Form DEFA14A IMMUNOMEDICS INC
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section
14(a) of
the Securities Exchange Act of 1934 (Amendment No. )
Filed by the Registrant ý
Filed by a Party other than the Registrant ¨
Check the appropriate box:
¨ Preliminary Proxy Statement
¨ Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
¨ Definitive Proxy Statement
ý Definitive Additional Materials
¨ Soliciting Material under §240.14a-12
| Immunomedics, Inc. |
| (Name of Registrant as Specified In Its Charter) |
| (Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
Payment of Filing Fee (Check the appropriate box):
ý No fee required.
¨ Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11.
| (1) | Title of each class of securities to which transaction applies: |
| (2) | Aggregate number of securities to which transaction applies: |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| (4) | Proposed maximum aggregate value of transaction: |
| (5) | Total fee paid: |
¨ Fee paid previously with preliminary materials.
¨ Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing.
| (1) | Amount Previously Paid: |
| (2) | Form, Schedule or Registration Statement No.: |
| (3) | Filing Party: |
| (4) | Date Filed: |

IMMUNOMEDICS, INC. Advanced Antibody - Based Therapeutics Oncology Autoimmune Diseases January 2017 2017 Investor R&D Day

Disc l aimer 2 Certain statements in this presentation constitute “forward - looking statements” of Immunomedics within the meaning of the Private Securities Litigation Reform Act of 1995 and constitute “forward - looking information” within the meaning of applicable U . S . securities laws . Such statements include, but are not limited to : our financial guidance ; statements concerning our clinical development programs and future plans, including regulatory and clinical plans and pathway and associated costs ; expected timing to commence and receive data from clinical trials, including our assumptions related to initiation of new studies, current and future study enrollment and timing to treat and re - treat patients ; statements concerning the potential benefits and success of our development programs ; and statements which contain language such as : “plan,” “potential,” “future,” “project,” “will,” “may,” “believe,” “intend,” “estimate,” “expect,” “anticipate,” “target” and similar expressions . Forward - looking statements are based on estimates and assumptions made by Immunomedics in light of its experience and its perception of historical trends, current conditions and expected future developments, as well as other factors that Immunomedics believes are appropriate in the circumstances, including but not limited to : general economic conditions, competition, clinical trial designs, progression of enrollment and development and interpretation of data . Forward - looking statements are predictions only which involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those expressed in such statements . Many such risks, uncertainties and other factors are taken into account as part of our assumptions underlying these forward - looking statements and include, among others, the following : the Company’s future operating results are uncertain and likely to fluctuate ; currency fluctuations ; uncertainties relating to the timing and results of the clinical development and commercialization of our products and technologies and the associated costs of these programs ; outcomes for our clinical trials may not be favorable or may be less favorable than interim results and/or previous trials ; there may be varying interpretations of data produced by one or more of our clinical trials ; the timing, expense and uncertainty associated with the regulatory approval process for products ; uncertainties regarding the impact of competitive products ; risks and uncertainties associated with the safety and effectiveness of our technology ; risks and uncertainties related to the scope, validity, and enforceability of our intellectual property rights and the impact of patents and other intellectual property of third parties ; and general economic conditions . These factors and others relating to Immunomedics are discussed in greater detail in the “Risk Factors” section of Immunomedics’ Annual Report on Form 10 - K, Quarterly Reports on Form 10 - Q, as well as in its other filings with the U . S . Securities and Exchange Commission . Given these uncertainties, assumptions and risk factors, investors are cautioned not to place undue reliance on such forward - looking statements . Immunomedics has no intention and assumes no obligation to update such information to reflect later events or developments, except as required by law . This presentation also contains “forward - looking information” that constitutes “financial outlooks” within the meaning of applicable U . S . securities laws . This information is provided to give investors general guidance on management's current expectations of certain factors affecting our business, including our financial results . Given the uncertainties, assumptions and risk factors associated with this type of information, including those described above, investors are cautioned that the information may not be appropriate for other purposes .
Important Additional Information 3 Immunomedics, Inc . (the “Company”), its directors and certain of its executive officers are deemed to be participants in the solicitation of proxies from Company stockholders in connection with the matters to be considered at the Company’s 2016 Annual Meeting . The Company has filed a definitive proxy statement and form of WHITE proxy card with the U . S . Securities and Exchange Commission (the “SEC”) in connection with any such solicitation of proxies from Company stockholders . COMPANY STOCKHOLDERS ARE STRONGLY ENCOURAGED TO READ THE DEFINITIVE PROXY STATEMENT (INCLUDING ANY AMENDMENTS AND SUPPLEMENTS), THE ACCOMPANYING WHITE PROXY CARD AND ANY OTHER RELEVANT DOCUMENTS THAT THE COMPANY FILES WITH THE SEC WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION . Information regarding the identity of the participants, and their direct or indirect interests, by security holdings or otherwise, is set forth in the proxy statement and other materials filed by the Company with the SEC . Stockholders will be able to obtain the proxy statement, any amendments or supplements to the proxy statement and other documents filed by the Company with the SEC for no charge at the SEC’s website at www . sec . gov . Copies will also be available at no charge at the Company’s website at www . immunomedics . com , by writing to Immunomedics, Inc . at 300 The American Road, Morris Plains, New Jersey 07950 , by calling the Company’s proxy solicitor, MacKenzie Partners, Inc . at ( 212 ) 929 - 5500 , or by calling Dr . Chau Cheng, Senior Director, Investor Relations & Corporate Secretary, ( 973 ) 605 - 8200 , extension 123 .

A g enda • Opening Remarks: Cynthia L. Sullivan, President and Chief Executive Officer • New Board Member Introductions: Brian A. Markison, Lead Independent Director • Overview of Clinical & Preclinical Programs, Platform Technologies, and Intellectual Property: David M. Goldenberg, Sc.D., M.D., Chairman, Chief Scientific Officer, and Chief Patent Officer • IMMU - 132 – Results in Urothelial Cancer : Scott T . Tagawa, M . D . , Richard A . Stratton Associate Professor in Hematology & Oncology ; Medical Director, Genitourinary Oncology Research Program, Division of Hematology & Medical Oncology, Weill Cornell Medical College, New York, NY • IMMU - 132 – Results in Non - Small - Cell and Small - Cell Lung Cancers: Ronald J. Scheff, M.D., Assistant Professor of Clinical Medicine, Weill Cornell Medical College; Medical Oncologist, New York - Presbyterian Hospital, New York, NY • IMMU - 132 – Results in Triple - Negative Breast Cancer: Linda T. Vahdat, M.D., MBA, Professor of Medicine, Weill Cornell Medical College; Co - Leader BC Program at Meyer Cancer Center, New York, NY • IMMU - 132 – Preclinical Development (PARP/Chemotherapy Combinations): Thomas Cardillo, Ph.D., Executive Director, Preclinical and Hybridoma Development • IMMU - 132 – Competitive Landscape and Commercial Assessment: Andrew Funderburk, Partner, and Earl Gillespie, Consultant, Health Advances • Q&A Session • IMMU - 132 – Manufacturing / Regulatory / Development Plan for TNBC: Edmund Rossi, Ph.D., Associate Vice President, Process Development and Manufacturing • IMMU - 130, Epratuzumab, Veltuzumab, Milatuzumab and IMMU - 114: William A. Wegener, Ph.D., M.D., Chief Medical Officer • IMMU - 140 – Overview: Thomas Cardillo, Ph.D., Executive Director, Preclinical and Hybridoma Development • Bispecific Antibodies and Immuno - Oncology Program: Edmund Rossi, Ph.D., Associate Vice President, Process Development and Manufacturing • Concluding Remarks: David M. Goldenberg, ScD., M.D., Chairman, Chief Scientific Officer, and Chief Patent Officer 4 • Q&A Session

Opening Remarks Cynthia L. Sullivan, President and Chief Executive Officer 5

Immunomedics – Corporate Information 6 • Clinical - stage biopharmaceutical company – Based in Morris Plains, New Jersey • History of approved products – CEA - Scan®; LeukoScan® • Management Leadership – David M. Goldenberg, ScD, MD, Chairman, Chief Science Officer and Chief Patent Officer – Cynthia L. Sullivan, President and Chief Executive Officer – Michael R. Garone, VP Finance and Chief Financial Officer – William A. Wegener, MD, PhD, Chief Medical Officer • Employees – 143 full time; 54 with M.D., Ph.D., or other advanced degrees – Ops/Manufacturing /QC /QA /Process Development ( 73 ), Finance & Administration ( 26 ), Clinical Research & Regulatory Affairs ( 24 ), R&D ( 20 )

Immunomedics – Corporate Information (Cont’d) 7 • Committed to be a leading, innovative biopharmaceutical company, dedicated to improving health and quality of life with novel therapeutics for the treatment of cancer, autoimmune and other serious diseases • Focus on developing monoclonal antibody - based products for the targeted treatment of cancer, autoimmune diseases and other serious diseases • All product candidates created, developed and produced in - house • Four bioreactor suites at Morris Plains headquarters – 2 each with 50 - L, 300 - L and 2500 - L stainless steel bioreactors – 2 each with 50 - L and 200 - L single - use bioreactors – Enough manufacturing capacity to support Phase 1 – 3 clinical trials • Validated first - in - class antibody - drug conjugate (ADC) platform technology for solid cancer therapy • Proprietary bispecific antibody and immuno - oncology platform technologies
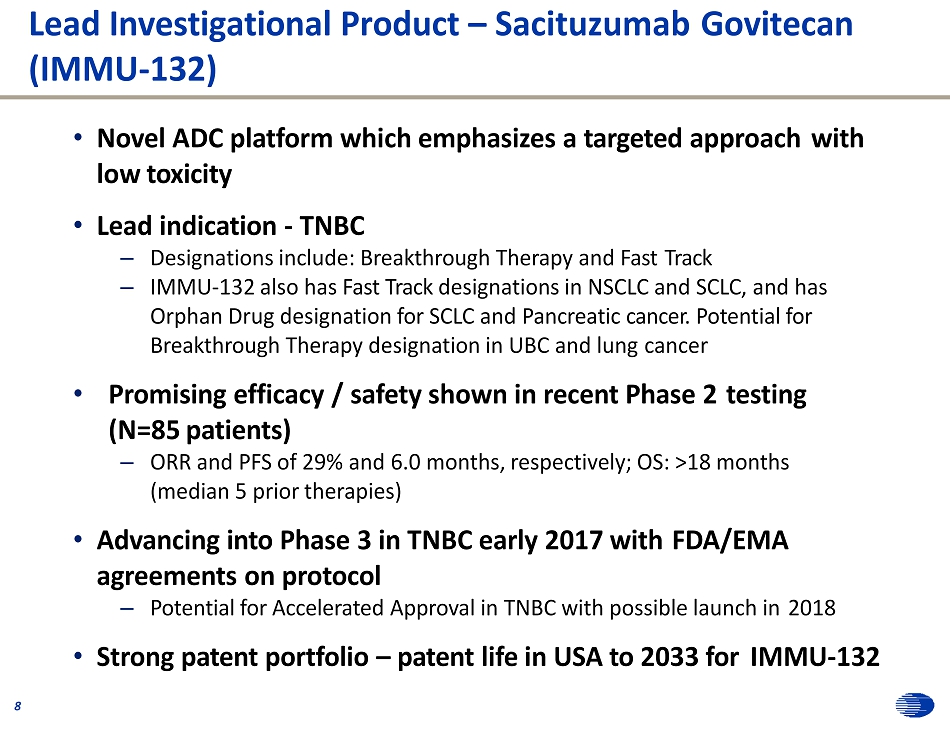
Lead Investigational Product – Sacituzumab Govitecan (IMMU - 132) 8 • Novel ADC platform which emphasizes a targeted approach with low toxicity • Lead indication - TNBC – Designations include: Breakthrough Therapy and Fast Track – IMMU - 132 also has Fast Track designations in NSCLC and SCLC, and has Orphan Drug designation for SCLC and Pancreatic cancer. Potential for Breakthrough Therapy designation in UBC and lung cancer • Promising efficacy / safety shown in recent Phase 2 testing (N=85 patients) – ORR and PFS of 29% and 6.0 months, respectively; OS: >18 months (median 5 prior therapies) • Advancing into Phase 3 in TNBC early 2017 with FDA/EMA agreements on protocol – Potential for Accelerated Approval in TNBC with possible launch in 2018 • Strong patent portfolio – patent life in USA to 2033 for IMMU - 132

