be considered U.S. source gain or loss for U.S. foreign tax credit purposes. Long-term capital gains of non-corporate taxpayers are eligible for reduced rates of taxation. The deductibility of capital losses is subject to limitations.
The Series A, Series B, Series C and Series F Warrants
Exercise of the Series A, Series B and Series F Warrants
A U.S. Holder may exercise Series A, Series B and Series F Warrants pursuant to the terms and provisions described in the section titled "Description of the Securities Being Distributed — Warrants." A U.S. Holder will generally not recognize gain or loss on the exercise of a Series A Warrant, a Series B Warrant, a Series F Warrant and the related receipt of a Series A Warrant Share, a Series B Warrant Share or a Series F Warrant Share. A U.S. Holder's initial tax basis in the Series A Warrant Share, the Series B Warrant Share or the Series F Warrant Share received on the exercise of a Series A Warrant, a Series B Warrant or a Series F Warrant should generally be equal to the sum of (a) the U.S. Holder's tax basis in such Series A Warrant, Series B Warrant or Series F Warrant plus (b) the exercise price paid by the U.S. Holder. A U.S. Holder's holding period for the Series A Warrant Share, the Series B Warrant Share or the Series F Warrant Share received on the exercise of a Series A Warrant, a Series B Warrant or a Series F Warrant should generally begin on the day after the date that the Series A Warrant, the Series B Warrant or the Series F Warrant is exercised.
In certain circumstances, a U.S. Holder may be permitted to undertake a cashless exercise of Series A Warrants, Series B Warrants and Series F Warrants into Series A, Series B and Series F Warrant Shares, respectively. The U.S. federal income tax treatment of a cashless exercise of Series A, Series B and Series F Warrants is unclear, and the tax consequences of a cashless exercise could differ from the consequences upon the exercise of a Series A, Series B and Series F Warrant described in the preceding paragraph. U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of Series A, Series B and Series F Warrants.
Exercise of the Series C Warrants
A U.S. Holder may exercise Series C Warrants pursuant to the terms and provisions described in the section titled "Description of the Securities Being Distributed — Warrants." A U.S. Holder will generally not recognize gain or loss on the exercise of a Series C Warrant and the related receipt of a Series C Unit comprised of a Series C Unit Share, a Series A Warrant and a Series B Warrant. A U.S. Holder's initial tax basis in the Series C Unit Share, Series A Warrant and the Series B Warrant received on the exercise of a Series C Warrant should generally be equal to the sum of (a) the U.S. Holder's tax basis in such Series C Warrant plus (b) the exercise price paid by the U.S. Holder, allocated to each of the Series C Unit Share, Series A Warrant and the Series B Warrant based on their respective fair market values at the time of exercise. A U.S. Holder's holding period for the Series C Unit Share received on the exercise of a Series C Warrant should generally begin on the day after the date that the Series C Warrant is exercised.
Sale, Exchange or Other Taxable Disposition
A U.S. Holder generally will recognize capital gain or loss on the sale, exchange or other taxable disposition of a Series A Warrant, a Series B Warrant, a Series C Warrant or a Series F Warrant in an amount equal to the
S-50
difference between the amount realized on such sale, exchange or other taxable disposition (or, if the amount realized is denominated in Canadian dollars, its U.S. dollar equivalent, generally, for U.S. Holders that use the cash method and for electing U.S. Holders that use accrual method, determined by reference to the spot rate of exchange on the date of settlement) and the holder's tax basis of such Series A Warrant, Series B Warrant, Series C Warrant or Series F Warrant. Such gain or loss will be a long-term capital gain or loss if the Series A Warrant, the Series B Warrant, the Series C Warrant or the Series F Warrant has been held for more than one year and will be short-term capital gain or loss if the holding period is equal to or less than one year. Such gain or loss generally will be considered U.S. source gain or loss for U.S. foreign tax credit purposes. Long-term capital gains of non-corporate taxpayers are eligible for reduced rates of taxation. The deductibility of capital losses is subject to limitations.
Lapse
Upon the lapse or expiration of a Series A Warrant, a Series B Warrant, a Series C Warrant or a Series F Warrant, a U.S. Holder will recognize a loss in an amount equal to its adjusted tax basis in the Series A Warrant, the Series B Warrant, the Series C Warrant or the Series F Warrant. Subject to the PFIC rules discussed below, any such loss should be a capital loss. Any capital loss recognized by a U.S. Holder will generally be treated as U.S. source loss for U.S. foreign tax credit purposes. The deductibility of capital losses is subject to limitations.
Certain Adjustments to the Series A, Series B, Series C and Series F Warrants
The number of Shares (or in the case of Series C Warrants, Series C Units) issuable upon exercise of the Series A, Series B, Series C or Series F Warrants and/or the exercise price per Series A, Series B and Series F Warrant Share or Series C Unit may be adjusted in certain circumstances. For U.S. federal income tax purposes, an adjustment to the number of Series A, Series B and Series F Warrant Shares or Series C Units that will be issued on the exercise of the Series A, Series B, Series C or Series F Warrants, or an adjustment to the exercise price of the Series A, Series B, Series C or Series F Warrants, may be treated as a constructive distribution to a U.S. Holder of the Series A, Series B, Series C and Series F Warrants if, and to the extent that, such adjustment has the effect of increasing such U.S. Holder's proportionate interest in the earnings and profits or assets of the Company, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to shareholders of the Company). Subject to the PFIC rules discussed below, any constructive distributions generally will be taxable as a distribution, as described above under "The Shares — Distributions." However, adjustments to the exercise price of the Series A, Series B, Series C and Series F Warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing the dilution of the interest of the holders of Warrants generally will not be considered to result in a constructive distribution to a U.S. Holder of Series A, Series B, Series C and Series F Warrants. U.S. Holders should carefully review the conversion rate adjustment provisions and consult their own tax advisors with respect to the tax consequences of any such adjustment.
Passive Foreign Investment Company Rules
A foreign corporation will be considered a PFIC for any taxable year in which (i) 75% or more of its gross income is "passive income" or (ii) 50% or more of the average quarterly value of its assets produce (or are held for the production of) "passive income." For this purpose, "passive income" generally includes interest, dividends, rents, royalties and certain gains. We currently do not believe that we were a PFIC in the preceding taxable year nor do we anticipate that we will be a PFIC in the current taxable year or in future taxable years. However, the determination as to whether we are a PFIC for any taxable year is based on the application of complex U.S. federal income tax rules, which are subject to differing interpretations, and is not determinable until after the end of such taxable year. Further, the determination is based in part on the mix, use and value of our assets, which values may be treated as changing for U.S. federal income tax purposes as our market capitalization changes. Because of the above described uncertainties, there can be no assurance that the IRS will not challenge the determination made by us concerning our PFIC status or that we will not be a PFIC for any taxable year. If we were classified as a PFIC in any taxable year during which a U.S. Holder owns our Shares or the Series A, Series B, Series C, Series D or Series F Warrants, certain adverse tax consequences could apply to such U.S. Holder. Certain elections may be available to U.S. Holders of Shares, but not Series A, Series B, Series C, Series D or Series F Warrants, that may mitigate some of the adverse consequences resulting from our
S-51
treatment as a PFIC. U.S. Holders should consult their own tax advisors regarding the application of the PFIC rules to their investments in the Units and whether to make an election or protective election.
Required Disclosure with Respect to Foreign Financial Assets
Certain U.S. Holders are required to report information relating to an interest in the Shares or Series A, Series B, Series C, Series D and Series F Warrants, subject to exceptions (including an exception for Shares or Series A, Series B, Series C, Series D and Series F Warrants held in accounts maintained by certain financial institutions), by attaching a completed IRS Form 8938, Statement of Specified Foreign Financial Assets, with their tax return for each year in which they hold an interest in the Shares or Series A, Series B, Series C, Series D or Series F Warrants. U.S. Holders should consult their own tax advisors regarding information reporting requirements relating to their ownership of the Shares or Series A, Series B, Series C, Series D and Series F Warrants.
Currency Conversion
Subject to certain exceptions that are not discussed herein, for purposes of the Income Tax Act (Canada) (the "Tax Act"), all amounts relating to the acquisition, holding or disposition of Units, including dividends on Shares, adjusted cost base and proceeds of dispositions must be determined in Canadian dollars based using the single daily exchange rate of the Bank of Canada on the particular date the particular amount arose or such other rate of exchange as acceptable to the CRA.
Non-Residents of Canada
The following portion of this summary is generally applicable to a Holder who, for purposes of the Tax Act and at all relevant times, is neither resident nor deemed to be resident in Canada and does not use or hold, and will not be deemed to use or hold, Shares or Warrants in a business carried on in Canada (each, a "Non-Resident Holder"). The term "US Holder", for the purposes of this summary, means a Non-Resident Holder who, for purposes of the Canada-U.S. Tax Convention, is at all relevant times a resident of the United States and is a "qualifying person" within the meaning of the Canada-U.S. Tax Convention. In some circumstances, persons deriving amounts through fiscally transparent entities (including limited liability companies) may be entitled to benefits under the Canada-U.S. Tax Convention. US Holders are urged to consult their own tax advisors to determine their entitlement to benefits under the Canada-U.S. Tax Convention based on their particular circumstances.
Special considerations, which are not discussed in this summary, may apply to a Non-Resident Holder that is an insurer that carries on an insurance business in Canada and elsewhere or an authorized foreign bank (as defined in the Tax Act). Such Non-Resident Holders should consult their own advisors.
Taxation of Dividends
Dividends paid or credited, or deemed to be paid or credited, to a Non-Resident Holder on the Shares will be subject to Canadian withholding tax under the Tax Act at the rate of 25% of the gross amount of the dividend. Such rate is generally reduced under the Canada-U.S. Tax Convention to 15% if the beneficial owner of such dividend is a U.S. Holder. The rate of withholding tax is further reduced to 5% if the beneficial owner of such dividend is a U.S. Holder that is a company that owns, directly or indirectly, at least 10% of the voting stock of the Company. In addition, under the Canada-U.S. Tax Convention, dividends may be exempt from such Canadian withholding tax if paid to certain U.S. Holders that are qualifying religious, scientific, literary, educational or charitable tax-exempt organizations or qualifying trusts, companies, organizations or arrangements operated exclusively to administer or provide pension, retirement or employee benefits or benefits for the self-employed under one or more funds or plans established to provide pension or retirement benefits or other employee benefits that are exempt from tax in the United States and that have complied with specific administrative procedures.
Disposition of Shares and Warrants
A Non-Resident Holder will not be subject to tax under the Tax Act in respect of any capital gain realized by such Non-Resident Holder on a disposition of Shares or Warrants, unless the Shares or Warrants constitute
S-52
"taxable Canadian property" (as defined in the Tax Act) of the Non-Resident Holder at the time of the disposition and are not "treaty-protected property" (as defined in the Tax Act) of the Non-Resident Holder at the time of the disposition.
Generally, as long as the Shares are then listed on a designated stock exchange (which currently includes the TSX), the Shares and Warrants will not constitute taxable Canadian property of a Non-Resident Holder, unless at any time during the 60-month period immediately preceding the disposition the following two conditions are met concurrently: (a) the Non-Resident Holder, persons with which the Non-Resident Holder does not deal at arm's length, partnerships whose members include, either directly or indirectly through one or more partnerships, the Non-Resident Holder or persons which do not deal at arm's length with the Non-Resident Holder, or any combination of them, owned 25% or more of the issued shares of any class or series of shares of the capital stock of the Company, and (b) more than 50% of the fair market value of the Shares was derived directly or indirectly, from one or any combination of real or immovable property situated in Canada, "Canadian resource properties", "timber resource properties" (each as defined in the Tax Act) and options in respect of or interests in, or for civil law rights in, any such property (whether or not such property exists).
See "Residents of Canada — Taxation of Capital Gains and Losses" for the consequences that will generally apply to a Non-Resident Holder if the Shares or Warrants are taxable Canadian property of the Non-Resident Holder and are not treaty-protected property of the Non-Resident Holder at the time of their disposition.
Non-Resident Holders whose Shares or Warrants are taxable Canadian property should consult their own advisors.
WHERE YOU CAN FIND MORE INFORMATION
We are required to file with the securities commission or authority in each of the applicable provinces of Canada annual and quarterly reports, material change reports and other information. In addition, we are subject to the informational requirements of the Exchange Act, and, in accordance with the Exchange Act, we also file reports with, and furnish other information to, the SEC. Under a multijurisdictional disclosure system adopted by the United States and Canada, these reports and other information (including financial information) may be prepared in accordance with the disclosure requirements of Canada, which differ in certain respects from those in the United States. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required to publish financial statements as promptly as U.S. companies.
You may read any document we file with the securities commissions and authorities of the provinces of Canada through SEDAR at www.sedar.com and any document we file with, or furnish to, the SEC at the SEC's public reference room at Station Place, 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at l-800-SEC-0330 for further information on the public reference rooms. Certain of our filings are also electronically available on EDGAR, and may be accessed at www.sec.gov.
AUDITORS, TRANSFER AGENT AND REGISTRAR
Grant Thornton LLP was reappointed as our auditor at our annual general meeting of shareholders held on June 13, 2017. Grant Thornton LLP is located at Suite 1600 – 333 Seymour Street, Vancouver, British Columbia, V6B 0A4, Canada. Grant Thornton LLP has reported on our fiscal December 31, 2016 and 2015 audited consolidated financial statements, which have been filed with the securities regulatory authorities and incorporated by reference herein. Grant Thornton LLP is independent with respect to the Company within the meaning of the Rules of Professional Conduct of the Institute of Chartered Accountants of British Columbia.
Our transfer agent and the registrar for our Common Shares in Canada is Computershare Investor Services Inc. located at 510 Burrard Street, 2nd Floor, Vancouver, British Columbia, Canada, V6C 3B9 and in the United States is Computershare Trust Company N.A. located at 740 – 350 Indiana St., Golden, Colorado, 80401.
S-53
William O'Neill, Steven Rubin and Jane Hsiao, in their capacity as directors of the Company, reside outside of Canada and have appointed the following agents for service of process in Canada:
| Name of Person | Name and Address of Agent | |
|---|---|---|
William O'Neill, Steven Rubin and Jane Hsiao |
Neovasc Inc. | |
|
Suite 5138 – 13562 Maycrest Way, Richmond, British Columbia, V6V 2J7 |
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
Certain legal matters related to our securities offered by this prospectus supplement will be passed upon on our behalf by Blake, Cassels & Graydon LLP, with respect to matters of Canadian law, and Skadden, Arps, Slate, Meagher & Flom LLP and Wilson Sonsini Goodrich & Rosati, with respect to matters of U.S. law, and Stikeman Elliott LLP, on behalf of the Underwriter with respect to matters of Canadian law and Goodwin Procter LLP with respect to matters of U.S. law. As of the date of this prospectus supplement, the partners and associates of Blake, Cassels & Graydon LLP and Stikeman Elliott LLP beneficially own, directly or indirectly, less than 1% of our outstanding Common Shares, respectively.
ENFORCEABILITY OF CIVIL LIABILITIES
We are a company continued under the CBCA. Most of our directors and officers and the experts named in this prospectus supplement, are residents of Canada or otherwise reside outside the United States, and all or a substantial portion of their assets may be, and a substantial portion of the Company's assets are, located outside the United States. We have appointed an agent for service of process in the United States (as set forth below), but it may be difficult for holders of securities who reside in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. We have been advised that a judgment of a U.S. court predicated solely upon civil liability under U.S. federal securities laws or the securities or "blue sky" laws of any state within the United States, would likely be enforceable in Canada if the United States court in which the judgment was obtained has a basis for jurisdiction in the matter that would be recognized by a Canadian court for the same purposes. We have also been advised, however, that there is substantial doubt whether an action could be brought in Canada in the first instance on the basis of the liability predicated solely upon U.S. federal securities laws.
We filed with the SEC, concurrently with our registration statement on Form F-10 of which this prospectus supplement and the accompanying base shelf prospectus are a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, we appointed CT Corporation System, 111 Eighth Avenue, New York, New York 10011 as our agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving us in a U.S. court arising out of or related to or concerning the offering of securities under the accompanying base shelf prospectus.
