Form 8-K PLURISTEM THERAPEUTICS For: May 27
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K
CURRENT REPORT PURSUANT
TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of report (Date of earliest event reported): May 27, 2015 (May 25, 2015)
PLURISTEM THERAPEUTICS INC.
(Exact Name of Registrant as Specified in Its Charter)
Nevada
(State or Other Jurisdiction of Incorporation)
|
001-31392
|
98-0351734
|
|
(Commission File Number)
|
(IRS Employer Identification No.)
|
|
MATAM Advanced Technology Park
Building No. 5
Haifa, Israel
|
31905
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
011 972 74 7107171
(Registrant’s Telephone Number, Including Area Code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
o
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
o
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
|
o
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
|
o
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
Item 7.01. Regulation FD Disclosure.
On May 25, 2015, the registrant issued a letter to its shareholders as of that date. The letter is furnished as Exhibit 99.1 to this Current Report on Form 8-K. On May 27, 2015, the registrant posted an updated presentation to its website. A copy of the presentation is furnished with this Current Report on Form 8-K as Exhibit 99.2 and is incorporated herein by reference.
Warning Concerning Forward Looking Statements
Exhibit 99.1 and Exhibit 99.2 to this Current Report on Form 8-K contain forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995 and federal securities laws. For example, forward-looking statements are being used when the registrant discusses moving closer to reaching the registrant’s objective to bring innovative, effective treatments to patients, when the registrant discusses its anticipation for new accomplishments in 2015, when the registrant discusses potential of approving its cells for the treatment of CLI via the Adaptive Pathway to significantly curtail the time and investment needed to bring this product to market, when the registrant discusses its anticipation that PLX-PAD cells could enter the market in 2018 to treat patients with the clearly defined subtype of CLI studied in the trial, when the registrant discusses achieving additional partnership for its CLI program over the next twelve months, when the registrant discusses its planned study of PLX-R18 in humans, the timing of its submission and related FDA and NIH approvals, when the registrant discusses the timing for completion of recruitment for its phase II IC trial, when the registrant discusses that PLX-PAD cells may potentially treat additional muscle indications, when the registrant discusses the timing for submission of Phase II study protocol to several national authorities for PLX-PAD cells in CLI and submission for Phase I/II study protocol to the PMDA, or when the registrant discusses timing for receipt of preliminary data from the Phase I trial in pulmonary arterial hypertension. These forward-looking statements and their implications are based on the current expectations of the management of the registrant only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: changes in technology and market requirements; we may encounter delays or obstacles in launching and/or successfully completing our clinical trials; our products may not be approved by regulatory agencies, our technology may not be validated as we progress further and our methods may not be accepted by the scientific community; we may be unable to retain or attract key employees whose knowledge is essential to the development of our products; unforeseen scientific difficulties may develop with our process; our products may wind up being more expensive than we anticipate; results in the laboratory may not translate to equally good results in real surgical settings; results of preclinical studies may not correlate with the results of human clinical trials; our patents may not be sufficient; our products may harm recipients; changes in legislation; inability to timely develop and introduce new technologies, products and applications; loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of the registrant to differ materially from those contemplated in such forward-looking statements. In addition, historic results of scientific research do not guarantee that the conclusions of future research would not suggest different conclusions or that historic results referred to in this letter would not be interpreted differently in light of additional research or otherwise. Also, while the company’s program was selected for the European Medicines Agency’s Adaptive Pathways pilot project, as well as recognized by the PMDA, these agencies are not bound by these communications and accordingly may change their position in the future due to reasons within or outside the control of the registrant Except as otherwise required by law, the registrant undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. For a more detailed description of the risks and uncertainties affecting the registrant, reference is made to the registrant's reports filed from time to time with the Securities and Exchange Commission.
Item 9.01. Financial Statements and Exhibits
(d) Exhibits
99.1 – Letter to the shareholders of Pluristem Therapeutics Inc. dated May 25, 2015.
99.2 – Presentation of Pluristem Therapeutics Inc. dated May 27, 2015.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
PLURISTEM THERAPEUTICS INC.
|
|||
|
Date: May 27, 2015
|
By:
|
/s/ Yaky Yanay
|
|
|
Name: Yaky Yanay
|
|||
|
Title: Chief Financial Officer, Secretary,
Chief Operating Officer and President
|
|||
Exhibit 99.1