C ompoun d Designations Phase 1 Phase 2 Phase 3 Development Plans (Assuming Sufficient Funds) IMMU - 132 (Sacituzumab govitecan) ▪ Breakthrough Therapy, Fast Track ▪ Completed enrollment of 100+ Phase 2 patients in 2016 ▪ Interim data submission and publishing in early 2017 ▪ Validation of commercial - scale production from outside CMOs ▪ Phase 3 enrollment early 2017 ▪ Submission of accelerated approval application with FDA in mid - 2017 ▪ Potential approval and subsequent launch in late 2017 / early 2018 ▪ Patient enrollment continues, potential Breakthrough Therapy designation ▪ Fast Track ▪ Upcoming conversation by end of January 2017 with FDA for potential Breakthrough Therapy designation ▪ Orphan Drug (US), Fast Track ▪ Patient enrollment continues, ongoing conversation with FDA for potential Breakthrough Therapy designation ▪ Orphan Drug (US & EU – Pancreatic Cancer) IMMU - 130 (Labetuzumab govitecan) ▪ Design and conduct Phase 3 Study IMMU - 102 (Epra t uzu m ab) ▪ Multiple Phase 1/2 trials completed ▪ Phase 3 in progress ▪ Partnership with Bayer ▪ Activity shown in 2 trials IMMU - 114 (An ti - HL A - D R) ▪ Also potential active in AML, ALL, and myeloma IMMU - 115 ( M il a t uzu m ab) ▪ Orphan Drug ▪ Ongoing RCT in moderate/severe lupus as a subq injection IMMU - 106 (Veltuzumab) ▪ Orphan Drug ▪ Multiple clinical trials completed; Sub - Q formulation Immunomedics Clinical Pipeline Triple Negative Breast Cancer (“TNBC”) Urothelial Cancer Non - small - cell Lung Cancer (“NSCLC”) Small - cell Lung Cancer (“SCLC”) Other Cancers (Prostatic, Endometrial and Common Breast Cancers) Colorectal Cancer (“CRC”) Non - Hodgkin’s Lymphoma (“NHL”) Pediatric Acute Lymphoblastic Leukemia (“ALL”) Sjogren’s Syndrome ALL; Emab - Thorium NHL, Chronic Lymphocytic Leukemia (“CLL”) Lupus, NHL, Multiple Myeloma (“MM”), Combined with veltuzumab NHL NHL, CLL, Idiopathic Thrombocytopenic Purpura (“ITP”), Pemphigus case study AD C Platform – S o li d T u m o rs m A b Platform – Blood Can c e rs & A u to i mm un e Dise a se s 9

New Board Member Introductions Brian A. Markison, Lead Independent Director 10

New Board Members 11 • Jason Aryeh – Founder & managing general partner, JALAA Equities, LP • Dr. Geoffrey Cox – Principal, Beacon Street Advisors, LLC • Robert Forrester – President & CEO, Verastem, Inc. • Bob Oliver – President & CEO, Otsuka America Pharma, Inc. • New independent directors bring significant, relevant experience to oversee IMMU - 132 strategic development and other value - creation processes – Assisted by Greenhill & Co., LLC – Exploring all strategic options, including licensing, asset sales, and other M&A activities • Retained experts for commercial assessment and manufacturing audit • Comprehensive process will result in significant near, short, and long - term value for ALL stockholders

Overview of Clinical & Preclinical Programs, Platform Technologies, and Intellectual Property 12 David M. Goldenberg, Sc.D., M.D., Chairman, Chief Scientific Officer, and Chief Patent Officer

Broad Technology Platforms 13 • Antibody - based technologies, involving – Unconjugated mAbs – Antibody - drug conjugates – Companion ImmunoPET imaging agents (IBC Pharmaceuticals, Inc.) – Bispecific antibodies (DNL®) – T - cell retargeting immuno - oncology • All internal science, with extensive patent coverage (>400)

Profile of Major mAbs 14 • Anti - CD22, epratuzumab – oncology and autoimmune disease* • Anti - CD20, veltuzumab – oncology and autoimmune disease* • Anti - CD74, milatuzumab – oncology and autoimmune disease* • Anti - CEACAM5, labetuzumab – oncology* • Anti - MUC5a,c, clivatuzumab – oncology* • Anti - Trop - 2, sacituzumab – oncology* • Anti - HLA - DR, IMMU - 114 – oncology and autoimmune disease* • Anti - CD19/CD3, (E1) - 3s – immuno - oncology • Anti - PD - 1 – immuno - oncology • Anti - PD - L1 – immuno - oncology • 22 - 20 - 20, bispecific mAb – oncology and autoimmune disease • 74 - 20 - 20, bispecific mAb – oncology and autoimmune disease • Anti - CEACAM6 – oncology *Clinically tested

Antibody - Drug Conjugates 15

• Unique approach to ADC therapeutics for cancer – Highly cancer - specific antibodies based on 30 years of experience – Utilize moderately potent payloads: increased therapeutic index • Proprietary linker for rapid payload release at or inside tumor – High drug - to - antibody ratio (~7.6:1) • SN - 38 payload – Active metabolite with more potency than its parent compound, irinotecan (a commonly used chemotherapeutic) – ADCs’ unique chemistry avoids low solubility and selectively delivers SN - 38 to the tumor • Two ADCs completed Phase 2: IMMU - 132 and IMMU - 130 • One ADC in advanced preclinical development: IMMU - 140 16 What Makes IMMU’s ADCs Different?
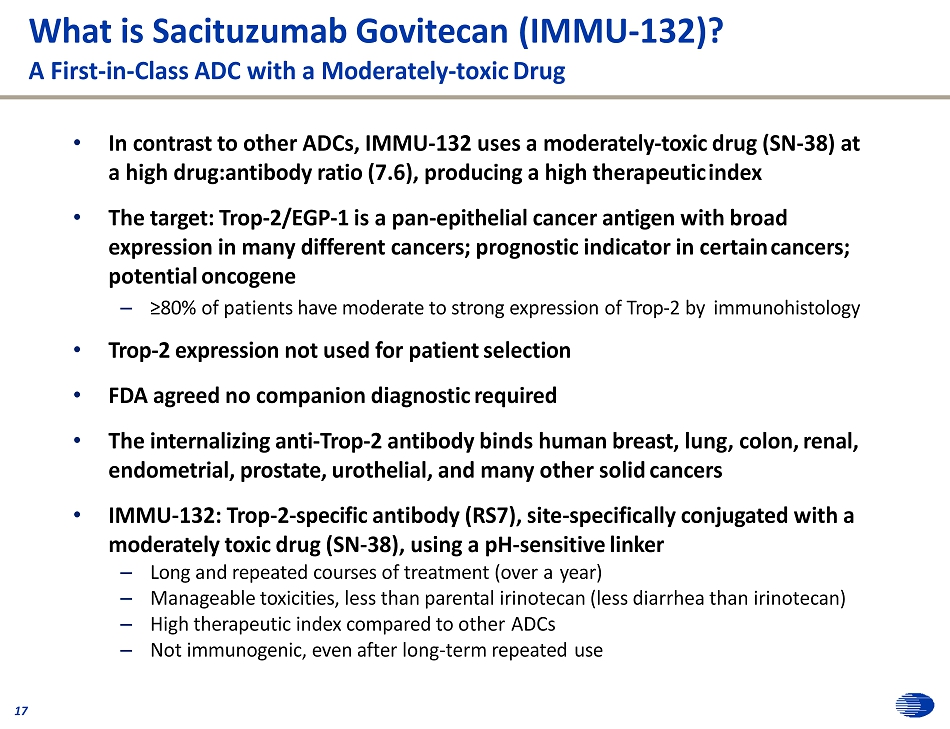
What is Sacituzumab Govitecan (IMMU - 132)? A First - in - Class ADC with a Moderately - toxic Drug 17 • In contrast to other ADCs, IMMU - 132 uses a moderately - toxic drug (SN - 38) at a high drug:antibody ratio (7.6), producing a high therapeutic index • The target: Trop - 2/EGP - 1 is a pan - epithelial cancer antigen with broad expression in many different cancers; prognostic indicator in certain cancers; potential oncogene – ≥80% of patients have moderate to strong expression of Trop - 2 by immunohistology • Trop - 2 expression not used for patient selection • FDA agreed no companion diagnostic required • The internalizing anti - Trop - 2 antibody binds human breast, lung, colon, renal, endometrial, prostate, urothelial, and many other solid cancers • IMMU - 132: Trop - 2 - specific antibody (RS7), site - specifically conjugated with a moderately toxic drug (SN - 38), using a pH - sensitive linker – Long and repeated courses of treatment (over a year) – Manageable toxicities, less than parental irinotecan (less diarrhea than irinotecan) – High therapeutic index compared to other ADCs – Not immunogenic, even after long - term repeated use

Novel linker and ADC construct Glucuronidation site protected while bound to IgG Immunomedics’ ADC Platform With SN - 38 Goldenberg et al, Oncotarget (2015) 6:22496 - 512 • Linker coupled to 20 th position stabilizes lactone ring − pH dependent release • Glucuronidation site (10 th position) is protected while bound to IgG − Much lower SN - 38G concentrations in serum than with irinotecan • Site - specific (99%) coupling to interchain thiols (N = 8) − Average of ~ 7.6 SN - 38 molecules/IgG − High doses of SN - 38 delivered 18

Key Clinical Points 19 • Novel ADC platform designed to address the limitations of first - generation ADCs: Therapeutic index appears to be improved – Active metabolite of irinotecan, SN - 38, can be conjugated to mAbs and provides higher SN - 38 doses in blood and tumor (~130 - fold in animal studies) than when irinotecan is given • Over 410 patients with diverse advanced cancers studied; >140 in TNBC, >100 in NSCLC + SCLC, > 40 urothelial cancer • Promising durable activity in relapsed/refractory metastatic TNBC, NSCLC, SCLC, and UC (post multiple prior lines of therapy): Some responses exceed 22 months • Acceptable safety profile in heavily pretreated patients with diverse solid cancers (median of 2 – 5 prior therapies) – Dose - limiting neutropenia, low rate of febrile neutropenia – Severe diarrhea rare (less glucuronidated SN - 38 released than irinotecan): ~10% • Repeated doses can be given over months without evoking interfering host anti - IMMU - 132 (anti - SN - 38 or anti - hRS7 antibodies)

Intellectual Property Highlights 20 • Company has over 300 US and over 400 foreign patents • In a recent ranking of IP strength by the Patent Board, Immunomedics was ranked No. 4 innovator in the biotechnology industry • Company awarded Thomas A . Edison Award by Research & Development Council of New Jersey in 2011 , 2012 , 2014 and 2016

IMMU - 132 Patent Portfolio 21 • 32 issued U.S. and 16 foreign patents covering composition of matter, synthesis and uses of IMMU - 132 • Company’s main composition of matter patents expire in 2023 in the U.S., and in 2029 in Europe – In addition, IMMU - 132 has patent coverage through 2033 for uses in various cancers at different dose schedules, and other proprietary features of the antibody - drug conjugate • Patent applications are being prosecuted in all major countries, with patents issued in Australia, Canada, China, Europe, Israel, Japan and South Korea • IMMU - 132 has potential for regulatory exclusivity of 12 years in the U.S. and 10 years in Europe – Moreover, the product has been granted Orphan Drug status in the US and EU for certain secondary indications

IMMU - 132 – Results in Urothelial Cancer 22 Scott T. Tagawa, M.D., Richard A. Stratton Associate Professor in Hematology & Oncology; Medical Director, Genitourinary Oncology Research Program, Division of Hematology & Medical Oncology Weill Cornell Medical College, New York, NY

Randomized Trials in Advanced Urothelial Carcinoma Study Response Rate Overall Survival Comments Cisplatin vs MVAC Loehrer et al JCO 1992 11 vs 36% p < 0.0001 8.8 vs 12.5 mo p = 0.0002 MVAC became standard of care CisCA vs MVAC Logothetis et al JCO 1990 46 vs 65% p < 0.05 10.0 vs 12.6 mo p = 0.0003 MVAC became standard of care MVAC vs FAP Siefker - Radtke et al JCO 2002 59 vs 42% p < 0.05 12.5 vs 12.5 mo p = 0.17 Alternative regimen not better than MVAC MVAC vs carbo/paclitaxel Dreicer et al Cancer 2004 36 vs 28% p = 0.63 15.4 vs 13.8 mo p = 0.65 Study terminated early for slow accrual; QOL measures equal both arms MVAC vs cis/docetaxel Bamias et al JCO 2004 54 vs 37% p = 0.017 14.2 vs 9.3 mo p = 0.026 Both arms + GCSF; MVAC + GCSF less toxic than historical controls MVAC vs Gem/Cis von der Maase et al JCO 2000 46 vs 49% p = 0.51 14.8 vs 13.8 mo p = 0.75 Negative for superiority of GC, but less toxic and less health - care utilization; long - term update still holds (von der Maase et al JCO 2005) MVAC vs HD MVAC Sternberg et al JCO 2001 58 vs 72% p = 0.06 14.1 vs 15.5 mo p = 0.122 HD MVAC less toxic; Survival advantage for HD MVAC at long - term follow up (p=0.042) (Sternberg et al Eu J Cancer 2006) Gem/Cis vs Paclitax/Cis/Gem Bellmunt et al, JCO 2012 43.6 vs 55.5% p = 0.003 12.7 vs 15.8 mo p = 0.085 Primary endpoint negative; response advantage at cost of toxicity (WBC (inc fever) vs plts); Subset analysis positive in eligible pts and bladder primary MCAVI vs Gem/Carbo DeSantis et al, JCO 2012 30.3 vs 41.2% p = 0.08 8.1 vs 9.3 mo p = 0.64 No significant difference in efficacy with higher toxicity with MCAVI (21.2 vs 9.3% 23

