Form 8-K Tonix Pharmaceuticals For: Jan 10
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (date of earliest event reported): January 10, 2017
TONIX PHARMACEUTICALS HOLDING CORP.
(Exact name of registrant as specified in its charter)
| Nevada | 001-36019 | 26-1434750 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
509 Madison Avenue, Suite 306, New York, New York 10022
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (212) 980-9155
Copy of correspondence to:
Marc J. Ross, Esq.
James M. Turner, Esq.
Sichenzia Ross Ference Kesner LLP
61 Broadway
New York, New York 10006
Tel: (212) 930-9700 Fax: (212) 930-9725
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
¨ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
| Item 7.01 | Regulation FD Disclosure. |
Tonix Pharmaceuticals Holding Corp. (the “Company”) intends to utilize an updated investor presentation to conduct meetings with investors, stockholders and analysts and at investor conferences, and which the Company intends to place on its website, which may contain non-public information. A copy of the presentation is filed as Exhibit 99.01.
The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.01, is furnished pursuant to, and shall not be deemed to be "filed" for the purposes of, Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section. The information contained in Item 7.01 of this Current Report shall not be incorporated by reference into any registration statement or any other document filed pursuant to the Securities Act of 1933, as amended, except as otherwise expressly stated in such filing. By filing this Current Report on Form 8-K and furnishing the information contained in this Item 7.01, including Exhibit 99.01, the Company makes no admission as to the materiality of any such information that it is furnishing.
| Item 8.01 | Other Events. |
On January 10, 2017, the Company issued a press release announcing that the Company presented details of its newly expanded product development pipeline at the 9th Annual Biotech Showcase Conference in San Francisco, California.
A copy of the press release that discusses this matter is filed as Exhibit 99.02 to, and incorporated by reference in, this report. The information in this Current Report is being furnished and shall not be deemed "filed" for the purposes of Section 18 of the Securities Exchange Act of 1934 or otherwise subject to the liabilities of that Section. The information in this Current Report shall not be incorporated by reference into any registration statement or other document pursuant to the Securities Act of 1933, except as shall be expressly set forth by specific reference in any such filing.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) | Exhibits. |
| 99.01 | Corporate Presentation by the Company for January 2017* | |
| 99.02 | Press release, dated January 10, 2017, issued by Tonix Pharmaceuticals Holding Corp.* |
* Furnished herewith.
| 2 |
SIGNATURE
Pursuant to the requirement of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| TONIX PHARMACEUTICALS HOLDING CORP. | ||
| Date: January 10, 2017 | By: | /s/ SETH LEDERMAN |
| Seth Lederman | ||
| Chief Executive Officer | ||
| 3 |
Exhibit 99.01