Dear fellow stockholders,
A common objective drives our efforts at Pluristem: to bring innovative, effective treatments to patients suffering from conditions with few or no adequate treatment options. With exciting progress made on many fronts since the start of 2015, Pluristem has moved closer to reaching this objective. We have accomplished a key element of our strategic plan to significantly shorten the time to commercialization of our PLX-PAD cells in critical limb ischemia (CLI), while also advancing development of PLX-R18, our second product. We have reported additional positive findings from our successful Phase II muscle trial, which already met its primary and secondary endpoints last year. Pluristem has also made meaningful additions to its robust patent portfolio. We anticipate new accomplishments in 2015, and look forward to sharing them with you.
Meaningful progress in executing our strategic plan
A key element of Pluristem’s strategic plan has been to reduce time to commercialization of our PLX-PAD cells in critical limb ischemia (CLI). To achieve this we applied to the new Adaptive Pathways pilot project in Europe and to the new regulatory pathway for regenerative therapy created under the Regenerative Medicine Law in Japan. In 2014, both Europe and Japan began offering unique opportunities to bring new products to market more quickly. Each country may now allow for limited commercialization of a product after a single successful initial trial, followed by further data collection and analysis after marketing has begun in order to evaluate the product for full marketing authorization. After the two programs were announced, we began working immediately to apply for the chance to commercialize PLX-PAD cells following a Phase II study and to circumvent the need for a long and expensive Phase III study. Building on the positive results of our two completed Phase I trials in CLI, we prepared and submitted applications to both new pathways.
On May 18, 2015, Pluristem announced that its PLX cell program in critical limb ischemia (CLI) was selected for the European Medicines Agency's Adaptive (EMA) Pathways pilot project, the goal of which is to improve timely access for patients to new medicines. Pluristem’s successful application could significantly curtail the time and financial investment needed to bring the product to market. The acceptance into the Adaptive Pathways will provide Pluristem with detailed guidance and frequent, high-level communications with the Adaptive Pathways discussion group and other relevant stakeholders while preparing a trial protocol for submission to the EMA. A successful phase II study could then be the basis for a limited marketing authorization of the PLX-PAD cells in the subset of CLI tested in the trial, and also the potential for expansion of the indication or even expansion to other indications with PLX-PAD. Pluristem’s manufacturing facility was approved in 2014 by EMA’s Qualified Person after inspection of the manufacturing facility and the 3D cell expansion technology platform. The Approved factory is capable of producing up to 150,000 doses per year and is ready to supply PLX-PAD as needed. Subject to a successful Phase II trial and with the EMA’s conditional approval, Pluristem anticipates that PLX-PAD cells could enter the market in 2018 to treat patients with the clearly defined subtype of CLI studied in the trial.
On May 13, 2015 the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) recognized the current quality and commercial-scale manufacturing methods of PLX-PAD cells for use in CLI trials in Japanese patients. This was a crucial step towards Pluristem’s acceptance into Japan’s new accelerated pathway for regenerative medicines. This pathway permits a regenerative medicine product to reach patients without having to complete lengthy and expensive Phase III clinical trials. An exploratory study whose results show that a product is safe and likely to be effective can be sufficient for the product to receive conditional, time-limited marketing authorization. Following approval, the product is subject to post-marketing safety monitoring in conjunction with surveillance and study to further confirm its efficacy and safety. The new Regenerative Medicine Law presents an exceptional opportunity for Pluristem, and we eagerly await the PMDA’s decision regarding our application to the pathway.
Opportunities for additional partnerships
Pluristem continues working to establish additional business collaborations with pharmaceutical companies. The new opportunities in Europe and Japan have translated into substantial interest from potential partners who would like to work together with us in both geographic regions. We are hopeful that we will achieve additional partnership for our CLI program over the next twelve months, and are also discussing partnerships for our muscle injury program and our emerging clinical program for PLX-R18, our second product. The first planned study of PLX-R18 in humans would be a Phase I trial to be submitted later this year. More details about the program are provided below.
Strong clinical progress
|
|
·
|
Recruitment for our multinational, double blind, randomized, placebo controlled Phase II trial in intermittent claudication(IC) has recruited 88 of a total of 150 patients. With 25 active sites recruiting in the U.S., Germany, Israel, and South Korea, we anticipate completion of recruitment by the first quarter 2016. The safety data collected in this trial could potentially be used to support a submission to either the EMA or the PMDA for limited marketing authorization of PLX-PAD in CLI. IC can progress to CLI, a more severe diagnosis, and the pathophysiology is similar for these two subtypes of peripheral artery disease.
|
|
|
·
|
In January 2014, we reported that a Phase II trial of PLX-PAD cells in muscle injury had clearly achieved both its primary and key secondary endpoints, with statistically significant findings of greatly increased muscle strength and muscle volume in patients whose muscle was injured and then treated with PLX cells versus placebo. Earlier this year Pluristem reported good safety data at 12 months follow up of patients from this trial, as well as findings showing that the contralateral, non-operated leg muscle of patients who had received cells in their injured leg was much stronger than the non-operated leg muscle of patients who had received placebo. This finding was also statistically significant. The implication of this latest finding is that PLX-PAD cells may potentially treat additional muscle indications such as muscle atrophy or muscle wasting; these can occur in multiple contexts such as rehabilitation after long periods of immobility.
|
|
|
·
|
