Form 8-K MYRIAD GENETICS INC For: May 31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): May 31, 2016
MYRIAD GENETICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 0-26642 | 87-0494517 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
320 Wakara Way
Salt Lake City, Utah 84108
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (801) 584-3600
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| ITEM 7.01 | Regulation FD Disclosure. |
On May 31 2016, Myriad Genetics, Inc. (“Myriad” or the “Company”) issued a press release relating to the Company’s acquisition of Sividon Diagnostics GmbH. On its conference call announcing the acquisition Myriad also delivered a slide presentation. A copy of the press release and slide presentation are furnished as Exhibit 99.1 and Exhibit 99.2 to this Current Report on Form 8-K and incorporated herein by reference. The press release and slide presentation will also be available under the “Investors –Events & Presentations” section of Myriad’s website at www.myriad.com.
FORWARD-LOOKING STATEMENTS
Exhibits 99.1 and 99.2 may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements relating to our business, goals, strategy and financial and operational outlook. These “forward-looking statements” are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by forward-looking statements. These risks and uncertainties include, but are not limited to: the risk that sales and profit margins of our existing molecular diagnostic tests and pharmaceutical and clinical services may decline or will not continue to increase at historical rates; risks related to our ability to transition from our existing product portfolio to our new tests; risks related to changes in the governmental or private insurers reimbursement levels for our tests or our ability to obtain reimbursement for our new tests at comparable levels to our existing tests; risks related to increased competition and the development of new competing tests and services; the risk that we may be unable to develop or achieve commercial success for additional molecular diagnostic tests and pharmaceutical and clinical services in a timely manner, or at all; the risk that we may not successfully develop new markets for our molecular diagnostic tests and pharmaceutical and clinical services, including our ability to successfully generate revenue outside the United States; the risk that licenses to the technology underlying our molecular diagnostic tests and pharmaceutical and clinical services tests and any future tests are terminated or cannot be maintained on satisfactory terms; risks related to delays or other problems with operating our laboratory testing facilities; risks related to public concern over our genetic testing in general or our tests in particular; risks related to regulatory requirements or enforcement in the United States and foreign countries and changes in the structure of the healthcare system or healthcare payment systems; risks related to our ability to obtain new corporate collaborations or licenses and acquire new technologies or businesses on satisfactory terms, if at all; risks related to our ability to successfully integrate and derive benefits from any technologies or businesses that we license or acquire; risks related to our projections about the potential market opportunity for our products; the risk that we or our licensors may be unable to protect or that third parties will infringe the proprietary technologies underlying our tests; the risk of patent-infringement claims or challenges to the validity of our patents; risks related to changes in intellectual property laws covering our molecular diagnostic tests and pharmaceutical and clinical services and patents or enforcement in the United States and foreign countries, such as the Supreme Court decision in the lawsuit brought against us by the Association for Molecular Pathology et al; risks of new, changing and competitive technologies and regulations in the United States and internationally; and other factors discussed under the heading “Risk Factors” contained in Item 1A of our most recent Annual Report on Form 10-K, which has been filed with the Securities and Exchange Commission, as well as any updates to those risk factors filed from time to time in our Quarterly Reports on Form 10-Q or Current Reports on Form 8-K. All information in the exhibits is as of the date of the exhibits, and Myriad undertakes no duty to update this information unless required by law.
Page 2
| ITEM 9.01 | Financial Statements and Exhibits. |
(d)
| Exhibit |
Description | |
| 99.1 | Press release dated May 31, 2016. | |
| 99.2 | Investor slide presentation dated May 31, 2016. | |
The exhibit(s) may contain hypertext links to information on our website or other parties’ websites. The information on our website and other parties’ websites is not incorporated by reference into this Current Report on Form 8-K and does not constitute a part of this Form 8-K.
In accordance with General Instruction B-2 of Form 8-K, the information set forth in Item 7.01 and in Exhibits 99.1 and 99.2 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section, and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Page 3
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| MYRIAD GENETICS, INC. | ||||||
| Date: May 31, 2016 | By: | /s/ R. Bryan Riggsbee | ||||
| R. Bryan Riggsbee | ||||||
| Executive Vice President, Chief Financial Officer | ||||||
Page 4
EXHIBIT INDEX
| Exhibit |
Description | |
| 99.1 | Press release dated May 31, 2016. | |
| 99.2 | Slide presentation dated May 31, 2016. | |
Page 5
Exhibit 99.1

|
News Release |
| Media Contact: | Ron Rogers | Investor Contact: | Scott Gleason | |||
| (908) 285-0248 | (801) 584-1143 | |||||
| [email protected] | [email protected] |
Myriad Acquires Sividon Diagnostics
Strengthens Market Leading Oncology Product Portfolio with Breast Cancer Prognostic Test EndoPredict®
SALT LAKE CITY, Utah, May 31, 2016 – Myriad Genetics, Inc. (NASDAQ: MYGN), a leader in molecular diagnostics and personalized medicine, today announced that it has acquired Sividon Diagnostics, a leading breast cancer prognostic company, for €35 million upfront with the potential for €15 million in additional performance-based milestones. The transaction closed on May 31, 2016. Myriad expects the deal to be neutral to both revenue and earnings in fiscal year 2017. A discussion on the details and strategy underlying the transaction will be provided on a conference call today at 4:30 pm EST.
