Form 8-K OXIGENE INC For: Jan 11
United States
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of report (Date of earliest event reported): January 11, 2016
OXiGENE, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 0-21990 | 13-3679168 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
701 Gateway Boulevard, Suite 210, South San Francisco, CA 94080
(Address of principal executive offices)
Registrant’s telephone number, including area code: (650) 635-7000
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the obligation of the registrant under any of the following provisions:
| ¨ | Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 8.01 | Other Events. |
The information attached as Exhibit 99.1 to this report for OXiGENE, Inc. (the “Company”), relating to the Company and its development programs, may be presented from time to time by the Company at various investor and analyst meetings.
| Item 9.01 | Financial Statements and Exhibits. |
The following exhibit is furnished with this report:
| Exhibit |
Description | |
| 99.1 | OXiGENE,Inc. Corporate Overview, as of January 11, 2016 | |
By filing this report, including the information contained in Exhibit 99.1 attached hereto, the Company makes no admission as to the materiality of any information in this report. The information contained in Exhibit 99.1 hereto is summary information that is intended to be considered in the context of the Company’s filings with the U.S. Securities and Exchange Commission (the “SEC”), including its Annual Report on Form 10-K filed on March 30, 2015, Quarterly Report on Form 10-Q filed on November 12, 2015, and other public announcements that the Company makes, by Current Report on Form 8-K, press release or otherwise, from time to time. The Company undertakes no duty or obligation to publicly update or revise the information contained in this report, although it may do so from time to time as it believes is appropriate. Any such updating may be made through the filing of other reports or documents with the SEC, through press releases, or through other public disclosure.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| OXiGENE, Inc. | ||||
| Date: January 11, 2016 | /S/ MATTHEW M. LOAR | |||
| By: Matthew M. Loar | ||||
| Chief Financial Officer | ||||

Corporate Overview William D. Schwieterman, MD President and Chief Executive Officer January 2016 Exhibit 99.1

This presentation contains forward-looking statements under the meaning of the Private Securities Litigation Reform Act of 1995. These statements give our current expectations or forecasts and use words such as “anticipate,” “estimate,” "expect," “believe,” and other words of similar meaning. Any or all of the forward-looking statements in this presentation may turn out to be wrong. They can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties including but not limited to, the efficacy of our product candidates, their efficacy at acceptable dosage levels, the ability to raise capital when needed and on reasonable terms, projections of potential commercial sales of company products, the results and progress of clinical trials, developing the necessary manufacturing processes and gaining all necessary regulatory approvals, both in the United States and internationally. Consequently, no forward-looking statement can be guaranteed and actual results may differ materially. Additional information concerning factors that could cause actual results to materially differ from those in the forward-looking statements are contained in our most recent reports to the Securities and Exchange Commission including our Form 10-Q, 8-K and 10-K reports. However, we undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise. We note these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. Safe Harbor Statement