S-54
Information has been incorporated by reference in this short form prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Company Secretary of Neovasc Inc. at Suite 5138 – 13562 Maycrest Way, Richmond, British Columbia, V6V 2J7, Canada, telephone: (604)270-4344, and are also available electronically at www.sedar.com.
SHORT FORM BASE SHELF PROSPECTUS
New Issue and Secondary Offering |
June 9, 2016 |

U.S.$200,000,000
Common Shares
Preferred Shares
Debt Securities
Subscription Receipts
Units
Warrants
This prospectus relates to the offering for sale from time to time, during the 25-month period that this prospectus, including any amendments hereto, remains effective, of the securities of Neovasc Inc. ("Neovasc" or the "Company") listed above in one or more series or issuances, with a total offering price of such securities, in the aggregate, of up to U.S.$200,000,000. The securities may be offered by us or by our securityholders. The securities may be offered separately or together, in amounts, at prices and on terms to be determined based on market conditions at the time of the sale and set forth in an accompanying prospectus supplement.
Our common shares are listed on the Toronto Stock Exchange ("TSX"), under the symbol "NVC", and on the NASDAQ Capital Market ("NASDAQ"), under the symbol "NVCN". On June 8, 2016, the last trading day before the date hereof, the closing price per share of our common shares was C$0.56 on the TSX and U.S.$0.44 on the NASDAQ. Unless otherwise specified in an applicable prospectus supplement, our preferred shares, debt securities, subscription receipts, units and warrants will not be listed on any securities or stock exchange or on any automated dealer quotation system. There is currently no market through which our securities, other than our common shares, may be sold and purchasers may not be able to resell such securities purchased under this prospectus. This may affect the pricing of our securities, other than our common shares, in the secondary market, the transparency and availability of trading prices, the liquidity of these securities and the extent of issuer regulation. See "Risk Factors".
All information permitted under securities legislation to be omitted from this prospectus will be contained in one or more prospectus supplements that will be delivered to purchasers together with this prospectus. Each prospectus supplement will be incorporated by reference into this prospectus for the purposes of securities legislation as of the date of the prospectus supplement and only for the purposes of the distribution of the securities to which the prospectus supplement pertains. You should read this prospectus and any applicable prospectus supplement carefully before you invest in any securities issued pursuant to this prospectus. Our securities may be sold pursuant to this prospectus through underwriters or dealers or directly or through agents designated from time to time at amounts and prices and other terms determined by us or any selling securityholders. In connection with any underwritten offering of securities, the underwriters may over-allot or effect transactions which stabilize or maintain the market price of the securities offered. Such transactions, if commenced, may discontinue at any time. See "Plan of Distribution". A prospectus supplement will set out the names of any underwriters, dealers, agents or selling securityholders involved in the sale of our securities, the amounts, if any, to be purchased by underwriters, the plan of distribution for such securities, including the net proceeds we expect to receive from the sale of such securities, if any, the amounts and prices at which such securities are sold and the compensation of such underwriters, dealers or agents.
Investment in the securities being offered is highly speculative and involves significant risks that you should consider before purchasing such securities. You should carefully review the risks outlined in this prospectus (including any prospectus supplement) and in the documents incorporated by reference as well as the information under the heading "Forward-Looking Statements" and consider such risks and information in connection with an investment in the securities. See "Risk Factors".
We are permitted under a multijurisdictional disclosure system adopted by the securities regulatory authorities in Canada and the United States to prepare this prospectus in accordance with the disclosure requirements of Canada. Prospective investors in the United States should be aware that such requirements are different from those of the United States. Financial statements incorporated by reference herein have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, and are subject to Canadian auditing and auditor independence standards, and thus may not be comparable to financial statements of United States companies.
Owning our securities may subject you to tax consequences both in Canada and the United States. Such tax consequences are not described in this prospectus and may not be fully described in any applicable prospectus supplement. You should read the tax discussion in any prospectus supplement with respect to a particular offering and consult your own tax advisor with respect to your own particular circumstances.
Your ability to enforce civil liabilities under the U.S. federal securities laws may be affected adversely because we are incorporated under the federal laws of Canada, most of our officers and directors and the experts named in this prospectus are Canadian residents, and a substantial portion of our assets and the assets of those officers, directors and experts are located outside of the United States.
Neither the U.S. Securities and Exchange Commission (the "SEC"), nor any state securities regulator has approved or disapproved the securities offered hereby or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offence.
No underwriter has been involved in the preparation of this prospectus or performed any review of the contents of this prospectus.
Our head office is located at Suite 5138 – 13562 Maycrest Way, Richmond, British Columbia, V6V 2J7 and our registered office is located at Suite 2600 – 595 Burrard Street, Vancouver, British Columbia, V7X 1L3, Canada.
Certain of the Company's directors reside outside of Canada and have appointed an agent for service of process in Canada. See "Agent for Service of Process".
Investors should rely only on the information contained in or incorporated by reference into this prospectus and any applicable prospectus supplement. The Company has not authorized anyone to provide investors with different information. Information contained on the Company's website shall not be deemed to be a part of this prospectus (including any applicable prospectus supplement) or incorporated by reference and should not be relied upon by prospective investors for the purpose of determining whether to invest in the securities. The Company will not make an offer of these securities in any jurisdiction where the offer or sale is not permitted. Investors should not assume that the information contained in this prospectus is accurate as of any date other than the date on the face page of this prospectus or any applicable prospectus supplement.
You should rely only on the information contained or incorporated by reference in this prospectus or any applicable prospectus supplement and on the other information included in the registration statement of which this prospectus forms a part. We have not authorized anyone to provide you with different or additional information. If anyone provides you with different or additional information, you should not rely on it. We are not making an offer to sell or seeking an offer to buy the securities offered pursuant to this prospectus in any jurisdiction where the offer or sale is not permitted. You should assume that the information contained in this prospectus or any applicable prospectus supplement is accurate only as of the date on the front of those documents and that information contained in any document incorporated by reference is accurate only as of the date of that document, regardless of the time of delivery of this prospectus or any applicable prospectus supplement or of any sale of our securities pursuant thereto. Our business, financial condition, results of operations and prospects may have changed since those dates.
Market data and certain industry forecasts used in this prospectus or any applicable prospectus supplement and the documents incorporated by reference in this prospectus or any applicable prospectus supplement were obtained from market research, publicly available information and industry publications. We believe that these sources are generally reliable, but the accuracy and completeness of this information is not guaranteed. We have not independently verified such information, and we do not make any representation as to the accuracy of such information.
In this prospectus and any prospectus supplement, unless otherwise indicated, all dollar amounts and references to "U.S.$" are to U.S. dollars and references to "C$" or "$" are to Canadian dollars. This prospectus and the documents incorporated by reference contain translations of some Canadian dollar amounts into U.S. dollars solely for your convenience. See "Exchange Rate Information".
In this prospectus and in any prospectus supplement, unless the context otherwise requires, references to "we", "us", "our" or similar terms, as well as references to "Neovasc" or the "Company", refer to Neovasc Inc., either alone or together with our subsidiaries.
The names Neovasc Reducer™, Tiara™ and Peripatch™ are our trademarks. Other trademarks, product names and company names appearing in this prospectus and any prospectus supplement and documents incorporated by reference in this prospectus and any prospectus supplement are the property of their respective owners.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the documents incorporated by reference herein, contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 and applicable Canadian securities laws. The words "expect", "anticipate", "may", "will", "estimate", "continue", "intend", "believe" and similar expressions are intended to identify such forward-looking statements. Forward-looking statements in this prospectus and the documents incorporated by reference herein include, but are not limited to, statements relating to:
- •
- the conduct or possible outcomes of any actual or threatened legal proceedings, including the Company's litigation with
CardiAQ Valve Technologies, Inc.;
- •
- the amount of estimated additional litigation expenses required to defend the Company in lawsuits filed by CardiAQ Valve
Technologies, Inc.;
- •
- our intention to expand the indications for which we may market Tiara™ (which does not have regulatory
approval and is not commercialized) and Reducer™ (which has CE Mark approval for sale in the European Union);
- •
- clinical development of our products, including the results of current and future clinical trials and studies;
- •
- our intention to apply for CE Mark approval for Tiara™ in the next two to three years;
- •
- our plans to develop and commercialize products, including the Tiara™, and the timing and cost of these development programs;
i
- •
- our strategy to refocus our business towards development and commercialization of the Neovasc Reducer™
and Tiara™;
- •
- our ability to replace declining revenues from the tissue business with revenues from the Neovasc Reducer™ and
Tiara™ in a timely manner;
- •
- whether we will receive, and the timing and costs of obtaining, regulatory approvals;
- •
- the cost of post-market regulation if we receive necessary regulatory approvals;
- •
- our ability to enroll patients in our clinical trials, studies and compassionate use cases in Canada, the
United States and in Europe;
- •
- our intention to continue directing a significant portion of our resources into sales expansion;
- •
- the expected decline of consulting services revenue in the long term as our consulting customers become contract
manufacturing customers;
- •
- our ability to get our products approved for use;
- •
- the benefits and risks of our products as compared to others;
- •
- our need for additional financing and our estimates regarding our capital requirements and future revenues, expenses and
profitability;
- •
- our estimates of the size of the potential markets for our products, including the anticipated market opportunity for the
Neovasc Reducer™;
- •
- our potential relationships with distributors and collaborators with acceptable development, regulatory and
commercialization expertise and the benefits to be derived from such collaborative efforts;
- •
- sources of revenues and anticipated revenues, including contributions from distributors and other third parties, product
sales, license agreements and other collaborative efforts for the development and commercialization of products;
- •
- our creation of an effective direct sales and marketing infrastructure for approved products we elect to market and
sell directly;
- •
- the rate and degree of market acceptance of our products;
- •
- the timing and amount of reimbursement for our products; and
- •
- the impact of foreign currency exchange rates.
Such statements reflect our current views with respect to future events and are subject to, and are necessarily based upon, a number of estimates and assumptions that, while considered reasonable by us, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies, many of which, with respect to future events, are subject to change. The material assumptions used by us to develop such forward-looking statements include, but are not limited to:
- •
- our regulatory and clinical strategies will continue to be successful;
- •
- our current positive interactions with regulatory agencies will continue;
- •
- recruitment to clinical trials and studies will continue;
- •
- the time required to enroll, analyze and report the results of our clinical studies will be consistent with projected
timelines;
- •
- current and future clinical trials and studies will generate the supporting clinical data necessary to achieve approval of
marketing authorization applications;
- •
- the regulatory requirements for approval of marketing authorization applications will be maintained;
- •
- our current good relationships with our suppliers and service providers will be maintained;
ii
- •
- our estimates of market size and reports reviewed by us are accurate;
- •
- our efforts to develop markets and generate revenue from Reducer will be successful;
- •
- genericisation of markets for Tiara and Reducer will develop; and
- •
- capital will be available on terms that are favourable to us.
Forward-looking statements are based on estimates and assumptions made by the Company in light of its experience and its perception of historical trends, current conditions and expected future developments, as well as other factors that the Company believes are appropriate in the circumstances. Many factors could cause the Company's actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation:
- •
- the conduct or possible outcomes of any actual or threatened legal proceedings, which are inherently uncertain;
- •
- the potential benefits of the Neovasc Reducer™ and Tiara™ as compared with other products;
successful enrollment of patients in studies and trials for the Neovasc Reducer™ and Tiara™;
- •
- results of the trials and studies for the Neovasc Reducer™ and Tiara™ that meet our expectations;
- •
- our receipt of any required local and institutional regulatory approvals and the timing and costs of obtaining
such approvals;
- •
- European enrollment in our clinical trials, studies and compassionate use cases and the success of applications
in Europe;
- •
- our ability to protect our intellectual property;
- •
- our ability to raise additional funding;
- •
- our retention and hiring of qualified employees in the future;
- •
- the manufacturing capacity of third-party manufacturers for our products;
- •
- the competition we face from other companies, research organizations, academic institutions and government agencies, and
the risks such competition pose to our products;
- •
- the success and pricing of other competing therapies that may become available;
- •
- the confidential information we possess about patients, customers and core business functions, and the information
technologies we use to protect it;
- •
- our ability to establish, maintain and defend intellectual property rights in our products;
- •
- government legislation in all countries that we already, or hope to, sell our products in, and its effect on our ability
to set prices, enforce patents and obtain product approvals or reimbursements;
- •
- changes in business strategy or development plans; and
- •
- general economic and business conditions, both nationally and in the regions in which we operate.
By their very nature, forward-looking statements or information involve known and unknown risks, uncertainties and other factors that may cause our actual results, events or developments, or industry results, to be materially different from any future results, events or developments expressed or implied by such forward-looking statements or information. In evaluating these statements, prospective purchasers should specifically consider various factors, including the risks outlined in the "Risk Factors" section herein and in documents incorporated by reference herein. These factors should be considered carefully, and readers should not place undue reliance on the Company's forward-looking statements. Should one or more of these risks or uncertainties or a risk that is not currently known to us materialize, or should assumptions underlying the forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements are made as of the date of this prospectus or, in the case of documents incorporated by reference in this prospectus, as of the date of such documents, and we do not intend, and do not
iii
assume any obligation, to update these forward-looking statements, except as required by law. Investors are cautioned that forward-looking statements are not guarantees of future performance and investors are cautioned not to put undue reliance on forward-looking statements due to their inherent uncertainty.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated by reference in this prospectus and not delivered with this prospectus may be obtained on request without charge from the Company Secretary of Neovasc Inc. at Suite 5138 – 13562 Maycrest Way, Richmond, British Columbia, V6V 2J7, Canada, telephone: (604) 270-4344 or by accessing the disclosure documents through the Internet on the Canadian System for Electronic Document Analysis and Retrieval, or SEDAR, at www.sedar.com. Documents filed with, or furnished to, the SEC are available through the SEC's Electronic Data Gathering and Retrieval System, or EDGAR, at www.sec.gov.
The following documents, filed with the securities commissions or similar regulatory authorities in certain provinces of Canada and filed with, or furnished to, the SEC are specifically incorporated by reference into, and form an integral part of, this prospectus:
- •
- our management information circular dated May 4, 2016, distributed in connection with our annual general meeting of
shareholders to be held on June 15, 2016;
- •
- our unaudited interim consolidated financial statements for the three months ended March 31, 2016 and 2015;
- •
- our management's discussion and analysis of financial condition and results of operations for the three months ended
March 31, 2016 and 2015;
- •
- our annual information form for the fiscal year ended December 31, 2015, dated as of March 29, 2016;
- •
- our audited annual consolidated financial statements for the fiscal years ended December 31, 2015 and 2014,
together with the notes thereto and the auditor's reports thereon;
- •
- our management's discussion and analysis of financial condition and results of operations for the fiscal years ended
December 31, 2015 and 2014; and
- •
- our material change report dated June 8, 2016 with respect to the jury verdict in litigation brought by CardiAQ.
Any documents of the type described in Section 11.1 of Form 44-101F1 Short Form Prospectuses filed by the Company with a securities commission or similar authority in any province of Canada subsequent to the date of this short form prospectus and prior to the expiry of this prospectus, or the completion of the issuance of securities pursuant hereto, will be deemed to be incorporated by reference into this prospectus.
Any template version of any "marketing materials" (as such term is defined in NI 44-101) filed after the date of a prospectus supplement and before the termination of the distribution of the securities offered pursuant to such prospectus supplement (together with this prospectus) is deemed to be incorporated by reference in such prospectus supplement.
In addition, to the extent that any document or information incorporated by reference into this prospectus is filed with, or furnished to, the SEC pursuant to the Securities Exchange Act of 1934, as amended (the "Exchange Act"), after the date of this prospectus, such document or information will be deemed to be incorporated by reference as an exhibit to the registration statement of which this prospectus forms a part (in the case of a report on Form 6-K, if and to the extent expressly provided therein).