“Sividon brings to Myriad the best-in-class breast cancer prognostic test and strengthens our market leading oncology portfolio of high value personalized medicine products,” said Mark C. Capone, president and CEO, Myriad Genetic Laboratories. “The EndoPredict® test will be the foundational product of our newly initiated kit-based strategy and allow Myriad to leverage its global oncology distribution to bring this important test to patients worldwide.”
“We are excited to be integrated with the global leader and pioneer in personalized medicine,” said Christoph Petry, CEO of Sividon Diagnostics. “Myriad has the reimbursement, regulatory, and commercial expertise to make this product very successful especially as we seek distribution in the United States and broader reimbursement coverage in Europe.”
Sividon Diagnostics was spun out of Siemens Healthcare Diagnostics in 2010 as part of a management buyout. Their core EndoPredict product is a kit based RNA expression test that evaluates 12 genes to assess the aggressiveness of breast cancer on a molecular level. The test is currently CE Marked on the Siemens Versant instrument, however, Myriad is transitioning the product to the Thermo Fisher QuantStudio platform as a key step in the international kit strategy. EndoPredict has been evaluated in 5 major studies incorporating more than 4,000 patients, utilized on a clinical basis in over 13,000 patients worldwide, and is extensively referenced in clinical guidelines across the globe. In a head-to head study, it has been shown to outperform the prognostic ability of the leading first generation test while providing definitive answers with no intermediate results.
Benefits of the Transaction
| • | Synergistic Product Within the 4in6 Strategy: EndoPredict evaluates the aggressiveness of breast cancer to help patients decide whether to safely forgo chemotherapy and will be added into our existing oncology commercial channel, creating significant opportunities for operating leverage. |
| • | Substantial Market Opportunity: Myriad believes the global market opportunity for EndoPredict is greater than $600 million with the majority of that market existing in major European countries, Canada, and the United States. We estimate that this market is less than 25 percent penetrated on a global basis and EndoPredict should benefit from a significant expansion in reimbursement in the coming years. |
| • | Best-in-Class Product: EndoPredict has been studied in approximately 4,000 patients and utilized in over 13,000 patients, and has consistently demonstrated the best ability to predict which patients are at low risk for distant metastases in both node negative and node positive patients. Additionally, the kit based format provides unique advantages in the marketplace and EndoPredict will be the foundational product in Myriad’s global kit based strategy. |
| • | Broadens Comprehensive Product Offering in Oncology: Myriad currently sells market leading tests in oncology for hereditary cancer and companion diagnostics. EndoPredict answers another important clinical question for breast cancer patients by identifying which can safely forgo chemotherapy. Oncology customers can increasingly rely on Myriad as a single source trusted advisor answering questions across the entire continuum of care with unmatched quality. |
Financing
Myriad intends to fund the transaction entirely through cash on hand. At the end of the fiscal third quarter Myriad had cash and cash equivalents of $286 million on hand.
Conference Call and Webcast
A conference call will be held today, Tuesday, May 31, 2016, at 4:30 p.m. EST to discuss Myriad’s acquisition of Sividon Diagnostics. The dial-in number for domestic callers is 1-888-224-7964. International callers may dial 1-303-223-4373. All callers will be asked to reference reservation number 21812274. An archived replay of the call will be available for seven days by dialing 1-800-633-8284 and entering the reservation number above. The conference call along with a slide presentation will also will be available through a live webcast at www.myriad.com.
2
About Myriad Genetics
Myriad Genetics Inc., is a leading personalized medicine company dedicated to being a trusted advisor transforming patient lives worldwide with pioneering molecular diagnostics. Myriad discovers and commercializes molecular diagnostic tests that: determine the risk of developing disease, accurately diagnose disease, assess the risk of disease progression, and guide treatment decisions across six major medical specialties where molecular diagnostics can significantly improve patient care and lower healthcare costs. Myriad is focused on three strategic imperatives: transitioning and expanding its hereditary cancer testing markets, diversifying its product portfolio through the introduction of new products and increasing the revenue contribution from international markets. For more information on how Myriad is making a difference, please visit the Company’s website: www.myriad.com.