Pluristem has made meaningful progress with the PLX-R18 program. This cell product targets hematological indications. Earlier this year we reported the U.S. National Institutes of Health’s (NIH) significant positive results from their second preclinical trial of PLX-R18 cells for the treatment of the bone marrow component of Acute Radiation Syndrome (ARS). After exposure to high levels of radiation, bone marrow is severely damaged and can lose its ability to produce white and red blood cells and platelets, which sometimes leads to death. In animals who were exposed to high levels of radiation, survival and recovery of bone marrow function were substantially increased in those who received PLX-R18 as compared to those who received placebo. This trial also described the mechanism of action of PLX-R18 cells, showing their secretion of therapeutic proteins, followed by the recovery sequence of progenitor and then mature hematopoietic cells, and finally production of normal levels of white and red cells and platelets and their successful migration into the bloodstream. The NIH trials have been in small animal models. We and the NIH representative will have a Pre-IND meeting with the FDA to agree on the design and the protocol of the large animal studies required to approve PLX-R18 for treatment of the bone marrow component of Acute Radiation Syndrome under the Animal Rule. We strongly believe that following potential FDA approval, The NIH will approve the financing of the large animal studies, having financed the small animal studies. Positive data from large animal studies would be sufficient for an application to the FDA for approval in this indication, since human efficacy trials are not feasible in ARS.
|
|
|
·
|
Building on data from more than 2 dozen animal trials studying PLX-R18 cells in hematologic indications, Pluristem is preparing a protocol for a Phase I trial to test these cells as a treatment for incomplete engraftment following hematopoietic cell transplantation. We anticipate submitting this protocol to the FDA by the end of 2015.
|
Expansion of our patent portfolio to support our strategy
Since the start of 2015, Pluristem has been granted key patents from Europe, Israel, South Africa, China, South Korea, Mexico and Russia. The European Medicines Agency's selection of Pluristem’s PLX-PAD program in CLI for the Adaptive Pathways pilot project, together with patent No. EP2200622, granted in 2014 and titled “Adherent Cells From Adipose or Placenta Tissues and Use Thereof in Therapy”, whose claims cover treatment of ischemia with placental-derived cells propagated using a 3D culture, place Pluristem in a unique position to move forward with its CLI program in Europe.
Additional activities
Other activities in the cell therapy arena also contribute to maintain our position as a leader in cell therapy. We continue to study the mechanism of action and capabilities of our cells and to share this knowledge with the scientific community and the cell therapy industry. Pluristem management has presented at 9 major scientific and industry conferences since January 2015. As active members in the Alliance for Regenerative Medicine and the International Society for Cellular Therapy, Pluristem continues to support the development of the regenerative medicine industry as a whole.
Over the next 12 months we are looking forward to continuing our momentum and achieving several additional milestones
|
·
|
Submission of a Phase II study protocol to several national authorities for PLX-PAD cells in critical limb ischemia, after having received advice during discussions with the EMA
|
|
·
|
Submission of a Phase I/II study protocol to the PMDA for PLX-PAD cells in critical limb ischemia
|
|
·
|
Submission of a Phase I study protocol to the FDA for PLX-R18 cells in incomplete engraftment following hematopoietic cell transplantation
|
|
·
|
Advancing a partnership with a pharmaceutical company to partner our CLI and/or Muscle injury programs.
|
|
·
|
Completion of the recruitment for our multinational Phase II study in IC
|
|
·
|
Preliminary data from the Phase I trial in pulmonary arterial hypertension being conducted by our partner United Therapeutics
|
We will continue to execute our long-term strategy to bring innovative and effective treatments to market in a timely fashion and to become a leader in the development and manufacture of cell therapies. We recognize and appreciate your continued support, and look forward to sharing upcoming achievements with you.
Sincerely,
Zami Aberman
Chairman and Chief Executive Officer
For Pluristem Conference presentation, May 25, 2015- http://www.pluristem.com/images/Pluristem_for_TASE_Combined.pdf
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995 and federal securities laws. For example, we are using forward-looking statements, when we discuss moving closer to reaching our objective to bring innovative, effective treatments to patients, when we discuss our anticipation for new accomplishments in 2015, when we discuss potential of approving our cells for the treatment of CLI via the Adaptive Pathway to significantly curtail the time and investment needed to bring this product to market, when we discuss our anticipation that PLX-PAD cells could enter the market in 2018 to treat patients with the clearly defined subtype of CLI studied in the trial, when we discuss achieving additional partnership for our CLI program over the next twelve months, when we discuss our planned study of PLX-R18 in humans, the timing of its submission and related FDA and NIH approvals, when we discuss the timing for completion of recruitment for our phase II IC trial, when we discuss that PLX-PAD cells may potentially treat additional muscle indications, when we discuss the timing for submission of Phase II study protocol to several national authorities for PLX-PAD cells in CLI and submission for Phase I/II study protocol to the PMDA, or when we discuss timing for receipt of preliminary data from the Phase I trial in pulmonary arterial hypertension. These forward-looking statements and their implications are based on the current expectations of the management of Pluristem only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: changes in technology and market requirements; we may encounter delays or obstacles in launching and/or successfully completing our clinical trials; our products may not be approved by regulatory agencies, our technology may not be validated as we progress further and our methods may not be accepted by the scientific community; we may be unable to retain or attract key employees whose knowledge is essential to the development of our products; unforeseen scientific difficulties may develop with our process; our products may wind up being more expensive than we anticipate; results in the laboratory may not translate to equally good results in real surgical settings; results of preclinical studies may not correlate with the results of human clinical trials; our patents may not be sufficient; our products may harm recipients; changes in legislation; inability to timely develop and introduce new technologies, products and applications; loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of Pluristem to differ materially from those contemplated in such forward-looking statements. In addition, historic results of scientific research do not guarantee that the conclusions of future research would not suggest different conclusions or that historic results referred to in this letter would not be interpreted differently in light of additional research or otherwise. Also, while the company’s program was selected for the European Medicines Agency’s Adaptive Pathways pilot project, as well as recognized by the PMDA, these agencies are not bound by these communications and accordingly may change their position in the future due to reasons within or outside the control of Pluristem. Except as otherwise required by law, Pluristem undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. For a more detailed description of the risks and uncertainties affecting Pluristem, reference is made to Pluristem's reports filed from time to time with the Securities and Exchange Commission.
Exhibit 99.2