Kaplan - Meier Curves for Overall Survival with Cisplatin - based Combination Chemotherapy von der Maase, H. et al. J Clin Oncol; 23:4602 - 4608 2005 24
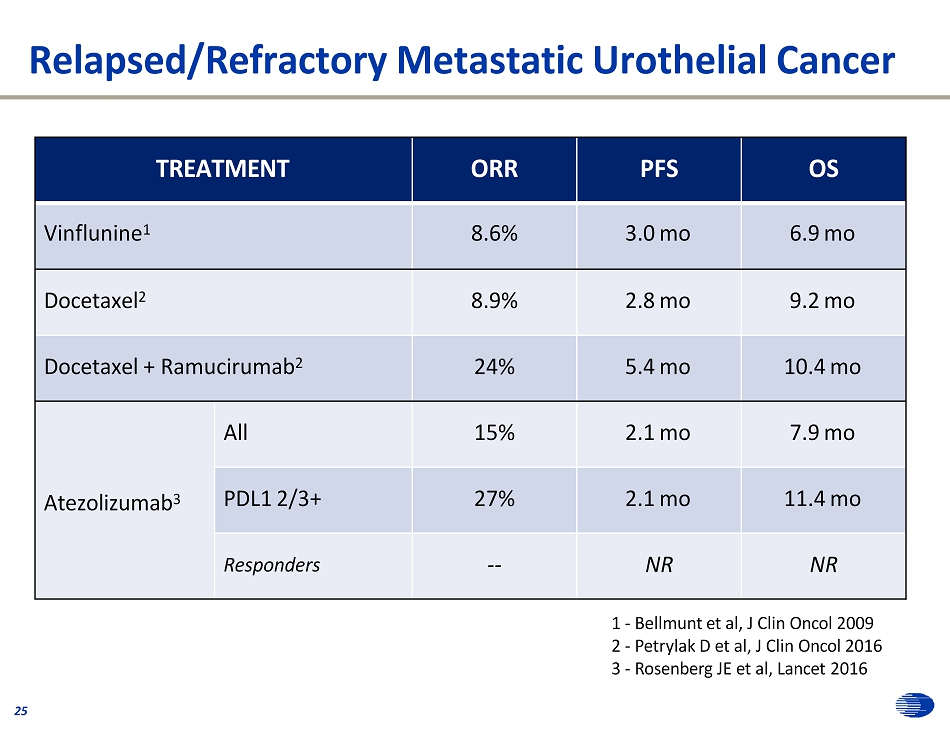
Relapsed/Refractory Metastatic Urothelial Cancer 25 1 - Bellmunt et al, J Clin Oncol 2009 2 - Petrylak D et al, J Clin Oncol 2016 3 - Rosenberg JE et al, Lancet 2016 TREATMENT ORR PFS OS Vinflunine 1 8.6% 3.0 mo 6.9 mo Docetaxel 2 8.9% 2.8 mo 9.2 mo Docetaxel + Ramucirumab 2 24% 5.4 mo 10.4 mo Atezolizumab 3 All 15% 2.1 mo 7.9 mo PDL1 2/3+ 27% 2.1 mo 11.4 mo Responders -- NR NR

Antibody - Drug Conjugates 26 • Antibody – Antigen characteristics – Antigen: Tumor specificity, expression – Antibody: Internalization, immunogenicity • Linker – Stability in circulation – Cleavable vs non - cleavable • Payload – Potency – Mechanism against tumor type Klute, Nackos et al; OncoTargets and Therapy 2014

Phase 1 IMMU - 132 - 01 Clinical Trial 1 (NCT01631552) 27 • Patients with metastatic treatment - refractory epithelial cancers • Treatment regimen: Days 1 and 8 every 21 days – Dose delays and reductions allowed to encourage treatment continuation – Treatment continued typically until progression or excess toxicities • Phase I examined 8, 10, 12, and 18 mg/kg – 12 mg/kg was declared the MTD (no DLT out of 9 patients at 12 mg/kg and two grade 4 dose limiting neutropenia out of 3 patients at 18 mg/kg) • Adverse events mostly limited to grade 1 - 2 – Neutropenia 56% (febrile neutropenia 11%), diarrhea 22%, hypokalemia 11%, DVT 11% at MTD • Early activity in advanced urothelial carcinoma 2 1 - Starodub et al, Clin Cancer Res 2015, 2 - Faltas et al, Clin Genitourin Cancer 2015

- 1 0 0 - 8 0 - 6 0 - 4 0 - 2 0 0 2 0 4 0 B e s t R e s p o n s e B e s t % c h a n g e i n T L f r o m b a s e l i n e C o m p le te re s p o n s e ( c o n f irm e d ) P a r t ia l r e s p o n s e ( c o n f ir m e d ) P a r t ia l r e s p o n s e ( p e n d in g ) P a r t ia l r e s p o n s e ( u n c o n f ir m e d ) S ta b le d is e a s e P r o g re s s io n 8 m g / k g ( a ll o th e rs , 1 0 m g /k g ) p r io r c h e c k p o in t in h ib ito r th e ra p y IMMU - 132 in Advanced Refractory Urothelial Cancers: Best Response 8 and 10 mg/kg; 27 assessable ( > 1 therapy cycle): RECIST 1.1 ORR (9/27) = 33% • 1 CR (confirmed) • 8 PR (confirmed) Other results • 1 PR (confirmation pending) • 12 Stable Disease (5pts ≥ 20%) • 5 Progression 81% showed tumor shrinkage from baseline CBR (PR+SD > 6 mos) = 59% ^ 27 - (5 pts continuing treatment) = 22 42 enrolled; 10 pts too early for 1 st assessment; 4 pts withdrew before 1 st assessment; 1 pt had 1 dose = 27 assessable for response Median # prior therapy: 2 (range, 1 – 5) 28

IMMU - 132: Duration of Response for PR + SD UBC patients Urothelial Cancers 22/27 pts (81%) with SD or better response KM - derived median duration of response for pts with PR + SD (N = 22): Median = 8.1 mos (95% CI = 6.9, 14.6) Median time to 1 st objective response = 1.9 mos (range, 1.7 to 5.7 mos; N = 10) 10/22 (45%) patients still ongoing Rx DoR for CR and PR pts only (N = 9) a Median = 7.5 mos (95% CI = 4.4, 12.9) a excludes 1 PR pt who is currently waiting for confirmation 0 1 8 C o m p le te r e s p o n s e ( c o n f ir m e d P a r t ia l r e s p o n s e ( c o n f ir m e d ) P a r t ia l r e s p o n s e ( p e n d in g ) S ta b le d is e a s e C o n tin u in g tr e a tm e n t O b je c t iv e r e s p o n s e f ir s t r e p o r te d 3 6 9 1 2 1 5 M o n th s fro m s ta rt o f tre a tm e n t 29

IMMU - 132: PFS and OS for Metastatic Urothelial Cancers ITT = all 32 - treated patients Median PFS = 7.2 months (95% CI = 4.9, 10.7) Median OS = 15.5 months (95% CI = 10.5, 17.2) 30

Case study 31 • 2008: diagnosis high grade non - invasive UC bladder transurethral resection, BCG • 2014 metastatic urothelial carcinoma to LN’s gem/cis +/ - bevacizumab gem/carbo • June, 2015 initiate IMMU - 132 Baselin e : Liver + L y mp h Node mets Com p l et e response in target lesio n s Remains on therapy maintaining response (and tolerating) 19 months after initiation
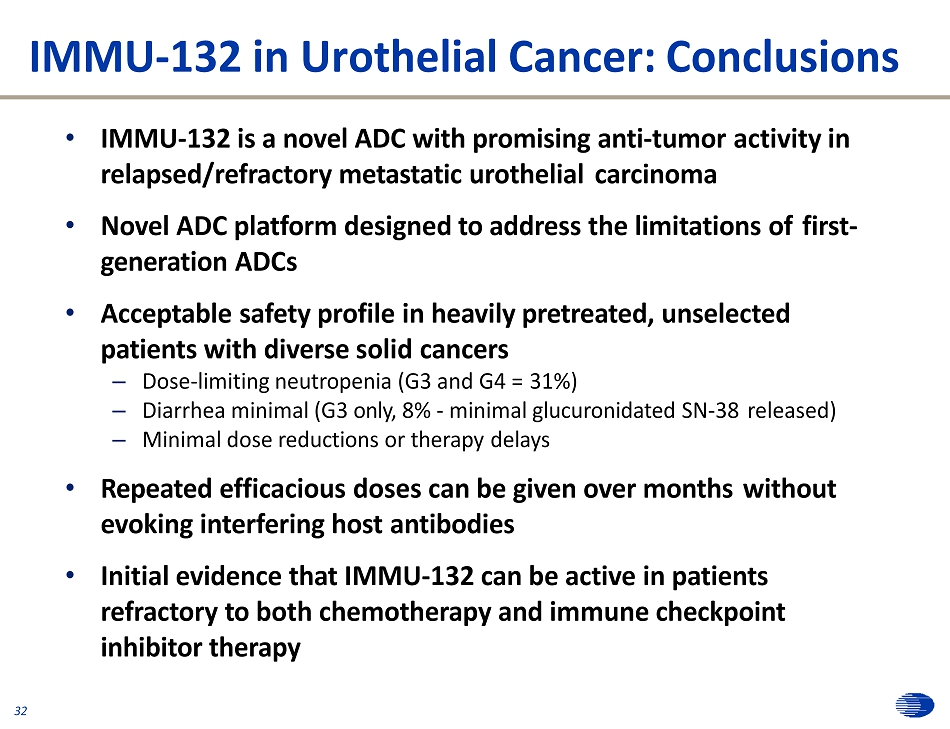
IMMU - 132 in Urothelial Cancer: Conclusions • IMMU - 132 is a novel ADC with promising anti - tumor activity in relapsed/refractory metastatic urothelial carcinoma • Novel ADC platform designed to address the limitations of first - generation ADCs • Acceptable safety profile in heavily pretreated, unselected patients with diverse solid cancers – Dose - limiting neutropenia (G3 and G4 = 31%) – Diarrhea minimal (G3 only, 8% - minimal glucuronidated SN - 38 released) – Minimal dose reductions or therapy delays • Repeated efficacious doses can be given over months without evoking interfering host antibodies • Initial evidence that IMMU - 132 can be active in patients refractory to both chemotherapy and immune checkpoint inhibitor therapy 32

IMMU - 132 – Results in Non - Small - Cell and Small - Cell Lung Cancers Ronald J. Scheff, M.D., Assistant Professor of Clinical Medicine, Weill Cornell Medical College; Medical Oncologist, New York - Presbyterian Hospital, New York, NY 33 Dra ft

Relapsed/Refractory Metastatic Lung Cancers 34 • Lung Cancer is the most common deadly form of cancer – Annually accounts for ~ 1 . 3 million cancer deaths worldwide and ~ 160 , 000 in U . S . (More deaths than colorectal, prostate and breast cancers combined) – NSCLC (85%), SCLC (~15%) • 5 - year survival rates for SCLC – 31%, 19%, 8% and 2% for stage I to IV, respectively • Antibodies to PD - 1 and PD - L1 have demonstrated prolonged tumor responses in metastatic NSCLC – Pembrolizumab is approved as 1 st line therapy – Atezolizumab, Nivolumab and Pembrolizumab are approved after a platinum - based chemotherapy with median OS of 12.6 - 13.8 months

Metastatic Non - Small - Cell Lung Cancer (mNSCLC) 35

IMMU - 132 in mNSCLC: Best Response By RECIST 1.1 47 response assessable; 45 with CT (8 and 10 mg/kg) ORR = 19% 64% had tumor shrinkage from baseline, including squamous pathology and prior immune checkpoint inhibitors Median duration of response: 8 months Clinical Benefit Rate (PR+SD > 4 mos) = 43% Median # prior therapy : 3 (range, 1 - 7) - 8 0 - 6 0 - 4 0 - 2 0 0 2 0 B e s t % c h a n g e i n t a r g e t l e s i o n s f r o m b a s e l i n e P a r t ia l r e s p o n s e ( c o n f ir m e d ) ( P R ) U n c o n f irm e d P R ( P R u ) ( s ta b le d is e a s e ) S ta b le d is e a s e P r o g r e s s io n 4 0 36 + S q u a m o u s c e ll h is to lo g y 8 m g / k g s ta r t in g d o s e P r io r c h e c k p o in t in h ib ito r T x + e a r ly C T a s s e s s m e n t a fte r 2 d o s e s

IMMU - 132: PFS and OS mNSCLC (8 or 10 mg/kg) ITT = all 54 - treated patients Median PFS = 5.2 months (95% CI = 2.6, 7.1) Median OS = 9.5 months (95% CI = 6.0, 16.7) 37

IMMU - 132 Benefit Versus Prior PD1/PD - L1 Rx Benefit 38 Age Sex # of Prior Tx CPI # line CPI Response to CPI Duration of CPI treatment (mos) IMMU - 132 Doses IMMU - 132 best response Best % change TL Histology PFS (mo) 78 M 2 Nivolumab 2 Yes 8 9 SD 0 SQ 1.2+ 63 F 6 Nivolumab 2 No 1 15 SD 7 AC 3.7+ 57 M 4 Nivolumab 4 Yes 8 6 PD 26 SQ 2 62 M 4 Avelumab 2 No 4 4 PD IER AC 1 77 F 1 Atezolizumab 1 Yes 23 8 SD - 13 AC 1.8+ 76 M 4 Atezolizumab 3 No 2 5 PD 21 SQ 2 61 F 4 Nivolumab 4 No 2 6 PD 36 AC 1.8 61 M 6 Atezolizumab 4 No 3 11 SD - 23 AC 3.1+ 66 F 2 Nivolumab 2 No 9 10 SD - 28* AC 2.7+ 69 M 3 Atezolizumab 3 No 2 7 SD 1 AC 1.6+ 74 M 3 Nivolumab 3 No 6 10 PRc - 50 SQ 3.4+ 67 F 3 Nivolumab 3 No 5 10 SD 10 AC 1.9+ 50 F 3 Nivolumab 3 Yes 9 5 SD 7 AC 1.5 • 4/13 IMMU - 132 pts responded (per investigator) to prior PD1/PD - L1 Rx and 6/13 had Rx ≥ 6 months • IMMU - 132: 1 PR (confirmed) + 2 SD with shrinkage of 23% and 28% (* converted to PR) noted Presented at ASCO 2016