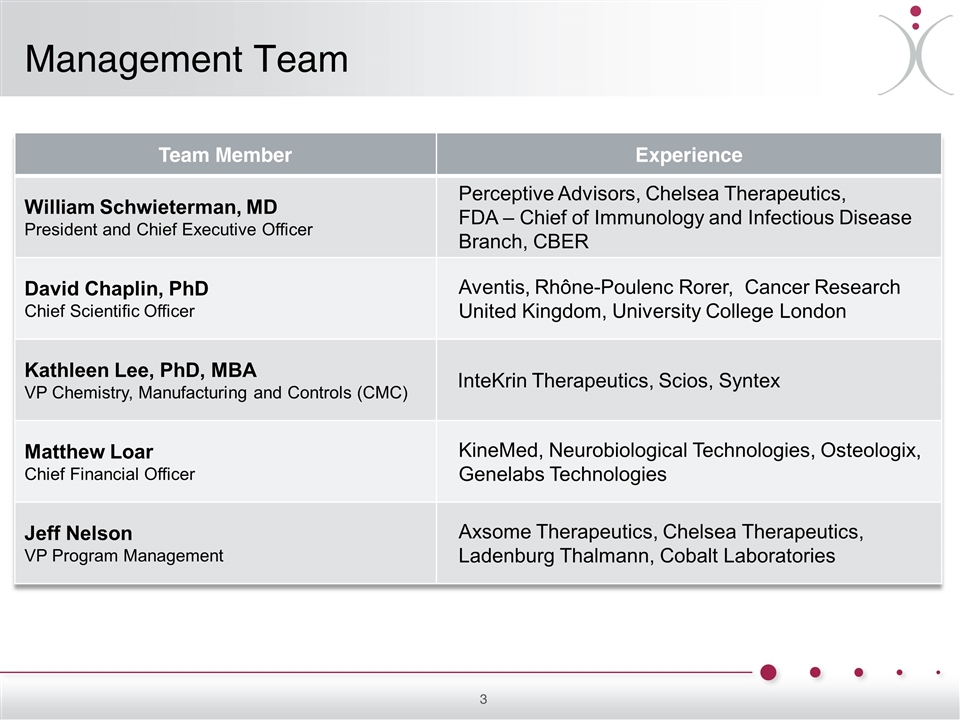
Management Team Team Member Experience William Schwieterman, MD President and Chief Executive Officer Perceptive Advisors, Chelsea Therapeutics, FDA – Chief of Immunology and Infectious Disease Branch, CBER David Chaplin, PhD Chief Scientific Officer Aventis, Rhône-Poulenc Rorer, Cancer Research United Kingdom, University College London Kathleen Lee, PhD, MBA VP Chemistry, Manufacturing and Controls (CMC) InteKrin Therapeutics, Scios, Syntex Matthew Loar Chief Financial Officer KineMed, Neurobiological Technologies, Osteologix, Genelabs Technologies Jeff Nelson VP Program Management Axsome Therapeutics, Chelsea Therapeutics, Ladenburg Thalmann, Cobalt Laboratories
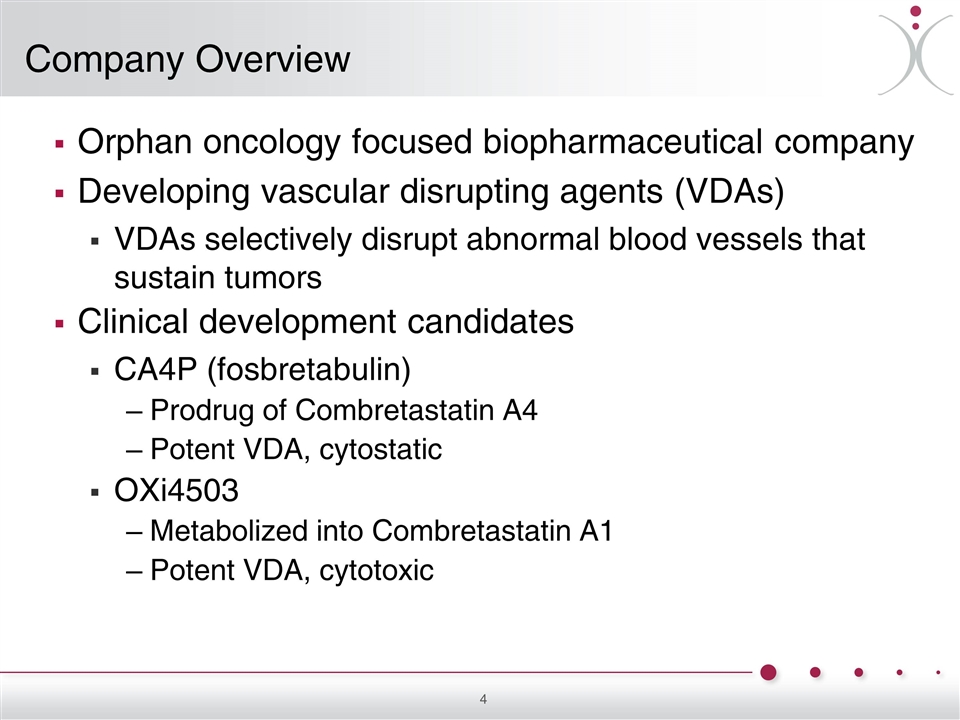
Company Overview Orphan oncology focused biopharmaceutical company Developing vascular disrupting agents (VDAs) VDAs selectively disrupt abnormal blood vessels that sustain tumors Clinical development candidates CA4P (fosbretabulin) Prodrug of Combretastatin A4 Potent VDA, cytostatic OXi4503 Metabolized into Combretastatin A1 Potent VDA, cytotoxic
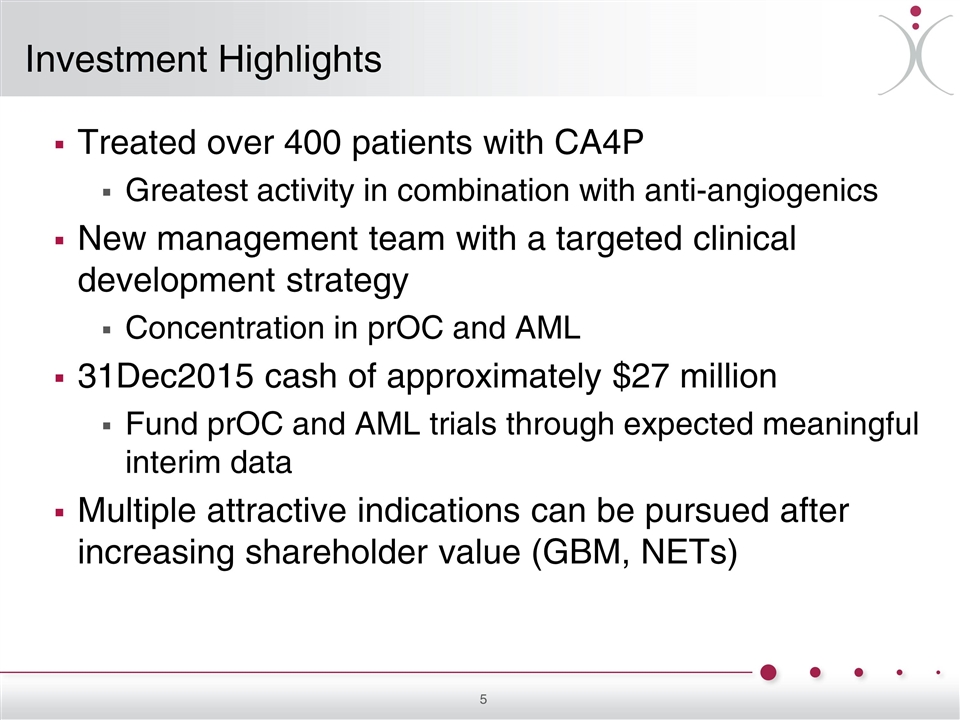
Investment Highlights Treated over 400 patients with CA4P Greatest activity in combination with anti-angiogenics New management team with a targeted clinical development strategy Concentration in prOC and AML 31Dec2015 cash of approximately $27 million Fund prOC and AML trials through expected meaningful interim data Multiple attractive indications can be pursued after increasing shareholder value (GBM, NETs)
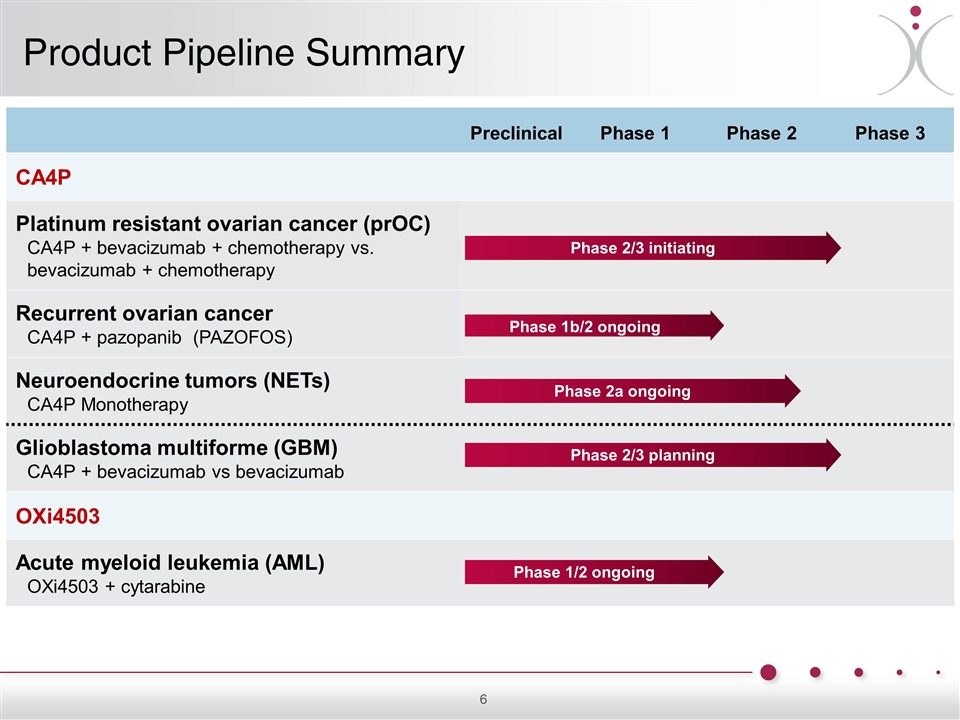
Preclinical Phase 1 Phase 2 Phase 3 CA4P Platinum resistant ovarian cancer (prOC) CA4P + bevacizumab + chemotherapy vs. bevacizumab + chemotherapy Recurrent ovarian cancer CA4P + pazopanib (PAZOFOS) Neuroendocrine tumors (NETs) CA4P Monotherapy Glioblastoma multiforme (GBM) CA4P + bevacizumab vs bevacizumab OXi4503 Acute myeloid leukemia (AML) OXi4503 + cytarabine Phase 1b/2 ongoing Phase 1/2 ongoing Phase 2a ongoing Phase 2/3 initiating Phase 2/3 planning Product Pipeline Summary
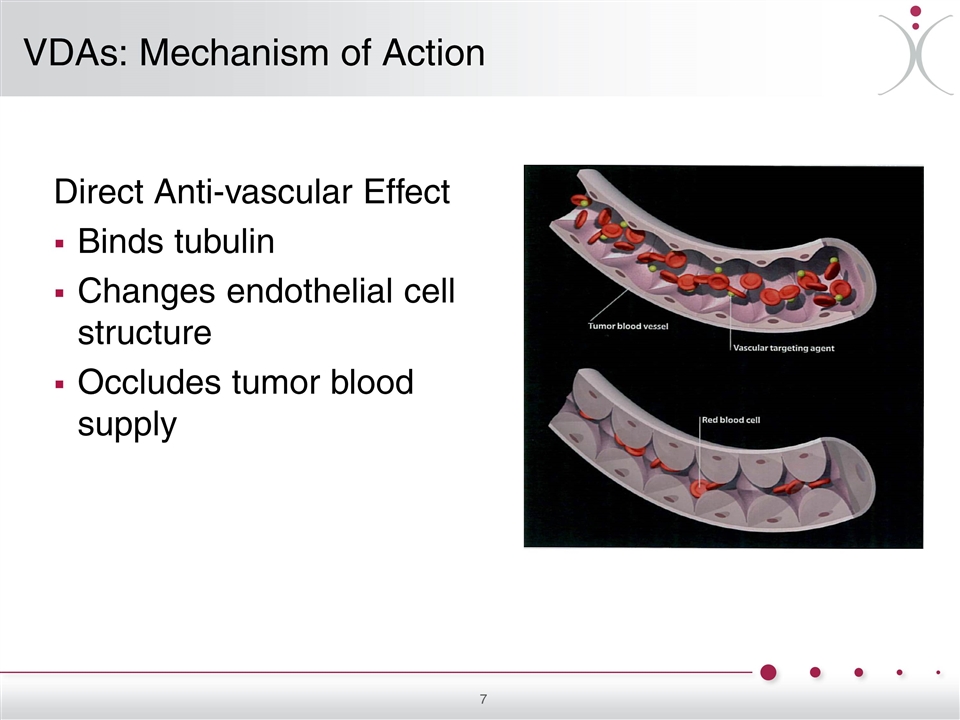
Direct Anti-vascular Effect Binds tubulin Changes endothelial cell structure Occludes tumor blood supply VDAs: Mechanism of Action
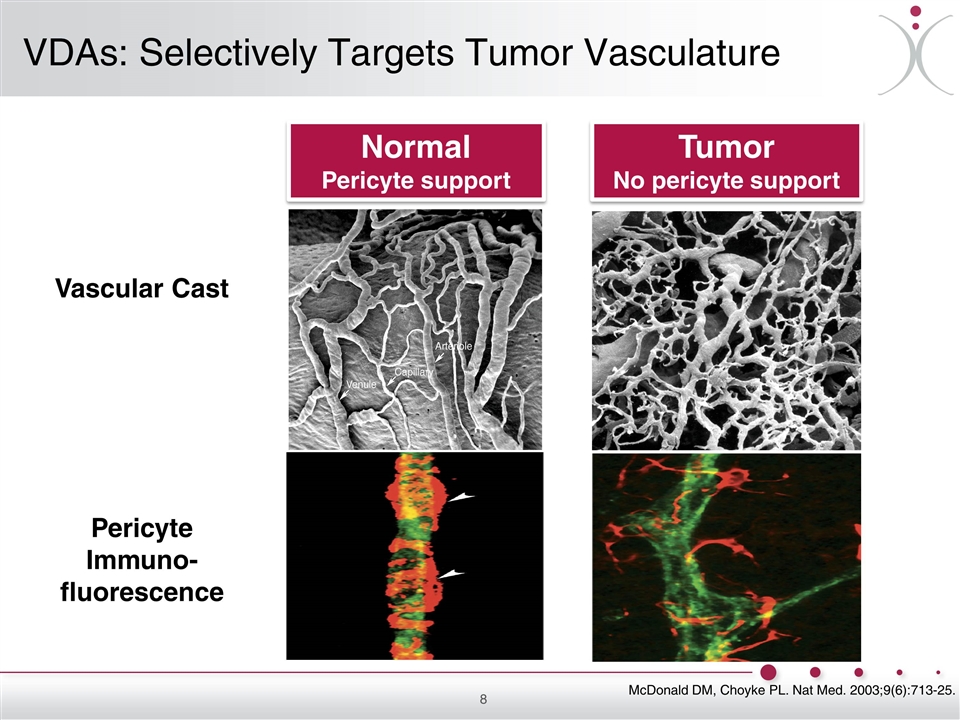
Normal Pericyte support Tumor No pericyte support Vascular Cast Pericyte Immuno-fluorescence VDAs: Selectively Targets Tumor Vasculature McDonald DM, Choyke PL. Nat Med. 2003;9(6):713-25.
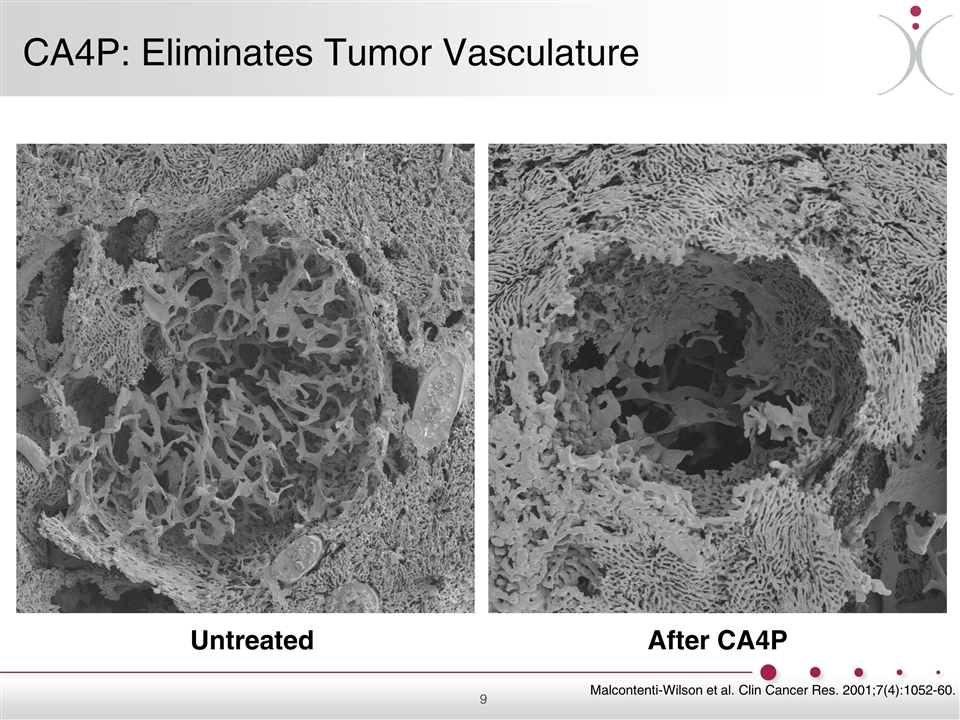
Untreated After CA4P CA4P: Eliminates Tumor Vasculature Malcontenti-Wilson et al. Clin Cancer Res. 2001;7(4):1052-60.
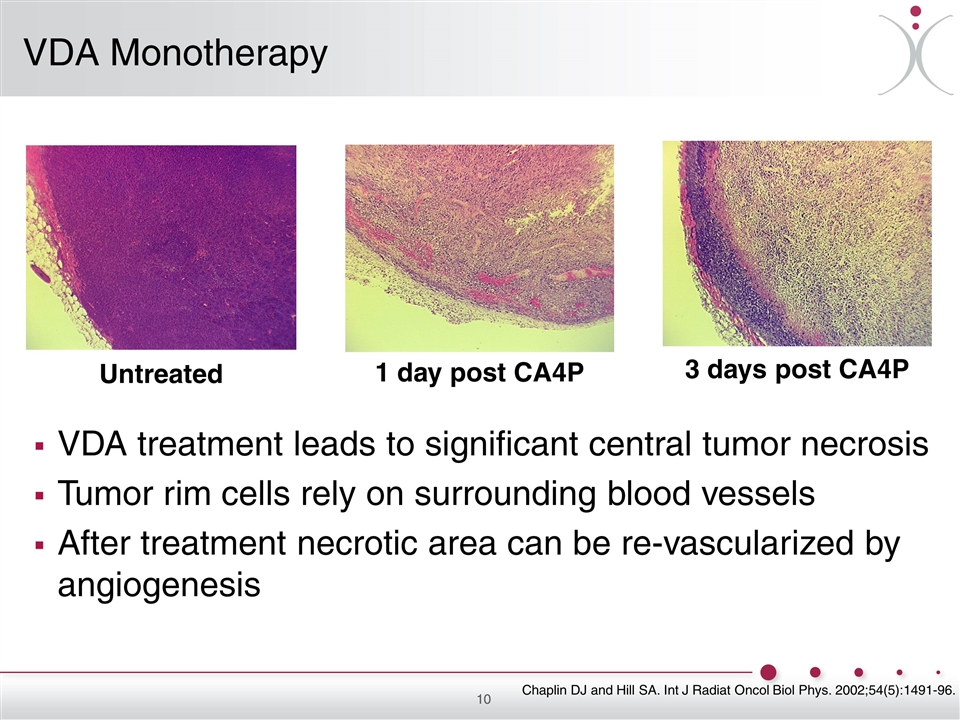
Untreated 1 day post CA4P 3 days post CA4P VDA treatment leads to significant central tumor necrosis Tumor rim cells rely on surrounding blood vessels After treatment necrotic area can be re-vascularized by angiogenesis VDA Monotherapy Chaplin DJ and Hill SA. Int J Radiat Oncol Biol Phys. 2002;54(5):1491-96.