1
Advancing cell therapeutic products for clinical use

Proprietary data of Pluristem Therapeutics Inc.
2
This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995
and federal securities laws. For example, we are using forward-looking statements when we discuss moving closer to reaching our objective to bring innovative,
effective treatments to patients, when we discuss the timing of receiving marketing approval for our product candidates, if at all, and the forecasted worldwide
sales of our product candidates, when we discuss the potential and timing of achieving regulatory approval for our product candidates via the EU Adaptive
Pathway or other expedited regulatory pathways, regarding future collaboration with other pharmaceutical companies, when we discuss anticipated milestones
regarding our regulatory approach and the development of current and future product candidates, including the timing and design of future clinical trials, and
whether such trials will be conducted at all. These forward-looking statements and their implications are based on the current expectations of the management
of Pluristem only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the
forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking
statements: changes in technology and market requirements; we may encounter delays or obstacles in launching and/or successfully completing our clinical
trials; our products may not be approved by regulatory agencies, our technology may not be validated as we progress further and our methods may not be
accepted by the scientific community; we may be unable to retain or attract key employees whose knowledge is essential to the development of our products;
unforeseen scientific difficulties may develop with our process; our products may wind up being more expensive than we anticipate; results in the laboratory
may not translate to equally good results in real surgical settings; results of preclinical studies may not correlate with the results of human clinical trials; our
patents may not be sufficient; our products may harm recipients; changes in legislation; inability to timely develop and introduce new technologies, products
and applications; loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of Pluristem to
differ materially from those contemplated in such forward-looking statements. In addition, historic results of scientific research do not guarantee that the
conclusions of future research would not suggest different conclusions or that historic results referred to in this presentation would not be interpreted
differently in light of additional research or otherwise. Also, while the company’s program was selected for the European Medicines Agency’s Adaptive
Pathways pilot project, as well as recognized by the PMDA, these agencies are not bound by these communications and accordingly may change their position
in the future due to reasons within or outside the control of Pluristem. Except as otherwise required by law, Pluristem undertakes no obligation to publicly
release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated
events. For a more detailed description of the risks and uncertainties affecting Pluristem, reference is made to Pluristem's reports filed from time to time with
the Securities and Exchange Commission.
and federal securities laws. For example, we are using forward-looking statements when we discuss moving closer to reaching our objective to bring innovative,
effective treatments to patients, when we discuss the timing of receiving marketing approval for our product candidates, if at all, and the forecasted worldwide
sales of our product candidates, when we discuss the potential and timing of achieving regulatory approval for our product candidates via the EU Adaptive
Pathway or other expedited regulatory pathways, regarding future collaboration with other pharmaceutical companies, when we discuss anticipated milestones
regarding our regulatory approach and the development of current and future product candidates, including the timing and design of future clinical trials, and
whether such trials will be conducted at all. These forward-looking statements and their implications are based on the current expectations of the management
of Pluristem only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the
forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking
statements: changes in technology and market requirements; we may encounter delays or obstacles in launching and/or successfully completing our clinical
trials; our products may not be approved by regulatory agencies, our technology may not be validated as we progress further and our methods may not be
accepted by the scientific community; we may be unable to retain or attract key employees whose knowledge is essential to the development of our products;
unforeseen scientific difficulties may develop with our process; our products may wind up being more expensive than we anticipate; results in the laboratory
may not translate to equally good results in real surgical settings; results of preclinical studies may not correlate with the results of human clinical trials; our
patents may not be sufficient; our products may harm recipients; changes in legislation; inability to timely develop and introduce new technologies, products
and applications; loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of Pluristem to
differ materially from those contemplated in such forward-looking statements. In addition, historic results of scientific research do not guarantee that the
conclusions of future research would not suggest different conclusions or that historic results referred to in this presentation would not be interpreted
differently in light of additional research or otherwise. Also, while the company’s program was selected for the European Medicines Agency’s Adaptive
Pathways pilot project, as well as recognized by the PMDA, these agencies are not bound by these communications and accordingly may change their position
in the future due to reasons within or outside the control of Pluristem. Except as otherwise required by law, Pluristem undertakes no obligation to publicly
release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated
events. For a more detailed description of the risks and uncertainties affecting Pluristem, reference is made to Pluristem's reports filed from time to time with
the Securities and Exchange Commission.
Forward Looking Statement

Proprietary data of Pluristem Therapeutics Inc.
3
• Cell therapy company (NasdaqCM: PSTI, TASE: PLTR)
• Using off-the-shelf placental expanded cells to achieve both local and
systemic therapeutic effects
systemic therapeutic effects
• First in class 3D cell culturing technology allowing for efficient, controlled
production of different cell products in commercial quantities - “the
process is the product”
production of different cell products in commercial quantities - “the
process is the product”
• Active with regulators in the U.S., EU, Japan, Korea, Australia and Israel
• Demonstrated clinical safety and significant efficacy in 3 clinical
studies (Two Phase I and one Phase I/II study)
studies (Two Phase I and one Phase I/II study)
Corporate Overview

Proprietary data of Pluristem Therapeutics Inc.
4
Strong Clinical Data
Muscle Injury following Total Hip Replacement N=20

Proprietary data of Pluristem Therapeutics Inc.
5
Correlation Between Improvement in the Muscle
Force of Injured and Contralateral Leg
Force of Injured and Contralateral Leg
Contralateral (non-operated)
Injured (operated)

Proprietary data of Pluristem Therapeutics Inc.
6
PLX Technology

Proprietary data of Pluristem Therapeutics Inc.
7
Culture conditions
PLX-R18
Hematological
PLX-PAD
Culture conditions
PLX-CNS
Neuronal
PLX-IMMUNE
Immunological
Human Placenta- A platform for distinct cell products
Four Distinct Products Derived from Placenta
Proprietary data of Pluristem Therapeutics Inc.
8
PLX-PAD CMC approved by 5 Regulatory agencies
Manufacturing facility and PLX-PAD CMC (3D culturing) for Phase II/III and
marketing*
marketing*
approved by FDA, PEI (Germany), EMA, Korean & Israeli regulatory agencies
Batch-to-Batch comparability confirmed,
multiple donors
* Subject to supplier approval