Metastatic Small - Cell Lung Cancer (mSCLC) 39

IMMU - 132 in mSCLC: Best Response By RECIST 1.1 8 and 10 mg/kg ORR = 16% 60% had tumor shrinkage from baseline Median Duration of Response (PR+SD): 5.2 months Clinical Benefit Rate (PR+SD > 4 mos) = 40% Median # prior therapy: 2 (range, 1 – 7) - 1 0 0 - 8 0 - 6 0 - 4 0 - 2 0 0 2 0 4 0 6 0 8 0 B e s t R e s p o n s e B e s t % c h a n g e i n T L f r o m b a s e l i n e P a r tia l r e s p o n s e ( c o n f ir m e d ) P a r tia l r e s p o n s e ( u n c o n f ir m e d ) S ta b le d is e a s e P r o g re s s io n 40 8 m g /m g ( a ll o th e rs , 1 0 m g /k g )

IMMU - 132: PFS and OS for mSCLC (8 or 10 mg/kg) ITT = all 53 - treated patients Median PFS = 3.7 months (95% CI = 2.0, 4.3) Median OS = 7.0 months (95% CI = 5.5, 8.3) 41

IMMU - 132 in Lung Cancer (NSCLC + SCLC): Adverse Events in Study Population (Regardless of Causality) 42 All Grades Grades ≥3 Adverse Event n % n % Nausea 70 65% 5 5% Diarrhoea 61 57% 9 8% Fatigue 50 47% 10 9% Neutropenia 43 40% 33 31% Vomiting 37 35% 2 2% Alopecia 33 31% 0 0% Anaemia 31 29% 5 5% Constipation 28 26% 1 5% Anorexia 28 26% 3 5% Abdominal pain 21 20% 3 5% Dehydration 19 18% 2 5% Hypomagnesaemia 19 18% 0 5% Weight decreased 17 16% 0 5% Dyspnoea 17 16% 3 5% Hypophosphataemia 14 13% 2 5% White blood cell decreased 14 13% 8 5% Hypokalaemia 13 12% 3 5% Pneumonia 12 11% 8 5% Oedema peripheral 11 10% 1 5% Dizziness 11 10% 1 5% Back pain 11 10% 3 3% Lymphopenia 10 9% 4 4% Hyponatraemia 9 8% 4 4% Blood alkalinephosphatase elevated 9 8% 4 4% Hypoxia 7 7% 5 5% Alanine aminotransferase elevated 6 6% 3 3% Febrile neutropenia 4 4% 3 3% Based on 107 patients with AEs in the database as of December 15, 2016. Table shows AEs regardless of causality occurring in >10% of patients (all grades) or >2 (grade ≥3) patients.

• 82 year old male initially diagnosed with NSCLC July 2012 (EGFR and ALK negative) • Prior therapies: 43 – Carboplatin – Abraxane – Ge m ci t abi ne – Carboplatin – Etoposide – Erlotinib – Vinorelbine (Jul - Nov 2012)/pemetrexed (Jul 2012 - Apr 2013) (May - Aug 2013) (Aug - Dec 2013 ) (Dec 2013 - Apr 2014 ) (Jun - Jul 2014 ) (Jul - Sep 2014 ) (Oct - Nov 2014 ) • IMMU - 132 Therapy (Dec 2014 – Nov 2015) – Started at 10 mg/kg, but reduced to 7.5 mg/kg for dose 3 • 32 doses given – Five target lesions (sum = 160 mm at baseline) • Lung x 1 • Liver x 2 • LN x 2 • 1 NTL in the bone of the spine – Confirmed partial response; PFS = 10.8 months (maximum tumor shrinkage was 53 %) Case Study: NSCLC, confirmed PR
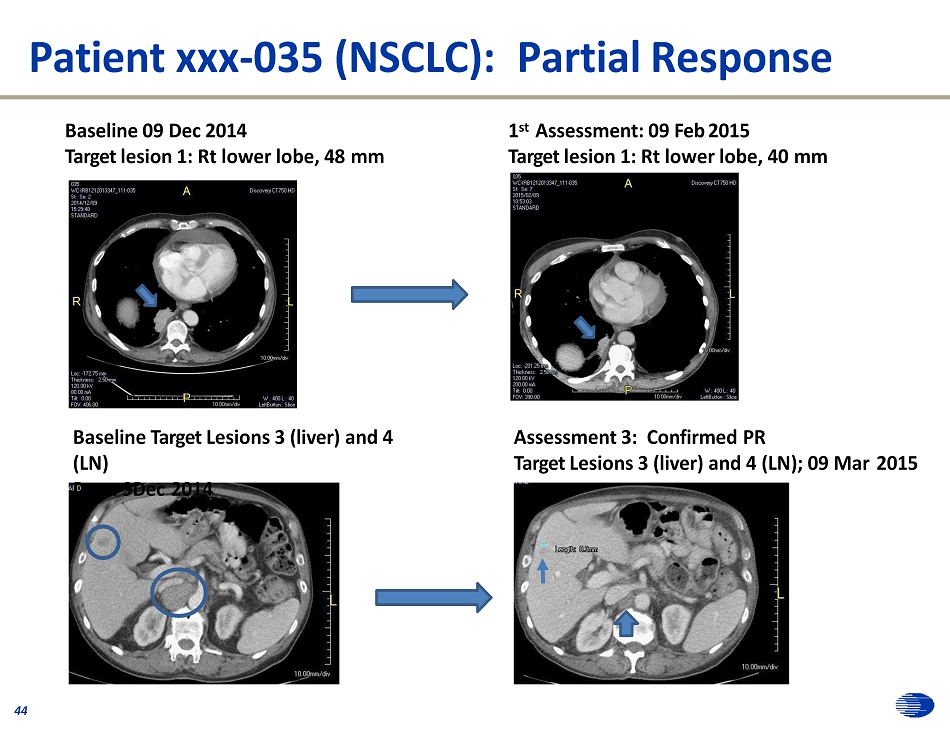
Patient xxx - 035 (NSCLC): Partial Response Baseline 09 Dec 2014 Target lesion 1: Rt lower lobe, 48 mm 1 st Assessment: 09 Feb 2015 Target lesion 1: Rt lower lobe, 40 mm Baseline Target Lesions 3 (liver) and 4 (LN) Da e: 9Dec 2014 Assessment 3: Confirmed PR Target Lesions 3 (liver) and 4 (LN); 09 Mar 2015 44

Case Study: SCLC, confirmed PR (PFS = 7.9 mos) 45 • 73 year old female initially diagnosed with SCLC July 2013 • Prior therapy – Carboplatin + Etoposide (6 cycles; Oct 2012 – Jan 2013); 11 - month response – Topotecan (Sep – Dec 2013); No response – Carboplatin + Etoposide (6 cycles; Nov 2013 – April 2014); 8 months response – Paclitaxel (May - June 2014); 1 month response • IMMU - 132 Therapy (Sept 2014) – Started at 10 mg/kg • 19 doses given – Five target lesions (sum = 230 mm at baseline) • Lung (21 mm), Axillary mass (60 mm), Adrenal mass (52 mm), Suprarenal mass (79 mm), retroperitoneal LN (18 mm short diameter) • 2 NTL, LN in chest and abdomen – Confirmed partial response; PFS = 7.9 months (maximum tumor shrinkage was 40%)

Patient 255 - 012 (SCLC): Partial Response Rt Lower Lobe Lt Axillary mass liver mass w i th Lt adrenal mass liver mass in view Responses in 4 of the 5 target lesions are shown Top row is baseline, bottom row is 1 st response assessment 8 weeks after treatment start 46

IMMU - 132 in Lung Cancer: Conclusions 47 • >50 patients enrolled to - date in each of NSCLC and SCLC • Patients very advanced (stage IV) and heavily pretreated, including having immune checkpoint inhibitors • Patients with either tumor showed ~60% shrinkage from baseline when treated with IMMU - 132 • Both cancers show response rates, PFS, and OS that appear to be improvements vs . current agents as a monotherapy in this setting • In NSCLC, several patients relapsing or refractive to a prior immune checkpoint inhibitor showed durable response • Safety profile similar to other indications: major toxicity manageable grade > 3 neutropenia

IMMU - 132 – Results in Triple - Negative Breast Cancer 48 Linda T. Vahdat, M.D., MBA, Professor of Medicine, Weill Cornell Medical College; Co - Leader BC Program at Meyer Cancer Center, New York, NY

Triple - Negative Breast Cancer Facts: 49 • ~15% of all breast cancer diagnosed • No optimal standard therapy in the adjuvant or metastatic setting • Metastatic TNBC – Median survival ~12 months – Short PFS - ~1.7 to 3.7 months • Large unmet need in the breast cancer community

N= 518 TNBC pts Setting: 0 - 1 prior lines of therapy Typical Clinical Scenario For Metastatic TNBC 50

Number of prior therapies – median (range) 5 (2 - 12) Prior chemotherapy drugs (>10%) Taxanes 67 (97%) Cyclophosphamide 63 (91%) Anthracyclines 58 (84%) Platinum agents 48 (70%) Fluoropyrimidine agents 34 (49%) Gemcitabine 30 (43%) Eribulin 27 (39%) Vinorelbine 13 (19%) Ixabepilone 8 (12%) Sites of Disease † Lymph node Lung Liver C he s t Bone Skin 43 (62%) 35 (51%) 30 (43%) 28 (41%) 21 (30%) 7 (10%) † As reported by baseline imaging RECIST 1.1 assessment. 53 IMMU - 132 in mTNBC: Prior Therapy and Sites of Disease

IMMU - 132 in mTNBC: Best Radiographic Response* ORR (25/85) = 29.4% • 2 CR (confirmed) • 23 PR (confirmed) • 3 PR (confirmatory CT pending) • 33 Stable Disease (10 pts ≥ 20% SD) • 21 progressive disease • 3 lack TL measurement at this time • 3 inevaluable for response; had 2 - 3 doses, but withdrew before 1 st CT CBR (CR+PR+SD > 6 Mos) = 44% CBR (CR+PR+SD > 4 Mos) = 57% - 1 0 0 - 8 0 - 6 0 - 4 0 - 2 0 0 2 0 4 0 6 0 8 0 B e s t R e s p o n s e B e s t % c h a n g e i n T L f r o m b a s e l i n e 7 9 / 8 5 re s p o n s e a s s e s s a b le p ts w h o c o m p le te d 1 tre a tm e n t c y c le a re re p re s e n te d 3 p ro g re s s e d , b u t T L m e a s u re m e n t u n a v a ila b le 3 d id n o t h a v e a C T re s p o n s e a s s e s s m e n t 54 O v e ra l l re s p o n s e a s s e s s m e n t d e s c r ip to r C o m p le te r e s p o n s e ( c o n f irm e d ) P a r tia l re s p o n s e ( c o n f ir m e d ) P a r tia l re s p o n s e ( p e n d in g ) P a r tia l re s p o n s e ( u n c o n f ir m e d ) S ta b le d is e a s e P r o g r e s s io n *Response measured by RECIST 1.1 (% Change From Baseline) in TNBC with > 2 Prior Lines for Metastatic Disease, Completed at Least 1 Therapy Cycle (10 mg/kg)

IMMU - 132: Duration of Response for CR+PR TNBC patients > 2 Prior Lines for Metastatic Disease, Completed 1 Cycle 10 mg/kg • For 25 pts with CR+PR: • Median duration of response = 10.8 mos (95% CI = 6.8, 12.7 mos) • Median time to 1 st response = 2.1 mos (range, 1.6 to 13.4 mos) • For 61 pts with CR+PR+SD: (including 3 PRu and 2 unconfirmed PR) • Median duration of response = 7.6 mos (95% CI = 6.1, 11.9) • 20 pts continuing treatment • 7 pts with PR • 3 with PRu • 10 SD 0 3 3 0 6 9 1 2 1 5 1 8 2 1 2 4 2 7 M o n th s fro m s ta rt o f tre a tm e n t C o m p le te r e s p o n s e ( c o n f irm e d ) P a r tia l re s p o n s e ( c o n f ir m e d ) P a r tia l re s p o n s e ( p e n d in g ) P a r tia l re s p o n s e ( u n c o n f ir m e d ) S ta b le d is e a s e C o n t in u in g tre a tm e n t P a r t ia l re s p o n s e 1 s t r e p o r te d 55

IMMU - 132 in mTNBC*: Progression - free Survival and Overall Survival *For patients with TNBC, > 2 Prior Therapy Lines for Metastatic Disease, 10 mg/kg ITT = all 89 - treated patients Median PFS = 6.0 months (95% CI = 5.0, 7.1) Median OS = 18.8 months (95% CI = 11.5, 20.6) 56

IMMU - 132 in mTNBC: Adverse Events in Study Population (Regardless of Causality) 57 All Grades Grades ≥3 Adverse Event n % n % Nausea 51 74% 5 7% Neutropenia 47 68% 27 39% Diarrhea 41 59% 9 13% Anemia 38 55% 10 14% Vomiting 35 51% 7 10% Fatigue 35 51% 6 9% Alopecia 31 45% 0 0% Constipation 26 38% 1 1% Rash 19 28% 1 1% Abdominal pain 18 26% 2 3% Leukopenia 17 25% 11 16% Dyspnea 16 23% 6 9% Back pain 16 23% 0 0% Anorexia 16 23% 0 0% Urinary tract infection 16 23% 2 3% Hypomagnesemia 15 22% 0 0% Dehydration 13 19% 4 6% Dizziness 13 19% 1 1% Headache 13 19% 1 1% Hyperglycemia 13 19% 1 1% Hypokalemia 13 19% 1 1% Hypophosphatemia 12 17% 7 10% Elevated alkaline phosphatase 11 16% 2 3% Arthralgia 11 16% 0 0% Febrile neutropenia 5 7% 5 7% Frequency of 69 patients with events regardless of causality occurring in >15% (all grades) or >3% (grade ≥3) of patients.