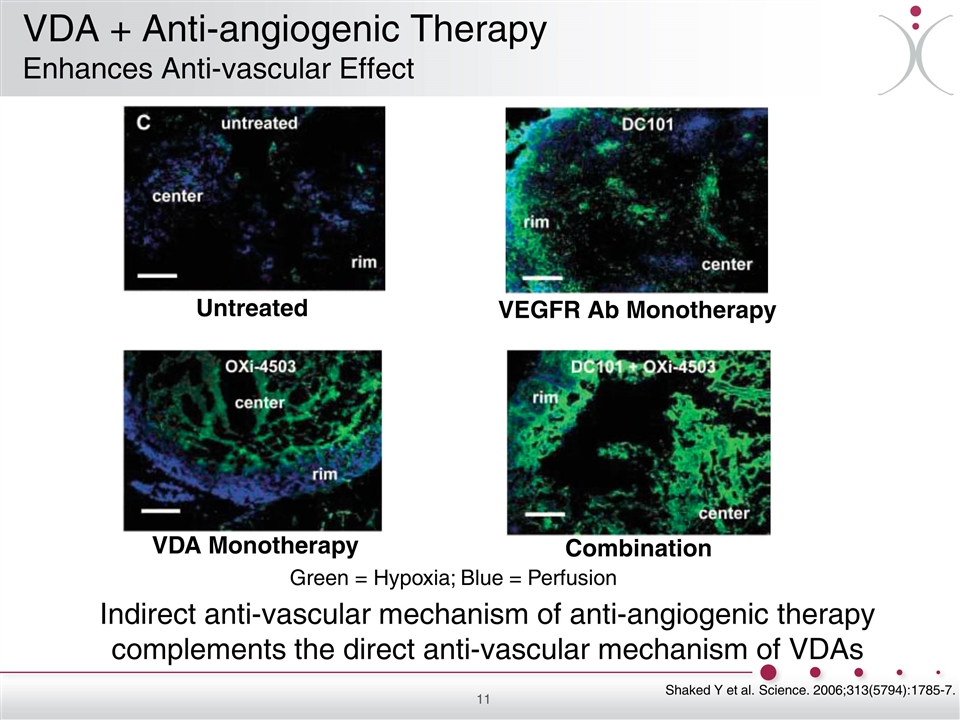
Green = Hypoxia; Blue = Perfusion Untreated VEGFR Ab Monotherapy VDA Monotherapy Combination Shaked Y et al. Science. 2006;313(5794):1785-7. Indirect anti-vascular mechanism of anti-angiogenic therapy complements the direct anti-vascular mechanism of VDAs VDA + Anti-angiogenic Therapy Enhances Anti-vascular Effect
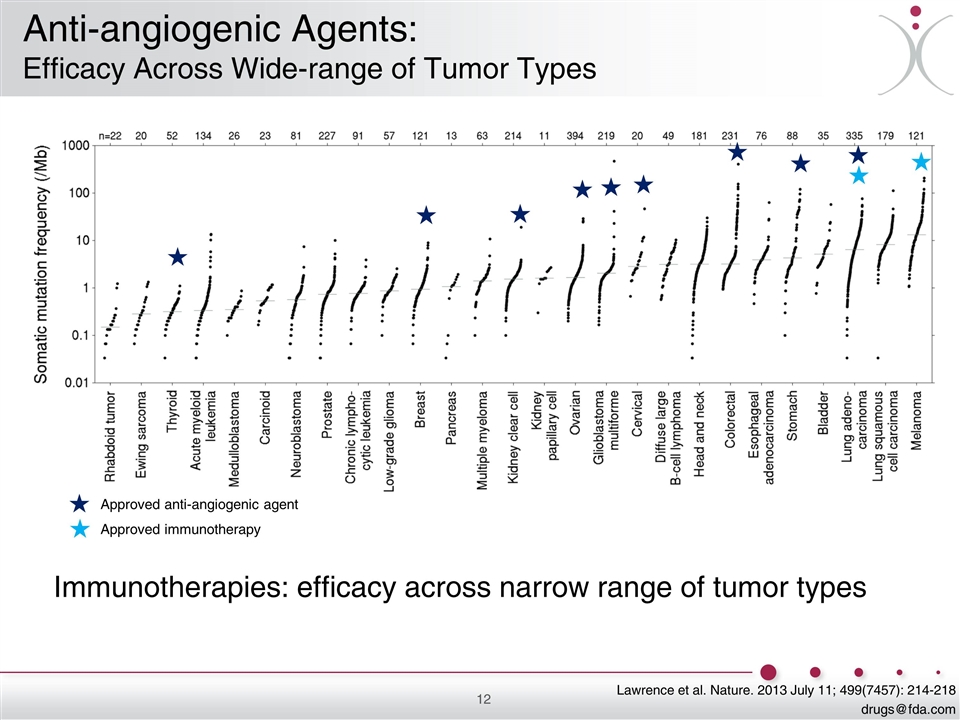
Lawrence et al. Nature. 2013 July 11; 499(7457): 214-218 [email protected] Immunotherapies: efficacy across narrow range of tumor types Approved anti-angiogenic agent Approved immunotherapy Anti-angiogenic Agents: Efficacy Across Wide-range of Tumor Types
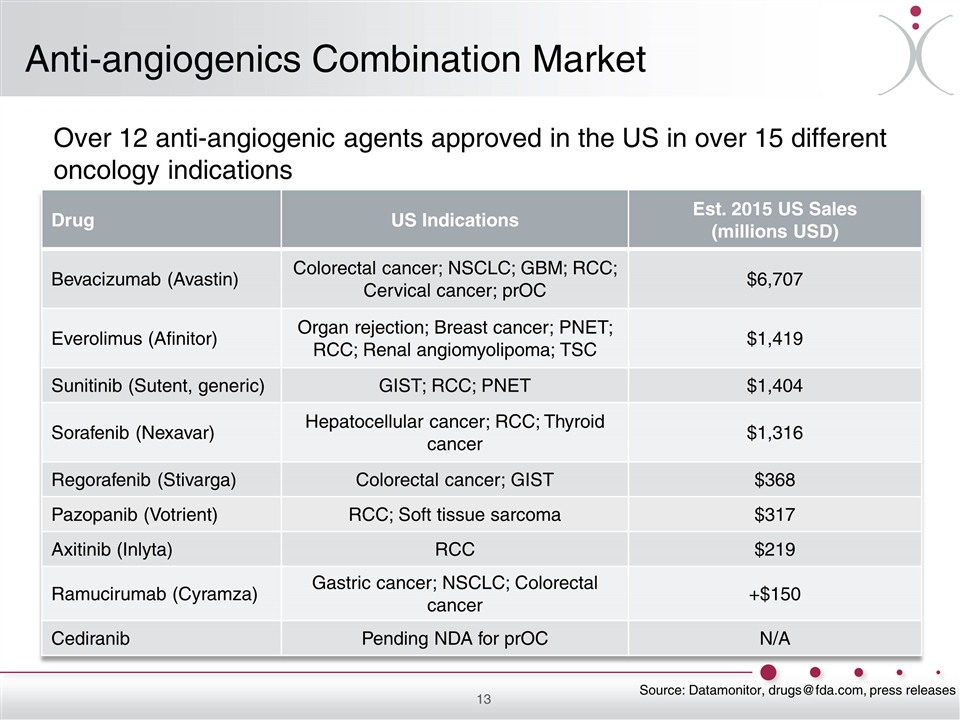
Anti-angiogenics Combination Market Over 12 anti-angiogenic agents approved in the US in over 15 different oncology indications Drug US Indications Est. 2015 US Sales (millions USD) Bevacizumab (Avastin) Colorectal cancer; NSCLC; GBM; RCC; Cervical cancer; prOC $6,707 Everolimus (Afinitor) Organ rejection; Breast cancer; PNET; RCC; Renal angiomyolipoma; TSC $1,419 Sunitinib (Sutent, generic) GIST; RCC; PNET $1,404 Sorafenib (Nexavar) Hepatocellular cancer; RCC; Thyroid cancer $1,316 Regorafenib (Stivarga) Colorectal cancer; GIST $368 Pazopanib (Votrient) RCC; Soft tissue sarcoma $317 Axitinib (Inlyta) RCC $219 Ramucirumab (Cyramza) Gastric cancer; NSCLC; Colorectal cancer +$150 Cediranib Pending NDA for prOC N/A Source: Datamonitor, [email protected], press releases

CA4P
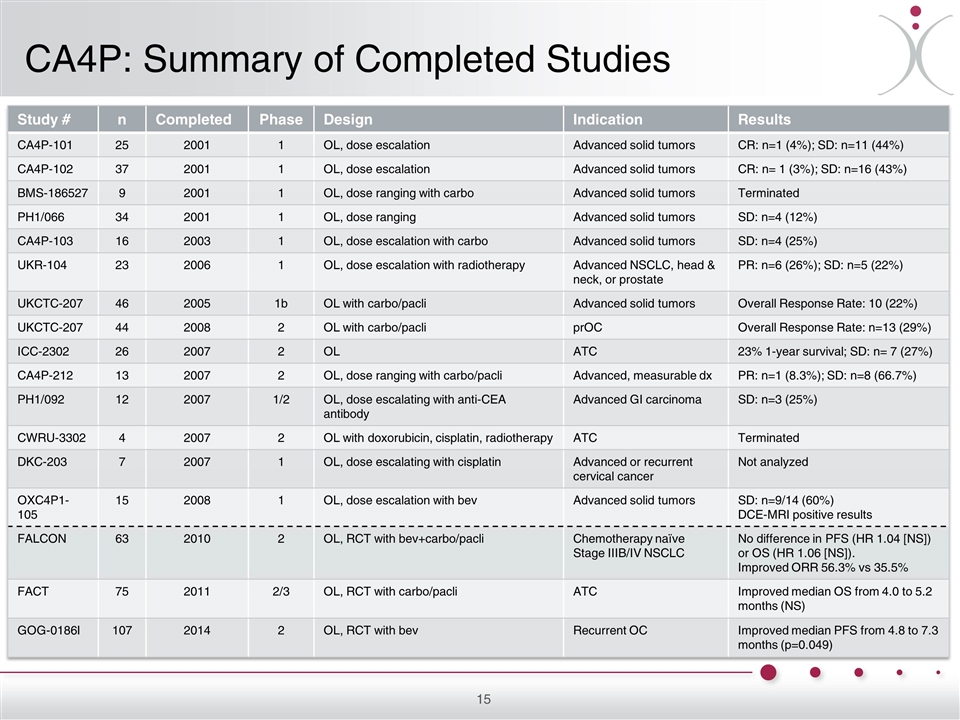
CA4P: Summary of Completed Studies Study # n Completed Phase Design Indication Results CA4P-101 25 2001 1 OL, dose escalation Advanced solid tumors CR: n=1 (4%); SD: n=11 (44%) CA4P-102 37 2001 1 OL, dose escalation Advanced solid tumors CR: n= 1 (3%); SD: n=16 (43%) BMS-186527 9 2001 1 OL, dose ranging with carbo Advanced solid tumors Terminated PH1/066 34 2001 1 OL, dose ranging Advanced solid tumors SD: n=4 (12%) CA4P-103 16 2003 1 OL, dose escalation with carbo Advanced solid tumors SD: n=4 (25%) UKR-104 23 2006 1 OL, dose escalation with radiotherapy Advanced NSCLC, head & neck, or prostate PR: n=6 (26%); SD: n=5 (22%) UKCTC-207 46 2005 1b OL with carbo/pacli Advanced solid tumors Overall Response Rate: 10 (22%) UKCTC-207 44 2008 2 OL with carbo/pacli prOC Overall Response Rate: n=13 (29%) ICC-2302 26 2007 2 OL ATC 23% 1-year survival; SD: n= 7 (27%) CA4P-212 13 2007 2 OL, dose ranging with carbo/pacli Advanced, measurable dx PR: n=1 (8.3%); SD: n=8 (66.7%) PH1/092 12 2007 1/2 OL, dose escalating with anti-CEA antibody Advanced GI carcinoma SD: n=3 (25%) CWRU-3302 4 2007 2 OL with doxorubicin, cisplatin, radiotherapy ATC