A prospectus supplement containing the specific terms of any offering of our securities will be delivered to purchasers of our securities together with this prospectus and will be deemed to be incorporated by reference in this prospectus as of the date of the prospectus supplement and only for the purposes of the offering of our securities to which that prospectus supplement pertains.
iv
Any statement contained in this prospectus or in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein or in any other subsequently filed document that also is or is deemed to be incorporated by reference herein modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement is not to be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of material fact or an omission to state a material fact that is required to be stated or is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
Upon our filing a new annual information form and the related annual financial statements and management's discussion and analysis with applicable securities regulatory authorities during the currency of this prospectus, the previous annual information form, the previous annual financial statements and management's discussion and analysis and all quarterly financial statements, supplemental information, material change reports and information circulars filed prior to the commencement of our financial year in which the new annual information form is filed will be deemed no longer to be incorporated into this prospectus for purposes of future offers and sales of our securities under this prospectus. Upon interim consolidated financial statements and the accompanying management's discussion and analysis being filed by us with the applicable securities regulatory authorities during the duration of this prospectus, all interim consolidated financial statements and the accompanying management's discussion and analysis filed prior to the new interim consolidated financial statements shall be deemed no longer to be incorporated into this prospectus for purposes of future offers and sales of securities under this prospectus.
References to our website in any documents that are incorporated by reference into this prospectus do not incorporate by reference the information on such website into this prospectus, and we disclaim any such incorporation by reference.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
The following documents have been or will be filed with the SEC as part of the registration statement of which this prospectus forms a part: (i) the documents listed under the heading "Documents Incorporated by Reference"; (ii) powers of attorney from our directors and officers; (iii) the consent of Grant Thornton LLP; and (iv) the form of indenture relating to the debt securities that may be issued under this prospectus.
The following table sets forth for each period indicated: (i) the noon exchange rates in effect at the end of the period; (ii) the high and low noon exchange rates during such period; and (iii) the average noon exchange rates for such period, for one Canadian dollar, expressed in U.S. dollars, as quoted by the Bank of Canada.
| |
Year Ended December 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
| |
U.S.$ |
U.S.$ |
U.S.$ |
|||||||
Closing |
0.7225 | 0.8620 | 0.9402 | |||||||
High |
0.8527 | 0.9422 | 1.0164 | |||||||
Low |
0.7148 | 0.8589 | 0.9348 | |||||||
Average |
0.7833 | 0.9058 | 0.9710 | |||||||
v
| |
Three Months Ended March 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
| |
U.S.$ |
U.S.$ |
U.S.$ |
|||||||
Closing |
0.7884 | 0.9050 | 0.9829 | |||||||
High |
0.8621 | 0.9444 | 1.0188 | |||||||
Low |
0.7793 | 0.8866 | 0.9669 | |||||||
Average |
0.8067 | 0.9069 | 0.9925 | |||||||
On June 8, 2016, the noon exchange rate as quoted by the Bank of Canada was C$1.00 = U.S.$0.7877.
vi
Neovasc was incorporated on November 2, 2000 under the laws of the Province of British Columbia and was continued to federal jurisdiction under the Canada Business Corporations Act ("CBCA") on April 19, 2002. Neovasc has five wholly owned subsidiaries, three of which are material: (i) Neovasc Tiara Inc. ("NTI"), a corporation incorporated under the federal laws of Canada; (ii) Neovasc Medical Ltd. ("NML"), a corporation incorporated under the laws of Israel; and (iii) Neovasc Medical Inc. ("NMI"), a corporation incorporated under the laws of British Columbia.
Our registered office is located at Suite 2600 – 595 Burrard Street, Vancouver, British Columbia, V7X 1L3, Canada and our head office and principal place of business are located at Suite 5138 – 13562 Maycrest Way, Richmond, British Columbia, V6V 2J7.
Our Business
Neovasc is a specialty medical device company that develops, manufactures and markets products for the rapidly growing cardiovascular marketplace. Its products include the Tiara technology in development for the transcatheter treatment of mitral valve disease and the Neovasc Reducer for the treatment of refractory angina.
In 2009, Neovasc started initial activities to develop novel technologies for catheter-based treatment of mitral valve disease. Based on the early positive results of these activities, the Company formally launched a program to develop the Tiara. Neovasc established a separate entity, NTI, in March 2013 to develop and own the intellectual property related to the Tiara (Neovasc has transferred all intellectual property related to Tiara to NTI). On February 3, 2014, Neovasc announced the first human implant of the Tiara under special access compassionate use exemptions. Subsequently sixteen additional patients have been implanted with the Tiara (twelve under compassionate use approvals in Canada and in Europe and four in the TIARA-I study) bringing the total number of patients treated with the device to seventeen as of this date. In December 2014, the Company announced that it had received approval from the U.S. Food and Drug Administration ("FDA") to initiate the TIARA-I study in the United States. The TIARA-I study is a multinational, multicenter early feasibility study being conducted to assess the safety and performance of the Tiara valve system in high risk surgical patients. The study will include up to 15 patients enrolled at centers in the United States and up to 15 patients at centers in Canada and Europe. The first European patient was enrolled in the study in Antwerp, Belgium in late-2014 and the first patient in the United States was enrolled in mid-2015. The Tiara is currently available in two sizes; additional sizes are under development. Following completion of the TIARA-I study the Company intends to continue advancing the Tiara to commercialization and will be undertaking additional studies to support authorization to affix the CE Mark and FDA approval as appropriate.
In July 2008, Neovasc acquired NML, a pre-commercial vascular device company based in Israel. NML developed and owned intellectual property related to a novel catheter-based treatment for refractory angina, a debilitating condition resulting from inadequate blood flow to the heart muscle. The Company estimates that there are approximately 620,000 refractory angina patients in the United States who are potential candidates for this treatment. The Company has completed development of the Reducer and obtained authorization to affix the CE Mark, which allows for marketing of the Reducer product in the European marketplace. The Company initiated commercial sales of the Reducer product in early-2015. In March 2014 the Company announced that results of its Coronary Sinus Reducer for Treatment of Refractory Angina clinical trial ("COSIRA") had been presented at the ACC.14 medical conference. The COSIRA trial was a sham-controlled randomized, double-blinded study of the Reducer device in 104 patients with moderate to severe refractory angina. The results presented at ACC.14 confirmed that the COSIRA study had met its primary endpoint demonstrating the efficacy of the Reducer device with statistical significance. The COSIRA trial results were published in the New England Journal of Medicine in February 2015.
Neovasc's business operations started in March 2002, with the acquisition of NMI. NMI manufactures a line of collagen-based surgical patch products made for use in cardiac reconstruction and vascular repair procedures as well as other surgeries. Neovasc, through NMI, also sells biological tissue to industry partners and other customers who incorporate this tissue into their own products such as transcatheter heart valves. Neovasc's biological products are made from chemically treated biocompatible pericardial tissue. In 2012, Neovasc sold the rights to manufacture a specific line of conventional surgical patch products to LeMaitre Vascular, Inc.
1
("LeMaitre") for U.S.$4.6 million. Neovasc has refocused its use of this treated pericardial tissue to constitute key components in third-party medical products, such as transcatheter heart valves. The Company also provides customers with consulting services related to the development of these products with specific expertise related to the transcatheter heart valve field as well as contract manufacturing services for these valves at all stages of development through to commercial scale production.
Our Strategy
The Company's core strategy is to focus on the continued development and commercialization of its products, the Tiara and Reducer, providing minimally invasive medical devices for a cardiovascular market that the Company believes is both growing and under-served by current treatment solutions.
Key elements of this strategy include:
- •
- continuing the Company's initial clinical experience of the Tiara, completing enrollment of a multi-center feasibility
study in 2016, and if the enrollment and results of the feasibility study are successful, initiating a CE Mark study in 2016;
- •
- continuing development of the Reducer, initiating a U.S. FDA investigational device exemption ("IDE") study in 2016
and supporting the successful COSIRA trial with additional experience through the Company's targeted commercial launch of Reducer in Europe; and
- •
- continuing efforts to support Neovasc customers through their regulatory pathways and commercialization of their products, with the view to ultimately supplying pericardial tissue and engaging in full scale manufacturing of their sub-components or complete transcatheter heart valves for commercial sale.
Our Products
Tiara
In the second quarter of 2011, the Company formally initiated a new project to develop the Tiara, a product for treating mitral valve disease. The Tiara is in preclinical / early clinical stage development to provide a minimally invasive transcatheter device for the millions of patients who experience mitral regurgitation as a result of mitral heart valve disease (in 2014 it was estimated that mitral regurgitation affects approximately 4.1 million people in the United States and the European Union). Mitral regurgitation is often severe and can lead to heart failure and death. Unmet medical need in these patients is high. Currently, a significant percentage of patients with severe mitral regurgitation are not good candidates for conventional surgical repair or replacement due to frailty or comorbidities. There are approximately 1.7 million patients suffering from significant mitral regurgitation in the United States. Currently there is no transcatheter mitral valve replacement device approved for use in any market.
Clinical experience to date has been primarily with the 35mm Tiara and the 32 French delivery system. First clinical use of the 40mm Tiara occurred in the fourth quarter of 2015 and first use of the 45mm Tiara is targeted for 2016. The additional sizes will allow Neovasc to expand treatment to a broader population of patients.
To date, seventeen patients have been implanted with Tiara in early feasibility and compassionate use cases and Neovasc believes that early results have been encouraging. The 30-day survival rate for the first twelve patients implanted with Tiara is 75% with one patient now over two years post implant. The Tiara has been successfully implanted in both functional and degenerative mitral regurgitation patients, as well as patients with pre-existing prosthetic aortic valves and mitral surgical rings.
The results from these early feasibility and compassionate use cases have been instrumental in helping to demonstrate the potential of the Tiara as well as refining the implantation procedure, patient selection criteria and the device itself. Careful patient selection continues to be critical as the Company and clinical community continue to learn more about treating this population of very sick patients.
While many challenges remain prior to achieving commercial production (including, but not limited to, positive clinical trial and study results and obtaining regulatory approval from the relevant authorities), the
2
Company believes the Tiara is being widely recognized as one of the leading devices exploring this new treatment option for patients who are unable or unsuited to receive an open heart surgical valve replacement or repair. There are several other transcatheter mitral valve replacement devices in development by third parties; some of which have been implanted in early feasibility type studies with varying results.
Neovasc believes that there are several unique attributes of the Tiara that may provide advantages over other approaches to mitral valve replacement. There is no certainty that the Tiara will successfully proceed through clinical testing and ultimately receive regulatory approval to treat these patients, nor is it possible to determine at this time if any of the other development stage devices will succeed in obtaining regulatory approval.
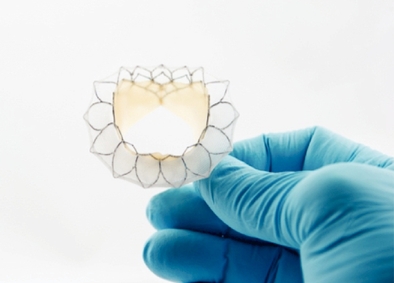
The Tiara valve is made up of two major components: the leaflets and skirt, which are made from the Company's Peripatch tissue, and the nitinol frame (to which the leaflets and skirt are attached), which is manufactured by a well-established specialty manufacturer in the medical device industry. If this supplier were unable to provide the nitinol frame in the future, it would seriously impact the further development of the Tiara. The Tiara delivery system is manufactured in-house by the Company using components that are readily available.
Regulatory Status
The Tiara is an early-stage development product without regulatory approvals in any country. The Company intends to continue to fund development of the product as cash flow allows and anticipates applying for CE Mark approval in Europe in the next two to three years. As at December 31, 2015, the Company has spent approximately U.S.$22.5 million developing the product and anticipates that it may require an additional U.S.$25-30 million as it moves forward to achieve CE Mark. There is no assurance that European regulatory approval will be granted in the time frame anticipated by management, or granted at any time in the future. There is no expectation that this product will be revenue-generating in the near term, although management believes that the product is addressing an important unmet clinical need and that the demand for the product is high.
On October 9, 2014 Neovasc announced that it has received conditional IDE approval from the U.S. FDA to initiate the U.S. arm of its TIARA-I study for the Company's Tiara. The TIARA-I study is a multinational, multicenter early feasibility study being conducted to assess the safety and performance of Neovasc's Tiara mitral valve system and implantation procedure in high-risk surgical patients suffering from severe mitral regurgitation. Severe mitral regurgitation is a critical condition that affects millions of patients and, if left untreated, can lead to heart failure or death. This FDA conditional approval allows clinical investigators to begin enrolling patients at participating U.S. medical centers once local hospital and related approvals are in place. This is an important step towards Tiara becoming one of the first transcatheter mitral valve replacement devices
3
available for treating U.S. patients. The TIARA-I study will enroll up to 30 patients globally and is being overseen by a multidisciplinary committee of internationally recognized physicians. Tiara has also been implanted under compassionate use approvals in Canada and implantations under similar approvals are anticipated in other countries in the future.
Reducer
The Reducer is a treatment for patients with refractory angina, a painful and debilitating condition that occurs when the coronary arteries deliver an inadequate supply of blood to the heart muscle, despite treatment with standard revascularization or cardiac drug therapies. It affects approximately 620,000 individuals in the United States who are not eligible for conventional treatments and typically lead severely restricted lives as a result of their disabling symptoms, and its incidence is growing. The Reducer provides relief of angina symptoms by altering blood flow in the heart's venous system, thereby increasing the perfusion of oxygenated blood to ischemic areas of the heart muscle.
The pain associated with refractory angina can make it difficult for patients to engage in routine activities, such as walking or climbing stairs. Using a catheter-based procedure, the Reducer is implanted in the coronary sinus, the major blood vessel that sends de-oxygenated blood from the heart muscle back to the right atrium of the heart. Pilot clinical studies demonstrate that the Reducer provides significant relief of chest pain in refractory angina patients. There are approximately 620,000 refractory angina patients in the United States who are potential candidates for the Reducer, either because they cannot be revascularized or because they are otherwise poorly managed using conventional medical therapies. These patients represent a substantial market opportunity for the Reducer product. If physicians adopt the Reducer for use in these refractory patients, it is expected that there will be a natural spillover into the broader recurrent angina market, which represents a substantially larger patient population.
The Reducer is targeting a currently untreatable patient population. A refractory patient by definition is resistant to other therapies. A patient who has refractory angina is not a surgical candidate, cannot benefit from existing interventional cardiology therapies and is not receiving adequate relief from available drug regimens to manage their chest pain. As such there are currently no direct competitors to the Reducer as the patient will have exhausted all other treatment options before a Reducer is considered. Once the Reducer is established as a standard of care for the refractory angina patient, Neovasc believes that the Reducer may also be considered for use in the larger population of recurrent angina patients (patients who are receiving repeat treatments for angina pain) and thus increase its market potential.
The Company has completed a COSIRA trial to assess the efficacy of the Reducer device. The COSIRA trial's primary endpoint was a two-class improvement six months after implantation in patients' ratings on the Canadian Cardiovascular Society ("CCS") angina grading scale, a four-class functional classification that is widely used to characterize the severity of angina symptoms and disability. Only patients with severe angina, CCS Class 3 or 4, were enrolled in the COSIRA trial. The COSIRA trial analysis showed that the study met the primary endpoint, with patients receiving the Reducer achieving a statistically significant improvement in CCS scores (two classes or better) compared to patients receiving a sham control (18 of 52 (34.6%) of the Reducer patients improved ³ 2 CCS classes compared to 8 of 52 (15.4%) of the control patients (p-value =0.024)). The analysis also showed that patients treated with the Reducer showed a statistically significant improvement of one or more CCS classes compared to the sham control patients (37 of 52 (71.2%) of the Reducer patients showed
4
this improvement compared to 22 of 52 (42.3%) of the control patients (p-value= 0.003)). The COSIRA trial results were published in the New England Journal of Medicine in February 2015.
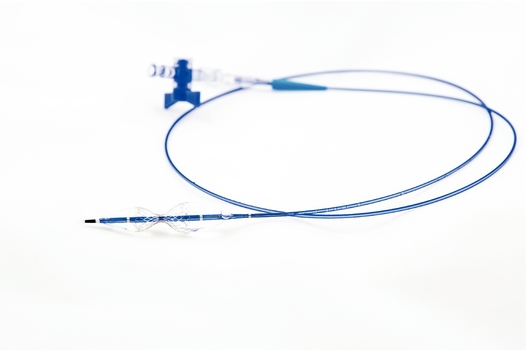
The Reducer is an hourglass-shaped, balloon-expandable, stainless steel, bare metal device, which is implanted in the coronary sinus, creating a restriction in venous outflow from the myocardium (the muscular layer of the heart wall). It is implanted using conventional percutaneous, or needle puncture, techniques. The Reducer is provided sterile and pre-loaded on a balloon catheter system. The system is 9 French sheath compatible and operates over a .035 inch guide wire. The implantation procedure is quick and requires minimal training. Once guide wire access to the coronary sinus is achieved, implantation typically takes less than 20 minutes.