Proprietary data of Pluristem Therapeutics Inc.
9

Proprietary data of Pluristem Therapeutics Inc.
10
Selected for EU
Adaptive Pathway -
potential for
marketing approval in
2018
Adaptive Pathway -
potential for
marketing approval in
2018
Proprietary data of Pluristem Therapeutics Inc.
11
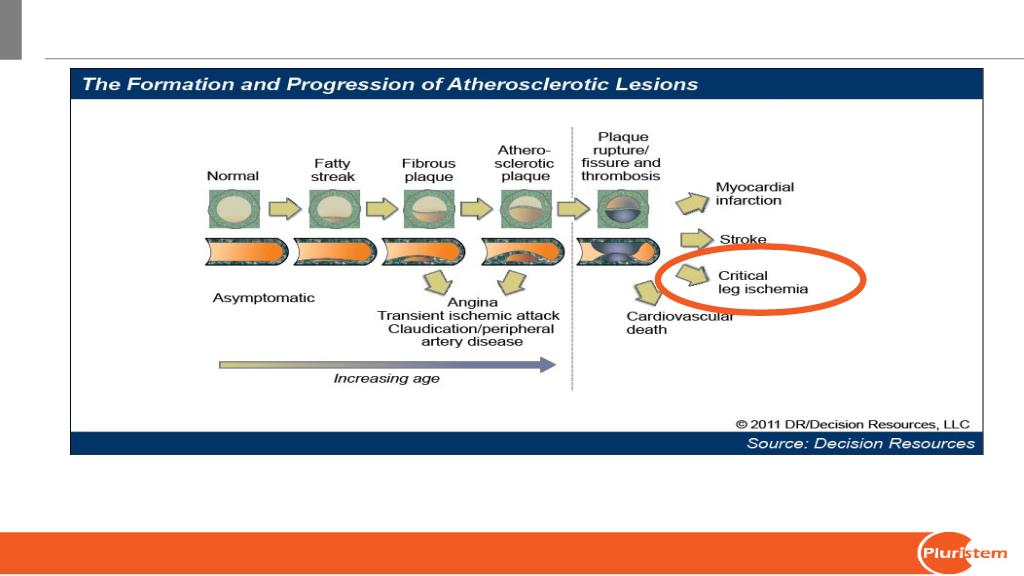
Progression of Atherosclerotic Lesions
From: Peripheral Arterial Disease. Source: Decision Resources
Created for Hillit Mannor Shachar, Pluristem Therapeutics. IP Address: (Unknown)
© 2011 Decision Resources, Inc.

Proprietary data of Pluristem Therapeutics Inc.
12
Therapeutic Angiogenesis by Growth Factors
From: Peripheral Arterial Disease. Source: Decision Resources
Created for Hillit Mannor Shachar, Pluristem Therapeutics. IP Address: (Unknown)
© 2011 Decision Resources, Inc.

Proprietary data of Pluristem Therapeutics Inc.
13
Proangiogenic VEGF, HGF, TGFb, Angiogenin, IL-8,
Angiopoietin, IL-6, MMP-1, TIMP-1, PAI-1, IGFBPs,
Angiopoietin, IL-6, MMP-1, TIMP-1, PAI-1, IGFBPs,
Immunomodulatory HGF, IDO, IL-28,
IL-22, IL-10RA,
Antifibrotic HGF, Decorin
Protrophic- LIF, Angiopoietin, b-NGF
PLX-PAD secretion profile

Proprietary data of Pluristem Therapeutics Inc.
14
Placebo
PLX-PAD treated
PLX-PAD increased blood flow in the tibia
Live Cellvizio imaging following IV
administration of FITC labeled Dextran
administration of FITC labeled Dextran

Proprietary data of Pluristem Therapeutics Inc.
15
• July 2009 - First CLI patient injected in Berlin (N=15)
• October 2010 - last CLI Patient injected in US (N=12)
• April 2012 - EMA declaration - Adaptive Pathways program
• May 2012 - End of 2 years patients safety follow-up in Europe
• April 2012 - EMA initiated the Adaptive Pathways program
• December 2012 - Initiation of IC Phase II clinical study (N=150)
• March 2014 - Pilot project launched by EMA
• May 2014 - Approval of the new facility, ready for Phase III and marketing
• May 2015 - PLX-PAD cells have been selected for the Adaptive Pathways pilot
project
project
PLX-PAD in Peripheral Artery Disease

Proprietary data of Pluristem Therapeutics Inc.
16
COMPANY CONFIDENTIAL
In the base scenario, PLX-PAD has the potential to achieve peak sales of ~$350M in the US
And ~ $290M in Europe
NPV - Base Scenario Peak sales in 2030
Peak worldwide sales: ~$700M
Peak US sales: ~$350M
Peak Europe sales: ~ $290M
NPV for CLI WW sales is: $2,100M
NPV for CLI Sales in Europe : $850M
Without the
EMA approval,
EMA approval,
Peak sales in
2033.
2033.
Reduce the
NPV by $200M
(~25%)
NPV by $200M
(~25%)

Proprietary data of Pluristem Therapeutics Inc.
17
COMPANY CONFIDENTIAL
In the upside scenario, PLX-PAD has the potential to achieve peak sales of ~$1.4B in the US and ~$1B in Europe
NPV Upside Scenario Peak sales in 2030
Peak worldwide sales: ~$2.7B
Peak US sales: ~$1,400M
Peak Europe sales: ~ $1,000M
NPV for CLI WW sales is: $8,300M
NPV for CLI Sales in Europe : $3,100M
Without the
EMA approval,
EMA approval,
Peak sales in
2033.
2033.
Reduce the
NPV by $700M
(~ 25%)
NPV by $700M
(~ 25%)