Terminated DKC-203 7 2007 1 OL, dose escalating with cisplatin Advanced or recurrent cervical cancer Not analyzed OXC4P1-105 15 2008 1 OL, dose escalation with bev Advanced solid tumors SD: n=9/14 (60%) DCE-MRI positive results FALCON 63 2010 2 OL, RCT with bev+carbo/pacli Chemotherapy naïve Stage IIIB/IV NSCLC No difference in PFS (HR 1.04 [NS]) or OS (HR 1.06 [NS]). Improved ORR 56.3% vs 35.5% FACT 75 2011 2/3 OL, RCT with carbo/pacli ATC Improved median OS from 4.0 to 5.2 months (NS) GOG-0186I 107 2014 2 OL, RCT with bev Recurrent OC Improved median PFS from 4.8 to 7.3 months (p=0.049)
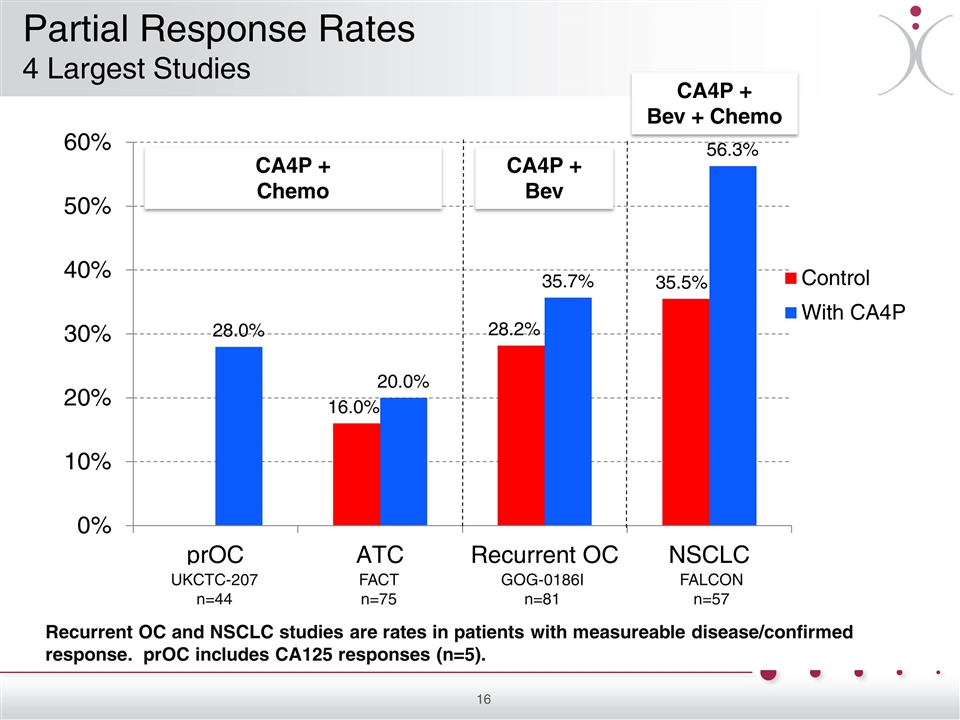
Recurrent OC and NSCLC studies are rates in patients with measureable disease/confirmed response. prOC includes CA125 responses (n=5). CA4P + Chemo CA4P + Bev + Chemo CA4P + Bev UKCTC-207 n=44 FACT n=75 GOG-0186I n=81 FALCON n=57 Partial Response Rates 4 Largest Studies
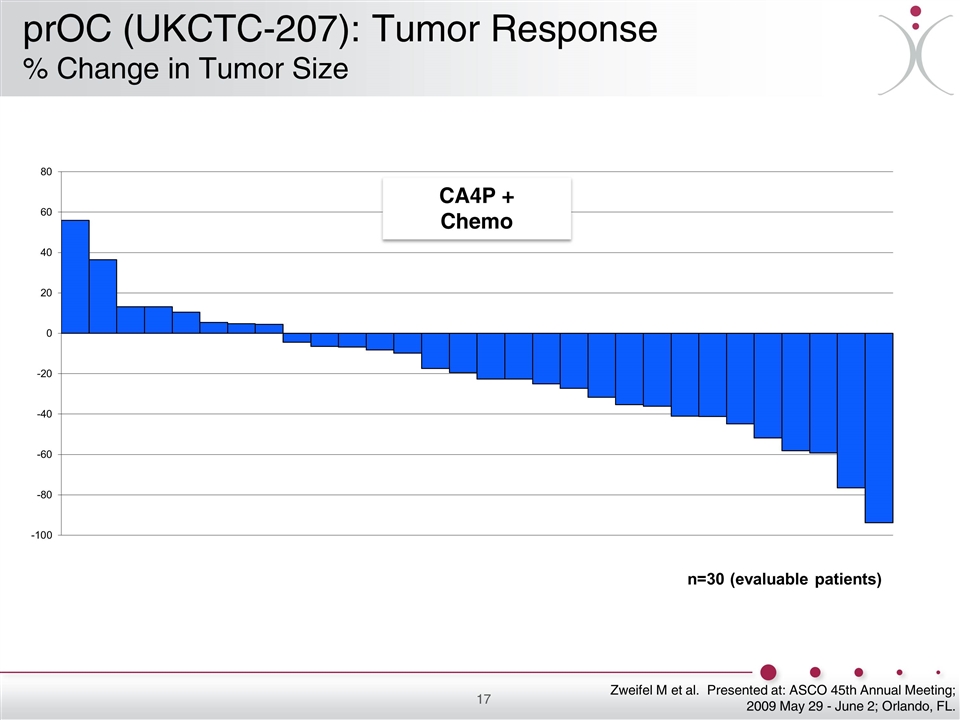
Zweifel M et al. Presented at: ASCO 45th Annual Meeting; 2009 May 29 - June 2; Orlando, FL. CA4P + Chemo prOC (UKCTC-207): Tumor Response % Change in Tumor Size
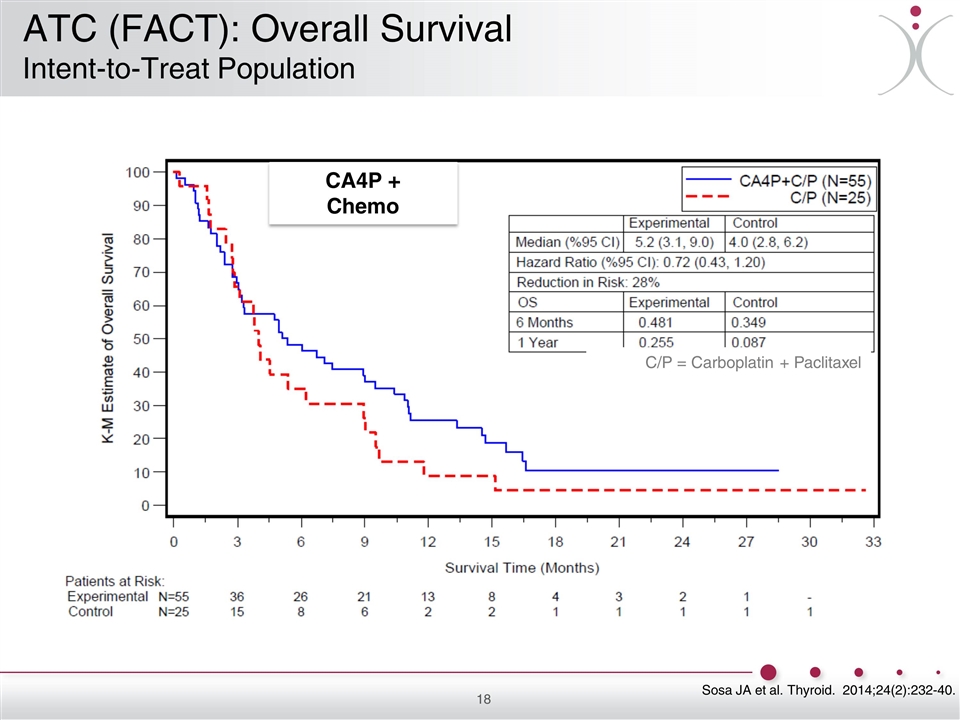
Sosa JA et al. Thyroid. 2014;24(2):232-40. C/P = Carboplatin + Paclitaxel CA4P + Chemo ATC (FACT): Overall Survival Intent-to-Treat Population
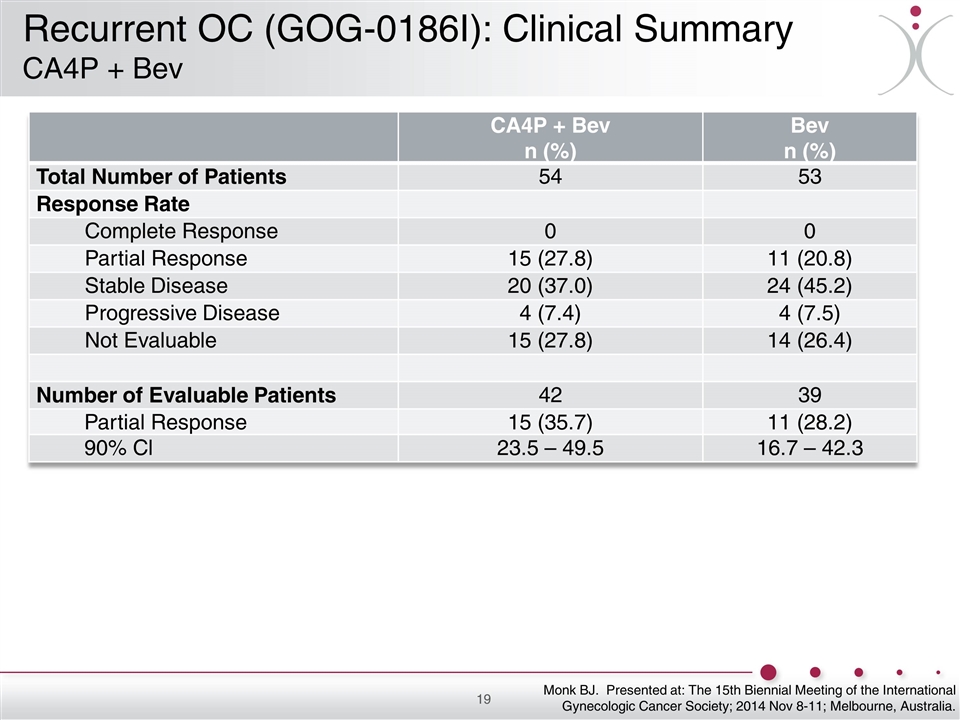
CA4P + Bev n (%) Bev n (%) Total Number of Patients 54 53 Response Rate Complete Response 0 0 Partial Response 15 (27.8) 11 (20.8) Stable Disease 20 (37.0) 24 (45.2) Progressive Disease 4 (7.4) 4 (7.5) Not Evaluable 15 (27.8) 14 (26.4) Number of Evaluable Patients 42 39 Partial Response 15 (35.7) 11 (28.2) 90% Cl 23.5 – 49.5 16.7 – 42.3 Monk BJ. Presented at: The 15th Biennial Meeting of the International Gynecologic Cancer Society; 2014 Nov 8-11; Melbourne, Australia. Recurrent OC (GOG-0186I): Clinical Summary CA4P + Bev
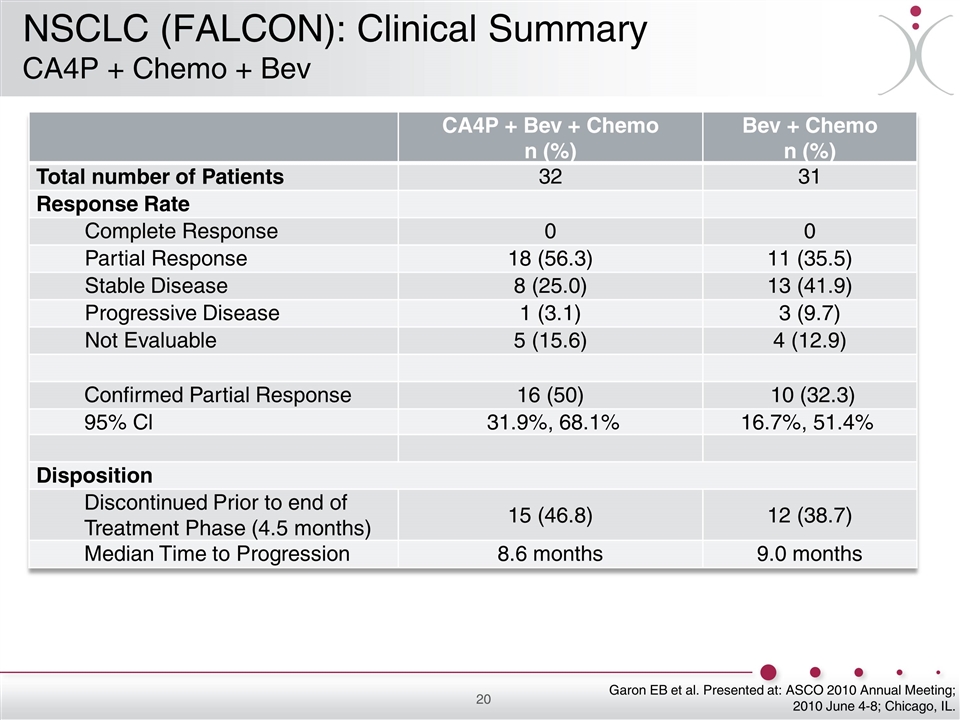
CA4P + Bev + Chemo n (%) Bev + Chemo n (%) Total number of Patients 32 31 Response Rate Complete Response 0 0 Partial Response 18 (56.3) 11 (35.5) Stable Disease 8 (25.0) 13 (41.9) Progressive Disease 1 (3.1) 3 (9.7) Not Evaluable 5 (15.6) 4 (12.9) Confirmed Partial Response 16 (50) 10 (32.3) 95% Cl 31.9%, 68.1% 16.7%, 51.4% Disposition Discontinued Prior to end of Treatment Phase (4.5 months) 15 (46.8) 12 (38.7) Median Time to Progression 8.6 months 9.0 months Garon EB et al. Presented at: ASCO 2010 Annual Meeting; 2010 June 4-8; Chicago, IL. NSCLC (FALCON): Clinical Summary CA4P + Chemo + Bev
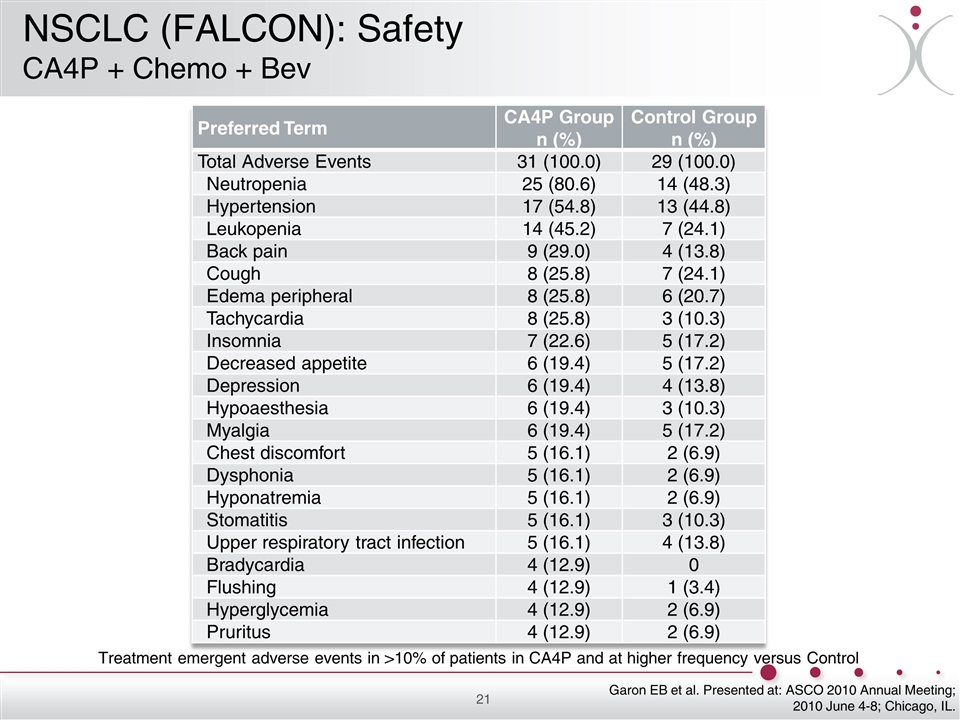