Following implantation, the Reducer is incorporated into the endothelial tissue and creates a permanent (but reversible) narrowing in the coronary sinus. The coronary sinus is narrowed from a typical diameter of 10-12mm to approximately 3mm at the site of implantation. This narrowing slightly elevates the venous outflow pressure, which restores a more normal ratio of epicardial to endocardial blood flow between the outer and inner layers of the ischemic areas of the heart muscle. This results in improved perfusion of the endocardium, which helps relieve ischemia and chest pain. The physiological mechanism behind this effect is well documented in medical literature.
The clinical utility of this approach was demonstrated by a number of analogous approaches used in the past that achieved positive clinical outcomes for angina patients by constricting or intermittently blocking the coronary sinus to improve perfusion to the heart muscle. However, these therapies required the use of highly invasive surgery, or leaving a catheter in the heart for a prolonged period, making them impractical or clinically unacceptable for use in modern medical practice. The Reducer was developed to deliver this therapy in a safe, simple and effective manner via a minimally invasive catheter that is consistent with contemporary medical practice.
The Reducer has demonstrated excellent results in multiple animal studies and in a clinical trial of fifteen patients suffering from chronic refractory angina who were followed for three years after implantation. The six-month results from this clinical trial were published in the Journal of the American College of Cardiology and three-year follow-up data was presented at the annual scientific meeting of the American College of Cardiology in March 2010. In this clinical trial, implantation of the Reducer resulted in significant clinical improvements in stress test and perfusion measurements, as well as in overall quality of life in the majority of the patients. These improvements were maintained for the three years of the study. During this period, the Reducer
5
appeared safe and well tolerated in these patients. More recently, the Company completed the COSIRA trial — a multi-center, double blinded sham controlled study intended to assess the safety and efficacy of the Reducer in a rigorous, controlled manner. The results of the COSIRA trial were positive and are discussed in more detail below.
Following this positive data from the COSIRA trial, the Company initiated a pilot launch of the Reducer in select European markets in early 2015. The Company has signed distribution agreements in Italy, Switzerland, the United Kingdom and Saudi Arabia and has initial sales into these countries. Launch of the product is also underway in select centers in Germany via direct sales. Based on the initial results from the targeted launch, Neovasc is presently developing an expanded sales plan and strategy for 2016 and beyond. It is anticipated that sales of the product in the United States would follow obtaining U.S. regulatory approval, if such approval is granted, as described further below.
Regulatory Status
The Reducer is approved for sale in Europe, having received CE Mark designation in November 2011. In preparation for product launch, Neovasc has completed development of the commercial-generation Reducer and the product is currently being transferred to commercial scale manufacture. The Company has completed the COSIRA trial that is expected to provide data to support broad commercialization of the Reducer product. The COSIRA trial is a double-blinded, randomized, sham controlled, multi-center trial of 104 patients at eleven clinical investigation sites. The study completed enrollment in early 2013 and on November 6, 2013, the Company reported topline results for its COSIRA trial assessing the efficacy and safety of the Reducer. In February 2015, the COSIRA trial results were published in the New England Journal of Medicine. As discussed above, the data shows that the Reducer achieved its primary endpoint, significantly improving the symptoms and functioning of patients disabled by previously untreatable refractory angina. The COSIRA trial also confirmed that the Reducer is safe and well tolerated. The safety and efficacy data from the randomized, controlled COSIRA trial is consistent with results seen in previous non-randomized pilot studies of the Reducer. Placement of the Reducer is performed using a minimally-invasive transvenous procedure that is similar to implanting a coronary stent and takes approximately 20 minutes. Neovasc has begun discussions with the FDA on the development of a randomized IDE trial in the United States. The Company expects to begin this trial in 2016. Marketing approval in the United States is expected about two to four years after the clinical trial begins. There is no assurance that regulatory approval in the United States will be granted in the time frame anticipated by management, or granted at any time in the future. The cost of the clinical trial in the United States is expected to be U.S.$15-20 million.
Tissue Products
Neovasc produces Peripatch, an advanced biological tissue product that is manufactured from pericardium, which is the protective sac that surrounds the heart of an animal. Neovasc uses its proprietary processes to convert raw pericardial tissue from animal sources into sheets of implantable tissue that can be incorporated into third-party medical devices (for example, for use as the material for artificial heart valve leaflets). Peripatch tissue retains the mechanical characteristics of natural tissue and is readily incorporated into the body without rejection. Peripatch tissue was originally developed to fabricate artificial heart valves and has a 25-year history of successful implantation for heart valve and other surgical applications. Peripatch tissue can be manufactured to meet the mechanical and biological characteristics required for a wide variety of applications, such as heart valve leaflets.
The product line includes Peripatch surgical patches, which are rectangular patches made from bovine tissue, applied as internal bandages to repair weak or damaged organs or vessels. On October 31, 2012, Neovasc amended its agreement with LeMaitre allowing LeMaitre to exercise its option to purchase certain specific rights to Neovasc's biological vascular surgical patch technology on an accelerated basis. Under the terms of the amendment, LeMaitre is permitted to use the Peripatch technology for the sole purpose of manufacturing surgical patches that it markets as its XenoSure™ surgical patch product line. Neovasc ceased manufacturing surgical patches for LeMaitre in the second quarter of 2015.
6
The Company also provides a range of custom Peripatch products to industry customers for incorporation into their own products, such as transcatheter heart valves and other specialty cardiovascular devices. These include Peripatch tissue fabricated from bovine and porcine sources and offered in a wide variety of shapes and sizes. Neovasc works closely with its industry customers to develop and supply tissue to meet their specific needs, such as for transcatheter heart valve leaflets. This often includes providing tissue in custom shapes or molded to three dimensional configurations. The Company also provides product development and specialized manufacturing services related to Peripatch tissue-based products such as transcatheter heart valves. The Company actively consults with a range of heart valve programs in order to refine their products and provide tissue to meet their needs and also provides transcatheter valve prototyping, pilot manufacture and commercial manufacture services to a range of customers.
Although the generic method of processing tissue in a way similar to the Peripatch is widely used, the Company's competitive position stems from its own proprietary process that is supported by a 25-year implant history for use as a surgical heart valve. A company that establishes its own process will have to go through a significant and costly series of studies to prove that their process produces tissue that is suitable as a medical device. The Peripatch product has already met these requirements and has already been validated through many years of successful use in multiple applications. Neovasc's customers make the decision to use the Company's tissue rather than take on the demanding and lengthy process of developing their own tissue processing operation. As stated elsewhere in this prospectus, Neovasc is not aware of any other company in the world that both provides such tissue and partners with customers to provide specialized heart valve development and manufacturing services.
The basic Peripatch technology was established over 25 years ago by a third party that was a predecessor company to NMI, when the material was used to fashion the leaflets and other components in surgical heart valves. Neovasc's processing of the material is a trade secret and proprietary to the Company. However, the use of the product in transcatheter minimally invasive heart valves and other medical devices such as artificial hearts are new uses for the technology. Appropriate testing is conducted to ensure the appropriateness and durability of the tissue for a new application before the medical device can be approved for use, and there is some additional risk when applying the technology to a new product or when amending to, or adding to, the fixation process to meet a new demand, such as for three dimensional shape setting of the tissue.
The supply of Peripatch products and the associated product development, consulting and specialized manufacturing services related to Peripatch tissue-based products represents 95% of the Company's current revenues.
Regulatory Status
While the Company does not maintain stand-alone marketing approval for its tissue products, a number of third-party products which incorporate Peripatch tissue are approved for sale (i.e. such products have obtained regulatory approval, such as a CE-Mark or Canadian medical device license) or have pending approvals in various markets. There is no assurance that further regulatory approvals for third-party products will be obtained.
Additional Products and Third-Party Sales
Neovasc provides consulting and original equipment manufacturing services to other medical device companies when these services fall within the scope of the Company's expertise and capabilities. These activities are substantially focused on providing specialized development and manufacturing services for industry customers who incorporate the Company's Peripatch tissue into their vascular device products such as heart valves. The goal of these activities is to drive near-term revenues as well as support development of a long-term revenue stream through the ongoing provision of tissue and manufacturing services to customers with commercially successful devices that incorporate Neovasc tissue. Revenue earned from various contract agreements varies throughout the year depending on customer needs.
7
Recent Developments
On June 6, 2014, Neovasc was named in a lawsuit filed by CardiAQ Valve Technologies, Inc. ("CardiAQ") in the U.S. District Court for the District of Massachusetts concerning intellectual property rights ownership, alleged unfair trade practices and an alleged breach of contract relating to Neovasc's transcatheter mitral valve technology, including Tiara. On May 19, 2016, following a trial in Boston, Massachusetts, a jury found in favor of CardiAQ, on CardiAQ's claims for relief for breach of contract, breach of the duty of honesty in contractual performance, and three of CardiAQ's six asserted trade secrets. The jury also issued advisory findings in favor of CardiAQ regarding its causes of action under Massachusetts Gen. Law. Ch. 93A and patent inventorship. The jury awarded US$70 million on the trade secret claim for relief, and no damages on the contractual claims for relief. On May 27, 2016, the Court granted Neovasc's motion for judgment as a matter of law on the Massachusetts Gen. Law. Ch. 93A claim. The Court will decide the patent inventorship claim following briefing that is expected to be completed in August 2016.
The Company and its legal counsel are reviewing the verdict, and the Company intends to explore its options regarding post-trial motions in the trial court and, potentially, the appellate process.
As of the date hereof, the Company is aware that a securities class action lawsuit has been filed in the United States District Court for the District of Massachusetts against the Company. The Company intends to vigorously defend itself in this lawsuit.
Investing in our securities involves a high degree of risk. In addition to the other information included, or incorporated by reference in this prospectus or any applicable prospectus supplement, you should carefully consider the risks described below before purchasing our securities. If any of the following risks actually occur, our business, financial condition and results of operations could materially suffer. As a result, the trading price of our securities, including our common shares, could decline, and you might lose all or part of your investment. The risks set out below are not the only risks we face; risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially and adversely affect our business, financial condition and results of operations. You should also refer to the other information set forth or incorporated by reference in this prospectus or any applicable prospectus supplement, including our consolidated financial statements and related notes.
Risks Relating to Our Business
Investors should carefully consider the risks described under the heading "Risk Factors" in our Annual Information Form and our other publicly filed documents which are incorporated herein by reference, as well as the risk factors described under the heading "Risk Factors" in any applicable prospectus supplement. See "Documents Incorporated by Reference".
The Company's litigation with CardiAQ could have a material adverse effect on its business and could potentially threaten the Company's ability to continue as a going concern.
On June 6, 2014, Neovasc was named in a lawsuit filed by CardiAQ in the U.S. District Court for the District of Massachusetts concerning intellectual property rights ownership, alleged unfair trade practices and an alleged breach of contract relating to Neovasc's transcatheter mitral valve technology, including Tiara. On May 19, 2016, following a trial in Boston, Massachusetts, a jury found in favor of CardiAQ, on CardiAQ's claims for relief for breach of contract, breach of the duty of honesty in contractual performance, and three of CardiAQ's six asserted trade secrets. The jury also issued advisory findings in favor of CardiAQ regarding its causes of action under Massachusetts Gen. Law. Ch. 93A and patent inventorship. The jury awarded US$70 million on the trade secret claim for relief, and no damages on the contractual claims for relief. On May 27, 2016, the Court granted Neovasc's motion for judgment as a matter of law on the Massachusetts Gen. Law. Ch. 93A claim. The Court will decide the patent inventorship claim following briefing that is expected to be completed in August 2016.
The Company and its legal counsel are reviewing the decision, and the Company intends to explore its options regarding post-trial motions in the trial court and, potentially, the appellate process.
8
As at March 31, 2016, the Company had US$50.4 million in current assets. Unless the Company is successful in post-trial motions and/or an appeal of the verdict, or otherwise is successful in reducing the amount of the US$70 million award, the Company will require additional financing in order to pay the damages and to continue to operate its business. There can be no assurance that such financing will be available on favorable terms, or at all.
In the cause of action relating to patent inventorship, CardiAQ claimed that two individuals should be added as inventors to a Neovasc patent. If the judge finds that these individuals should be added as inventors, and such a decision is upheld in subsequent proceedings, then it will materially impact the Company's competitive advantage, because CardiAQ will have the ability to practice, assign, and/or license the patent.
On June 23, 2014, CardiAQ also filed a complaint against Neovasc in Germany requesting that Neovasc assign its right to one of its European patent applications to CardiAQ. On July 7, 2014, the Company was made aware through a press release issued by CardiAQ of a stay in proceedings for Neovasc's European patent application that is the subject of the German lawsuit. This stay of proceedings was granted without an opportunity for Neovasc to respond to CardiAQ's allegations. The Company requested that the stay be lifted, but the request was denied by the European Patent office pending resolution of the German lawsuit. Neovasc filed its response to the German lawsuit in December 2014. The court in Munich is expected to render its decision after a hearing currently scheduled for August 2016.
The Company intends to continue to vigorously defend itself in the litigation with CardiAQ and so the outcome of these matters, including whether the Company will be required to pay some or all of the jury award of US$70 million is not currently determinable. Litigation is inherently uncertain. Therefore, until these matters have been resolved to their ultimate conclusion by the appropriate courts, the Company cannot give any assurances as to the outcome. In addition, the Company's litigation with CardiAQ has been, and is expected to continue to be, costly and time-consuming could divert the attention of management and key personnel from the Company's business operations. If the Company is unsuccessful in its defense of these claims, including any appeal of the verdict in the Massachusetts litigation, or is unable to settle the claims in a manner satisfactory to the Company, it may be faced with significant monetary damages that could exceed its resources, loss of intellectual property rights and damage to its competitive position, which would have a material adverse effect on the Company's business and could potentially threaten the Company's ability to continue as a going concern.
The Company may be subject to lawsuits that could divert its resources and result in the payment of significant damages and other remedies.
As of the date hereof, the Company is aware that a securities class action lawsuit has been filed in the United States District Court for the District of Massachusetts against the Company. The Company intends to vigorously defend itself in this lawsuit. The Company may, in the future, become the subject of further lawsuits. Litigation resulting from these claims could be costly and time-consuming and could divert the attention of management and key personnel from our business operations. We cannot assure that we will succeed in defending any of these claims and those judgments will not be entered against us with respect to the litigation resulting from such claims. If we are unsuccessful in our defense of these claims or unable to settle the claims in manner satisfactory to us, we may be faced with significant monetary damages or injunctive relief against us that could have a material adverse effect on our business and financial condition.
We have a history of negative operating cash flow and may continue to experience negative operating cash flow.
We had negative operating cash flow for the financial years ended December 31, 2015 and December 31, 2014. We anticipate that we will continue to have negative cash flow until such time, if at all, that profitable commercial production is achieved with either the Reducer or Tiara products. To the extent that we have negative cash flow in future periods we may need to allocate a portion of our cash reserves to fund such negative cash flow. We may also be required to raise additional funds through the issuance of equity or debt securities. There can be no assurance that additional capital or other types of financing will be available when needed or that these financings will be on terms favourable to us.
9
Risks Relating to the Offering
There is currently no market through which our securities, other than our common shares, may be sold.
There is currently no market through which our securities, other than our common shares, may be sold and, unless otherwise specified in the applicable prospectus supplement, our preferred shares, debt securities, subscription receipts, units and warrants will not be listed on any securities or stock exchange or any automated dealer quotation system. As a consequence, purchasers may not be able to resell preferred shares, debt securities, subscription receipts, units or warrants purchased under this prospectus. This may affect the pricing of our securities, other than our common shares, in the secondary market, the transparency and availability of trading prices, the liquidity of these securities and the extent of issuer regulation. There can be no assurance that an active trading market for our securities, other than our common shares, will develop or, if developed, that any such market will be sustained.
Our common share price has experienced volatility and may be subject to fluctuation in the future based on market conditions.