Proprietary data of Pluristem Therapeutics Inc.
18
PLX-PAD CLI program status in Europe
• 3-4 years earlier in a multi-billion market
• Manufacturing facility approved and inspected by European Qualified Person for
Phase III and marketing
Phase III and marketing
• Granted European patent for the use of PLX in ischemic disease
• Higher probability for a large pharma deal
• EMA new approach to expand the use of PLX-PAD to additional indications
within PAD and other ischemic indications
within PAD and other ischemic indications

Proprietary data of Pluristem Therapeutics Inc.
19

Proprietary data of Pluristem Therapeutics Inc.
20
Zami Aberman
Chairman & CEO
Chairman & CEO
Efrat Livne-Hadass
VP Human Resources
VP Human Resources
Racheli Ofir, Ph.D.
VP Research & Intellectual Property
Sagi Moran
VP Operations
VP Operations
Erez Egozi
VP Finance
Karine Kleinhaus, M.D., MPH
Divisional VP, North America
Divisional VP, North America
Yaky Yanay
President & COO
President & COO
Hillit Mannor Shachar, M.D., M.B.A.
VP Business Development
Ohad Karnieli, Ph.D., M.B.A.
VP Technology & Manufacturing
Esther Lukasiewicz Hagai, M.D., Ph.D.
VP Clinical & Medical Affairs
Orly Amiran
VP Quality Assurance
Management Team

Proprietary data of Pluristem Therapeutics Inc.
21
New Regulatory Approaches
for Accelerated Approval
of
PLX-PAD in Critical Limb Ischemia
for Accelerated Approval
of
PLX-PAD in Critical Limb Ischemia
Dr Esther Lukasiewicz-Hagai, MD, PhD
VP Medical and Clinical Affairs

Proprietary data of Pluristem Therapeutics Inc.
22
• Pluristem is highly committed to provide early access of PLX-PAD
to Critical limb ischemia (CLI) patients all over the world
to Critical limb ischemia (CLI) patients all over the world
• Pluristem is taking advantage of each new regulatory opportunity
to achieve this goal:
to achieve this goal:
Ø In Europe: New Adaptive Pathways pilot project of EMA
May 2015: PLX-PAD cells have been selected for the Adaptive Pathways pilot
project
project
Ø In Japan: Accelerated Pathway for Regenerative Medicine
April 2015: PLX-PAD quality and manufacturing processes agreed with PMDA
for use in clinical studies
for use in clinical studies
Introduction

Proprietary data of Pluristem Therapeutics Inc.
23
Completed, ongoing and planned studies in PAOD
• 2 completed Phase I studies in Critical Limb Ischemia (n= 27)
• 1 ongoing Phase 2 study in Intermittent claudication (n=150)
• 2 planned studies in CLI:
ü 1 Phase II/III in Europe via Adaptive Pathways
ü1 Phase I/II in Japan through Expedited Approval for Regenerative
Therapy
Therapy

Proprietary data of Pluristem Therapeutics Inc.
24
Completed Phase I Studies in CLI
• Two open label, dose-escalation, Phase I studies in patients with CLI Rutherford
category 4 (pain at rest) or 5 (minor tissue loss)
category 4 (pain at rest) or 5 (minor tissue loss)
|
PLX-PAD dose
|
U.S. study n=12
( 8 Ruth 4/4 Ruth 5)
|
German study n=15
(9 Ruth 4/6 Ruth 5)
|
|
200x 106
|
-
|
Single course
50 injections (n=3)
|
|
300x 106
|
Single course
30 injections (n=5)
|
Single course
50 injections (n=6)
|
|
600x 106
|
2 courses of 300 106 at 2 weeks
apart (30 injections per course) (n=7)
|
Single course
50 injections
(n=6)
|
• 12 months FU in US study and 24 months FU in German study

Proprietary data of Pluristem Therapeutics Inc.
25
Event Rate
USA data (AFS- 100%)
Germany data (AFS- 73%)
Average (85%)
Published historical data
Strong Clinical Data from 2 Phase I/II Critical Limb Ischemia Trials(N=27)
12 month - 85% Amputation-Free Survival (AFS)
Proprietary data of Pluristem Therapeutics Inc.
26
• Good Safety profile
• Trends in efficacy with improvement from baseline in:
– Transcutaneous Oxygen Pressure (limb perfusion)
– Quality of Life
– Pain score
Main studies results
Pre-Treatment
8 Weeks After Treatment

Proprietary data of Pluristem Therapeutics Inc.
27
EMA Adaptive Pathways

Proprietary data of Pluristem Therapeutics Inc.
28
EMA Adaptive Pathways - Background
Pilot project launched by EMA on March 19th, 2014 :
• European initiative intended to grant earlier access to drugs meant to
treat debilitating and/or life-threatening diseases with unmet
treat debilitating and/or life-threatening diseases with unmet
medical need
• Only for Product at an early stage of clinical development (during or
prior to Phase II)
prior to Phase II)