Preferred Term CA4P Group n (%) Control Group n (%) Total Adverse Events 31 (100.0) 29 (100.0) Neutropenia 25 (80.6) 14 (48.3) Hypertension 17 (54.8) 13 (44.8) Leukopenia 14 (45.2) 7 (24.1) Back pain 9 (29.0) 4 (13.8) Cough 8 (25.8) 7 (24.1) Edema peripheral 8 (25.8) 6 (20.7) Tachycardia 8 (25.8) 3 (10.3) Insomnia 7 (22.6) 5 (17.2) Decreased appetite 6 (19.4) 5 (17.2) Depression 6 (19.4) 4 (13.8) Hypoaesthesia 6 (19.4) 3 (10.3) Myalgia 6 (19.4) 5 (17.2) Chest discomfort 5 (16.1) 2 (6.9) Dysphonia 5 (16.1) 2 (6.9) Hyponatremia 5 (16.1) 2 (6.9) Stomatitis 5 (16.1) 3 (10.3) Upper respiratory tract infection 5 (16.1) 4 (13.8) Bradycardia 4 (12.9) 0 Flushing 4 (12.9) 1 (3.4) Hyperglycemia 4 (12.9) 2 (6.9) Pruritus 4 (12.9) 2 (6.9) Treatment emergent adverse events in >10% of patients in CA4P and at higher frequency versus Control Garon EB et al. Presented at: ASCO 2010 Annual Meeting; 2010 June 4-8; Chicago, IL. NSCLC (FALCON): Safety CA4P + Chemo + Bev

Recurrent Ovarian Cancer (GOG-0186I)

CA4P combined with bevacizumab Bevacizumab (Avastin) 15 mg/kg IV q3 wks (n=54) Bevacizumab 15 mg/kg+CA4P 60 mg/m2 IV q3 wks (n=53) Patient Population: 2nd- and 3rd-line ovarian cancer Both platinum-sensitive and platinum-resistant Primary endpoint: PFS n=107 (67 sites); randomized controlled study Ongoing follow-up for overall survival Monk BJ. Presented at: The 15th Biennial Meeting of the International Gynecologic Cancer Society; 2014 Nov 8-11; Melbourne, Australia. Phase 2 Study in Recurrent Ovarian Cancer Study GOG-0186I
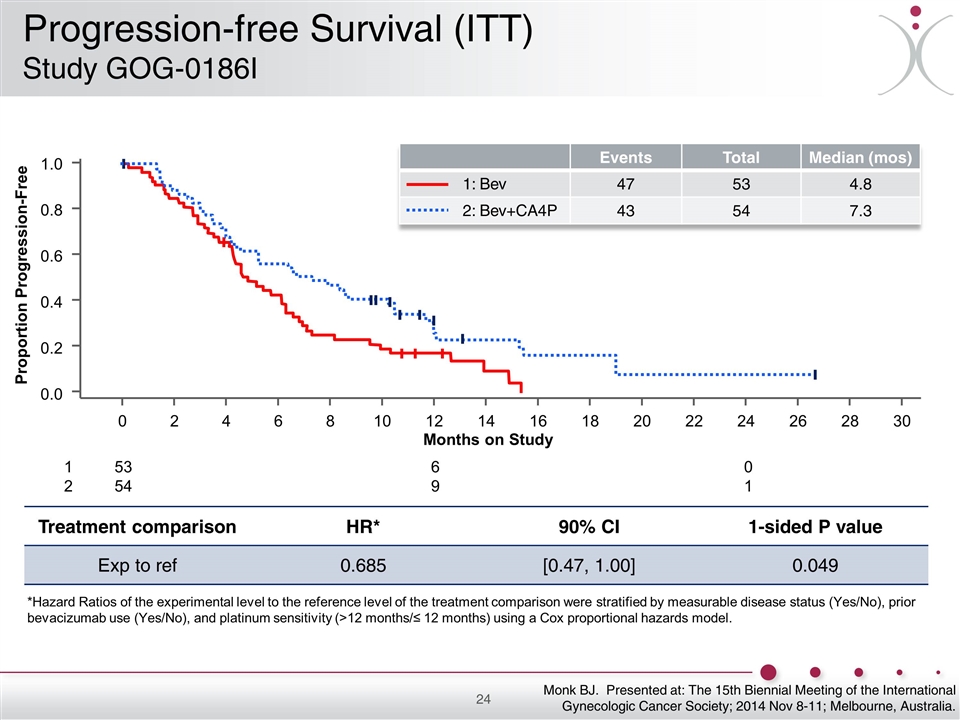
Treatment comparison HR* 90% CI 1-sided P value Exp to ref 0.685 [0.47, 1.00] 0.049 *Hazard Ratios of the experimental level to the reference level of the treatment comparison were stratified by measurable disease status (Yes/No), prior bevacizumab use (Yes/No), and platinum sensitivity (>12 months/≤ 12 months) using a Cox proportional hazards model. 53 54 1 2 6 9 0 1 Events Total Median (mos) 47 53 4.8 43 54 7.3 1: Bev 2: Bev+CA4P Months on Study 1.0 0.8 0.6 0.4 0.2 0.0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 Proportion Progression-Free Monk BJ. Presented at: The 15th Biennial Meeting of the International Gynecologic Cancer Society; 2014 Nov 8-11; Melbourne, Australia. Progression-free Survival (ITT) Study GOG-0186I
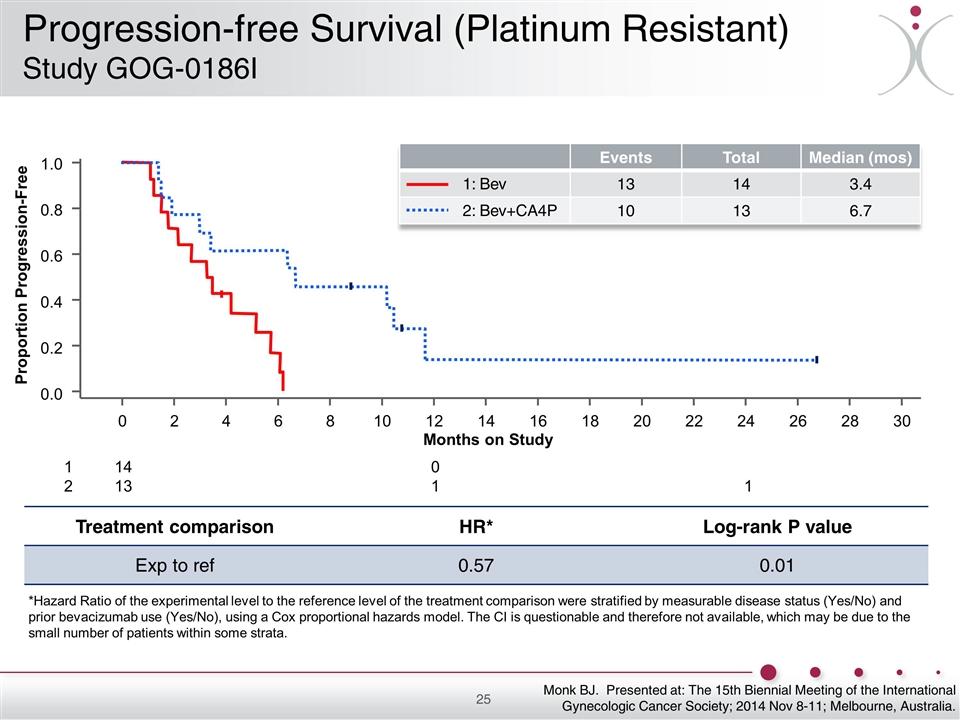
Treatment comparison HR* Log-rank P value Exp to ref 0.57 0.01 *Hazard Ratio of the experimental level to the reference level of the treatment comparison were stratified by measurable disease status (Yes/No) and prior bevacizumab use (Yes/No), using a Cox proportional hazards model. The CI is questionable and therefore not available, which may be due to the small number of patients within some strata. 14 13 1 2 0 1 1 Months on Study 1.0 0.8 0.6 0.4 0.2 0.0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 Proportion Progression-Free Monk BJ. Presented at: The 15th Biennial Meeting of the International Gynecologic Cancer Society; 2014 Nov 8-11; Melbourne, Australia. Events Total Median (mos) 13 14 3.4 10 13 6.7 1: Bev 2: Bev+CA4P Progression-free Survival (Platinum Resistant) Study GOG-0186I