The market prices for the securities of medical device companies, including our own, have historically been highly volatile. The market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of any particular company. In addition, because of the nature of our business, certain factors such as our announcements, competition from new therapeutic products or technological innovations, government regulations, fluctuations in our operating results, results of clinical trials, public concern regarding the safety of drugs generally, general market conditions and developments in patent and proprietary rights can have an adverse impact on the market price of our common shares. For example, since June 1, 2015, the closing price of our common shares on the TSX has ranged from a low of C$0.50 to a high of C$9.15 and on the NASDAQ has ranged from a low of U.S.$0.37 to a high of U.S.$7.63.
Any negative change in the public's perception of our prospects could cause the price of our securities, including the price of our common shares, to decrease dramatically. Furthermore, any negative change in the public's perception of the prospects of medical device companies in general could depress the price of our securities, including the price of our common shares, regardless of our results. Following declines in the market price of a company's securities, securities class-action litigation is often instituted. Litigation of this type, if instituted, could result in substantial costs and a diversion of our management's attention and resources.
Future issuances of equity securities by us or sales by our existing shareholders may cause the price of our securities to fall.
The market price of our equity securities could decline as a result of issuances of securities by us or sales by our existing shareholders of common shares in the market, or the perception that these sales could occur, during the currency of this prospectus. Sales of our common shares by shareholders pursuant to this prospectus or otherwise might also make it more difficult for us to sell equity securities at a time and price that we deem appropriate. With an additional sale or issuance of equity securities, investors will suffer dilution of their voting power and may experience dilution in earnings per share.
You may be unable to enforce actions against us, certain of our directors and officers, or the experts named in this prospectus under U.S. federal securities laws.
We are a company continued under the federal laws of Canada. Most of our directors and officers, as well as the experts named in this prospectus, reside principally in Canada. Because all or a substantial portion of our assets and the assets of these persons are located outside of the United States, it may not be possible for you to effect service of process within the United States upon us or those persons. Furthermore, it may not be possible for you to enforce against us or those persons in the United States, judgments obtained in U.S. courts based upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States. There is doubt as to the enforceability, in original actions in Canadian courts, of liabilities based upon U.S. federal securities laws and as to the enforceability in Canadian courts of judgments of U.S. courts obtained in actions based upon the civil liability provisions of the U.S. federal securities laws. Therefore, it may not be possible to enforce those actions against us, certain of our directors and officers or the experts named in this prospectus.
10
The debt securities will be unsecured and will rank equally in right of payment with all of our other future unsecured debt.
The debt securities will be unsecured and will rank equally in right of payment with all of our other existing and future unsecured debt. The debt securities will be effectively subordinated to all of our existing and future secured debt to the extent of the assets securing such debt. If we are involved in any bankruptcy, dissolution, liquidation or reorganization, the secured debt holders would, to the extent of the value of the assets securing the secured debt, be paid before the holders of unsecured debt securities, including the debt securities. In that event, a holder of debt securities may not be able to recover any principal or interest due to it under the debt securities.
We will have broad discretion in the use of the net proceeds of an offering of our securities and may not use them to effectively manage our business.
We will have broad discretion over the use of the net proceeds from an offering of our securities. Because of the number and variability of factors that will determine our use of such proceeds, our ultimate use might vary substantially from our planned use. You may not agree with how we allocate or spend the proceeds from an offering of our securities. We may pursue acquisitions, collaborations or clinical trials that do not result in an increase in the market value of our securities, including the market value of our common shares, and may increase our losses.
We do not intend to pay dividends in the foreseeable future.
We have never declared or paid any dividends on our common shares. We intend, for the foreseeable future, to retain our future earnings, if any, to finance our commercial activities and further research and the expansion of our business. The payment of future dividends, if any, will be reviewed periodically by our board of directors and will depend upon, among other things, conditions then existing including earnings, financial conditions, cash on hand, financial requirements to fund our commercial activities, development and growth, and other factors that our board of directors may consider appropriate in the circumstances.
We may be treated as a passive foreign investment company, which could result in adverse U.S. federal income tax consequences for U.S. investors.
U.S. investors should be aware that they could be subject to certain adverse U.S. federal income tax consequences in the event that we are classified as a passive foreign investment company ("PFIC") for U.S. federal income tax purposes. The determination of whether we are a PFIC for a taxable year depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations, and the determination will depend on the composition of our income, expenses and assets from time to time and the nature of the activities performed by our officers and employees. Prospective investors should carefully read the tax discussion in any applicable prospectus supplement for more information and consult their own tax advisors regarding the likelihood and consequences of the Company being treated as a PFIC for U.S. federal income tax purposes, including the advisability of making certain elections that may mitigate certain possible adverse U.S. federal income tax consequences but may result in an inclusion in gross income without receipt of such income.
Unless we otherwise indicate in a prospectus supplement, we currently intend to use the net proceeds from the sale of our securities to advance our business objectives outlined above under "Our Strategy". More detailed information regarding the use of proceeds from the sale of securities, including any determinable milestones at the applicable time, will be described in any applicable prospectus supplement.
11
The following table sets forth information in respect of our common shares that we issued upon the exercise of options granted under our incentive stock option plan during the previous twelve month period:
Exercise Date
|
Number of Shares | Exercise Price | |||||
|---|---|---|---|---|---|---|---|
May 12, 2015 |
2,000 | C$1.45 | |||||
May 12, 2015 |
4,000 | C$2.49 | |||||
May 12, 2015 |
400 | C$6.50 | |||||
May 20, 2015 |
11,264 | C$0.01 | |||||
June 12, 2015 |
2,688 | C$0.01 | |||||
June 12, 2015 |
200 | C$1.00 | |||||
June 12, 2015 |
1,600 | C$1.45 | |||||
June 12, 2015 |
280 | C$6.50 | |||||
July 9, 2015 |
6,000 | C$1.00 | |||||
July 24, 2015 |
700 | C$2.49 | |||||
August 6, 2015 |
900 | C$2.49 | |||||
August 21, 2015 |
1,250 | C$1.00 | |||||
October 16, 2015 |
12,000 | C$2.49 | |||||
October 16, 2015 |
2,000 | C$2.50 | |||||
November 10, 2015 |
200 | C$0.97 | |||||
November 23, 2015 |
4,500 | C$0.97 | |||||
November 23, 2015 |
100,000 | C$1.00 | |||||
December 10, 2015 |
19,450 | C$0.97 | |||||
December 14, 2015 |
66,000 | C$1.00 | |||||
December 14, 2015 |
7,200 | C$1.45 | |||||
January 5, 2016 |
6,798 | C$0.01 | |||||
January 21, 2016 |
65,000 | C$1.00 | |||||
March 9, 2016 |
600 | C$2.49 | |||||
April 25, 2016 |
1,000 | C$1.00 | |||||
May 3, 2016 |
1,000 | C$1.45 | |||||
May 3, 2016 |
5,000 | C$1.00 | |||||
May 13, 2016 |
11,000 | C$1.45 | |||||
Total |
333,030 | ||||||
The following table sets forth information in respect of options to acquire our common shares that we granted under our incentive stock option plan during the previous twelve month period.
Grant Date
|
Number of Options | Grant Price | |||||
|---|---|---|---|---|---|---|---|
May 14, 2015 |
4,500 | C$8.45 | |||||
June 1, 2015 |
70,500 | C$8.43 | |||||
July 1, 2015 |
97,500 | C$8.55 | |||||
August 1, 2015 |
103,500 | C$7.92 | |||||
September 1, 2015 |
28,500 | C$7.38 | |||||
October 1, 2015 |
68,500 | C$7.13 | |||||
November 2, 2015 |
12,000 | C$7.19 | |||||
December 1, 2015 |
75,000 | C$6.70 | |||||
December 28, 2015 |
356,630 | C$5.19 | |||||
February 1, 2016 |
109,061 | C$4.94 | |||||
April 1, 2016 |
35,000 | C$5.47 | |||||
May 1, 2016 |
26,000 | C$3.99 | |||||
Total |
886,691 | ||||||
12
No other common shares, preferred shares, debt securities or warrants, or securities exchangeable or convertible into common shares, preferred shares, debt securities or warrants have been issued during the twelve month period preceding the date of this prospectus.
Our common shares are listed on the TSX in Canada (trading symbol: NVC). The following table sets forth, for the periods indicated, the reported high and low prices (in Canadian dollars) and volume traded on the TSX.
Month
|
High | Low | Close | Volume | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
June, 2015 |
C$ | 9.15 | C$ | 8.07 | C$ | 8.55 | 38,258 | ||||||
July, 2015 |
C$ | 8.96 | C$ | 6.55 | C$ | 7.92 | 41,755 | ||||||
August, 2015 |
C$ | 7.69 | C$ | 5.00 | C$ | 7.38 | 37,678 | ||||||
September, 2015 |
C$ | 8.63 | C$ | 6.96 | C$ | 7.13 | 54,212 | ||||||
October, 2015 |
C$ | 8.48 | C$ | 5.23 | C$ | 7.19 | 78,667 | ||||||
November, 2015 |
C$ | 7.35 | C$ | 5.31 | C$ | 6.70 | 102,061 | ||||||
December, 2015 |
C$ | 6.89 | C$ | 4.81 | C$ | 6.25 | 53,172 | ||||||
January, 2016 |
C$ | 6.91 | C$ | 4.20 | C$ | 4.94 | 32,665 | ||||||
February, 2016 |
C$ | 5.505 | C$ | 4.40 | C$ | 5.40 | 35,898 | ||||||
March, 2016 |
C$ | 6.07 | C$ | 4.90 | C$ | 5.47 | 107,838 | ||||||
April, 2016 |
C$ | 5.73 | C$ | 3.63 | C$ | 3.99 | 132,556 | ||||||
May, 2016 |
C$ | 4.25 | C$ | 0.50 | C$ | 0.53 | 1,687,331 | ||||||
June 1 – 8, 2016 |
C$ | 0.73 | C$ | 0.51 | C$ | 0.56 | 1,697,468 | ||||||
Our Common Shares began trading on NASDAQ on May 21, 2014. The following table sets forth, for the periods indicated, the reported high, low and closing prices (in U.S. dollars) and volume traded on NASDAQ.
Month
|
High | Low | Close | Volume | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
June, 2015 |
U.S.$ | 7.63 | U.S.$ | 6.55 | U.S.$ | 6.84 | 1,310,214 | ||||||
July, 2015 |
U.S.$ | 6.94 | U.S.$ | 5.03 | U.S.$ | 5.90 | 852,264 | ||||||
August, 2015 |
U.S.$ | 6.33 | U.S.$ | 3.66 | U.S.$ | 5.67 | 1,161,751 | ||||||
September, 2015 |
U.S.$ | 6.57 | U.S.$ | 4.60 | U.S.$ | 4.91 | 928,040 | ||||||
October, 2015 |
U.S.$ | 6.48 | U.S.$ | 3.74 | U.S.$ | 5.55 | 1,326,278 | ||||||
November, 2015 |
U.S.$ | 5.74 | U.S.$ | 4.00 | U.S.$ | 4.98 | 740,632 | ||||||
December, 2015 |
U.S.$ | 5.03 | U.S.$ | 3.41 | U.S.$ | 4.50 | 1,035,303 | ||||||
January, 2016 |
U.S.$ | 4.94 | U.S.$ | 2.88 | U.S.$ | 3.42 | 588,256 | ||||||
February, 2016 |
U.S.$ | 4.09 | U.S.$ | 3.01 | U.S.$ | 3.91 | 408,787 | ||||||
March, 2016 |
U.S.$ | 4.68 | U.S.$ | 3.77 | U.S.$ | 4.18 | 598,118 | ||||||
April, 2016 |
U.S.$ | 4.31 | U.S.$ | 2.95 | U.S.$ | 3.23 | 1,091,204 | ||||||
May, 2016 |
U.S.$ | 3.49 | U.S.$ | 0.37 | U.S.$ | 0.39 | 12,467,478 | ||||||
June 1 – 8, 2016 |
U.S.$ | 0.59 | U.S.$ | 0.40 | U.S.$ | 0.44 | 21,991,323 | ||||||
If we offer debt securities having a term to maturity in excess of one year or preferred shares under this prospectus and any applicable prospectus supplement, the applicable prospectus supplement will include earnings coverage ratios giving effect to the issuance of such securities.
Since March 31, 2015, the date of our financial statements for the most recently completed financial period, there have been no material changes in our consolidated share and loan capital other than as outlined under "Prior Sales". For information on the issuance of shares pursuant to the exercise of options pursuant to our incentive stock option plan and common share purchase warrants, see "Prior Sales".
13
Our authorized share capital consists of an unlimited number of common shares and an unlimited number of preferred shares. As of the date of this prospectus, we had 66,855,345 common shares and nil preferred shares of any series issued and outstanding. In addition, as of the date of this prospectus, there were 8,185,090 common shares issuable upon the exercise of outstanding stock options and no outstanding common share purchase warrants.
Common Shares
All of our common shares are of the same class and, once issued, rank equally as to entitlement to dividends, voting powers (one vote per common share) and participation in assets upon dissolution, liquidation or winding-up. No common shares have been issued subject to call or assessment. Our common shares contain no pre-emptive or conversion rights and have no provisions for redemption or purchase for cancellation, surrender, or sinking or purchase funds. Provisions as to the modification, amendment or variation of such rights or provisions are contained in our articles and by-laws and in the CBCA.
Preferred Shares
We may issue our preferred shares from time to time in one or more series. The terms of each series of preferred shares, including the number of shares, the designation, rights, preferences, privileges, priorities, restrictions, conditions and limitations, will be determined at the time of creation of each such series by our board of directors, without shareholder approval, provided that all preferred shares will rank equally within their class as to dividends and distributions in the event of our dissolution, liquidation or winding-up.
DESCRIPTION OF DEBT SECURITIES
In this description of debt securities, "we", "us", "our" or "Neovasc" refer to Neovasc Inc., but not to its subsidiaries. This section describes the general terms that will apply to any debt securities issued pursuant to this prospectus. We may issue debt securities in one or more series under an indenture, or the indenture, to be entered into between us and one or more trustees. The indenture will be subject to and governed by the United States Trust Indenture Act of 1939, as amended (the "Trust Indenture Act") and the CBCA. A copy of the form of the indenture will be filed with the SEC as an exhibit to the registration statement of which this prospectus forms a part. The following description sets forth certain general terms and provisions of the debt securities and is not intended to be complete. For a more complete description, prospective investors should refer to the indenture and the terms of the debt securities. If debt securities are issued, we will describe in the applicable prospectus supplement the particular terms and provisions of any series of the debt securities and a description of how the general terms and provisions described below may apply to that series of the debt securities. Prospective investors should rely on information in the applicable prospectus supplement and not on the following information to the extent that the information in such prospectus supplement is different from the following information.
We may issue debt securities and incur additional indebtedness other than through the offering of debt securities pursuant to this prospectus.
General
The indenture will not limit the aggregate principal amount of debt securities that we may issue under the indenture and will not limit the amount of other indebtedness that we may incur. The indenture will provide that we may issue debt securities from time to time in one or more series and may be denominated and payable in U.S. dollars, Canadian dollars or any foreign currency. Unless otherwise indicated in the applicable prospectus supplement, the debt securities will be our unsecured obligations. The indenture will also permit us to increase the principal amount of any series of the debt securities previously issued and to issue that increased principal amount.