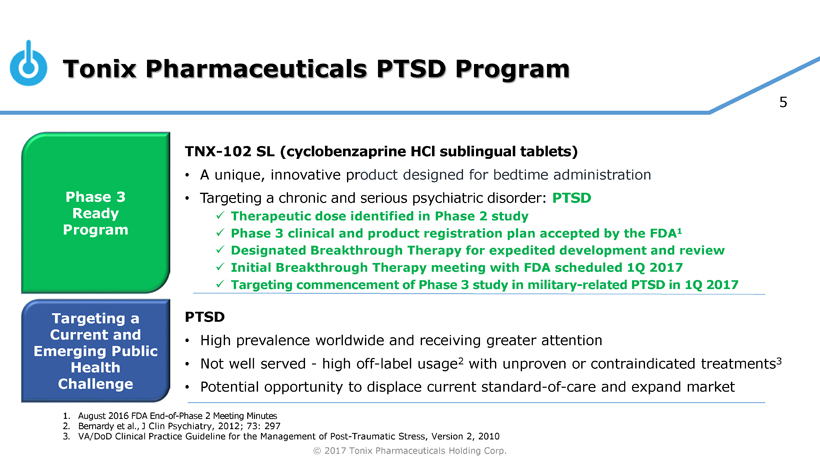
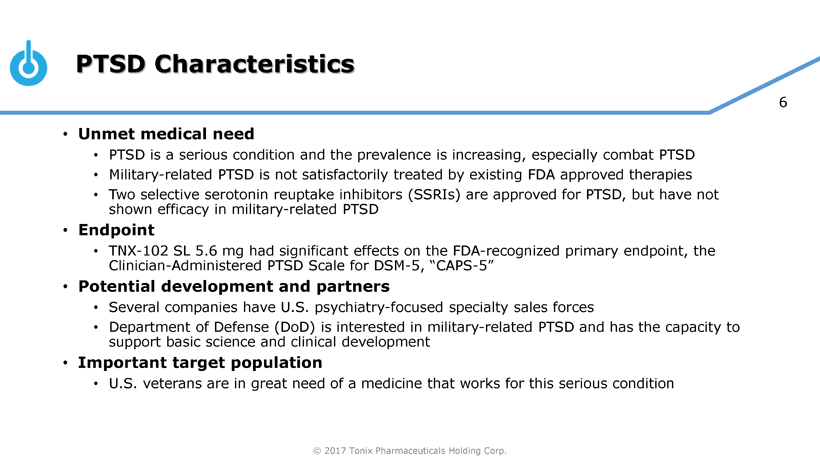