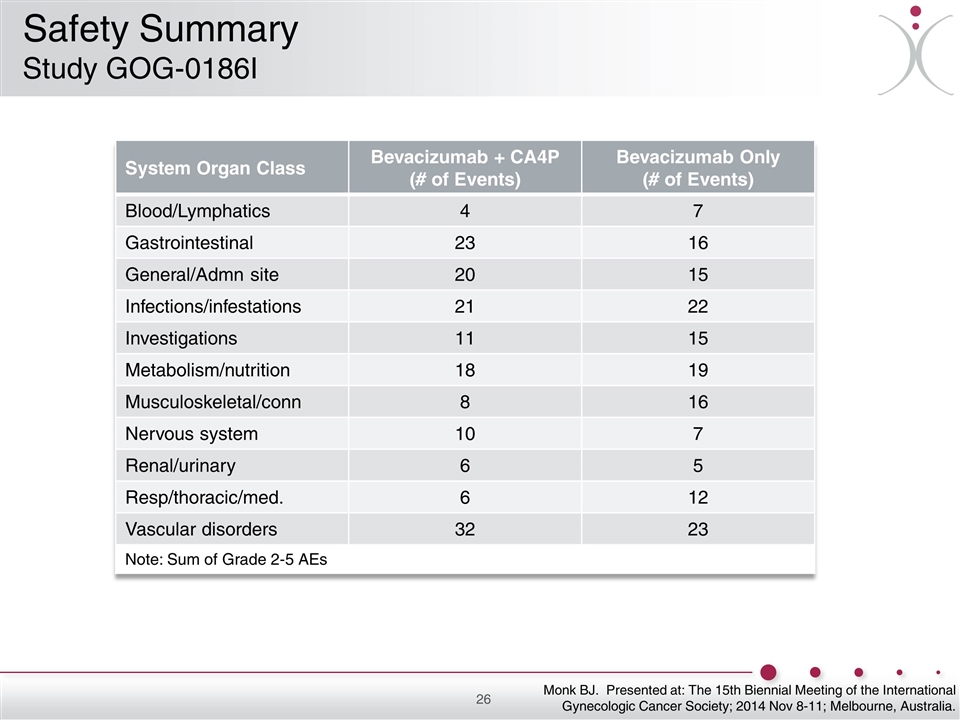
System Organ Class Bevacizumab + CA4P (# of Events) Bevacizumab Only (# of Events) Blood/Lymphatics 4 7 Gastrointestinal 23 16 General/Admn site 20 15 Infections/infestations 21 22 Investigations 11 15 Metabolism/nutrition 18 19 Musculoskeletal/conn 8 16 Nervous system 10 7 Renal/urinary 6 5 Resp/thoracic/med. 6 12 Vascular disorders 32 23 Note: Sum of Grade 2-5 AEs Monk BJ. Presented at: The 15th Biennial Meeting of the International Gynecologic Cancer Society; 2014 Nov 8-11; Melbourne, Australia. Safety Summary Study GOG-0186I
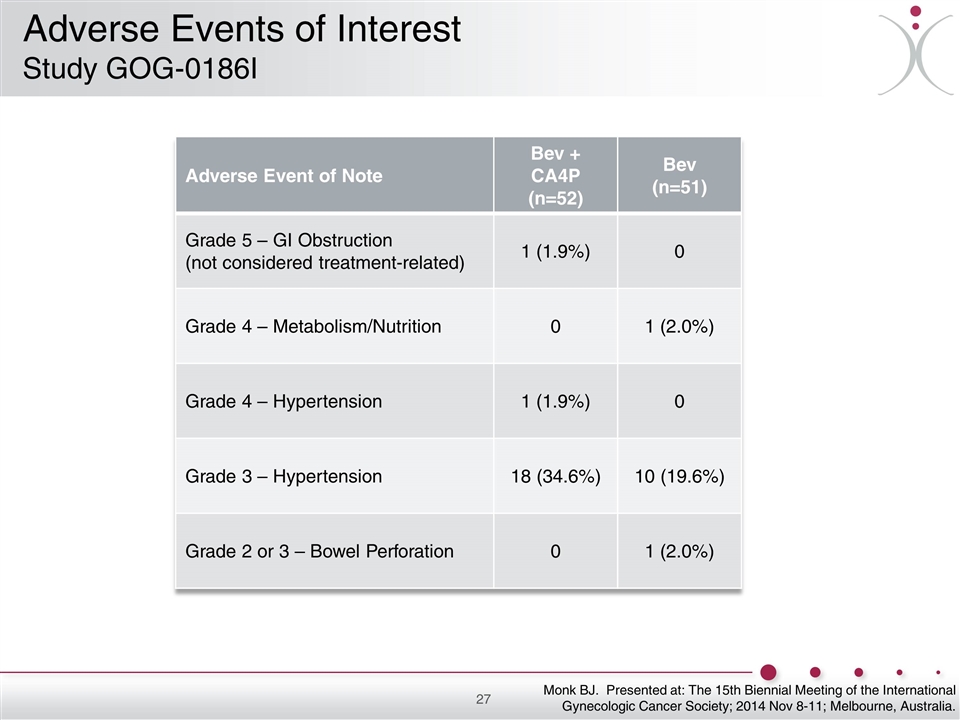
Adverse Event of Note Bev + CA4P (n=52) Bev (n=51) Grade 5 – GI Obstruction (not considered treatment-related) 1 (1.9%) 0 Grade 4 – Metabolism/Nutrition 0 1 (2.0%) Grade 4 – Hypertension 1 (1.9%) 0 Grade 3 – Hypertension 18 (34.6%) 10 (19.6%) Grade 2 or 3 – Bowel Perforation 0 1 (2.0%) Monk BJ. Presented at: The 15th Biennial Meeting of the International Gynecologic Cancer Society; 2014 Nov 8-11; Melbourne, Australia. Adverse Events of Interest Study GOG-0186I
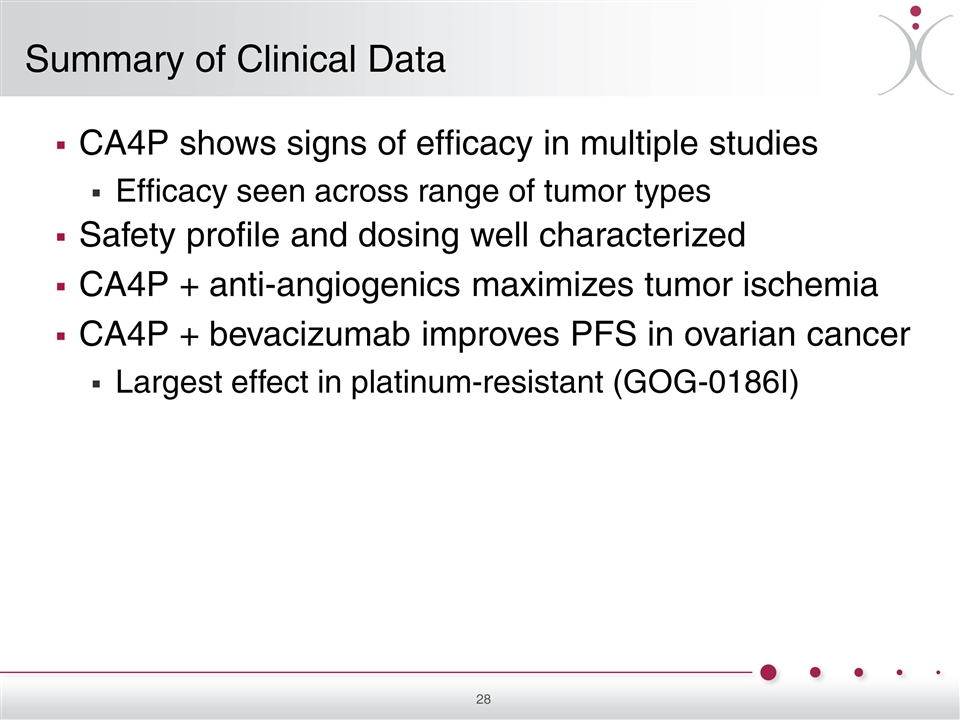
CA4P shows signs of efficacy in multiple studies Efficacy seen across range of tumor types Safety profile and dosing well characterized CA4P + anti-angiogenics maximizes tumor ischemia CA4P + bevacizumab improves PFS in ovarian cancer Largest effect in platinum-resistant (GOG-0186I) Summary of Clinical Data
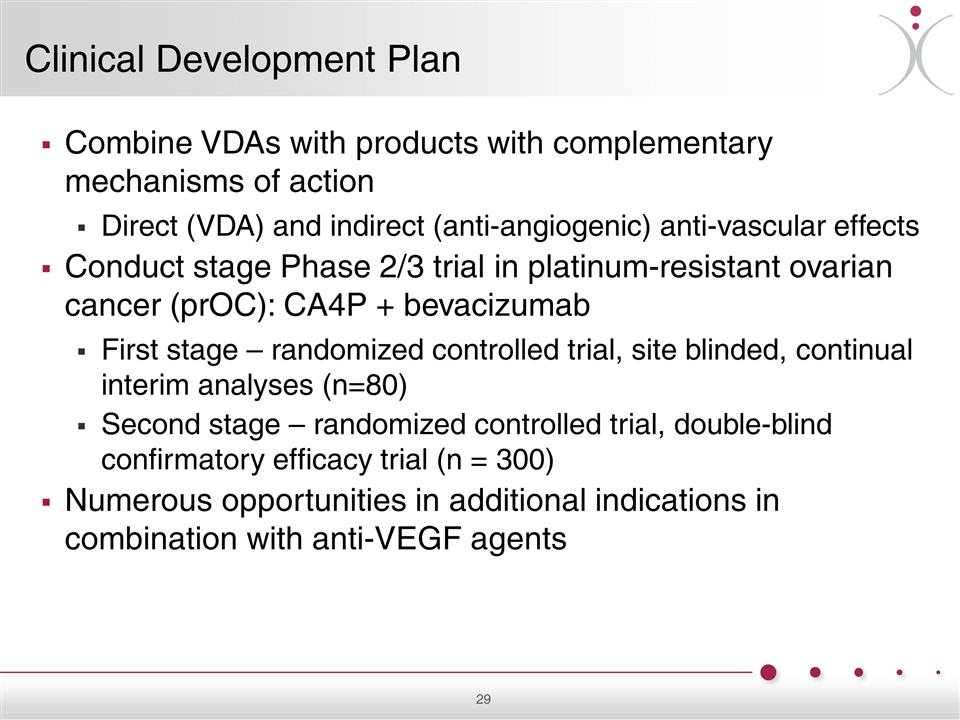
Combine VDAs with products with complementary mechanisms of action Direct (VDA) and indirect (anti-angiogenic) anti-vascular effects Conduct stage Phase 2/3 trial in platinum-resistant ovarian cancer (prOC): CA4P + bevacizumab First stage – randomized controlled trial, site blinded, continual interim analyses (n=80) Second stage – randomized controlled trial, double-blind confirmatory efficacy trial (n = 300) Numerous opportunities in additional indications in combination with anti-VEGF agents Clinical Development Plan
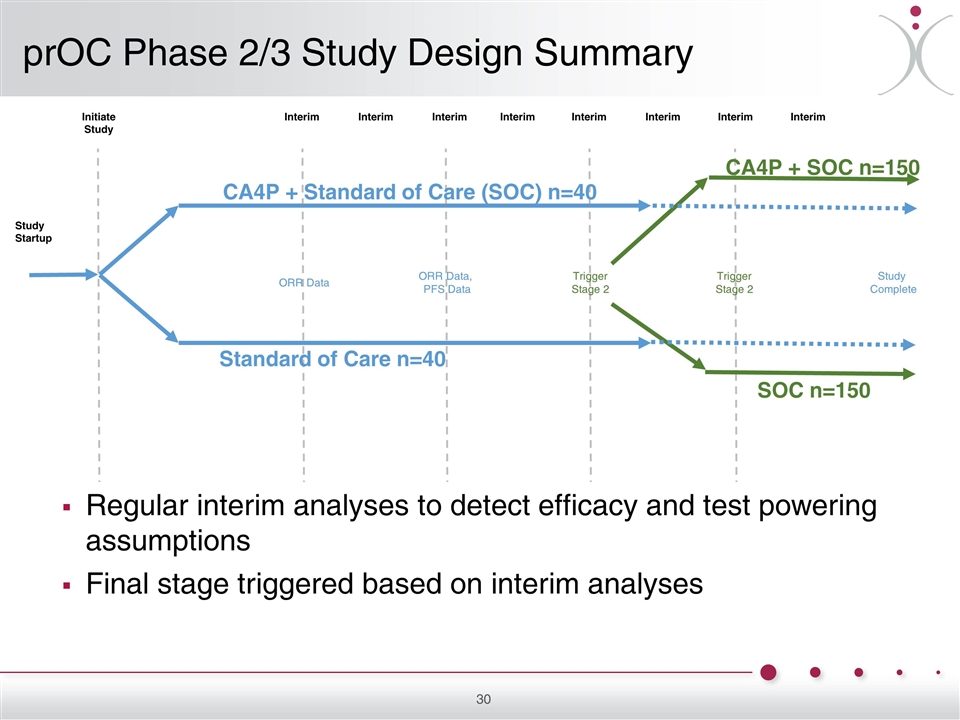
Initiate Study Interim Interim Interim Interim Interim Interim Interim Interim Study Startup Standard of Care n=40 CA4P + Standard of Care (SOC) n=40 Study Complete CA4P + SOC n=150 SOC n=150 ORR Data, PFS Data ORR Data Trigger Stage 2 Trigger Stage 2 Regular interim analyses to detect efficacy and test powering assumptions Final stage triggered based on interim analyses prOC Phase 2/3 Study Design Summary

