Form 8-K THERAVANCE INC For: Oct 26
�
�
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
�
�
FORM�8-K
�
�
Current Report Pursuant
to Section�13 or 15(d)�of the
Securities Exchange Act of 1934
�
Date of Report (Date of earliest event Reported): October�26, 2014
�
�
THERAVANCE,�INC.
(Exact Name of Registrant as Specified in its Charter)
�
�
|
Delaware |
� |
000-30319 |
� |
94-3265960 |
�
951 Gateway Boulevard
South San Francisco, California 94080
(650) 238-9600
(Addresses, including zip code, and telephone numbers, including area code, of principal executive offices)
�
�
Check the appropriate box below if the Form�8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
�
o����������� Written communications pursuant to Rule�425 under the Securities Act (17 CFR 230.425)
�
o����������� Soliciting material pursuant to Rule�14a-12 under the Exchange Act (17 CFR 240.14a-12)
�
o����������� Pre-commencement communications pursuant to Rule�14d-2(b)�under the Exchange Act (17 CFR 240.14d-2(b))
�
o����������� Pre-commencement communications pursuant to Rule�13e-4(c)�under the Exchange Act (17 CFR 240.13e-4(c))
�
�
�
�
Item 8.01 Other Events.
�
On October�26, 2014 at CHEST 2014 in Austin, Texas, GlaxoSmithKline plc (GSK) presented data from two Phase�3 studies comparing the efficacy and safety of ANORO��(umeclidinium/vilanterol �UMEC/VI�) once daily versus ADVAIR� (fluticasone/salmeterol combination �FSC�) twice daily in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD) and infrequent COPD exacerbations.� ANORO��is a once-daily combination treatment comprising two bronchodilators, UMEC, a long-acting muscarinic antagonist (LAMA), and VI, a long-acting beta2�agonist (LABA), in a single inhaler, the ELLIPTA�.� UMEC/VI has been developed under the 2002 LABA collaboration between�Glaxo Group Limited�and Theravance,�Inc.� The slide presentation is filed as Exhibit�99.1 to this report and is incorporated herein by reference.