Dramatic Response in Chest Wall Disease to IMMU - 132 Before treatment with IMMU - 132 After 2 cycles of IMMU - 132 58 y.o. with Stage 2 TNBC diagnosed in 2011 (ACTdd). Recurred in 2014 with an IBTR ( Tcarbo). CW nodules 3/2015: received Ixabepilone+ xeloda, eribulin, casodex ( AR+). 58
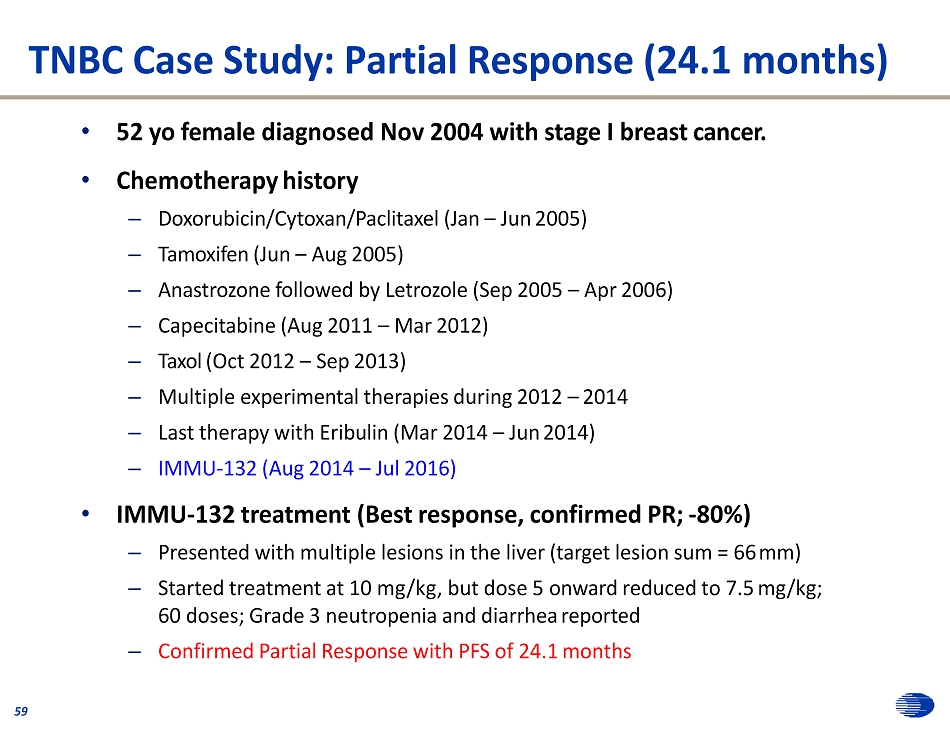
TNBC Case Study: Partial Response (24.1 months) 59 • 52 yo female diagnosed Nov 2004 with stage I breast cancer. • Chemotherapy history – Doxorubicin/Cytoxan/Paclitaxel (Jan – Jun 2005) – Tamoxifen (Jun – Aug 2005) – Anastrozone followed by Letrozole (Sep 2005 – Apr 2006) – Capecitabine (Aug 2011 – Mar 2012) – Taxol (Oct 2012 – Sep 2013) – Multiple experimental therapies during 2012 – 2014 – Last therapy with Eribulin (Mar 2014 – Jun 2014) – IMMU - 132 (Aug 2014 – Jul 2016) • IMMU - 132 treatment (Best response, confirmed PR; - 80%) – Presented with multiple lesions in the liver (target lesion sum = 66 mm) – Started treatment at 10 mg/kg, but dose 5 onward reduced to 7.5 mg/kg; 60 doses; Grade 3 neutropenia and diarrhea reported – Confirmed Partial Response with PFS of 24.1 months

Patient xxx - 010: TNBC TL #1 TL #1 Baseline: 28Jul14 60 1 st assessment: 22Sep14 Baseline: 28Jul14 1 st assessment: 22Sep14 8 - week assessment: - 51% decrease in index lesions TL #2 TL #2

IMMU - 132 in mTNBC: Conclusions 61 • In advanced, heavily pre - treated TNBC patients IMMU - 132 is very active: – Confirmed Response Rate ( CR+PR) = 29.4% with CBR (+SD≥6mos) = 44% – Median progression - free survival = 6 months – Median overall survival = 18.8 months • IMMU - 132 has a good therapeutic index: – Grade 3/4 neutropenia (39%), is manageable; few patients develop febrile neutropenia (7%) – Grade 3/4 diarrhea (13%) is less than with parental drug of SN - 38, irinotecan • This ADC can be given in repeated cycles for many months, without causing an immune response • Next step: use in earlier disease settings

IMMU - 132 – Preclinical Development (PARP/Chemo Combinations) 62 Thomas Cardillo, Ph.D., Executive Director, Preclinical and Hybridoma Development

Antitumor Activity of IMMU - 132 Alone and Combined with Chemotherapy 63 • Preclinically, IMMU - 132 has demonstrated efficacy against a wide range of Trop - 2 - expressing solid tumors (e.g., NSCLC, TNBC, pancreatic, gastric, colon, and urinary bladder) • In TNBC tumor - xenografts, when IMMU - 132 is combined with the clinically approved PARPi, olaparib (for BRCA1/2 - mutated ovarian cancer), as well as with talazoparib, there is a significant antitumor effect above that observed with monotherapy, in both BRCA1/2 - mutated and wild - type tumors (Cardillo et al, Clin Cancer Res. January 2017) • Combinations with other currently approved therapeutics (microtubule inhibitors and carboplatin) likewise demonstrate significant antitumor effects above single agent therapy in TNBC disease - models • All these combinations were well tolerated by the animals with no overt signs of toxicity

Tumor Growth Inhibition of Combined IMMU - 132 and Olaparib in Human TNBC Models: BRCA1/2 - and PTEN - Defective Tumors 0 . 0 0 0 . 7 5 1 . 0 0 1 . 2 5 O la p a r ib ( 5 0 m g / k g ) C o n t r o l A D C ( 2 5 0 g ) S a l i n e 0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 T im e P o s t - T u m o r T ra n s p la n t ( d a y s ) T u m o r V o l u m e s “ S D ( c m 3 ) O la p a r ib p lu s C o n t r o l A D C 0 . 5 0 IM M U - 1 3 2 ( 2 5 0 g ) O la p a r i b p lu s IM M U - 1 3 2 0 . 2 5 A D C A d m in is tra t io n O la p a r ib A d m in is tra t io n H C C 1 8 0 6 0 . 5 0 0 . 7 5 1 . 0 0 O la p a r ib ( 5 0 m g / k g ) C o n t r o l A D C ( 2 0 0 g ) O la p a r ib p lu s C o n t r o l A D C S a l i n e T u m o r V o l u m e s “ S D ( c m 3 ) IM M U - 1 3 2 ( 2 0 0 g ) O la p a r i b p lu s IM M U - 1 3 2 0 . 2 5 A D C A d m in is tra tio n O la p a r ib A d m in is tra tio n 0 . 0 0 0 1 4 2 8 4 2 5 6 7 0 8 4 9 8 1 1 2 T im e P o s t - T u m o r T ra n s p la n t ( d a y s ) M D A - M B - 4 6 8 (N=9 - 10) 64 (N=9 - 10)

Summary of in vitro and in vivo results of IMMU - 132 combinations with various chemotherapeutics in human TNBC 65 Chemotherapeutic In Vitro Synergy with IMMU - 132 In Vivo Combination Superior to M o no t h e r a p y PARP Inhibitors Ol a p a r ib * Yes † Yes T a l a z oparib * Yes ‡ Yes Ru c aparib * Yes n.d. Microtubule Inhibitors P acli t a x el n.d. † Yes Eribulin Mesylate n.d. † Yes Platinum - Based C a rbop l a tin n.d. ‡ Yes * Synergistic growth inhibition in both BRCA1/2 - mutated and - wild - type human TNBC tumor lines. † Improved efficacy demonstrated in mice bearing BRCA1/2 - mutated and - wild - type TNBC tumor xenografts. ‡ Only tested in mice bearing BRCA1/2 - mutated TNBC tumors thus far. n.d. Not Done
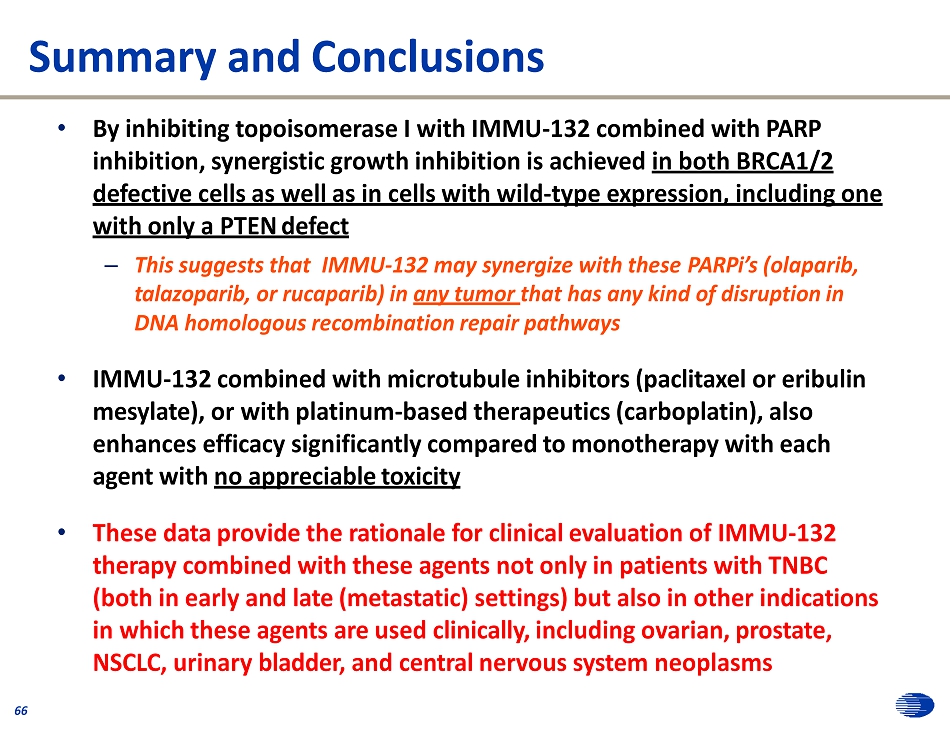
Summary and Conclusions 66 • By inhibiting topoisomerase I with IMMU - 132 combined with PARP inhibition, synergistic growth inhibition is achieved in both BRCA1/2 defective cells as well as in cells with wild - type expression, including one with only a PTEN defect – This suggests that IMMU - 132 may synergize with these PARPi’s (olaparib, talazoparib, or rucaparib) in any tumor that has any kind of disruption in DNA homologous recombination repair pathways • IMMU - 132 combined with microtubule inhibitors (paclitaxel or eribulin mesylate), or with platinum - based therapeutics (carboplatin), also enhances efficacy significantly compared to monotherapy with each agent with no appreciable toxicity • These data provide the rationale for clinical evaluation of IMMU - 132 therapy combined with these agents not only in patients with TNBC (both in early and late (metastatic) settings) but also in other indications in which these agents are used clinically, including ovarian, prostate, NSCLC, urinary bladder, and central nervous system neoplasms

IMMU - 132 – Competitive Landscape and Commercial Assessment 67 Andrew Funderburk, Partner, and Earl Gillespie, Consultant, Health Advances

Q&A Session 68

IMMU - 132 Manufacturing / Regulatory / 69 Development Plan for TNBC Edmund Rossi, Ph.D., Associate Vice President, Process Development and Manufacturing

Phase 3/Commercial Production of IMMU - 132 BSP Conjugation Fill and L y oph i li z a t i on 200 mg/vial JMPS CL2A - SN - 38 IMMU hRS7 - 80 - 36 - 35 IgG 70