Proprietary data of Pluristem Therapeutics Inc.
29
EMA Adaptive Pathways - General principles:
• Early approval of a drug for a restricted patient population followed by progressive
adaptations of the marketing authorization to expand access to the drug to broader
patient populations based on data gathered from its use and additional studies
adaptations of the marketing authorization to expand access to the drug to broader
patient populations based on data gathered from its use and additional studies
AND/OR
• Early regulatory approval (e.g. conditional approval) with collection of post-approval
confirmatory data on the drug's use in patients
confirmatory data on the drug's use in patients
• Involves balancing the importance of timely patient access with the need for
adequate, evolving evidence on a drugs’ risks and benefits
adequate, evolving evidence on a drugs’ risks and benefits
• Builds on regulatory processes already in place within the existing European Union
legal framework
legal framework
• Early discussion between a wide range of stakeholders to explore ways of optimizing
development pathways: HTA bodies, payers, patients’ organizations, physicians’
organizations, academic and researchers
development pathways: HTA bodies, payers, patients’ organizations, physicians’
organizations, academic and researchers

Proprietary data of Pluristem Therapeutics Inc.
30
Adaptive vs. traditional Pathway to Market
Phase II RCT
CLI (niche population)
Conditional MA
in niche population
2016
2017
2018
2019
2020
2021
Confirmation for the CLI subgroup (Phase III/post-
marketing registries) + Additional studies in broader
populations of CLI patients
marketing registries) + Additional studies in broader
populations of CLI patients
Full MA
Phase II RCT
Two Phase III RCTs
Full MA
Adaptive Pathways
Traditional Pathway

Proprietary data of Pluristem Therapeutics Inc.
31
Adaptive Pathways for PLX-PAD in CLI
• Severely debilitating and life-threatening disease: overall 30% of CLI patients have
amputations and 25% die by 1 year after diagnosis (higher rates in CLI patients that
cannot undergo revascularization)
amputations and 25% die by 1 year after diagnosis (higher rates in CLI patients that
cannot undergo revascularization)
• Unmet medical need: CLI patients who are not eligible for revascularization or have
failed revascularization have no treatment option
failed revascularization have no treatment option
• Phase I data with PLX-PAD in CLI patients with no option for revascularization
showing good safety profile and trends of efficacy
showing good safety profile and trends of efficacy
• Safety data from 150 patients enrolled into IC study to support initial approval in CLI
• Initial Phase II study to get conditional approval in a selected population of CLI
patients with high unmet medical need , then post-marketing extension to more
CLI patients
patients with high unmet medical need , then post-marketing extension to more
CLI patients
• A surrogate endpoint will most probably be accepted for initial approval
(reducing length of the study)
Proprietary data of Pluristem Therapeutics Inc.
32
Clinical development of PLX-PAD
Subgroup of CLI patients at high risk
(Phase II trial /Surrogate endpoint)
Initial approval in selected subgroup of
CLI patients
CLI patients
Expansion to broader CLI population (RCT / RWD)
Expansion to additional indication/s Based on:
-subgroup data/secondary endpoint
- Common clinical endpoints
- Common Mechanism of Action
Final/Additional Approval
Confirmation for selected subgroup of CLI patients
(Phase III/registries of CLI patients treated with PLX-PAD)
Supportive Real-World Data (RWD)
CLI registries

Proprietary data of Pluristem Therapeutics Inc.
33
Expedited approval for
regenerative therapy in Japan
regenerative therapy in Japan

Proprietary data of Pluristem Therapeutics Inc.
34
Background
New Japanese regulations from Nov 2014 to accelerate
development of drugs in the field of regenerative therapy:
development of drugs in the field of regenerative therapy:
• Only for Regenerative therapy
• Seriously-debilitating / Life-threatening indication
• Unmet medical need

Proprietary data of Pluristem Therapeutics Inc.
35
Accelerated vs. traditional approval process
• Conditional time-limited approval can be obtained based on proof of safety and “limited proof of
efficacy” (Phase I/II, surrogate endpoint)
efficacy” (Phase I/II, surrogate endpoint)
• Up to 7 years to confirm efficacy post conditional approval
• Condition to have patients treated post conditional approval signed an ICF and registered in a post
marketing registry for pharmacovigilance
marketing registry for pharmacovigilance

Proprietary data of Pluristem Therapeutics Inc.
36
Clinical development of PLX-PAD for CLI
Phase I/II trial
(limited proof of efficacy)
Time-limited conditional approval
Final/Additional Approval
Registry of patients treated with
PLX-PAD for pharmacovigilance

Proprietary data of Pluristem Therapeutics Inc.
37
|
|
2015
|
2016
|
2017
|
2018
|
2019
|
2020
|
2021
|
|||||||
|
EMA initiation
|
PIP
Waiver |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ATMP
Class. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SA on
CMC |
SA with
EMA/HTA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phase II/III
(EU)
|
|
CTA Subm.
|
Phase II
|
Phase III/Long term follow up
Registry of patients treated with PLX-PAD
|
|
|
|
|||||||
|
MA
(EMA)
|
|
|
|
|
|
|
CMA
|
|
|
|
Presub.
meeting |
MAA
subm. |
MA
Approval |
|
|
PMDA initiation
|
R&D
meeting |
Pre Phase I/II
meeting |
|
|
|
|
|
|
|
|
|
|
|
|
|
Phase I/II
(Japan)
|
|
CTN
submission
|
Phase I/II
|
Safety
follow up |
Confirmation of efficacy
Registry of Patients treated with PLX-PAD
|
|
|
|
||||||
|
Conditional Time-
Limited Authorization Application (PMDA) |
|
|
|
|
Pre-NDA
meetings |
CAA
subm. |
CAA
Approval |
|
|
|
|
|
|
|
|
Marketing
Authorization Application (PMDA)
|
|
|
|
|
|
|
|
|
|
|
Pre-NDA
Meeting |
NDA
Subm. |
NDA
Approv. |
|

Proprietary data of Pluristem Therapeutics Inc.
38
Conclusion
In the framework of the 2 new regulatory approaches that exist
in Europe and in Japan , Pluristem plan to initiate 2 clinical
studies in CLI in 2016 with the aim of obtaining initial approval
already in 2018
in Europe and in Japan , Pluristem plan to initiate 2 clinical
studies in CLI in 2016 with the aim of obtaining initial approval
already in 2018

Proprietary data of Pluristem Therapeutics Inc.
39
• Time to Market (TTM)- we will be focus on adaptive pathways
Ø Europe
Ø Japan
Ø Our goal is to file early/conditional approval by the end of
2017/early 2018
2017/early 2018
• Launching PLX-R18 studies for hematology and ARS
• Building platform for new products- serum free based
• Entering into Life changing indication
• Entering into licensing deal
So, what’s next?