�
Item 9.01 Financial Statements and Exhibits.
�
|
� |
(d) |
Exhibits. |
�
|
� |
Exhibit |
� |
Description |
|
� |
Exhibit�99.1 |
� |
Efficacy and Safety of Umeclidinium/Vilanterol (UMEC/VI) Once Daily (OD) vs Fluticasone/Salmeterol Combination (FSC) Twice Daily (BD) in Patients With Moderate-to-Severe COPD and Infrequent COPD Exacerbations |
�
�
SIGNATURE
�
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
�
�
|
� |
THERAVANCE,�INC. | |
|
� |
� | |
|
Date: October�27, 2014 |
By: |
/s/ Michael W. Aguiar |
|
� |
� |
Michael W. Aguiar |
|
� |
� |
Chief Executive Officer |
�
�
EXHIBIT�INDEX
�
|
Exhibit�No. |
� |
Description |
|
99.1 |
� |
Efficacy and Safety of Umeclidinium/Vilanterol (UMEC/VI) Once Daily (OD) vs Fluticasone/Salmeterol Combination (FSC) Twice Daily (BD) in Patients With Moderate-to-Severe COPD and Infrequent COPD Exacerbations |
�
Exhibit 99.1
�
|
|
Efficacy and safety of umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol (FSC) combination twice daily (BD) in patients with moderate-to-severe COPD and infrequent COPD exacerbations James F. Donohue, MD, FCCP Department of Medicine, University of North Carolina, North Carolina, USA Presentation No: 73A |
�
|
|
Disclosures Jim Donohue has the following statement of interest: served as consultant to Almirall, AstraZeneca, Boehringer Ingelheim, Elevation Pharmaceuticals, Forest Laboratories, GSK, Mylan, Novartis, Pearl Pharmaceuticals, Pfizer and Sunovion served as a member of Drug Safety Monitoring Boards for the NIH, Novartis, Otsuka, Pearl and Teva |
�
|
|
Off-label discussion declaration I will not be discussing off-label uses of the treatments presented here |
�
|
|
Primary objective To compare the efficacy and safety of once-daily UMEC/VI 62.5/25mcg with twice-daily FSC 250/50mcg over 12 weeks in patients with moderate-to-severe COPD with a history of infrequent COPD exacerbations Two studies were conducted DB2114930 (NCT01817764) DB2114951 (NCT01879410) COPD: chronic obstructive pulmonary disease; FSC: fluticasone propionate/salmeterol; UMEC: umeclidinium; VI: vilanterol |