14
The applicable prospectus supplement for any series of debt securities that we offer will describe the specific terms of the debt securities and may include, but is not limited to, any of the following:
- •
- the title of the debt securities;
- •
- the aggregate principal amount of the debt securities;
- •
- the percentage of principal amount at which the debt securities will be issued;
- •
- whether payment on the debt securities will be senior or subordinated to our other liabilities or obligations;
- •
- whether the payment of the debt securities will be guaranteed by any other person;
- •
- the date or dates, or the methods by which such dates will be determined or extended, on which we may issue the debt
securities and the date or dates, or the methods by which such dates will be determined or extended, on which we will pay the principal and any premium on the debt securities and the portion
(if less than the principal amount) of debt securities to be payable upon a declaration of acceleration of maturity;
- •
- whether the debt securities will bear interest, the interest rate (whether fixed or variable) or the method of determining
the interest rate, the date from which interest will accrue, the dates on which we will pay interest and the record dates for interest payments, or the methods by which such dates will be determined
or extended;
- •
- the place or places we will pay principal, premium, if any, and interest and the place or places where debt securities can
be presented for registration of transfer or exchange;
- •
- whether and under what circumstances we will be required to pay any additional amounts for withholding or deduction for
Canadian taxes with respect to the debt securities, and whether and on what terms we will have the option to redeem the debt securities rather than pay the additional amounts;
- •
- whether we will be obligated to redeem or repurchase the debt securities pursuant to any sinking or purchase fund or other
provisions, or at the option of a holder and the terms and conditions of such redemption;
- •
- whether we may redeem the debt securities at our option and the terms and conditions of any such redemption;
- •
- the denominations in which we will issue any registered debt securities, if other than denominations of U.S.$1,000 and any
multiple of U.S.$l,000 and, if other than denominations of U.S.$5,000, the denominations in which any unregistered debt security shall be issuable;
- •
- whether we will make payments on the debt securities in a currency or currency unit other than U.S. dollars or by
delivery of our common shares or other property;
- •
- whether payments on the debt securities will be payable with reference to any index or formula;
- •
- whether we will issue the debt securities as global securities and, if so, the identity of the depositary for the global
securities;
- •
- whether we will issue the debt securities as unregistered securities (with or without coupons), registered securities
or both;
- •
- the periods within which and the terms and conditions, if any, upon which we may redeem the debt securities prior to
maturity and the price or prices of which and the currency or currency units in which the debt securities are payable;
- •
- any changes or additions to events of default or covenants;
- •
- the applicability of, and any changes or additions to, the provisions for defeasance described under "Defeasance" below;
- •
- whether the holders of any series of debt securities have special rights if specified events occur;
15
- •
- any mandatory or optional redemption or sinking fund or analogous provisions;
- •
- the terms, if any, for any conversion or exchange of the debt securities for any other securities;
- •
- rights, if any, on a change of control;
- •
- provisions as to modification, amendment or variation of any rights or terms attaching to the debt securities; and
- •
- any other terms, conditions, rights and preferences (or limitations on such rights and preferences) including covenants and events of default which apply solely to a particular series of the debt securities being offered which do not apply generally to other debt securities, or any covenants or events of default generally applicable to the debt securities which do not apply to a particular series of the debt securities.
Unless stated otherwise in the applicable prospectus supplement, no holder of debt securities will have the right to require us to repurchase the debt securities and there will be no increase in the interest rate if we become involved in a highly leveraged transaction or we have a change of control.
We may issue debt securities bearing no interest or interest at a rate below the prevailing market rate at the time of issuance, and offer and sell these securities at a discount below their stated principal amount. We may also sell any of the debt securities for a foreign currency or currency unit, and payments on the debt securities may be payable in a foreign currency or currency unit. In any of these cases, we will describe certain Canadian federal and U.S. federal income tax consequences and other special considerations in the applicable prospectus supplement.
We may issue debt securities with terms different from those of debt securities previously issued and, without the consent of the holders thereof, we may reopen a previous issue of a series of debt securities and issue additional debt securities of such series (unless the reopening was restricted when such series was created).
Ranking and Other Indebtedness
Unless otherwise indicated in an applicable prospectus supplement, our debt securities will be unsecured obligations and will rank equally with all of our other unsecured and unsubordinated debt from time to time outstanding and equally with other securities issued under the indenture. The debt securities will be structurally subordinated to all existing and future liabilities, including trade payables, of our subsidiaries.
Our board of directors may establish the extent and manner, if any, to which payment on or in respect of a series of debt securities will be senior or will be subordinated to the prior payment of our other liabilities and obligations and whether the payment of principal, premium, if any, and interest, if any, will be guaranteed by any other person and the nature and priority of any security.
Debt Securities in Global Form
The Depositary and Book-Entry
Unless otherwise specified in the applicable prospectus supplement, a series of the debt securities may be issued in whole or in part in global form as a "global security" and will be registered in the name of and be deposited with a depositary, or its nominee, each of which will be identified in the applicable prospectus supplement relating to that series. Unless and until exchanged, in whole or in part, for the debt securities in definitive registered form, a global security may not be transferred except as a whole by the depositary for such global security to a nominee of the depositary, by a nominee of the depositary to the depositary or another nominee of the depositary or by the depositary or any such nominee to a successor of the depositary or a nominee of the successor.
The specific terms of the depositary arrangement with respect to any portion of a particular series of the debt securities to be represented by a global security will be described in the applicable prospectus supplement relating to such series. We anticipate that the provisions described in this section will apply to all depositary arrangements.
16
Upon the issuance of a global security, the depositary therefor or its nominee will credit, on its book entry and registration system, the respective principal amounts of the debt securities represented by the global security to the accounts of such persons, designated as "participants", having accounts with such depositary or its nominee. Such accounts shall be designated by the underwriters, dealers or agents participating in the distribution of the debt securities or by us if such debt securities are offered and sold directly by us. Ownership of beneficial interests in a global security will be limited to participants or persons that may hold beneficial interests through participants. Ownership of beneficial interests in a global security will be shown on, and the transfer of that ownership will be effected only through, records maintained by the depositary therefor or its nominee (with respect to interests of participants) or by participants or persons that hold through participants (with respect to interests of persons other than participants). The laws of some states in the United States may require that certain purchasers of securities take physical delivery of such securities in definitive form.
So long as the depositary for a global security or its nominee is the registered owner of the global security, such depositary or such nominee, as the case may be, will be considered the sole owner or holder of the debt securities represented by the global security for all purposes under the indenture. Except as provided below, owners of beneficial interests in a global security will not be entitled to have a series of the debt securities represented by the global security registered in their names, will not receive or be entitled to receive physical delivery of such series of the debt securities in definitive form and will not be considered the owners or holders thereof under the indenture.
Any payments of principal, premium, if any, and interest, if any, on global securities registered in the name of a depositary or its nominee will be made to the depositary or its nominee, as the case may be, as the registered owner of the global security representing such debt securities. None of us, the trustee or any paying agent for the debt securities represented by the global securities will have any responsibility or liability for any aspect of the records relating to or payments made on account of beneficial ownership interests of the global security or for maintaining, supervising or reviewing any records relating to such beneficial ownership interests.
We expect that the depositary for a global security or its nominee, upon receipt of any payment of principal, premium, if any, or interest, if any, will credit participants' accounts with payments in amounts proportionate to their respective beneficial interests in the principal amount of the global security as shown on the records of such depositary or its nominee. We also expect that payments by participants to owners of beneficial interests in a global security held through such participants will be governed by standing instructions and customary practices, as is now the case with securities held for the accounts of customers registered in "street name", and will be the responsibility of such participants.
Discontinuance of Depositary's Services
If a depositary for a global security representing a particular series of the debt securities is at any time unwilling or unable to continue as depositary and a successor depositary is not appointed by us within 90 days, we will issue such series of the debt securities in definitive form in exchange for a global security representing such series of the debt securities. If an event of default under the indenture has occurred and is continuing, debt securities in definitive form will be printed and delivered upon written request by the holder to the trustee. In addition, we may at any time and in our sole discretion determine not to have a series of the debt securities represented by a global security and, in such event, will issue a series of the debt securities in definitive form in exchange for all of the global securities representing that series of debt securities.
Debt Securities in Definitive Form
A series of the debt securities may be issued in definitive form, solely as registered securities, solely as unregistered securities or as both registered securities and unregistered securities. Registered securities will be issuable in denominations of U.S.$1,000 and integral multiples of U.S.$1,000 and unregistered securities will be issuable in denominations of U.S.$5,000 and integral multiples of U.S.$5,000 or, in each case, in such other denominations as may be set out in the terms of the debt securities of any particular series. Unless otherwise indicated in the applicable prospectus supplement, unregistered securities will have interest coupons attached.
Unless otherwise indicated in the applicable prospectus supplement, payment of principal, premium, if any, and interest, if any, on the debt securities (other than global securities) will be made at the office or agency of
17
the trustee, or at our option we can pay principal, interest, if any, and premium, if any, by check mailed or delivered to the address of the person entitled at the address appearing in the security register of the trustee or electronic funds wire or other transmission to an account of the person entitled to receive payments. Unless otherwise indicated in the applicable prospectus supplement, payment of interest, if any, will be made to the persons in whose name the debt securities are registered at the close of business on the day or days specified by us.
At the option of the holder of debt securities, registered securities of any series will be exchangeable for other registered securities of the same series, of any authorized denomination and of a like aggregate principal amount and tenor. If, but only if, provided in an applicable prospectus supplement, unregistered securities (with all unmatured coupons, except as provided below, and all matured coupons in default) of any series may be exchanged for registered securities of the same series, of any authorized denominations and of a like aggregate principal amount and tenor. In such event, unregistered securities surrendered in a permitted exchange for registered securities between a regular record date or a special record date and the relevant date for payment of interest shall be surrendered without the coupon relating to such date for payment of interest, and interest will not be payable on such date for payment of interest in respect of the registered security issued in exchange for such unregistered security, but will be payable only to the holder of such coupon when due in accordance with the terms of the indenture. Unless otherwise specified in an applicable prospectus supplement, unregistered securities will not be issued in exchange for registered securities.
The applicable prospectus supplement may indicate the places to register a transfer of the debt securities in definitive form. Except for certain restrictions set forth in the indenture, no service charge will be payable by the holder for any registration of transfer or exchange of the debt securities in definitive form, but we may, in certain instances, require a sum sufficient to cover any tax or other governmental charges payable in connection with these transactions.
We shall not be required to:
- •
- issue, register the transfer of or exchange any series of the debt securities in definitive form during a period beginning
at the opening of business 15 days before any selection of securities of that series of the debt securities to be redeemed and ending on the relevant redemption date if the debt securities for
which such issuance, registration or exchange is requested may be among those selected for redemption;
- •
- register the transfer of or exchange any registered security in definitive form, or portion thereof, called for
redemption, except the unredeemed portion of any registered security being redeemed in part;
- •
- exchange any unregistered security called for redemption except to the extent that such unregistered security may be
exchanged for a registered security of that series and like tenor; provided that such registered security will be simultaneously surrendered for redemption with written instructions for payment
consistent with the provisions of the indenture; or
- •
- issue, register the transfer of or exchange any of the debt securities in definitive form which have been surrendered for repayment at the option of the holder, except the portion, if any, thereof not to be so repaid.
Merger, Amalgamation or Consolidation
The indenture will provide that we may not consolidate with or amalgamate or merge with or into any other person, enter into any statutory arrangement with any person or convey, transfer or lease our properties and assets substantially as an entirety to another person, unless among other items:
- •
- we are the surviving person, or the resulting, surviving or transferee person, if other than us, is organized and existing
under the laws of the United States, any state thereof or the District of Columbia, Canada, or any province or territory thereof, or, if the amalgamation, merger, consolidation, statutory
arrangement or other transaction would not impair the rights of holders, any other country;
- •
- the successor person (if not us) assumes all of our obligations under the debt securities and the indenture; and
18
- •
- we or such successor person will not be in default under the indenture immediately after the transaction.
When such a person assumes our obligations in such circumstances, subject to certain exceptions, we shall be discharged from all obligations under the debt securities and the indenture.
Provision of Financial Information
We will file with the trustee, within 20 days after we file or furnish them with the SEC, copies of our annual reports and of the information, documents and other reports (or copies of such portions of any of the foregoing as the SEC may by rules and regulations prescribe) which we are required to file or furnish with the SEC pursuant to Section 13 or 15(d) of the Exchange Act.
Notwithstanding that we may not remain subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, we will continue to provide the trustee:
- •
- within 20 days after the time periods required for the filing or furnishing of such forms by the SEC, annual
reports on Form 40-F or Form 20-F, as applicable, or any successor form; and
- •
- within 20 days after the time periods required for the filing of such forms by the SEC, reports on Form 6-K (or any successor form), which, regardless of applicable requirements shall, at a minimum, contain such information required to be provided in quarterly reports under the laws of Canada or any province thereof to security holders of a company with securities listed on the TSX, whether or not we have any of the debt securities listed on such exchange. Each of such reports, to the extent permitted by the rules and regulations of the SEC, will be prepared in accordance with Canadian disclosure requirements and generally accepted accounting principles provided, however, that we shall not be obligated to file or furnish such reports with the SEC if the SEC does not permit such filings.
Events of Default
Unless otherwise specified in the applicable prospectus supplement relating to a particular series of debt securities, the following is a summary of events which will, with respect to any series of the debt securities, constitute an event of default under the indenture with respect to the debt securities of that series:
- •
- we fail to pay principal of, or any premium on, any debt security of that series when it is due and payable;
- •
- we fail to pay interest or any additional amounts payable on any debt security of that series when it becomes due and
payable, and such default continues for 30 days;
- •
- we fail to make any required sinking fund or analogous payment for that series of debt securities;
- •
- we fail to observe or perform any of the covenants described in the section "— Merger,
Amalgamation or Consolidation" for a period of 30 days;
- •
- we fail to comply with any of our other agreements in the indenture that affect or are applicable to the debt securities
for 60 days after written notice by the trustee or to us and the trustee by holders of at least 25% in aggregate principal amount of the outstanding debt securities of any series affected
thereby;
- •
- a default (as defined in any indenture or instrument under which we or one of our subsidiaries has at the time of the indenture relating to this prospectus or will thereafter have outstanding any indebtedness) has occurred and is continuing, or we or any of our subsidiaries has failed to pay principal amounts with respect to such indebtedness at maturity and such event of default or failure to pay has resulted in such indebtedness under such indentures or instruments being declared due, payable or otherwise being accelerated, in either event so that an amount in excess of the greater of U.S.$5,000,000 and 2% of our shareholders' equity will be or become due, payable and accelerated upon such declaration or prior to the date on which the same would otherwise have become due, payable and accelerated, or the accelerated indebtedness, and such acceleration will not be rescinded or annulled, or such event of default or failure to pay under such indenture or instrument will not be remedied or cured, whether by payment or otherwise, or waived by the holders of such accelerated indebtedness, then (i) if the accelerated
19
- •
- certain events involving our bankruptcy, insolvency or reorganization; and
- •
- any other event of default provided for in that series of debt securities.
indebtedness will be as a result of an event of default which is not related to the failure to pay principal or interest on the terms, at the times, and on the conditions set out in any such indenture or instrument, it will not be considered an event of default for the purposes of the indenture governing the debt securities relating to this prospectus until 30 days after such indebtedness has been accelerated, or (ii) if the accelerated indebtedness will occur as a result of such failure to pay principal or interest or as a result of an event of default which is related to the failure to pay principal or interest on the terms, at the times, and on the conditions set out in any such indenture or instrument, then (A) if such accelerated indebtedness is, by its terms, non-recourse to us or our subsidiaries, it will be considered an event of default for purposes of the indenture governing the debt securities relating to this prospectus; or (B) if such accelerated indebtedness is recourse to us or our subsidiaries, any requirement in connection with such failure to pay or event of default for the giving of notice or the lapse of time or the happening of any further condition, event or act under such indenture or instrument in connection with such failure to pay or event of default will be applicable together with an additional seven days before being considered an event of default for the purposes of the indenture relating to this prospectus;
A default under one series of debt securities will not necessarily be a default under another series. The trustee may withhold notice to the holders of the debt securities of any default, except in the payment of principal or premium, if any, or interest, if any, if in good faith it considers it in the interests of the holders to do so.
If an event of default for any series of debt securities occurs and continues, the trustee or the holders of at least 25% in aggregate principal amount of the debt securities of that series, subject to any subordination provisions, may require us to repay immediately:
- •
- the entire principal and interest and premium, if any, of the debt securities of the series; or
- •
- if the debt securities are discounted securities, that portion of the principal as is described in the applicable prospectus supplement.
If an event of default relates to events involving our bankruptcy, insolvency or reorganization, the principal of all debt securities will become immediately due and payable without any action by the trustee or any holder. Subject to certain conditions, the holders of a majority of the aggregate principal amount of the debt securities of the affected series can rescind this accelerated payment requirement. If debt securities are discounted securities, the applicable prospectus supplement will contain provisions relating to the acceleration of maturity of a portion of the principal amount of the discounted securities upon the occurrence or continuance of an event of default.
Other than its duties in case of a default, the trustee is not obligated to exercise any of the rights or powers that it will have under the indenture at the request, order or direction of any holders, unless the holders offer the trustee reasonable indemnity. If they provide this reasonable indemnity, the holders of a majority in aggregate principal amount of any series of debt securities may, subject to certain limitations, direct the time, method and place of conducting any proceeding or any remedy available to the trustee, or exercising any power conferred upon the trustee, for any series of debt securities.