Proprietary data of Pluristem Therapeutics Inc.
40

Proprietary data of Pluristem Therapeutics Inc.
41
|
Partner
|
Indication
|
Deal structure
|
|
|
Upfront payment of $7M, additional
$48M in milestones, cells supply (cost +) and royalties in gross margin |
|
|
|
IC, CLI
South Korea only
|
JV following marketing authorization
of the Korean authorities |
|
|
Acute Radiation Syndrome
|
U.S. National Institutes of Health to
support development of Pluristem's PLX-R18 |
Pluristem keeps IP and manufacturing rights in all collaborations
Collaborations
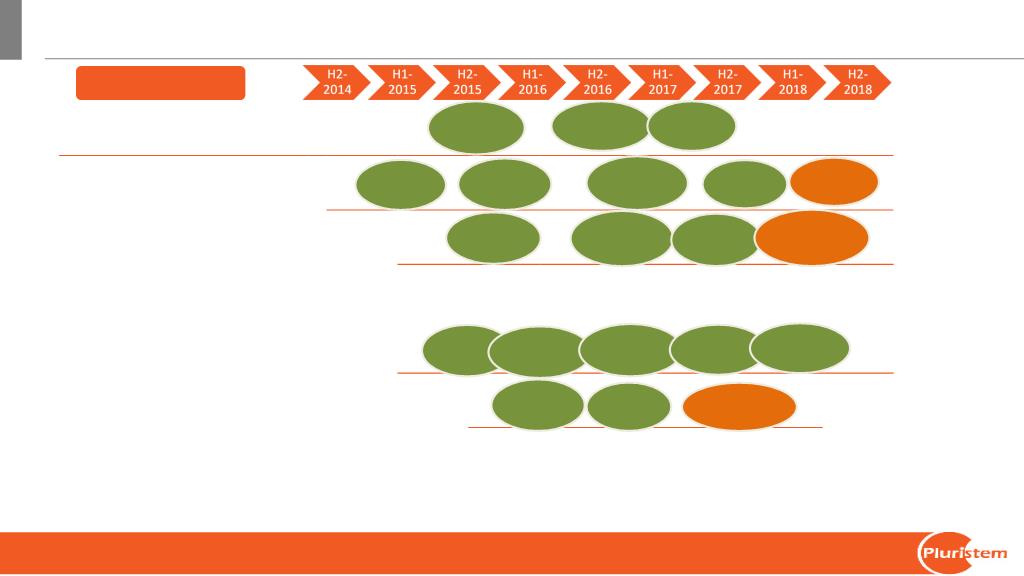
Proprietary data of Pluristem Therapeutics Inc.
42
|
IC
|
|||||||||
|
CLI (Adaptive EU)
|
|||||||||
|
CLI (Japan)
|
|
|
|
|
|||||
|
Orthopedic indication
|
|
|
|
|
|
|
|
|
|
|
Hematology
|
|
|
|
||||||
|
ARS
|
|
|
|
|
|
||||
|
PAH
|
|
|
|
|
|
|
|
|
|
|
Preeclampsia
|
|
|
|
|
|
|
|
|
|
End of
enrollment
enrollment
Proprietary data of Pluristem Therapeutics Inc.
Company milestones
indication
End of
enrollment
enrollment
Data Readout
Licensing deal
Study
initiation
initiation
Adaptive
Pathway
approval
Pathway
approval
Initial
approval
approval
Data
Readout
Readout
End of
enrollment
enrollment
Data
Readout
Study initiation
Conditional
approval
approval
We will seek to include the indication under the Adaptive licensing
pathway- based on discussion with EMA
pathway- based on discussion with EMA
IND
Study
initiation
End of
enrollment
enrollment
Data
Readout
Adaptive or
phase II/III
phase II/III
Pivotal study
Data
Readout
Readout
BLA application
UT will pursue IND upon completion of the phase I
We will seek to include preeclampsia under the Adaptive licensing
pathway- based on discussion with EMA
pathway- based on discussion with EMA

Proprietary data of Pluristem Therapeutics Inc.
43
Proprietary data of Pluristem Therapeutics Inc.
43
indication
|
Technologies
|
|
|
|
|
|||||
|
New products
(Tox ready)
|
|
|
|
|
Thawing
device
Plurispheare
MP4
NewPro1
PLX-R18
MP5
NewPro2
NewPro3
Company milestones

Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Epique Realty Sets New Industry Standard with Epique PowerCON 2024 Event FREE For Epique Agents
- Nationwide Insurance to Showcase How they are Driving Proactive Risk Management Using iLearningEngines’ Enterprise AI Platform at the 2024 Insurance Innovators Conference
- SNOW Shareholders – Lead Plaintiff Deadline Set for April 29, 2024 – Contact Robbins LLP for More Information
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share