�
|
|
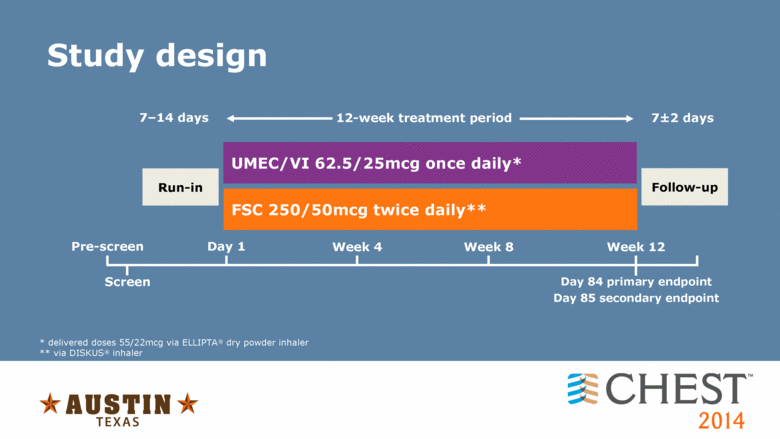
Study design * delivered doses 55/22mcg via ELLIPTA� dry powder inhaler ** via DISKUS� inhaler Week 4 Day 1 Pre-screen Screen Week 8 Week 12 Day 84 primary endpoint Day 85 secondary endpoint Follow-up Run-in FSC 250/50mcg twice daily** 7�14 days 12-week treatment period 7�2 days UMEC/VI 62.5/25mcg once daily* |
�
|
|
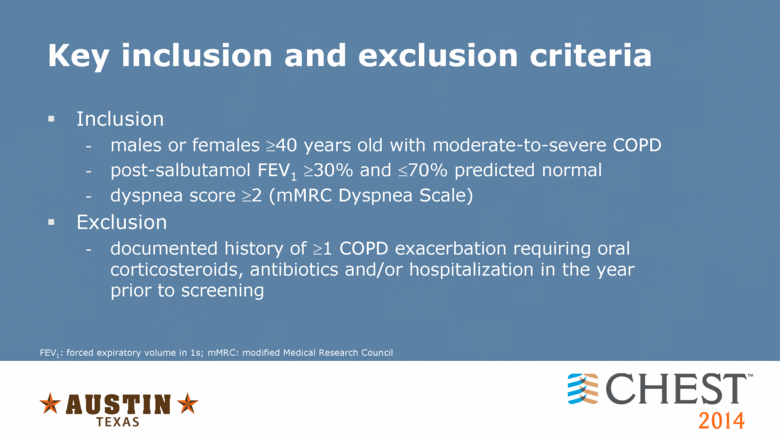
Key inclusion and exclusion criteria Inclusion males or females 40 years old with moderate-to-severe COPD post-salbutamol FEV1 30% and 70% predicted normal dyspnea score 2 (mMRC Dyspnea Scale) Exclusion documented history of 1 COPD exacerbation requiring oral corticosteroids, antibiotics and/or hospitalization in the year prior to screening FEV1: forced expiratory volume in 1s; mMRC: modified Medical Research Council |
�
|
|
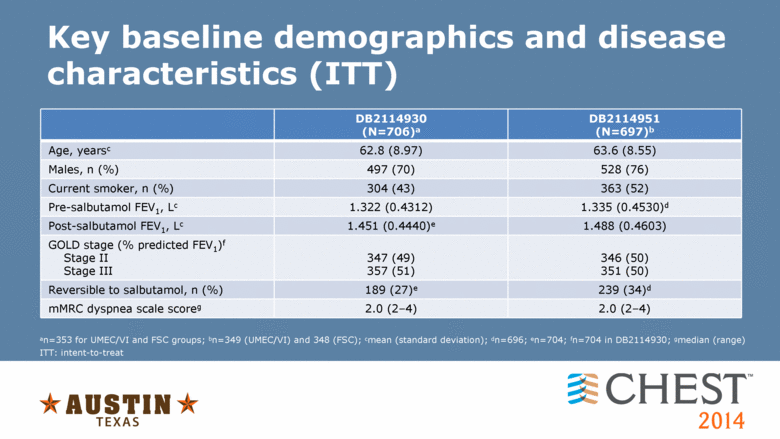
an=353 for UMEC/VI and FSC groups; bn=349 (UMEC/VI) and 348 (FSC); cmean (standard deviation); dn=696; en=704; fn=704 in DB2114930; gmedian (range) Key baseline demographics and disease characteristics (ITT) DB2114930 (N=706)a DB2114951 (N=697)b Age, yearsc 62.8 (8.97) 63.6 (8.55) Males, n (%) 497 (70) 528 (76) Current smoker, n (%) 304 (43) 363 (52) Pre-salbutamol FEV1, Lc 1.322 (0.4312) 1.335 (0.4530)d Post-salbutamol FEV1, Lc 1.451 (0.4440)e 1.488 (0.4603) GOLD stage (% predicted FEV1)f Stage II Stage III 347 (49) 357 (51) 346 (50) 351 (50) Reversible to salbutamol, n (%) 189 (27)e 239 (34)d mMRC dyspnea scale scoreg 2.0 (2�4) 2.0 (2�4) ITT: intent-to-treat |
�
|
|
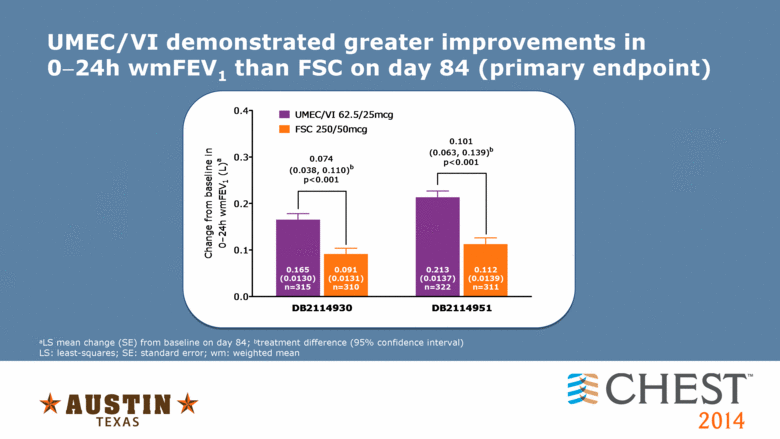