IMMU - 132 Manufacturing Timeline L o t 71 2016 Q2 2016 Q3 2016 Q4 2017 Q1 2017 Q2 2017 Q3 2017 Q4 I g G S N 38 DP I M MU Eng (2.4 kg) I0 E M fg C omp S ta b ili ty → Clin (2.4 kg) I1 1 M fg Rel Clin (2.4 kg) I2 1 , 2 M fg Rel C omp S ta b ili ty → Clin (2.4 kg) I3 2 M fg Rel Clin (2.4 kg) I4 4 M f g Rel Co m p S tab ili ty → Clin (2.4 kg) I5 4 , 5 M f g Rel Clin (2.4 kg) I6 5 M f g Rel Vali dation V i rus Upstream Process Controls J M PS Clin (450 g) J1 E Mfg Rel C omp S ta b ili ty → Clin (480 g) J2 1,2 Mfg Rel Clin (340 g) J3 2 M fg Rel C omp S ta b ili ty → Clin (300 - 500 g) J4 3 M fg Rel Clin (300 - 500 g) J5 4 M fg Rel Clin (300 - 500 g) J6 5 M fg Rel Vali dation Method Validation B S P Eng (9,000 vls) I0 J1 E C on L y o Rel DS / D P Comp S ta b ili ty → Clin (16,000 vls) I1 , I2 J 1 , J 2 1 Con Con 1 2 L y o Rel DS / D P Comp S ta b ili ty → Clin (16,000 vls) I2 , I3 J 2 , J 3 2 Con 1 & 2 L y o Rel Clin (16,000 vls) I4 , I5 J5 4 C on 1 & 2 L y o Rel Clin (16,000 vls) I5 , I6 J6 5 C on 1 & 2 L y o Rel Validation Process Controls IgG , hRS7 IgG intermediate SN38 , CL2A - SN38 activated drug DP , IMMU - 132 drug product Eng , Engineering lot Clin , Clinical lot Mfg , Manufacture Comp , Comparability Lyo , Lyophilization Rel , Release date Con , Conjugation

Regulatory Status Update 72 Designations Indication Breakthrough Fast Track Orphan Accelerated Approval TNBC x x Planned UC Pending Submission SCLC Pending Submission * x x NSCLC Pending Submission x Pancreatic x Regulatory Progress ▪ Received Scientific Advice from the EMA on confirmatory Phase 3 protocol ▪ Reached an agreement with SPA in place with FDA on confirmatory Phase 3 protocol ▪ FDA has recently clarified what supporting data would be needed in relapsed/refractory TNBC to file for a BLA under the FDA’s Accelerated Approval (“AA”) pathway ▪ Plans to submit CMC briefing book to the FDA and discuss non - clinical aspects of the BLA, including CMC data related to CMO engineering runs ▪ In mid - 2017, the Company plans to submit the BLA under the agency’s AA pathway with FDA decision expected in late 2017/early 2018 (if the AA is granted) ▪ CRO identified for the confirmatory Phase 3 TNBC trial * Preliminary Advice Meeting held with FDA on 10/19/2016
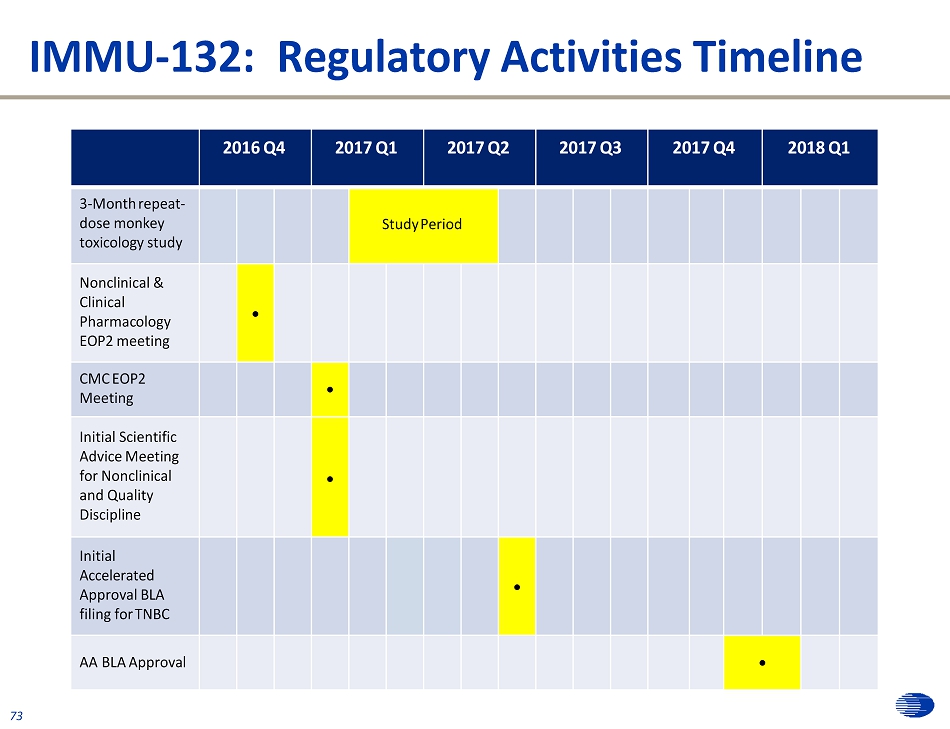
IMMU - 132: Regulatory Activities Timeline 73 2016 Q4 2017 Q1 2017 Q2 2017 Q3 2017 Q4 2018 Q1 3 - Month repeat - dose monkey toxicology study Study Period Nonclinical & Clinical P h arma c ol o gy EOP2 meeting CMC EOP2 Meeting Initial Scientific Advice Meeting for Nonclinical and Quality Discipline Initial Accelerated Approval BLA filing for TNBC AA BLA Approval

• Single - arm Phase 2 Basket (diverse cancers) study – Enrolled 100 assessable TNBC patients with at least 2 prior therapies required for BLA submission – Patient follow - up continuing – Third - party “blinded” confirmation of response assessment ongoing – high concordance to - date • Phase 3 confirmatory RCT – Validation of commercial - scale manufacturing by outside CMOs – Enrollment planned to begin in early 2017 – SPA with FDA; protocol accepted by EMA – Out - licensing expected to be consummated before trial launch • Accelerated approval application – Submission is expected in mid - 2017 74 IMMU - 132: Updated Clinical Development in TNBC

Phase 3 Confirmatory Trial Design for IMMU - 132: TNBC ▪ Primary endpoint is PFS ▪ Two arms: IMMU - 132 vs physician’s choice of 1 from 4 chemotherapies ▪ 328 patients to be enrolled, 1:1 randomization Designed to Replicate Success Attention to Execution ▪ Trial will be conducted under a SPA and is expected to take ~ 3 years ▪ Key powering considerations: - 99% powering for PFS Phase 3 Design Control Arm 164 patients to receive physician’s choice of 1 from 4 chemotherapies (Eribulin, Capecitabine, Gemcitabine and Vinorelbine) Primary Endpoint: PFS Treatment Arm 164 patients to receive IMMU - 132 328 patients with mTNBC failed 2+ prior lines of treatment Randomized 1:1 75

IMMU - 130, Epratuzumab, Veltuzumab, Milatuzumab and IMMU - 114 William A. Wegener, M.D., Ph.D., Chief Medical Officer 76

IMMU - 130 (labetuzumab govitecan) 77 Mechanism of Action • IMMU - 130 binds CEACAM5 on colorectal tumors and other cancers • IMMU - 130 releases SN - 38 locally and internally for diffusion into cancer cells

Phase 1/2 Trial of IMMU - 130 in Patients with Refractory/Relapsing Metastatic Colorectal Cancer 78 • Multicenter study – Richard Goldberg, OSU Cancer Center, Columbus, OH – Steven Cohen, E. Dotan, Fox Chase Cancer Center, Philadelphia, PA – Wells Messersmith, C. Lieu, U. Colorado Cancer Center, Aurora, CO – John Marshall, Georgetown U. Hospital, Washington, DC – Randolph Hecht, UCLA Jonsson Cancer Center, Los Angeles, CA – Jordan Berlin, Vanderbilt - Ingram Cancer Center, Nashville, TN – Michael Guarino, Helen F. Graham Cancer Center, Newark, DE – Alexander Starodub, Indiana U. Center for Cancer Care, Goshen, IN • Heavily - pretreated patients: median 5 prior treatments (range 1 - 13). All relapsed or refractory to irinotecan • IMMU - 130 given weeks 1 & 2 of 3 - week treatment cycles • 4 dosing cohorts evaluated (4 or 6 mg/kg BIW, 8 or 10 mg/kg QW) • Final results in 91 patients in preparation for publication

PR n(%) Median PFS (mo.) Median OS (mo.) 8 mg/kg QW (n = 16) 0 4.8 7.5 10 mg/kg QW (n = 15) 0 4.6 9.2 4 mg/kg BIW (n = 18) 0 2.1 6.0 6 mg/kg BIW (n = 16) 1 (6%), confirmed 3.7 7.3 Regorafenib (3 rd line) 1% 2.0 6.4 Treatment Response by RECIST 1.1 (AACR 2015) • Partial response (88% shrinkage of lung and liver lesions) • Plasma CEA decreased 21.0 to 2.4 ng/mL • Response for 13 months before patient took a drug holiday, comparable response when resumed treatment (spanned 2 yrs) 7 0

Humanized B - cell Antibodies 8 0 • Epratuzumab (anti - CD22) • Veltuzumab (anti - CD20)

Epratuzumab (hLL2) 81 • CD22 expressed across different NHL histologies, and pediatric/adult ALL • High affinity binding to CD22 (0.7 nM) induces rapid CD22 internalization • May block natural ligands and elicit signals which leads to inhibition of BCR • Induces phosphorylation of cytoplasmic tail of CD22 which ultimately leads to SHP activation • Well Tolerated. Infusion Time ~1 hour • No Significant Toxicity in Current Clinical Trials (>2000 pts)

Epratuzumab/Rituximab Combination Studies (N=136) 82 Study Design Population Treatment Pts 128 Singl e - Ce nt er Refractory or Recurrent NHL 360 mg/m 2 hLL2 followed by 375 mg/m 2 rituximab once weekly for 4 doses 22 138 Multi - Ce nt er Recurrent or Refractory NHL 360 mg/m 2 hLL2 followed by 375 mg/m 2 rituximab once weekly for 4 doses 49 08EU Multi - Center Refractory or Recurrent NHL 360 mg/m 2 hLL2 followed by 375 mg/m 2 rituximab once weekly for 4 doses 65

E/R Combination Response Rates 83 Study Population OR CR/CRu Single - Center #128 1 FCL (N=15) 67% 60% Multi - Center #138 2 FCL (N=41) 61% 24% Multi - Center #08EU 3 FCL (N=33) 64% 24% Rituximab Phase 2* FCL (N=60) 38% 10% Rituximab Phase 2* FCL/SLL (N=166) 48% 6% Single - Center #128 1 DLBCL (N=5) 80% 60% Multi - Center #08EU 3 DLBCL (N=15) 47% 33% Rituximab Phase 2 † DLBCL (N=54) 31% 9% 1 Leonard et al., JCO 2005; 23:5044 - 51; 2 Emmanouilides et al., Cancer 2008; 113:2714 - 23 3 Strauss et al., JCO 2006; 24:3880 - 6 *Rituximab package Insert data; † Coiffier et al, Blood 1998 92:1927 - 32.
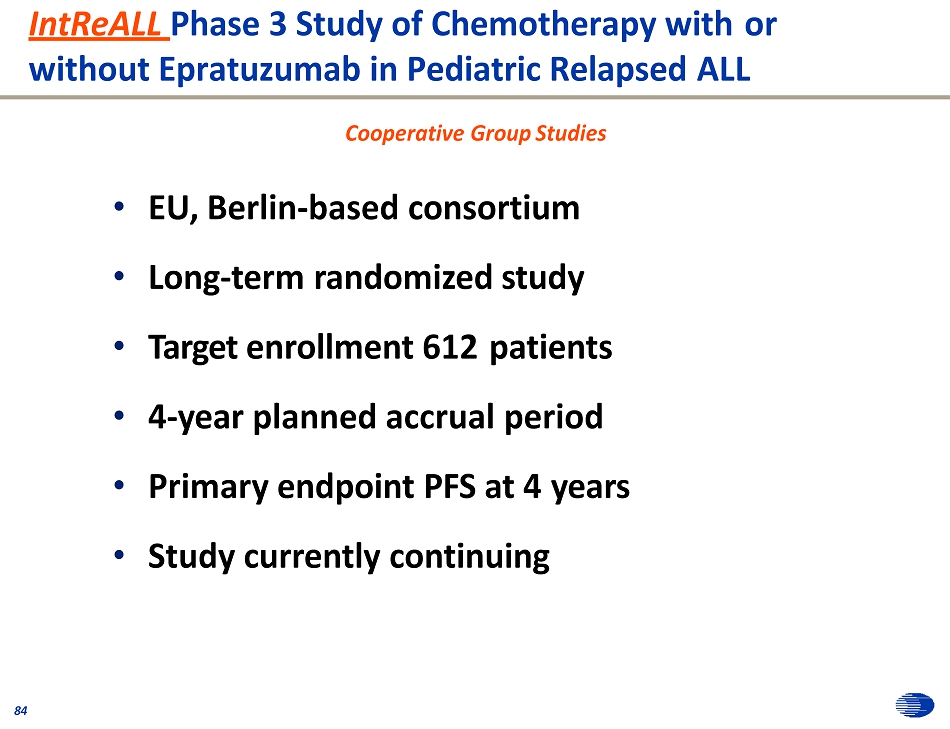
IntReALL Phase 3 Study of Chemotherapy with or without Epratuzumab in Pediatric Relapsed ALL 8 8 4 4 • EU, Berlin - based consortium • Long - term randomized study • Target enrollment 612 patients • 4 - year planned accrual period • Primary endpoint PFS at 4 years • Study currently continuing Cooperative Group Studies

Veltuzumab: Oncology and Autoimmune Markets 85 • 2 nd generation anti - CD20 antibody • Structural differences from rituximab* – Humanized antibody (rituximab is chimeric) – Binding site differs from rituximab by one amino acid in VH - CDR3, and has different framework regions • Functional differences from rituximab (comparative preclinical studies)* – Binds CD20 3X longer – Increased complement dependent cytotoxicity (CDC) – Improved survival in lymphoma xenograft models *Goldenberg et al., Blood, 2009 , 113: 1062 - 70.