We will be required to furnish to the trustee a statement annually as to our compliance with all conditions and covenants under the indenture and, if we are not in compliance, we must specify any defaults. We will also be required to notify the trustee as soon as practicable upon becoming aware of any event of default.
No holder of a debt security of any series will have any right to institute any proceeding with respect to the indenture, or for the appointment of a receiver or a trustee, or for any other remedy, unless:
- •
- the holder has previously given to the trustee written notice of a continuing event of default with respect to the debt securities of the affected series;
20
- •
- the holders of at least 25% in principal amount of the outstanding debt securities of the series affected by an event of
default have made a written request, and the holders have offered reasonable indemnity, to the trustee to institute a proceeding as trustee; and
- •
- the trustee has failed to institute a proceeding, and has not received from the holders of a majority in aggregate principal amount of the outstanding debt securities of the series affected by an event of default a direction inconsistent with the request, within 60 days after their notice, request and offer of indemnity.
However, such above-mentioned limitations do not apply to a suit instituted by the holder of a debt security for the enforcement of payment of the principal of or any premium, if any, or interest on such debt security on or after the applicable due date specified in such debt security.
Defeasance
When we use the term "defeasance", we mean discharge from some or all of our obligations under the indenture. Unless otherwise specified in the applicable prospectus supplement, if we deposit with the trustee sufficient cash or government securities to pay the principal, interest, if any, premium, if any, and any other sums due to the stated maturity date or a redemption date of the debt securities of a series, then at our option:
- •
- we will be discharged from the obligations with respect to the debt securities of that series; or
- •
- we will no longer be under any obligation to comply with certain restrictive covenants under the indenture, and certain events of default will no longer apply to us.
If this happens, the holders of the debt securities of the affected series will not be entitled to the benefits of the indenture except for registration of transfer and exchange of debt securities and the replacement of lost, stolen or mutilated debt securities. These holders may look only to the deposited fund for payment on their debt securities.
To exercise our defeasance option, we must deliver to the trustee:
- •
- an opinion of counsel in the United States to the effect that the holders of the outstanding debt securities of the
affected series will not recognize a gain or loss for U.S. federal income tax purposes as a result of a defeasance and will be subject to U.S. federal income tax on the same amounts, in
the same manner and at the same times as would have been the case if the defeasance had not occurred;
- •
- an opinion of counsel in Canada or a ruling from the Canada Revenue Agency to the effect that the holders of the
outstanding debt securities of the affected series will not recognize income, or a gain or loss for Canadian federal, provincial or territorial income or other tax purposes as a result of a defeasance
and will be subject to Canadian federal, provincial or territorial income tax and other tax on the same amounts, in the same manner and at the same times as would have been the case had the defeasance
not occurred; and
- •
- a certificate of one of our officers and an opinion of counsel, each stating that all conditions precedent provided for relating to defeasance have been complied with.
If we are to be discharged from our obligations with respect to the debt securities, and not just from our covenants, the U.S. opinion must be based upon a ruling from or published by the United States Internal Revenue Service or a change in law to that effect.
In addition to the delivery of the opinions described above, the following conditions must be met before we may exercise our defeasance option:
- •
- no event of default or event that, with the passing of time or the giving of notice, or both, shall constitute an event of
default shall have occurred and be continuing for the debt securities of the affected series;
- •
- we are not an "insolvent person" within the meaning of applicable bankruptcy and insolvency legislation; and
- •
- other customary conditions precedent are satisfied.
21
Modification and Waiver
Modifications and amendments of the indenture may be made by us and the trustee with the consent of the holders of a majority in aggregate principal amount of the outstanding debt securities of each series affected by the modification. However, without the consent of each holder affected, no modification may:
- •
- change the stated maturity of the principal of, premium, if any, or any installment of interest, if any, on any
debt security;
- •
- reduce the principal, premium, if any, or rate of interest, if any, or any obligation to pay any additional amounts;
- •
- reduce the amount of principal of a debt security payable upon acceleration of its maturity;
- •
- change the place or currency of any payment;
- •
- affect the holder's right to require us to repurchase the debt securities at the holder's option;
- •
- impair the right of the holders to institute a suit to enforce their rights to payment;
- •
- adversely affect any conversion or exchange right related to a series of debt securities;
- •
- change the percentage of debt securities required to modify the indenture or to waive compliance with certain provisions
of the indenture; or
- •
- reduce the percentage in principal amount of outstanding debt securities necessary to take certain actions.
The holders of a majority in principal amount of outstanding debt securities of any series may on behalf of the holders of all debt securities of that series waive, insofar as only that series is concerned, past defaults under the indenture and compliance by us with certain restrictive provisions of the indenture. However, these holders may not waive a default in any payment on any debt security or compliance with a provision that cannot be modified without the consent of each holder affected.
We may modify the indenture without the consent of the holders to:
- •
- evidence our successor under the indenture;
- •
- add covenants or surrender any right or power for the benefit of holders;
- •
- add events of default;
- •
- provide for unregistered securities to become registered securities under the indenture and make other such changes to
unregistered securities that in each case do not materially and adversely affect the interests of holders of outstanding securities;
- •
- establish the forms of the debt securities;
- •
- appoint a successor trustee under the indenture;
- •
- add provisions to permit or facilitate the defeasance or discharge of the debt securities as long as there is no material
adverse effect on the holders;
- •
- cure any ambiguity, correct or supplement any defective or inconsistent provision, make any other provisions in each case
that would not materially and adversely affect the interests of holders of outstanding securities and related coupons, if any;
- •
- comply with any applicable laws of the United States and Canada in order to effect and maintain the qualification
of the indenture under the Trust Indenture Act; or
- •
- change or eliminate any provisions where such change takes effect when there are no securities outstanding under the indenture.
22
Governing Law
The indenture and the debt securities will be governed by and construed in accordance with the laws of the State of New York.
The Trustee
The trustee under the indenture or its affiliates may provide banking and other services to us in the ordinary course of their business.
The indenture will contain certain limitations on the rights of the trustee, as long as it or any of its affiliates remains our creditor, to obtain payment of claims in certain cases or to realize on certain property received on any claim as security or otherwise. The trustee and its affiliates will be permitted to engage in other transactions with us. If the trustee or any affiliate acquires any conflicting interest and a default occurs with respect to the debt securities, the trustee must eliminate the conflict or resign.
Resignation of Trustee
The trustee may resign or be removed with respect to one or more series of the debt securities and a successor trustee may be appointed to act with respect to such series. In the event that two or more persons are acting as trustee with respect to different series of debt securities, each such trustee shall be a trustee of a trust under the indenture separate and apart from the trust administered by any other such trustee, and any action described herein to be taken by the "trustee" may then be taken by each such trustee with respect to, and only with respect to, the one or more series of debt securities for which it is trustee.
Consent to Service
In connection with the indenture, we will designate and appoint CT Corporation System, 111 Eighth Avenue, New York, New York, 10011, as our authorized agent upon which process may be served in any suit or proceeding arising out of or relating to the indenture or the debt securities that may be instituted in any U.S. federal or New York state court located in the Borough of Manhattan, in the City of New York, or brought by the trustee (whether in its individual capacity or in its capacity as trustee under the indenture), and will irrevocably submit to the non-exclusive jurisdiction of such courts.
Enforceability of Judgments
Since all or substantially all of our assets, as well as the assets of some of our directors and officers, are outside the United States, any judgment obtained in the United States against us or certain of our directors or officers, including judgments with respect to the payment of principal on the debt securities, may not be collectible within the United States.
We have been advised that the laws of the Province of British Columbia and the federal laws of Canada applicable therein permit an action to be brought against us in a court of competent jurisdiction in the Province of British Columbia on any final and conclusive judgment in personam of any federal or state court located in the State of New York, or a New York Court, which is subsisting and unsatisfied for a sum certain with respect to the enforcement of the indenture and the debt securities that is not impeachable as void or voidable under the internal laws of the State of New York if: (1) the New York Court rendering such judgment had jurisdiction over the judgment debtor, as recognized by the courts of the Province of British Columbia (and submission by us in the indenture to the jurisdiction of the New York Court will be sufficient for that purpose); (2) proper service of process in respect of the proceedings in which such judgment was obtained was made in accordance with New York law; (3) such judgment was not obtained by fraud or in a manner contrary to natural justice and the enforcement thereof would not be inconsistent with public policy, as such terms are understood under the laws of the Province of British Columbia, the federal laws of Canada or contrary to any order made by the Attorney General of Canada and under the Foreign Extraterritorial Measures Act (Canada) or by the Competition Tribunal under the Competition Act (Canada); (4) the enforcement of such judgment would not be contrary to the laws of general application limiting the enforcement of creditors' rights, including bankruptcy, reorganization, winding-up, moratorium and similar laws, and does not constitute, directly or indirectly, the enforcement of
23
foreign laws which a court in the Province of British Columbia would characterize as revenue, expropriatory or penal laws; (5) in an action to enforce a default judgment, the judgment does not contain a manifest error on its face; (6) the action to enforce such judgment is commenced within the appropriate limitation period; (7) interest payable on the debt securities is not characterized by a court in the Province of British Columbia as interest payable at a criminal rate within the meaning of Section 347 of the Criminal Code (Canada); and (8) the judgment does not conflict with another final and conclusive judgment in the same cause of action; except that a court in the Province of British Columbia may stay an action to enforce a foreign judgment if an appeal of a judgment is pending or time for appeal has not expired; and except that any court in the Province of British Columbia may give judgment only in Canadian dollars.
We have been advised that there is doubt as to the enforceability in Canada by a court in original actions, or in actions to enforce judgments of U.S. courts, of civil liabilities predicated solely upon the U.S. federal securities laws.
General
This section describes the general terms that will apply to any warrants for the purchase of common shares, or equity warrants, or for the purchase of debt securities, or debt warrants.
We may issue warrants independently or together with other securities, and warrants sold with other securities may be attached to or separate from the other securities. Warrants will be issued under one or more warrant indentures or warrant agency agreements to be entered into by us and one or more banks or trust companies acting as warrant agent.
This summary of some of the provisions of the warrants is not complete. The statements made in this prospectus relating to any warrant agreement and warrants to be issued under this prospectus are summaries of certain anticipated provisions thereof and do not purport to be complete and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable warrant agreement. You should refer to the warrant indenture or warrant agency agreement relating to the specific warrants being offered for the complete terms of the warrants. A copy of any warrant indenture or warrant agency agreement relating to an offering or warrants will be filed by us with the securities regulatory authorities in applicable Canadian offering jurisdictions and the United States after we have entered into it.
The applicable prospectus supplement relating to any warrants that we offer will describe the particular terms of those warrants and include specific terms relating to the offering.
Original purchasers of warrants (if offered separately) will have a contractual right of rescission against us in respect of the exercise of such warrant. The contractual right of rescission will entitle such original purchasers to receive, upon surrender of the underlying securities acquired upon exercise of the warrant, the total of the amount paid on original purchase of the warrant and the amount paid upon exercise, in the event that this prospectus (as supplemented or amended) contains a misrepresentation, provided that: (i) the exercise takes place within 180 days of the date of the purchase of the warrant under the applicable prospectus supplement; and (ii) the right of rescission is exercised within 180 days of the date of purchase of the warrant under the applicable prospectus supplement. This contractual right of rescission will be consistent with the statutory right of rescission described under section 131 of the Securities Act (British Columbia), and is in addition to any other right or remedy available to original purchasers under section 131 of the Securities Act (British Columbia) or otherwise at law.
Original purchasers are further advised that in certain provinces the statutory right of action for damages in connection with a prospectus misrepresentation is limited to the amount paid for the security that was purchased under a prospectus, and therefore a further payment at the time of exercise may not be recoverable in a statutory action for damages. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser's province for the particulars of these rights, or consult with a legal advisor.
24
Equity Warrants
The particular terms of each issue of equity warrants will be described in the applicable prospectus supplement. This description will include, where applicable:
- •
- the designation and aggregate number of equity warrants;
- •
- the price at which the equity warrants will be offered;
- •
- the currency or currencies in which the equity warrants will be offered;
- •
- the date on which the right to exercise the equity warrants will commence and the date on which the right
will expire;
- •
- the number of common shares that may be purchased upon exercise of each equity warrant and the price at which and currency
or currencies in which the common shares may be purchased upon exercise of each equity warrant;
- •
- the terms of any provisions allowing or providing for adjustments in (i) the number and/or class of shares that may
be purchased, (ii) the exercise price per share or (iii) the expiry of the equity warrants;
- •
- whether we will issue fractional shares;
- •
- whether we have applied to list the equity warrants or the underlying shares on a stock exchange;
- •
- the designation and terms of any securities with which the equity warrants will be offered, if any, and the number of the
equity warrants that will be offered with each security;
- •
- the date or dates, if any, on or after which the equity warrants and the related securities will be transferable
separately;
- •
- whether the equity warrants will be subject to redemption and, if so, the terms of such redemption provisions;
- •
- material U.S. and Canadian federal income tax consequences of owning the equity warrants; and
- •
- any other material terms or conditions of the equity warrants.
Debt Warrants
The particular terms of each issue of debt warrants will be described in the related prospectus supplement. This description will include, where applicable:
- •
- the designation and aggregate number of debt warrants;
- •
- the price at which the debt warrants will be offered;
- •
- the currency or currencies in which the debt warrants will be offered;
- •
- the designation and terms of any securities with which the debt warrants are being offered, if any, and the number of the
debt warrants that will be offered with each security;
- •
- the date or dates, if any, on or after which the debt warrants and the related securities will be transferable separately;
- •
- the principal amount of debt securities that may be purchased upon exercise of each debt warrant and the price at which
and currency or currencies in which that principal amount of debt securities may be purchased upon exercise of each debt warrant;
- •
- the date on which the right to exercise the debt warrants will commence and the date on which the right
will expire;
- •
- the minimum or maximum amount of debt warrants that may be exercised at any one time;
- •
- whether the debt warrants will be subject to redemption, and, if so, the terms of such redemption provisions;
25
- •
- material U.S. and Canadian federal income tax consequences of owning the debt warrants; and
- •
- any other material terms or conditions of the debt warrants.
Prior to the exercise of their warrants, holders of warrants will not have any of the rights of holders of the securities subject to the warrants.
Neovasc may issue units, which may consist of one or more common shares, warrants or any combination of securities as is specified in the relevant prospectus supplement. In addition, the relevant prospectus supplement relating to an offering of units will describe all material terms of any units offered, including, as applicable:
- •
- the designation and aggregate number of units being offered;
- •
- the price at which the units will be offered;
- •
- the designation, number and terms of the securities comprising the units and any agreement governing the units;
- •
- the date or dates, if any, on or after which the securities comprising the units will be transferable separately;
- •
- whether it will apply to list the units on any exchange;
- •
- material U.S. and Canadian income tax consequences of owning the units, including, how the purchase price paid for the
units will be allocated among the Securities comprising the units; and
- •
- any other material terms or conditions of the units.
DESCRIPTION OF SUBSCRIPTION RECEIPTS
We may issue subscription receipts, which will entitle holders thereof to receive, upon satisfaction of certain release conditions and for no additional consideration, common shares, warrants or any combination thereof. Subscription receipts will be issued pursuant to one or more subscription receipt agreements (each, a "Subscription Receipt Agreement"), each to be entered into between the Company and an escrow agent (the "Escrow Agent") that will be named in the relevant prospectus supplement. Each Escrow Agent will be a financial institution organized under the laws of Canada or a province thereof and authorized to carry on business as a trustee. If underwriters or agents are used in the sale of any subscription receipts, one or more of such underwriters or agents may also be a party to the subscription agreement governing the subscription receipts sold to or through such underwriter or agent.
The following description sets forth certain general terms and provisions of subscription receipts that may be issued hereunder and is not intended to be complete. The statements made in this prospectus relating to any Subscription Receipt Agreement and subscription receipts to be issued thereunder are summaries of certain anticipated provisions thereof and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable Subscription Receipt Agreement. Prospective investors should refer to the Subscription Receipt Agreement relating to the specific subscription receipts being offered for the complete terms of the subscription receipts. We will file a copy of any Subscription Receipt Agreement relating to an offering of subscription receipts with the securities regulatory authorities in Canada and the United States after it has entered into it.