UMEC/VI demonstrated greater improvements in 024h wmFEV1 than FSC on day 84 (primary endpoint) aLS mean change (SE) from baseline on day 84; btreatment difference (95% confidence interval) LS: least-squares; SE: standard error; wm: weighted mean 0.0 0.1 0.2 0.3 0.4 UMEC/VI 62.5/25mcg FSC 250/50mcg 0.074 (0.038, 0.110) b p<0.001 0.165 (0.0130) n=315 DB2114930 DB2114951 Change from baseline in 0�24h wmFEV 1 (L) a 0.091 (0.0131) n=310 0.213 (0.0137) n=322 0.112 (0.0139) n=311 0.101 (0.063, 0.139) b p<0.001 |
�
|
|
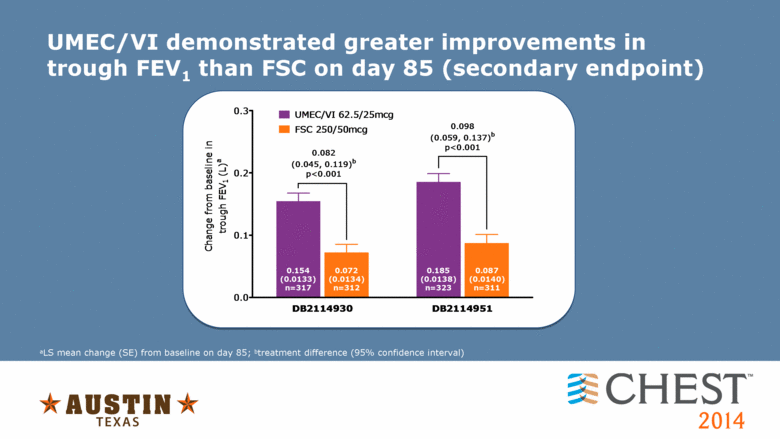
UMEC/VI demonstrated greater improvements in trough FEV1 than FSC on day 85 (secondary endpoint) aLS mean change (SE) from baseline on day 85; btreatment difference (95% confidence interval) 0.0 0.1 0.2 0.3 UMEC/VI 62.5/25mcg FSC 250/50mcg 0.082 (0.045, 0.119) b p<0.001 0.154 (0.0133) n=317 DB2114930 DB2114951 Change from baseline in trough FEV 1 (L) a 0.072 (0.0134) n=312 0.185 (0.0138) n=323 0.087 (0.0140) n=311 0.098 (0.059, 0.137) b p<0.001 |
�
|
|
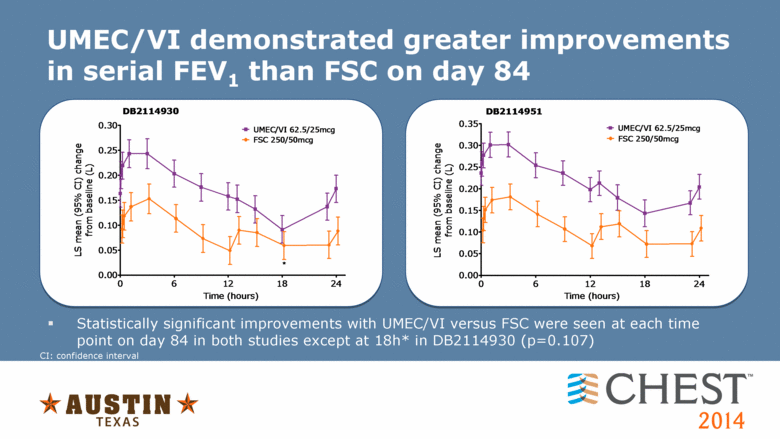
UMEC/VI demonstrated greater improvements in serial FEV1 than FSC on day 84 Statistically significant improvements with UMEC/VI versus FSC were seen at each time point on day 84 in both studies except at 18h* in DB2114930 (p=0.107) CI: confidence interval 0.00 0.05 0.10 0.15 0.20 0.25 0.30 UMEC/VI 62.5/25mcg FSC 250/50mcg 0 6 12 18 24 DB2114930 * Time (hours) LS mean (95% CI) change from baseline (L) 0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 UMEC/VI 62.5/25mcg FSC 250/50mcg 0 6 12 18 24 DB2114951 Time (hours) LS mean (95% CI) change from baseline (L) |
�
|
|
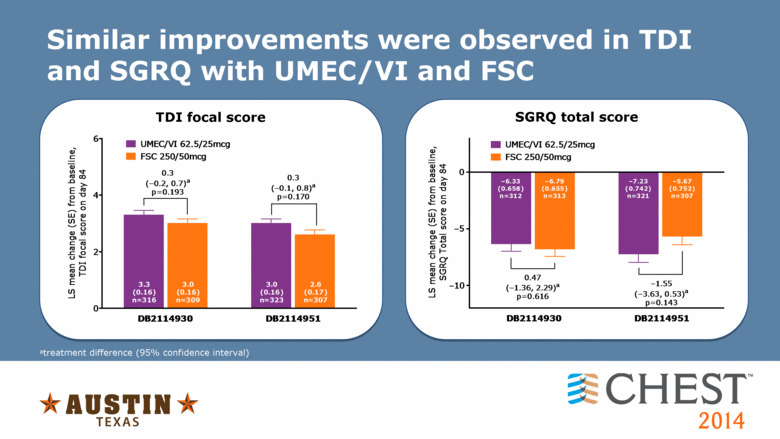
Similar improvements were observed in TDI and SGRQ with UMEC/VI and FSC atreatment difference (95% confidence interval) TDI focal score SGRQ total score UMEC/VI 62.5/25mcg FSC 250/50mcg 0.47 (�1.36, 2.29) a p=0.616 DB2114930 DB2114951 LS mean change (SE) from baseline, SGRQ Total score on day 84 �5.67 (0.752) n=307 �1.55 (�3.63, 0.53) a p=0.143 �7.23 (0.742) n=321 �6.79 (0.655) n=313 �6.33 (0.658) n=312 0 �5 �10 |