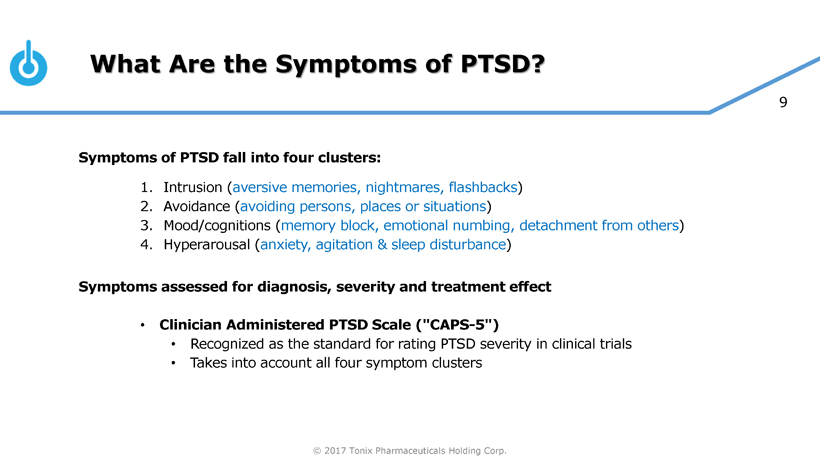






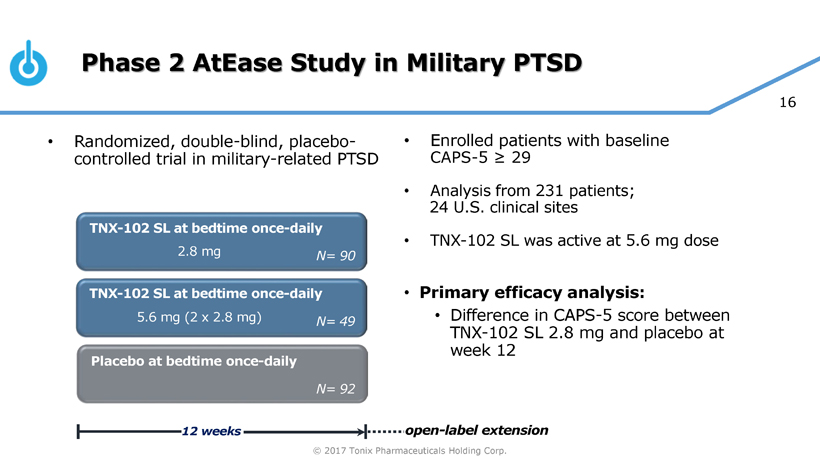

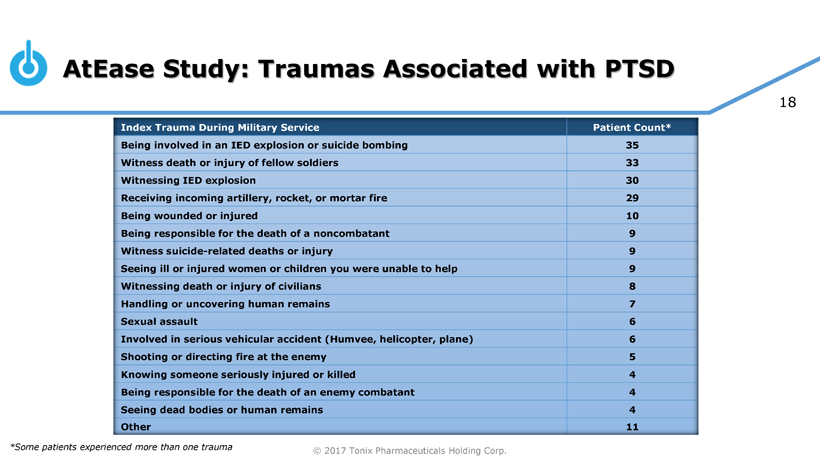
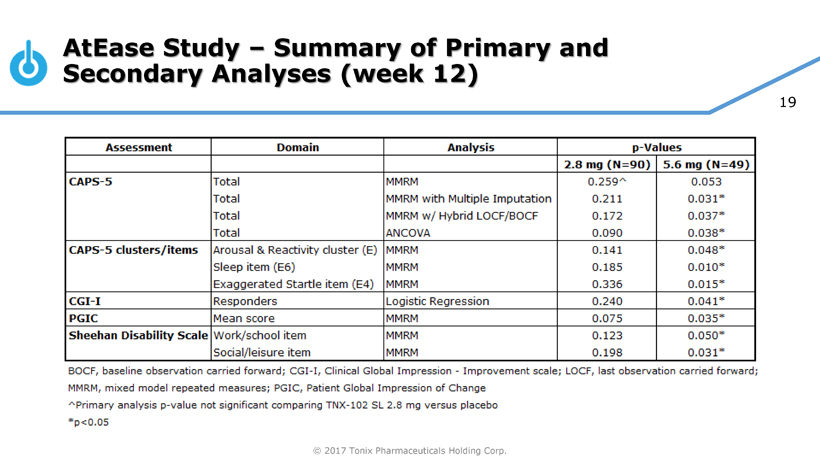