OXi4503
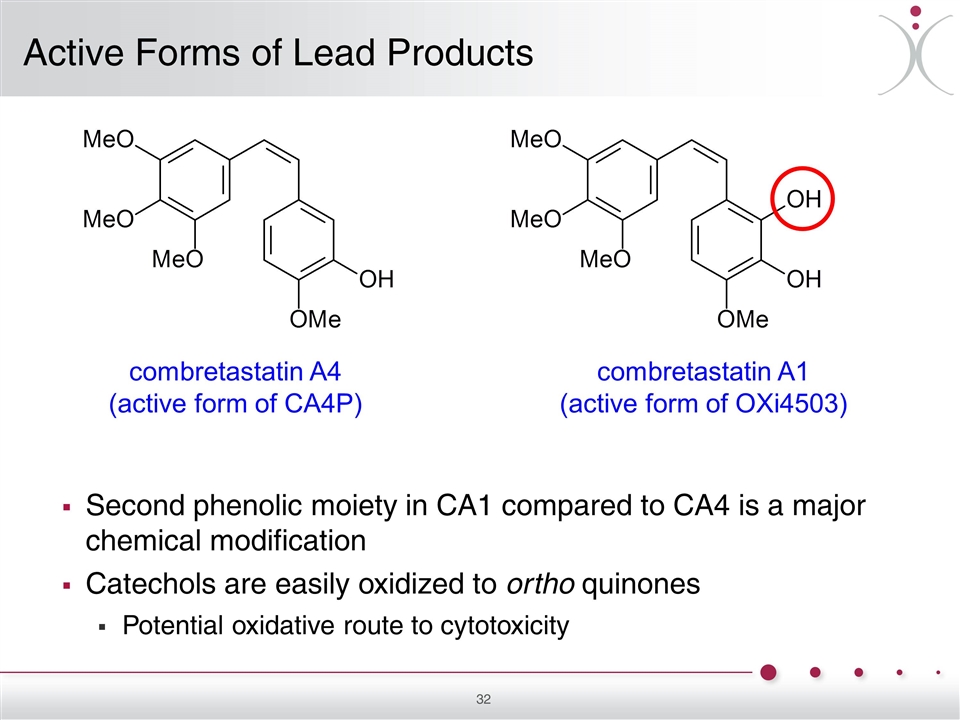
combretastatin A4 (active form of CA4P) combretastatin A1 (active form of OXi4503) Active Forms of Lead Products Second phenolic moiety in CA1 compared to CA4 is a major chemical modification Catechols are easily oxidized to ortho quinones Potential oxidative route to cytotoxicity
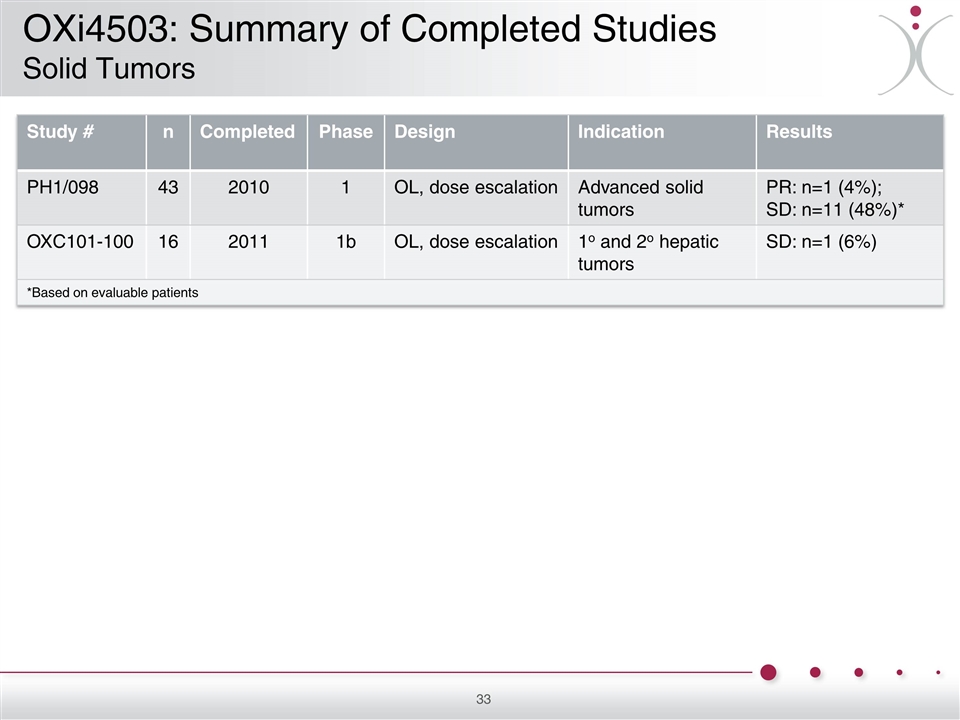
Study # n Completed Phase Design Indication Results PH1/098 43 2010 1 OL, dose escalation Advanced solid tumors PR: n=1 (4%); SD: n=11 (48%)* OXC101-100 16 2011 1b OL, dose escalation 1o and 2o hepatic tumors SD: n=1 (6%) *Based on evaluable patients OXi4503: Summary of Completed Studies Solid Tumors
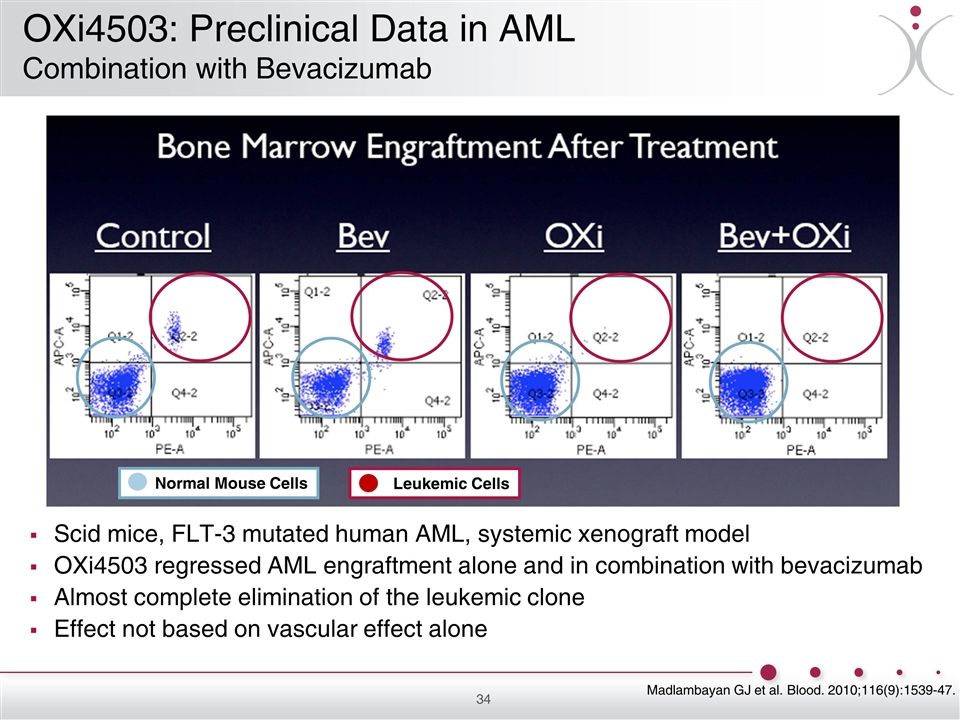
Scid mice, FLT-3 mutated human AML, systemic xenograft model OXi4503 regressed AML engraftment alone and in combination with bevacizumab Almost complete elimination of the leukemic clone Effect not based on vascular effect alone Normal Mouse Cells Leukemic Cells Madlambayan GJ et al. Blood. 2010;116(9):1539-47. OXi4503: Preclinical Data in AML Combination with Bevacizumab
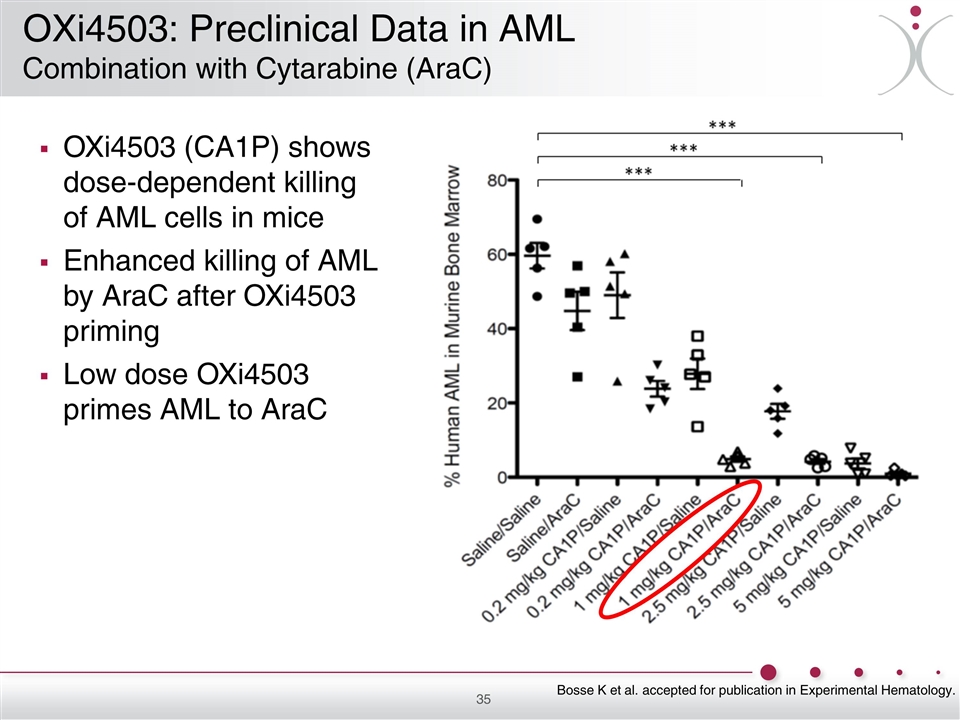
OXi4503 (CA1P) shows dose-dependent killing of AML cells in mice Enhanced killing of AML by AraC after OXi4503 priming Low dose OXi4503 primes AML to AraC Bosse K et al. accepted for publication in Experimental Hematology. OXi4503: Preclinical Data in AML Combination with Cytarabine (AraC)
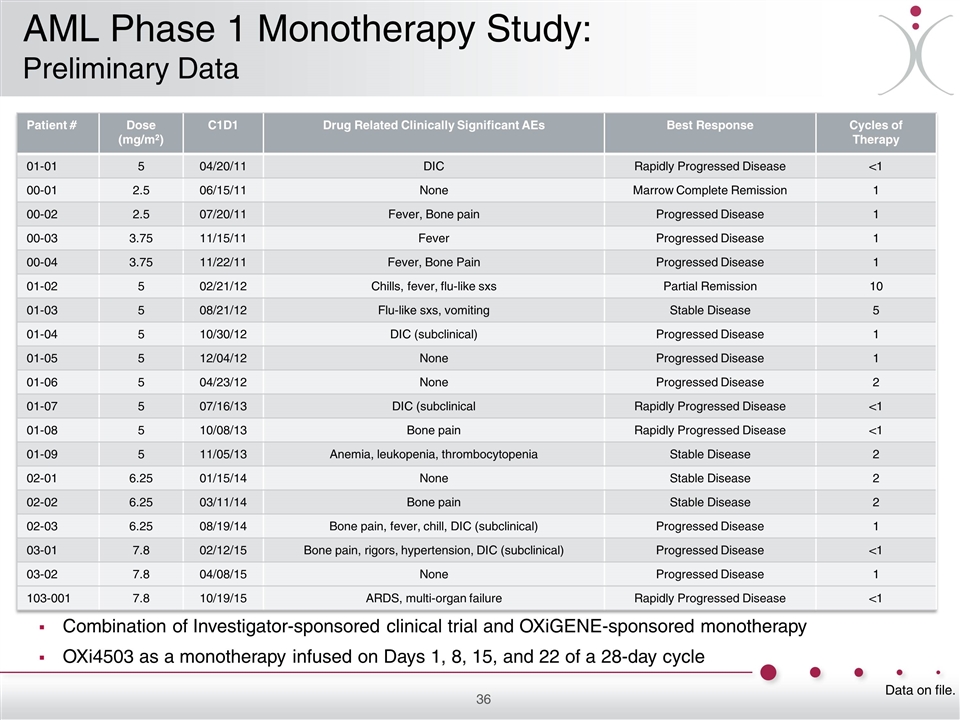
Combination of Investigator-sponsored clinical trial and OXiGENE-sponsored monotherapy OXi4503 as a monotherapy infused on Days 1, 8, 15, and 22 of a 28-day cycle Patient # Dose (mg/m2) C1D1 Drug Related Clinically Significant AEs Best Response Cycles of Therapy 01-01 5 04/20/11 DIC Rapidly Progressed Disease <1 00-01 2.5 06/15/11 None Marrow Complete Remission 1 00-02 2.5 07/20/11 Fever, Bone pain Progressed Disease 1 00-03 3.75 11/15/11 Fever Progressed Disease 1 00-04 3.75 11/22/11 Fever, Bone Pain Progressed Disease 1 01-02 5 02/21/12 Chills, fever, flu-like sxs Partial Remission 10 01-03 5 08/21/12 Flu-like sxs, vomiting Stable Disease 5 01-04 5 10/30/12 DIC (subclinical) Progressed Disease 1 01-05 5 12/04/12 None Progressed Disease 1 01-06 5 04/23/12 None Progressed Disease 2 01-07 5 07/16/13 DIC (subclinical Rapidly Progressed Disease <1 01-08 5 10/08/13 Bone pain Rapidly Progressed Disease <1 01-09 5 11/05/13 Anemia, leukopenia, thrombocytopenia Stable Disease 2 02-01 6.25 01/15/14 None Stable Disease 