General
The prospectus supplement and the Subscription Receipt Agreement for any subscription receipts that we may offer will describe the specific terms of the subscription receipts offered. This description may include, but may not be limited to, any of the following, if applicable:
- •
- the designation and aggregate number of subscription receipts being offered;
- •
- the price at which the subscription receipts will be offered;
26
- •
- the designation, number and terms of the common shares, warrants or a combination thereof to be received by the holders of
subscription receipts upon satisfaction of the release conditions, and any procedures that will result in the adjustment of those numbers;
- •
- the conditions (the "Release Conditions") that must be met in order for holders of subscription receipts to
receive, for no additional consideration, the common shares, warrants or a combination thereof;
- •
- the procedures for the issuance and delivery of the common shares, warrants or a combination thereof to holders of
subscription receipts upon satisfaction of the Release Conditions;
- •
- whether any payments will be made to holders of subscription receipts upon delivery of the common shares, warrants or a
combination thereof upon satisfaction of the Release Conditions;
- •
- the identity of the Escrow Agent;
- •
- the terms and conditions under which the Escrow Agent will hold all or a portion of the gross proceeds from the sale of
subscription receipts, together with interest and income earned thereon (collectively, the "Escrowed Funds"), pending satisfaction of the Release Conditions;
- •
- the terms and conditions pursuant to which the Escrow Agent will hold common shares, warrants or a combination thereof
pending satisfaction of the Release Conditions;
- •
- the terms and conditions under which the Escrow Agent will release all or a portion of the Escrowed Funds to the Company
upon satisfaction of the Release Conditions;
- •
- if the subscription receipts are sold to or through underwriters or agents, the terms and conditions under which the
Escrow Agent will release a portion of the Escrowed Funds to such underwriters or agents in payment of all or a portion of their fees or commissions in connection with the sale of the subscription
receipts;
- •
- procedures for the refund by the Escrow Agent to holders of subscription receipts of all or a portion of the subscription
price of their subscription receipts, plus any pro rata entitlement to interest earned or income generated on such amount, if the Release Conditions are not satisfied;
- •
- any contractual right of rescission to be granted to initial purchasers of subscription receipts in the event that this
prospectus, the prospectus supplement under which subscription receipts are issued or any amendment hereto or thereto contains a misrepresentation;
- •
- any entitlement of Neovasc to purchase the subscription receipts in the open market by private agreement
or otherwise;
- •
- whether Neovasc will issue the subscription receipts as global securities and, if so, the identity of the depository for
the global securities;
- •
- whether Neovasc will issue the subscription receipts as bearer securities, as registered securities or both;
- •
- provisions as to modification, amendment or variation of the Subscription Receipt Agreement or any rights or terms of the
subscription receipts, including upon any subdivision, consolidation, reclassification or other material change of the common shares, warrants or other Neovasc securities, any other reorganization,
amalgamation, merger or sale of all or substantially all of the Company's assets or any distribution of property or rights to all or substantially all of the holders of common shares;
- •
- whether Neovasc will apply to list the subscription receipts on any exchange;
- •
- material U.S. and Canadian federal income tax consequences of owning the subscription receipts; and
- •
- any other material terms or conditions of the subscription receipts.
Rights of Holders of Subscription Receipts Prior to Satisfaction of Release Conditions
The holders of subscription receipts will not be, and will not have the rights of, shareholders of Neovasc. Holders of subscription receipts are entitled only to receive common shares, warrants or a combination thereof on exchange of their subscription receipts, plus any cash payments, all as provided for under the Subscription
27
Receipt Agreement and only once the Release Conditions have been satisfied. If the Release Conditions are not satisfied, holders of subscription receipts shall be entitled to a refund of all or a portion of the subscription price thereof and all or a portion of the pro rata share of interest earned or income generated thereon, all as provided in the Subscription Receipt Agreement.
Escrow
The Subscription Receipt Agreement will provide that the Escrowed Funds will be held in escrow by the Escrow Agent, and such Escrowed Funds will be released to the Company (and, if the subscription receipts are sold to or through underwriters or agents, a portion of the Escrowed Funds may be released to such underwriters or agents in payment of all or a portion of their fees in connection with the sale of the subscription receipts) at the time and under the terms specified by the Subscription Receipt Agreement. If the Release Conditions are not satisfied, holders of subscription receipts will receive a refund of all or a portion of the subscription price for their subscription receipts, plus their pro-rata entitlement to interest earned or income generated on such amount, if provided for in the Subscription Receipt Agreement, in accordance with the terms of the Subscription Receipt Agreement. Common shares or warrants may be held in escrow by the Escrow Agent and will be released to the holders of subscription receipts following satisfaction of the Release Conditions at the time and under the terms specified in the Subscription Receipt Agreement.
Modifications
The Subscription Receipt Agreement will specify the terms upon which modifications and alterations to the subscription receipts issued thereunder may be made by way of a resolution of holders of subscription receipts at a meeting of such holders or consent in writing from such holders. The number of holders of subscription receipts required to pass such a resolution or execute such a written consent will be specified in the Subscription Receipt Agreement.
The Subscription Receipt Agreement will also specify that the Company may amend any Subscription Receipt Agreement and the subscription receipts, without the consent of the holders of the subscription receipts, to cure any ambiguity, to cure, correct or supplement any defective or inconsistent provision, or in any other manner that will not materially and adversely affect the interests of the holder of outstanding subscription receipts or as otherwise specified in the Subscription Receipt Agreement.
The foregoing summary of certain of the principal provisions of the securities is a summary of anticipated terms and conditions only and is qualified in its entirety by the description in the applicable prospectus supplement under which any securities are being offered.
CERTAIN INCOME TAX CONSIDERATIONS
The applicable prospectus supplement may describe certain Canadian federal income tax consequences to an investor who is a non-resident of Canada or to an investor who is a resident of Canada of acquiring, owning and disposing of any of our securities offered thereunder. The applicable prospectus supplement may also describe certain U.S. federal income tax consequences of the acquisition, ownership and disposition of any of our securities offered thereunder by an initial investor who is a U.S. person (within the meaning of the U.S. Internal Revenue Code), including, to the extent applicable, such consequences relating to debt securities payable in a currency other than the U.S. dollar, issued at an original issue discount for U.S. federal income tax purposes or containing early redemption provisions or other special items.
Our common shares may be sold under this prospectus by way of a secondary offering by or for the account of certain of our securityholders. The prospectus supplement that we will file in connection with any offering of our common shares by selling securityholders will include the following information:
- •
- the names of the selling securityholders;
- •
- the number or amount of our common shares owned, controlled or directed by each selling securityholder;
28
- •
- the number or amount of our common shares being distributed for the account of each selling securityholder;
- •
- the number or amount of securities to be owned by the selling securityholders after the distribution and the percentage
that number or amount represents of the total number of our outstanding securities; and
- •
- whether our common shares are owned by the selling securityholders both of record and beneficially, of record only or beneficially only.
New Issue
We may issue our securities offered by this prospectus for cash or other consideration (i) to or through underwriters, dealers, placement agents or other intermediaries, (ii) directly to one or more purchasers or (ii) in connection with acquisitions of assets or shares or another entity or company.
Each prospectus supplement with respect to our securities being offered will set forth the terms of the offering, including:
- •
- the name or names of any underwriters, dealers or other placement agents;
- •
- the number and the purchase price of, and form of consideration for, our securities;
- •
- any proceeds to us; and
- •
- any commissions, fees, discounts and other items constituting underwriters', dealers' or agents' compensation.
Our securities may be sold, from time to time, in one or more transactions at a fixed price or prices which may be changed or at market prices prevailing at the time of sale, at prices related to such prevailing market price or at negotiated prices, including sales in transactions that are deemed to be "at the market distributions" as defined in National Instrument 44-102 — Shelf Distributions, including sales made directly on the TSX, NASDAQ or other existing trading markets for the securities. The prices at which the securities may be offered may vary as between purchasers and during the period of distribution. If, in connection with the offering of securities at a fixed price or prices, the underwriters have made a bona fide effort to sell all of the securities at the initial offering price fixed in the applicable prospectus supplement, the public offering price may be decreased and thereafter further changed, from time to time, to an amount not greater than the initial offering price fixed in such prospectus supplement, in which case the compensation realized by the underwriters will be decreased by the amount that the aggregate price paid by purchasers for the securities is less than the gross proceeds paid by the underwriters to the Company.
Only underwriters named in the prospectus supplement are deemed to be underwriters in connection with our securities offered by that prospectus supplement.
Under agreements which may be entered into by us, underwriters, dealers and agents who participate in the distribution of our securities may be entitled to indemnification by us against certain liabilities, including liabilities under the U.S. Securities Act of 1933, as amended, or the U.S. Securities Act, and applicable Canadian securities legislation, or to contribution with respect to payments which such underwriters, dealers or agents may be required to make in respect thereof. The underwriters, dealers and agents with whom we enter into agreements may be customers of, engage in transactions with, or perform services for, us in the ordinary course of business.
No underwriter or dealer involved in an "at the market distribution" as defined under applicable Canadian securities legislation, no affiliate of such underwriter or dealer and no person acting jointly or in concert with such underwriter or dealer has over-allotted, or will over allot, our securities in connection with an offering of our securities or effect any other transactions that are intended to stabilize the market price of our securities.
In connection with any offering of our securities, other than an "at the market distribution", the underwriters may over-allot or effect transactions which stabilize or maintain the market price of our securities
29
offered at a level above that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time.
Secondary Offering
This prospectus may also, from time to time, relate to the offering of our common shares by certain selling securityholders.
The selling securityholders may sell all or a portion of our common shares beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If our common shares are sold through underwriters or broker-dealers, the selling securityholders will be responsible for underwriting discounts or commissions or agent's commissions. Our common shares may be sold by the selling securityholders in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions, as follows:
- •
- on any national securities exchange or quotation service on which the securities may be listed or quoted at the time
of sale;
- •
- in the over-the-counter market;
- •
- in transactions otherwise than on these exchanges or systems or in the over-the-counter market;
- •
- through the writing of options, whether such options are listed on an options exchange or otherwise;
- •
- ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
- •
- block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of
the block as principal to facilitate the transaction;
- •
- purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
- •
- an exchange distribution in accordance with the rules of the applicable exchange;
- •
- privately negotiated transactions;
- •
- short sales;
- •
- sales pursuant to Rule 144 under the U.S. Securities Act;
- •
- broker-dealers may agree with the selling securityholders to sell a specified number of such shares at a stipulated price
per share;
- •
- a combination of any such methods of sale; and
- •
- any other method permitted pursuant to applicable law.
If the selling securityholders effect such transactions by selling our common shares to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling securityholders or commissions from purchasers of our common shares for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved). In connection with sales of our common shares or otherwise, the selling securityholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of our common shares in the course of hedging in positions they assume. The selling securityholders may also sell our common shares short and deliver our common shares covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling securityholders may also loan or pledge our common shares to broker-dealers that in turn may sell such shares.
The selling securityholders may pledge or grant a security interest in some or all of the common shares owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell our common shares from time to time pursuant to this prospectus or any supplement
30
to this prospectus filed under General Instruction II.L. of Form F-10 under the U.S. Securities Act, amending, if necessary, the list of selling securityholders to include, pursuant to a prospectus amendment or prospectus supplement, the pledgee, transferee or other successors in interest as selling securityholders under this prospectus. The selling securityholders also may transfer and donate our common shares in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The selling securityholders and any broker-dealer participating in the distribution of our common shares may be deemed to be "underwriters" within the meaning of the U.S. Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the U.S. Securities Act. At the time a particular offering of our common shares is made, a prospectus supplement, if required, will be distributed which will identify the selling securityholders and provide the other information set forth under "Selling Securityholders", set forth the aggregate amount of our common shares being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling securityholders and any discounts, commissions or concessions allowed or reallowed or paid to broker-dealers.
Under the securities laws of some states, our common shares may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states our common shares may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any securityholder will sell any or all of our common shares registered pursuant to the registration statement, of which this prospectus forms a part.
The selling securityholders and any other person participating in such distribution will be subject to applicable provisions of Canadian securities legislation and the Exchange Act and the rules and regulations thereunder, including, without limitation, Regulation M under the Exchange Act, which may limit the timing of purchases and sales of any of our common shares by the selling securityholders and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of our common shares to engage in market-making activities with respect to our common shares. All of the foregoing may affect the marketability of our common shares and the ability of any person or entity to engage in market-making activities with respect to our common shares.
Once sold under the shelf registration statement, of which this prospectus forms a part, our common shares will be freely tradable in the hands of person other than our affiliates.
AUDITORS, TRANSFER AGENT AND REGISTRAR
Grant Thornton LLP was appointed as our auditor at our annual general meeting of shareholders held on June 12, 2003. Grant Thornton LLP is located at Suite 1600 – 333 Seymour Street, Vancouver, British Columbia, V6B 0A4, Canada. Grant Thornton LLP has reported on our fiscal December 31, 2015 and 2014 audited consolidated financial statements, which have been filed with the securities regulatory authorities and incorporated by reference herein. Grant Thornton LLP is independent with respect to the Company within the meaning of the Rules of Professional Conduct of the Institute of Chartered Accountants of British Columbia.
Our transfer agent and the registrar for our common shares in Canada is Computershare Investor Services Inc. located at 510 Burrard Street, 2nd Floor, Vancouver, British Columbia, Canada, V6C 3B9.
31
William O'Neill, Steven Rubin and Jane Hsiao, directors of the Company, reside outside of Canada and have appointed the following agent for service of process in Canada:
| Name of Person | Name and Address of Agent | |
|---|---|---|
William O'Neill, Steven Rubin and Jane Hsiao |
Neovasc Inc. Suite 5138-13562 Maycrest Way Richmond, British Columbia V6V 2J7 |
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
Certain legal matters related to our securities offered by this prospectus will be passed upon on our behalf by Blake, Cassels & Graydon LLP, with respect to matters of Canadian law, and Skadden, Arps, Slate, Meagher & Flom LLP, with respect to matters of U.S. law. As of the date of this prospectus, the partners and associates of Blake, Cassels & Graydon LLP beneficially own, directly or indirectly, less than 1% of our outstanding common shares.
WHERE YOU CAN FIND MORE INFORMATION
We are required to file with the securities commission or authority in each of the applicable provinces of Canada annual and quarterly reports, material change reports and other information. In addition, we are subject to the informational requirements of the Exchange Act, and, in accordance with the Exchange Act, we also file reports with, and furnish other information to, the SEC. Under a multijurisdictional disclosure system adopted by the United States and Canada, these reports and other information (including financial information) may be prepared in accordance with the disclosure requirements of Canada, which differ in certain respects from those in the United States. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required to publish financial statements as promptly as U.S. companies.
You may read any document we file with or furnish to the securities commissions and authorities of the provinces of Canada through SEDAR and any document we file with, or furnish to, the SEC at the SEC's public reference room at Station Place, 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at l-800-SEC-0330 for further information on the public reference rooms. Certain of our filings are also electronically available on EDGAR, and may be accessed at www.sec.gov.
ENFORCEABILITY OF CIVIL LIABILITIES
We are a company continued under the CBCA. Most of our directors and officers, and the experts named in this prospectus, are residents of Canada or otherwise reside outside the United States, and all or a substantial portion of their assets may be, and a substantial portion of the Company's assets are, located outside the United States. We have appointed an agent for service of process in the United States (as set forth below), but it may be difficult for holders of securities who reside in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. We have been advised that a judgment of a U.S. court predicated solely upon civil liability under U.S. federal securities laws or the securities or "blue sky" laws of any state within the United States, would likely be enforceable in Canada if the United States court in which the judgment was obtained has a basis for jurisdiction in the matter that would be recognized by a
32
Canadian court for the same purposes. We have also been advised, however, that there is substantial doubt whether an action could be brought in Canada in the first instance on the basis of the liability predicated solely upon U.S. federal securities laws.
We filed with the SEC, concurrently with our registration statement on Form F-10 of which this prospectus is a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, we appointed CT Corporation System, 111 Eighth Avenue, New York, New York 10011 as our agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving us in a U.S. court arising out of or related to or concerning the offering of securities under this prospectus.
33