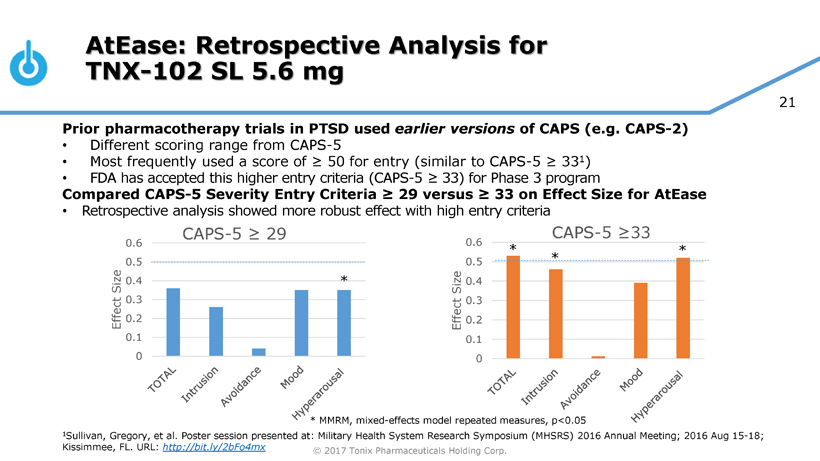

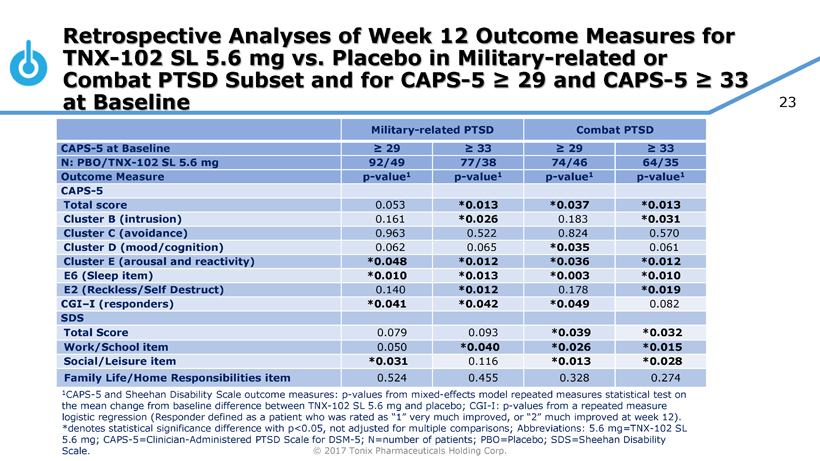


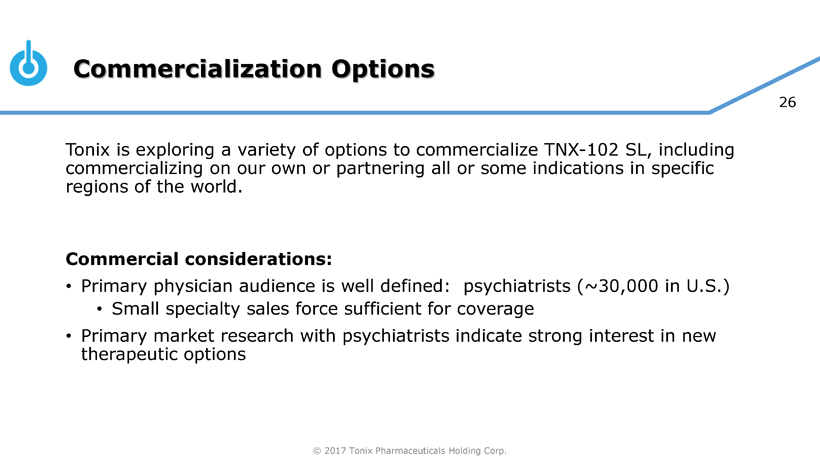




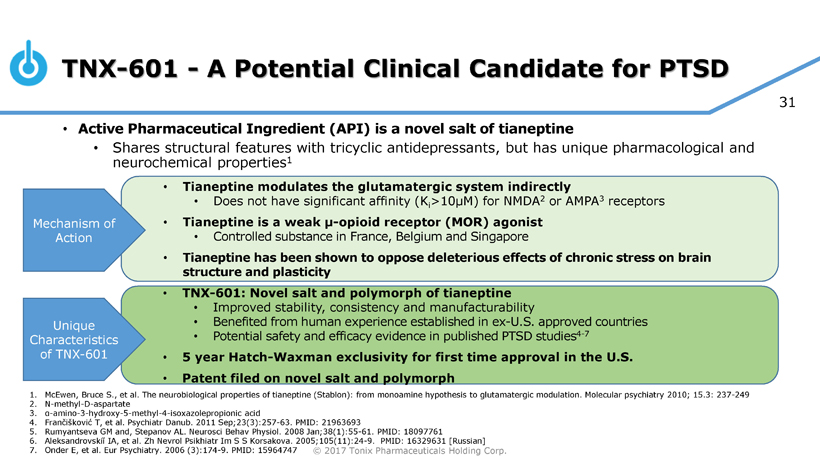

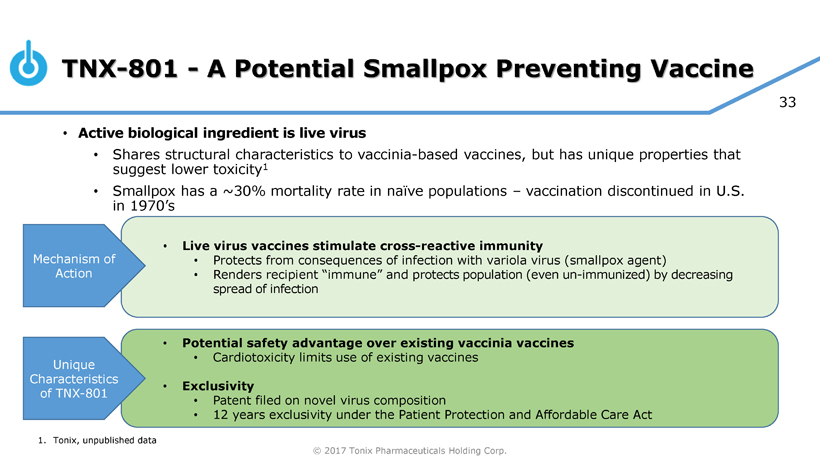



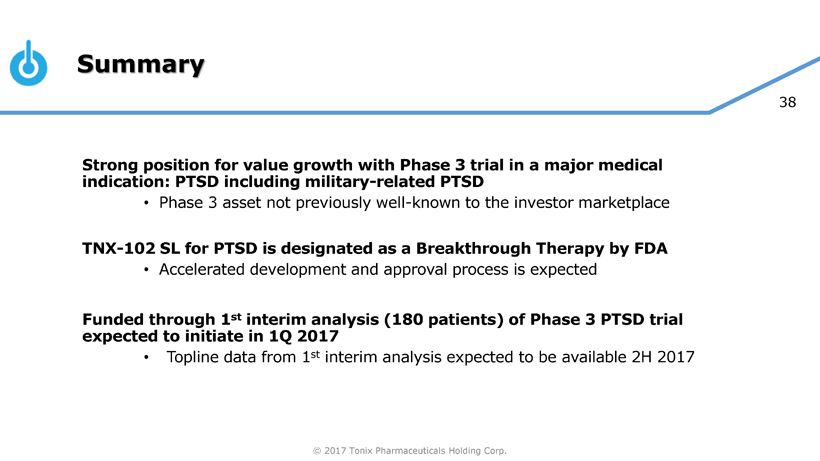

Exhibit 99.02

Tonix Pharmaceuticals Presented Details of Newly Expanded Product Development Pipeline at 9th Annual Biotech Showcase Conference
Development Programs Include Phase 3-Ready Breakthrough Therapy TNX-102 SL, Designed for Bedtime Dosing for PTSD, IND Drug Candidate TNX-601 Designed for Daytime Dosing for PTSD, and Smallpox Preventing Vaccine TNX-801 with Priority Review Voucher Potential
Pivotal Study of TNX-102 SL in Military-Related PTSD Set to Begin this Quarter
NEW YORK, Jan 10, 2017 – Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix), a company that is developing innovative pharmaceutical products to address current and emerging public health challenges, today provided details of the company’s recently expanded product pipeline.
Seth Lederman, M.D., Tonix’s president and chief executive officer, presenting at the 9th Annual Biotech Showcase Conference in San Francisco, CA, said, “We are poised to begin, in the first quarter of 2017, the Phase 3 HONOR study of TNX-102 SL for military-related posttraumatic stress disorder (PTSD). We are confident that we can report our first topline result by the fourth quarter of 2017.” Dr. Lederman continued, “While TNX-102 SL development remains our top priority, we are fortunate to have the resources to pursue in parallel two additional internally-developed programs that could be of tremendous value in addressing some public health challenges that can have major positive implications to our healthcare system.”
New Development Programs
TNX-601 for PTSD
Leveraging its expertise in PTSD, Tonix is developing TNX-601 as a first line PTSD monotherapy. TNX-601 is a novel oral formulation of tianeptine designed for daytime dosing. TNX-601 is at the pre-IND (Investigational New Drug) stage of development. Tianeptine’s reported pro-cognitive and anxiolytic effects, as well as its ability to reduce excessive stress responses, suggest that it can be used to treat PTSD by a different mechanism of action than that of TNX-102 SL, which was designed for bedtime administration.