�
|
|
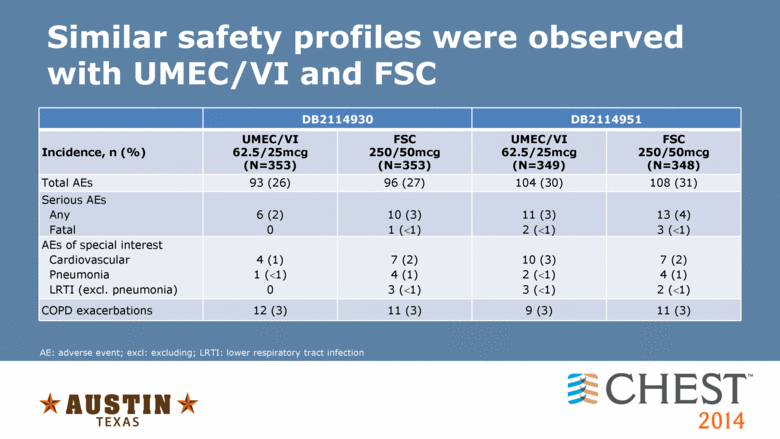
Similar safety profiles were observed with UMEC/VI and FSC DB2114930 DB2114951 Incidence, n (%) UMEC/VI 62.5/25mcg (N=353) FSC 250/50mcg (N=353) UMEC/VI 62.5/25mcg (N=349) FSC 250/50mcg (N=348) Total AEs 93 (26) 96 (27) 104 (30) 108 (31) Serious AEs Any Fatal 6 (2) 0 10 (3) 1 (1) 11 (3) 2 (1) 13 (4) 3 (1) AEs of special interest Cardiovascular Pneumonia LRTI (excl. pneumonia) 4 (1) 1 (1) 0 7 (2) 4 (1) 3 (1) 10 (3) 2 (1) 3 (1) 7 (2) 4 (1) 2 (1) COPD exacerbations 12 (3) 11 (3) 9 (3) 11 (3) AE: adverse event; excl: excluding; LRTI: lower respiratory tract infection |
�
|
|
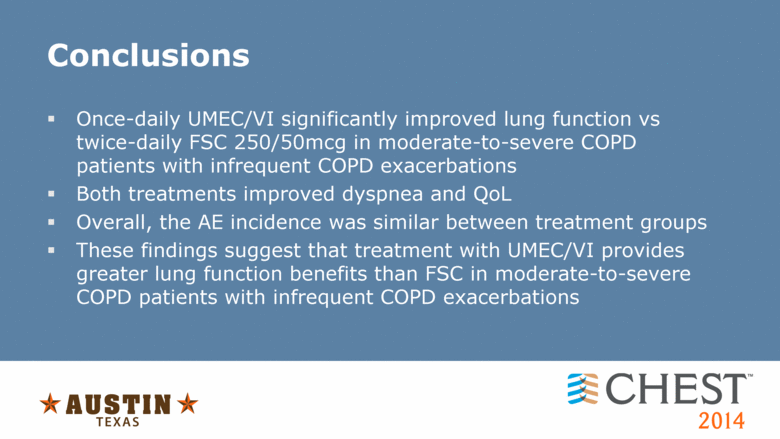
Conclusions Once-daily UMEC/VI significantly improved lung function vs twice-daily FSC 250/50mcg in moderate-to-severe COPD patients with infrequent COPD exacerbations Both treatments improved dyspnea and QoL Overall, the AE incidence was similar between treatment groups These findings suggest that treatment with UMEC/VI provides greater lung function benefits than FSC in moderate-to-severe COPD patients with infrequent COPD exacerbations |
�
|
|
Acknowledgments Patients, investigators and staff at the 63 study centers in Argentina, Chile, Greece, Peru, Romania, Ukraine and the USA (DB2114930) and at the 71 study centers in Chile, Mexico, Norway, Romania, Ukraine, Russian Federation, South Africa and the USA (DB2114951) Funded by GSK (DB2114930 / NCT01817764; DB2114951 / NCT01879410) |
�
�
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Share Buyback Transaction Details April 18 – April 24, 2024
- PolyU forms global partnership with ZEISS Vision Care to expand impact and accelerate market penetration of patented myopia control technology
- NETA Auto: The First Car from the Indonesian Factory Powers up Global Strategy
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share