Veltuzumab: NHL IV Phase 1/2 Trial • 82 patients • Adults CD20 + B - cell NHL, failed ≥1 prior therapy • Veltuzumab infusions once weekly X 4 wks • 5 Dose levels: 80, 120, 200, 375, 750 mg/m 2 • Rapid infusions & limited mild moderate reactions • No other significant safety issues • Treatment responses comparable to rituximab - even at 80 mg/m 2 86
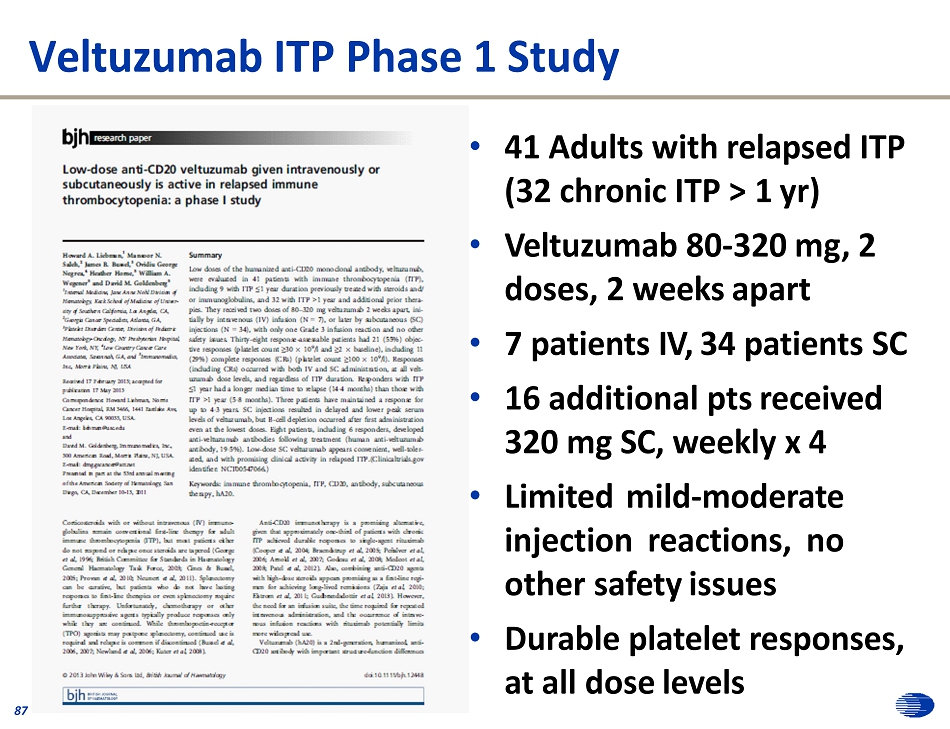
Veltuzumab ITP Phase 1 Study • 41 Adults with relapsed ITP (32 chronic ITP > 1 yr) • Veltuzumab 80 - 320 mg, 2 doses, 2 weeks apart • 7 patients IV, 34 patients SC • 16 additional pts received 320 mg SC, weekly x 4 • Limited mild - moderate injection reactions, no other safety issues • Durable platelet responses, at all dose levels 87

Veltuzumab Conclusions 88 • Anti - CD20 market well established including autoimmune indications • Preclinical evidence of superiority (binding duration, in vivo efficacy) vs. rituximab: lower dose potent clinically • Promising clinical experience in > 200 patients, oncology and autoimmune • Improved SC dosing safe, effective and convenient • SC dosing expands market to offices without infusion suites • Ready for controlled trials in oncology (e.g., combination therapy in CLL) and autoimmune disease (e.g., ITP and MS)
Humanized Antigen - Presenting Cell Antibodies 89 • Milatuzumab (anti - CD74) • IMMU - 114 (anti - HLA - DR)

IMMU - 115 (Milatuzumab) 90 • Targets CD74 on antigen - presenting cells (APC), including B - cells and dendritic cells • Clinical and pharmacodynamic activity in heme malignancies – Alone (Kaufman et al, Br J Haematol. 2013 163:478 - 86) and combined with anti - CD20 veltuzumab (Christian et al. Br J Haematol. 2015;169:701 - 10) • Dysregulation of APCs may occur in autoimmune disease • Phase 1b study in patients with systemic lupus erythematosus (SLE) and moderately active disease* – IMMU - 115 given by subcutaneous injection (SC) for 4 consecutive weeks – SLE Disease activity assessed by BILAG2004 and SELENA - SLEDAI every 4 weeks until week 24 – Initial results presented at EULAR 2016 for first 10 patients *Study supported by grant from US Department of Defense

SC IMMU - 115 Appears Safe with Evidence of Treatment Efficacy in SLE • All 10 patients decreased initial disease activity in at least one body system • Suppression of disease activity extended 24 weeks in most patients • Injection reactions were mild - moderate • No other safety issues emerged • As such, a double - blind placebo - controlled expansion phase is underway in order to confirm the activity of SC IMMU - 115 in this population Mean Disease Activity Scores Disease Activity by Body System (BILAG B = 5, C = 1, D = E = 0) 91

IMMU - 114 – 2 nd Generation anti - HLA - DR antibody • Human Leukocyte - Antigen - DR (HLA - DR) - appealing target for therapy – Significantly higher expression than CD20 and other typical B - cell markers in NHL, CLL, other hematologic malignancies – Also expressed in solid tumors (e.g., melanoma, NSCLC, neuroblastoma, gastric, hepatic, breast, pancreatic) • Prior anti - HLA - DR antibodies (IgG 1 , IV) failed – limiting toxicities, such as severe infusion reactions ( Lin et al, Leuk Lymphoma, 2009 ) • IMMU - 114 was engineered to minimize toxicity - a novel humanized IgG 4 anti - HLA - DR antibody, it lacks effector - cell functions (ADCC, CDC) but retain binding with a broad range of direct antitumor effects (Stein R et al., Blood . 2010;115:5180 - 90). • IMMU - 114 also concentrated for SC injection - to further minimize toxicity 92

IMMU - 114 – Initial Phase 1 Study • Initial study in relapsed/refractory NHL and CLL after failing rituximab - containing therapies is showing encouraging efficacy with no severe reactions as seen previously (ClinicalTrials.gov, NCT01728207) • Results recently presented at Pan Pacific Lymphoma Conference 2016 (Stephens et al) showed objective evidence of treatment activity in 8/16 (50%) currently assessable patients (FL x 4, CLL x 3, DLBCL) • Treatment cycles now given repeatedly to determine appropriate dosing for next studies 93

IMMU - 140 – Overview Thomas Cardillo, Ph.D., Executive Director, Preclinical and Hybridoma Development 94
Antitumor Activity of IMMU - 140 in ALL, AML, MM, and CLL Hematopoietic Disease Models 95 • IMMU - 140 makes use of the same CL2A - SN - 38 conjugation methodology as used for IMMU - 132 to attach 6 - 8 SN - 38 molecules per IMMU - 114 molecule • IMMU - 140 provides an added benefit of a dual - therapeutic through the direct antitumor activity of IMMU - 114 (e.g., pERK1/2, apoptosis inducing factor) and the cytotoxic effect of SN - 38 (via caspase - cascade and PARP cleavage) • Using IMMU - 140 to target difficult - to - treat ALL, AML, and CLL disease models results in an added, superior antitumor effect above that achieved with IMMU - 114 alone. IMMU - 140 also provide an improvement in survival in a MM model when compared to IMMU - 114 therapy that approaches significance • The dual - therapeutic potential of IMMU - 140 ( via independent activity of IMMU - 114 and SN - 38), allows for the ability to treat a range of hematopoietic and solid human cancers clinically

0 1 4 2 8 4 2 5 6 7 0 8 4 9 8 1 1 2 0 2 5 5 0 7 5 1 0 0 S a l i n e IM M U - 1 1 4 ( 5 0 0 g ) C o n t r o l Ig G ( 5 0 0 g ) C o n t r o l A D C ( 5 0 0 g ) IM M U - 1 4 0 vs . c o n tr o ls ( P - v a l u e ) 3 7 d a y s < 0 . 0 0 0 1 M e d ia n S u rv iv a l 6 6 . 5 d a y s n . a . 2 3 d a y s < 0 . 0 0 0 1 2 6 d a y s < 0 . 0 0 0 1 I n j e c t C e l l s < 0 . 0 0 0 1 2 2 . 5 d a y s T im e P o s t - C e l l In o c u la t io n ( D a y s ) P e r c e n t S u r v i v a l T h e r a p y 0 4 2 0 2 5 5 0 7 5 1 0 0 M e d ia n S u rv iv a l 5 0 0 g vs . c o n tr o ls ( P - v a l u e ) 7 1 4 2 1 2 8 3 5 T im e P o s t - C e l l In o c u la t io n ( D a y s ) P e r c e n t S u r v i v a l C e l ls I n j e c t e d i . v . T h e r a p y 2 5 0 g vs . c o n tr o ls ( P - v a l u e ) IM M U - 1 4 0 ( 5 0 0 g ) IM M U - 1 4 0 ( 2 5 0 g ) 3 7 d a y s 2 7 d a y s n . a . 0 . 0 0 2 1 n . a . n . a . C o n t r o l A D C ( 5 0 0 g ) 2 1 d a y s 0 . 0 0 3 1 0 . 0 8 4 7 1 5 d a y s 0 . 0 0 3 1 0 . 0 0 3 1 C o n t r o l A D C ( 2 5 0 g ) IM M U - 1 1 4 ( 5 0 0 g ) 1 5 d a y s 0 . 0 0 1 5 0 . 0 0 4 1 S a l in e C o n t r o l 1 4 d a y s 0 . 0 0 3 1 0 . 0 0 3 1 96 Therapeutic Efficacy of IMMU - 140 versus IMMU - 114 in a Mouse Xenograft Disease Model of Human ALL (MN - 60) and AML (MOLM - 14) (N=10) IM M U - 1 4 0 ( 5 0 0 g ) MN - 60 (ALL) • Both IMMU - 140 and IMMU - 114 provided a survival benefit compared to all controls ( P <0.001). • However, IMMU - 140 provided a significantly improved survival benefit above that achieved with IMMU - 114 therapy ( P <0.0001; log - rank) with 3 mice still alive and tumor - free when the study ended on day 105. • Therapy well tolerated in the mice. MN - 60 MOLM - 14 (AML) • Saline control and IMMU - 114 treated mice succumbed to disease progression quickly. • Mice treated with IMMU - 140 had a greater than 1.5 - fold increase in survival ( P =0.0031). • IMMU - 140 therapy was significantly better than control ADC ( P =0.0031). • A dose - reduction to 250 g hL243 - SN - 38 still provided a greater than 80% improvement in survival compared to saline and control ADC at the same dose ( P =0.0031) (N= 5 ) MO L M - 14