Tonix has discovered a novel salt and polymorph of tianeptine that may provide improved stability, consistency, and manufacturability as compared to known forms of tianeptine. Currently there is no tianeptine-containing product approved in the U.S., though tianeptine sodium (amorphous) has been available in Europe, Asia, and Latin America for the treatment of depression since 1987.
TNX-801 (live virus vaccine) for Smallpox Prevention
TNX-801 is a novel, live virus vaccine that Tonix is developing as a potential smallpox-preventing vaccine for the national stockpile and potentially for widespread immunization. Vaccination against smallpox was discontinued in the U.S. in the 1970’s yet smallpox continues to represent a material threat to national security. Tonix currently is developing a good manufacturing practice-quality vaccine to support an IND application.
The newly-discovered and -characterized active biological component shares structural characteristics with vaccinia-based vaccines, but TNX-801 has unique properties that Tonix believes may suggest lower toxicity and potential safety advantages over existing vaccinia-based vaccines, which have been associated with adverse side effects such as myopericarditis.
The development and approval of TNX-801 are expected to benefit from the regulatory pathway referred to as the “Animal Rule”. Under 21 CFR 601 Subpart H, the U.S. Food and Drug Administration (FDA) may grant marketing approval for a biological product for which safety has been established in humans, and for which the requirements for efficacy are met based on adequate and well-controlled animal studies, but where human efficacy studies are not ethical or feasible.
Tonix believes that, under the recently-passed 21st Century Cures Act, TNX-801 qualifies as a medical countermeasure and therefore could be eligible for a Priority Review Voucher (PRV) upon FDA approval. PRVs are transferrable by their recipients, who are permitted to monetize the transaction. Priority Review Vouchers previously have been sold for up to $350 million.