2 02-02 6.25 03/11/14 Bone pain Stable Disease 2 02-03 6.25 08/19/14 Bone pain, fever, chill, DIC (subclinical) Progressed Disease 1 03-01 7.8 02/12/15 Bone pain, rigors, hypertension, DIC (subclinical) Progressed Disease <1 03-02 7.8 04/08/15 None Progressed Disease 1 103-001 7.8 10/19/15 ARDS, multi-organ failure Rapidly Progressed Disease <1 Data on file. AML Phase 1 Monotherapy Study: Preliminary Data
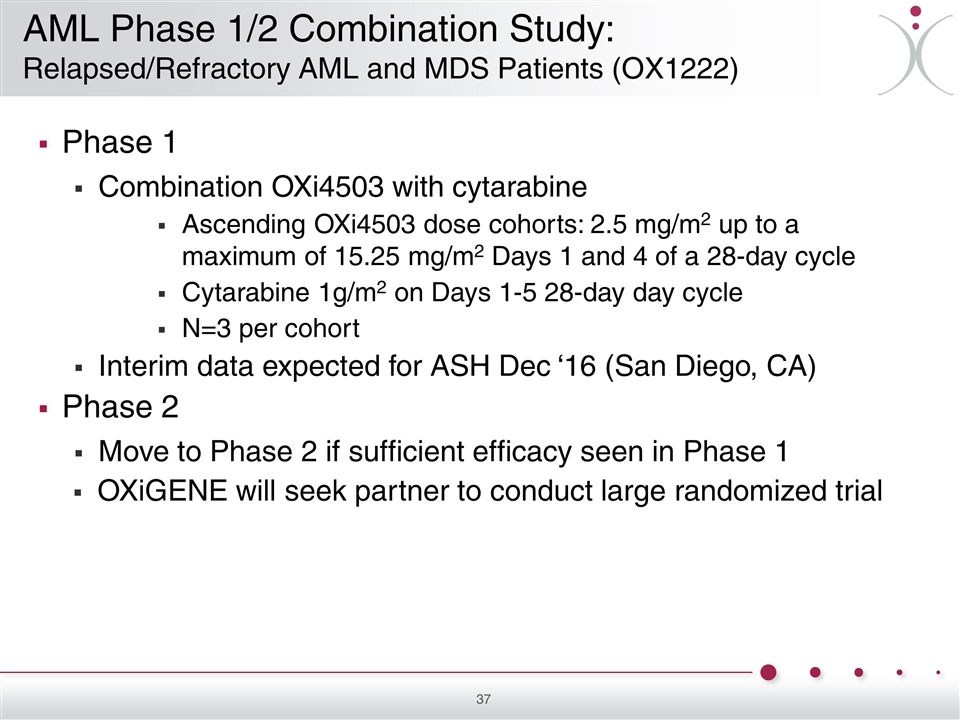
Phase 1 Combination OXi4503 with cytarabine Ascending OXi4503 dose cohorts: 2.5 mg/m2 up to a maximum of 15.25 mg/m2 Days 1 and 4 of a 28-day cycle Cytarabine 1g/m2 on Days 1-5 28-day day cycle N=3 per cohort Interim data expected for ASH Dec ‘16 (San Diego, CA) Phase 2 Move to Phase 2 if sufficient efficacy seen in Phase 1 OXiGENE will seek partner to conduct large randomized trial AML Phase 1/2 Combination Study: Relapsed/Refractory AML and MDS Patients (OX1222)

Stock listed on Nasdaq Capital Market: OXGN $27 million cash at 31Dec2015 Sufficient to get to meaningful clinical data 2H2017 prOC trial interim analysis AML Phase 1 combination trial completed No preferred stock or debt outstanding Approximately 26.5 million shares outstanding Additional 11.7 million warrants and options outstanding at average exercise price of $2.51 Finances
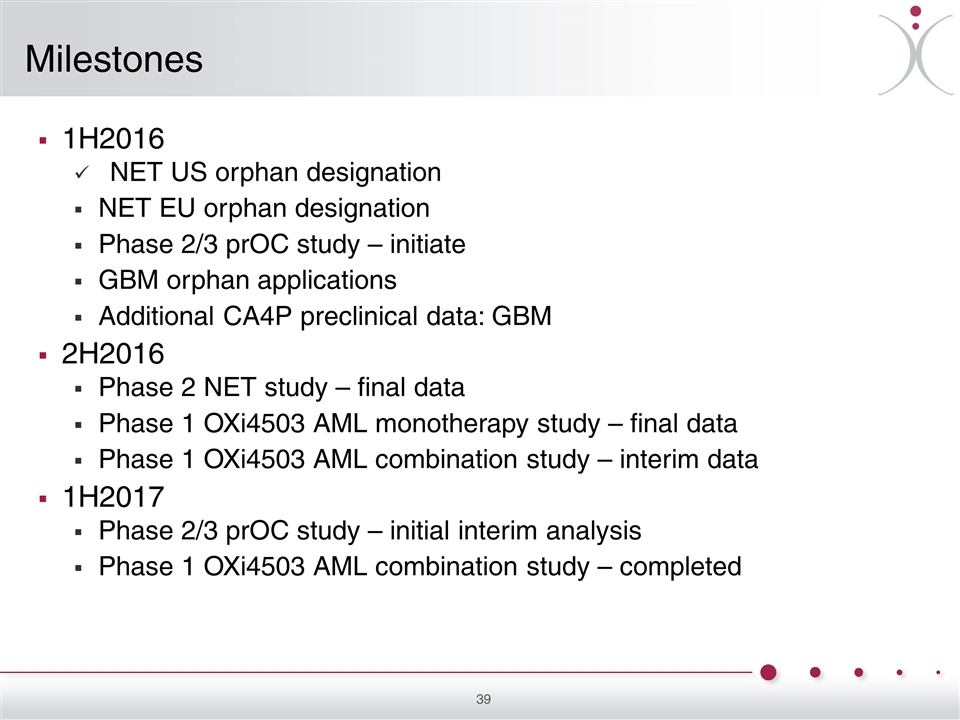
1H2016 NET US orphan designation NET EU orphan designation Phase 2/3 prOC study – initiate GBM orphan applications Additional CA4P preclinical data: GBM 2H2016 Phase 2 NET study – final data Phase 1 OXi4503 AML monotherapy study – final data Phase 1 OXi4503 AML combination study – interim data 1H2017 Phase 2/3 prOC study – initial interim analysis Phase 1 OXi4503 AML combination study – completed Milestones
Summary Orphan oncology focused biopharmaceutical company Treated over 400 patients with CA4P Greatest activity in combination with anti-angiogenics New management team with a targeted clinical development strategy Concentration in prOC and AML Resources fund trials through expected meaningful data Multiple attractive indications can be pursued after increasing shareholder value (GBM, NETs)

www.oxigene.com 650-635-7000
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Granite Creek Announces Investment in The District Communications Group (DCG)
- Pixalate Releases Top 100 Connected TV (CTV) Bundle ID Rankings For Global Open Programmatic Advertising on Roku, Amazon Fire TV, Samsung Smart TV, and Apple TV in March 2024
- Smart Lock Market to Cross USD 9.63 Billion by 2031 Driven by Growing Demand for Convenience and Advanced Security
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share