0 1 4 2 8 9 8 1 1 2 1 2 6 1 4 0 0 2 5 5 0 7 5 1 0 0 M e d ia n C o m p a ra b le IM M U - 1 4 0 vs . 4 2 5 6 7 0 8 4 T im e ( D a y s ) P e r c e n t S u r v i v a l i . v . C e lls I n je c t e d T h e r a p y S u rv iv a l IM M U - 1 1 4 IM M U - 1 4 0 ( 5 0 0 g ; i . p . ) > 1 3 3 d a y s n . a . IM M U - 1 4 0 ( 2 5 0 g ; i . p . ) > 1 3 3 d a y s n . a . IM M U - 1 4 0 ( 1 0 0 g ; i . p . ) 1 0 0 . 5 d a y s n . a . IM M U - 1 1 4 ( 5 0 0 g ; s . c . ) 1 0 8 d a y s 0 . 0 4 9 3 IM M U - 1 1 4 ( 2 5 0 g ; s . c . ) 7 8 . 5 d a y s 0 . 0 2 8 9 IM M U - 1 1 4 ( 1 0 0 g ; s . c . ) 1 0 4 d a y s 0 . 3 8 5 8 h 6 7 9 - S N - 3 8 ( 5 0 0 g ; i . p . ) 4 4 . 5 d a y s < 0 . 0 0 2 3 * S a l in e C o n t r o l 4 1 d a y s < 0 . 0 0 0 1 * * B o t h IM M U - 1 4 0 a n d IM M U - 1 1 4 s ig n i f ic a n t ly b e t t e r t h a n c o n t r o l s 97 Therapeutic Efficacy of IMMU - 140 versus IMMU - 114 in a Mouse Xenograft Disease Model of Human MM (CAG) and CLL (JVM - 3) CAG (MM) • There is a >151 - d MST in mice treated with IMMU - 140 which is significantly better than saline, bortezomib, or ADC controls (P<0.0001). • While not significant, IMMU - 140 does provide an ~60% improvement in survival when compared to IMMU - 114 therapy (MST=94.5 d, P=0.0612). • Bortezomib therapy combined with IMMU - 114 or IMMU - 140 did not improve survival above monotherapy. JVM - 3 (CLL) • Both IMMU - 140 and IMMU - 114 therapy provide a significant survival benefit when compared to control ADC or saline ( P <0.0023). • Importantly, there is a significant survival benefit in mice treated with the two highest doses of IMMU - 140 vs. comparable IMMU - 114 doses ( P <0.0493). 2 5 5 0 7 5 1 0 0 S a l in e C o n t r o l IM M U - 1 1 4 ( 5 0 0 g ) IM M U - 1 4 0 ( 5 0 0 g ) IM M U - 1 1 4 + B o r t e z o m i b IM M U - 1 4 0 + B o r t e z o m i b h M N - 1 4 ( 5 0 0 g ) + B o r t e z o m i b B o r t e z o m i b ( 1 5 g ; 0 . 8 9 m g / k g ) M e d ia n S u r v iv a l 9 4 . 5 d a y s 0 . 0 6 1 2 > 1 5 1 d a y s n . a . 3 2 . 5 d a y s < 0 . 0 0 0 1 1 3 1 d a y s 0 . 1 5 5 4 > 1 5 1 d a y s 0 . 9 7 7 6 3 2 d a y s < 0 . 0 0 0 1 3 2 d a y s < 0 . 0 0 0 1 4 2 5 6 7 0 8 4 9 8 1 1 2 1 2 6 1 4 0 1 5 4 T im e ( D a y s ) P e r c e n t S u r v i v a l I n je c t e d i . v . C e lls M A b T h e r a p y i . p . C o n t r o l A D C ( 5 0 0 g ) + B o r t e z o m i b 3 2 . 5 d a y s < 0 . 0 0 0 1 0 0 1 4 2 8 B o r t e z o m i b T h e r a p y i . v . IM M U - 1 4 0 v s . c o n tr o ls ( P - v a l u e ) C A G (N= 9 - 10) JV M - 3 (N=10)

• IMMU - 140 provides an added benefit of a dual - therapeutic through the direct antitumor activity mediated by the IMMU - 114 HLA - DR - binding moiety (p - ERK1/2 and AIF signaling) and the added cytotoxic effect of SN - 38 delivery to the cells (caspase cascade and PARP cleavage) • IMMU - 140 antibody - drug conjugate shows higher potency than naked IMMU - 114 pre - clinically in ALL, AML, and CLL and an added, if not significant, survival benefit in experimental MM • IMMU - 140 has activity in malignant melanoma; HLA - DR expressed by several solid cancers • IMMU - 140 therapy was well tolerated with not observable toxicities in the form of weight loss and no treatment related deaths • The dual - therapeutic potential of SN - 38 - conjugated IMMU - 114 (IMMU - 140) allows for the ability to treat a range of HLA - DR - positive hematopoietic and solid cancers, and therefore warrants further clinical development 98 Summary and Conclusions

Bispecific Antibodies and Immuno - Oncology Programs 99 Edmund Rossi, Ph.D., Associate Vice President, Process Development and Manufacturing

Bispecific Antibodies Platform Technology 100 • Dock - and - Lock® (DNL®) for improved therapy of cancers and immune diseases • DNL BsMabs for immuno - oncology (T - cell retargeting for solid cancer therapy)

AD2 = C G QIEYLAKQIVDNAIQQA G C ( AKAP - IS : 0.4 nM) DDD2 = C GHIQIPPGLTELLQGYTVEVLRQQPPDLVEFAVEYFTRLREARA (DDD - RII ) 101 Dock - and - Lock® Proprietary Technology

Features of DNL 102 • One of the two modules is always present in two copies • The method is modular and versatile • Ease of preparation • Site - specific conjugation • Quantitative yields • Homogeneous final product of defined composition, retained bioactivities of each parental module, and in vivo stability

22* - (20) - (20) (PLoS ONE 2014, 9: e98315) Ep r a t u z u m a b 103 Veltuzumab V eltuzum a b The conjugation of Fab - DDD2 to C L - AD2 - IgG retains a fully functional Fc

22* - (20) - (20) Potently Kills Daudi Cells, But Not Normal B Cells 104

Potential Indications for CD22 - CD20 Targeting 105 • B - cell non - Hodgkin lymphomas (FL and DLBCL), chronic lymphocytic leukemia, acute lymphoblastic leukemia (children) • B - cell - related autoimmune diseases: rheumatoid arthritis, systemic lupus erythematosus, Sjogren’s syndrome, psoriasis, granulomatosis, various arteritis indications, immune thrombocytopenia, multiple sclerosis, etc.

C L C L C C H 1 H 1 V L V V H L V H DDD2 DDD2 H H H H H H AD2 Okt 3 s c F v - A D 2 DNL (19) - 3s 106 Trivalent Bispecific Antibodies (Fab) 2 x scFv for Redirected T - Cell Killing hA19 - Fab - DDD2
Bispecific Antibodies for T - cell Retargeting Immunotherapy 107 • CD19 target for lymphomas and leukemias • Trop - 2 target for solid cancers • CEACAM5 target for solid cancers

(X) - 3s Bispecific Antibodies Redirect T - cells to Bind Cancer Cell 108 and Kill Cancer Cells T T T T ( X ) - 3s
(X) - 3s Bispecific Antibodies Redirect T - cells to Bind and Kill Cancer Cells 109

Da u d i - 8 100 90 80 70 60 50 40 30 20 10 0 - 1 3 ( 22 ) - 3s ( 19 ) - 3s ( 20 ) - 3s - 12 - 11 - 10 - 9 Log Concentration (M) % S p eci f ic L ysis Rossi et al, MAbs, 2014; 6(2):381 - 91 110 (X) - 3s Trivalent Bispecific Antibodies Mediate Potent Redirected T - cell Lysis of NHL Cells in an ex - vivo Assay

Each regimen significantly inhibited tumor growth; >60% of mice were tumor - free at the end of the study 111 (19) - 3s Mediated Effective T - cell Therapy of Human Burkitt Lymphoma Xenografts (Raji) at Three Dosing Schedules

(E1) - 3s: A Bispecific Antibody for T - Cell Redirected Killing of Various Solid Cancers 112 • (X) - 3s design produced with DNL – Bivalent binding of Trop - 2 on many types of human epithelial cancers • Potent activity with reduced cytokine release – Potentially allowing for higher dose with less side - effects – First clinical study planned for second - half 2017

NCI - N87 Gastric Carcinoma Cells (green) CD8 + T Cells ( y ell o w / o r a n g e) (E1) - 3s (E1) - 3s (19) - 3s TF12 (19) - 3s Binds T cells but not tumor 113 TF12 Anti - Trop - 2 bsAb binds tumor, but not T cells Rossi et al, Mol Cancer Ther, 2014; 13(10):2341 - 51 (E1) - 3s Effectively Targets T - cells to Solid Cancer Cells Expressing Trop - 2

0 1 4 2 8 8 4 9 8 0 2 5 5 0 7 5 1 0 0 4 2 5 6 7 0 T im e ( D a y s ) P e r c e n t S u r v i v a l 9 8 1 1 2 1 2 6 0 2 5 5 0 7 5 1 0 0 ( E 1 ) - 3 s + P E G A S Y S ( E 1 ) - 3 s P E G A S Y S U n t re a te d 0 1 4 2 8 4 2 5 6 7 0 8 4 T im e ( D a y s ) P e r c e n t S u r v i v a l • Low doses (50 μg) of (E1) - 3s significantly improved survival • Efficacy was enhanced with the addition of interferon alpha 114 Rossi et al, Mol Cancer Ther, 2014; 13(10):2341 - 51 Capan - 1 (Pancreatic) NCI - N87 (Gastric) (E1) - 3s Effectively Mediated T - cell Killing of Pancreatic and Gastric Cancer Xenografts, which was Enhanced with Interferon Alpha

- 1 2 - 9 0 500 1000 1500 - 11 - 10 log [bsAb], M [ I L - 6 ] , pg / m L - 1 2 - 9 0 500 1000 1500 2000 [ I L - 2 ] , pg / m L - 1 2 - 9 0 250 500 750 1000 - 11 - 10 log [bsAb], M [ TNF - ] , p g / m L - 11 - 10 log [bsAb], M IFN - - 1 2 - 9 0 20000 40000 60000 1250 80000 - 11 - 10 log [bsAb], M [ I FN - ] , p g / m L - 1 2 - 9 0 2000 4000 6000 I L - 2 I L - 6 I L - 1 0 2500 2000 8000 115 - 11 - 10 log [bsAb], M TFN - [ I L - 10 ] , pg / m L E1 - BiTE (E1) - 3s Rossi et al, Mol Cancer Ther, 2014; 13(10):2341 - 51 The (X) - 3s Design is Less Likely to Induce Cytokine Release Syndrome Compared to Other bsAb Formats (e.g., BiTE )

(E1) - 3s Effectively Redirects T - cell Killing of Breast (including TNBC) and Prostate Cancers - 1 3 - 9 - 8 0 2 0 4 0 6 0 8 0 1 0 0 - 1 2 - 1 1 - 1 0 lo g [ ( E 1 ) - 3 s ] , M % U n t r e a t e d M D A - M B - 2 3 1 ( T N B C ) H C C 3 8 ( T N B C ) B T - 2 0 ( T N B C ) H C C 1 9 5 4 ( B r e a s t) P C - 3 ( P r o s ta te ) 116

(E1) - 3s Effectively Mediated T - cell Killing of Triple - negative Breast Cancer Xenografts, which was Enhanced with a Checkpoint Inhibitor Antibody 0 6 3 0 2 5 5 0 7 5 O u t - G ro w th C o n tro l ( M D A - M B - 2 3 1 c e ll o n ly ) U n tre a te d C o n tro l ( T u m o r c e lls + T c e lls ) ( E 1 ) - 3 s c P D - 1 1 0 0 M e d ian S u rv iv a l 4 9 d a y s 3 1 d a y s 2 8 d a y s 3 5 d a y s 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 T im e P o s t - T u m o r In o c u la t io n ( d a y s ) P e r c e n t S u r v i v a l ( E 1 ) - 3 s + c P D - 1 4 2 d a y s • Low doses (50 μg) of (E1) - 3s significantly improved survival • Efficacy was enhanced with the addition of a checkpoint inhibitor 117

• (E1) - 3s effectively induced T - cell - mediated killing of Trop - 2 - expressing pancreatic, gastric, colorectal, prostate and breast cancer cells ( in vitro and in vivo results) • Reduced cytokine release and potentially reduced side effects • IFN - α potentiates retargeted T - cell killing of cancer cells • Checkpoint inhibitors can enhance retargeted T - cell therapy • (X) - 3s bsAbs targeting different tumor - associated antigens have also shown very potent activity (e.g., against CEACAM5) • This T - cell retargeting may be an improved second - generation technology for immunotherapy of solid cancers 118 T - cell Retargeting Conclusions

Concluding Remarks David M. Goldenberg, Sc.D., M.D., Chairman, Chief Scientific Officer, and Chief Patent Officer 119

Key Programs 120 • New ADC technology using a moderately toxic drug conjugated at a high drug:antibody ratio – IMMU - 132 : TNBC, UBC, NSCLC, SCLC, followed by other metastatic breast cancers, metastatic endometrial and prostate cancers – IMMU - 130: CRC and other CEA - expressing cancers (head - and - neck squamous, mucinous ovarian, gastric, esophageal) – IMMU - 140: AML, ALL, MM, CLL, melanoma, gastric ca., glioblastoma multiforme

Key Programs (cont’d) 121 • Unconjugated humanized antibodies – Epratuzumab, veltuzumab, milatuzumab, IMMU - 114 • Immuno - Oncology – Bispecific antibody T - cell retargeting (Trop - 2 and CEACAM5 targets for solid cancers) – Combination agents including alpha - interferon and immune checkpoint inhibitors

How Can We Further Improve IMMU - 132 Results? 122 • Overcome tumor resistance • Modify therapy to include maintenance schedules and earlier treatment settings • Combination therapies: Immuno - oncology; PARP inhibitors; DNA - modifiers IMMUNOMEDICS SCIENTISTS ARE ADDRESSING THESE IMPORTANT GOALS AND ARE SOLVING HOW TO OVERCOME RESISTANCE

Thank You 123 We hope you share our commitment to excellence in science as the basis for creating and advancing novel therapies in challenging diseases with unmet therapeutic needs

Q&A Session 124
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- NorthStar Appoints Peter Pfreundschuh to Board of Managers
- AxonDAO Goes Multichain with Arbitrum
- Form 8.3 - DS Smith Plc
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share