Webcast Details
The 9th Annual Biotech Showcase Conference presentation, in which the expanded pipeline was described, was webcast live and will remain available for 90 days following the presentation. To access the webcast, please visit the Events tab of the Investor Relations section of Tonix’s website at www.tonixpharma.com.
*TNX-102 SL (cyclobenzaprine HCl sublingual tablets) is an Investigational New Drug and has not been approved for any indication.
About Tonix Pharmaceuticals Holding Corp.
Tonix is developing innovative pharmaceutical products to address current and emerging public health challenges, with two programs focusing on PTSD. TNX-102 SL is ready to start Phase 3 clinical trials and TNX-601 is in pre-IND stage of development. PTSD is a serious condition characterized by chronic disability, inadequate treatment options especially for military-related PTSD and overall high utilization of healthcare services creating significant economic burden. TNX-102 SL was recently granted Breakthrough Therapy designation by the FDA for the treatment of PTSD. Other development efforts include TNX-801, a potential smallpox preventing vaccine.
This press release and further information about Tonix can be found at www.tonixpharma.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, substantial competition; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2015, as filed with the Securities and Exchange Commission (the “SEC”) on March 3, 2016, and future periodic reports filed with the SEC on or after the date hereof. All of Tonix's forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date hereof.
Contacts
Jessica Smiley
Investor Relations
(212) 980-9155 x185
Edison Advisors (investors)
Tirth Patel
(646) 653-7035
MSLGROUP Boston (media)
Sherry Feldberg
(781) 684-0770
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Tonix Pharmaceuticals Announces Presentation at Planet MicroCap Showcase
- F3 Uranium to Present at the OTC Metals & Mining Virtual Investor Conference on April 30th
- Exodus Movement, Inc. to Present at the Blockchain & Digital Asset Virtual Investor Conference April 25th
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share