Myriad, the Myriad logo, BART, BRACAnalysis, Colaris, Colaris AP, myPath, myRisk, Myriad myRisk, myRisk Hereditary Cancer, myChoice, myPlan, BRACAnalysis CDx, Tumor BRACAnalysis CDx, myChoice HRD, Vectra and Prolaris are trademarks or registered trademarks of Myriad Genetics, Inc. or its wholly owned subsidiaries in the United States and foreign countries. MYGN-F, MYGN-G
Safe Harbor Statement
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements relating to the acquisition of Sividon Diagnostics for €35 million upfront with the potential for €15 million in additional performance-based milestones; the Company’s expectation that the deal to be neutral to both revenue and earnings in fiscal year 2017; the EndoPredict test being the foundational product of the Company’s newly initiated kit-based strategy and allowing the Company to leverage its global oncology distribution to bring this important test to patients worldwide; the transitioning of the EndoPredict test to the Thermo Fisher QuantStudio platform as a key step in the international kit strategy; the EndoPredict test’s outperformance in its prognostic ability in a head-to head study; the Company’s estimate that the EndoPredict test market is less than 25 percent penetrated on a global basis and the EndoPredict test should benefit from a significant expansion in reimbursement in the coming years; the EndoPredict test being the foundational product in Myriad’s global kit based strategy; and the Company’s strategic directives under the caption “About Myriad Genetics.” These “forward-looking statements” are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those described or implied in the forward-looking statements. These risks include, but are not limited to: the risk that
3
sales and profit margins of our existing molecular diagnostic tests and pharmaceutical and clinical services may decline or will not continue to increase at historical rates; risks related to our ability to transition from our existing product portfolio to our new tests; risks related to changes in the governmental or private insurers’ reimbursement levels for our tests or our ability to obtain reimbursement for our new tests at comparable levels to our existing tests; risks related to increased competition and the development of new competing tests and services; the risk that we may be unable to develop or achieve commercial success for additional molecular diagnostic tests and pharmaceutical and clinical services in a timely manner, or at all; the risk that we may not successfully develop new markets for our molecular diagnostic tests and pharmaceutical and clinical services, including our ability to successfully generate revenue outside the United States; the risk that licenses to the technology underlying our molecular diagnostic tests and pharmaceutical and clinical services tests and any future tests are terminated or cannot be maintained on satisfactory terms; risks related to delays or other problems with operating our laboratory testing facilities; risks related to public concern over our genetic testing in general or our tests in particular; risks related to regulatory requirements or enforcement in the United States and foreign countries and changes in the structure of the healthcare system or healthcare payment systems; risks related to our ability to obtain new corporate collaborations or licenses and acquire new technologies or businesses on satisfactory terms, if at all; risks related to our ability to successfully integrate and derive benefits from any technologies or businesses that we license or acquire, including but not limited to our acquisition of a healthcare clinic in Germany; risks related to our projections about the potential market opportunity for our products; the risk that we or our licensors may be unable to protect or that third parties will infringe the proprietary technologies underlying our tests; the risk of patent-infringement claims or challenges to the validity of our patents; risks related to changes in intellectual property laws covering our molecular diagnostic tests and pharmaceutical and clinical services and patents or enforcement in the United States and foreign countries, such as the Supreme Court decision in the lawsuit brought against us by the Association for Molecular Pathology et al; risks of new, changing and competitive technologies and regulations in the United States and internationally; and other factors discussed under the heading “Risk Factors” contained in Item 1A of our Annual report on Form 10-K for the fiscal year ended June 30, 2016, which has been filed with the Securities and Exchange Commission, as well as any updates to those risk factors filed from time to time in our Quarterly Reports on Form 10-Q or Current Reports on Form 8-K.
###
4
Myriad Genetics Acquisition of Sividon Diagnostics 05/31/2016 Exhibit 99.2

Forward Looking Statements Some of the information presented here today may contain projections or other forward-looking statements regarding future events or the future financial performance of the Company. These statements are based on management’s current expectations and the actual events or results may differ materially and adversely from these expectations. We refer you to the documents the Company files from time to time with the Securities and Exchange Commission, specifically, the Company’s annual reports on Form 10-K, its quarterly reports on Form 10-Q, and its current reports on Form 8-K. These documents identify important risk factors that could cause the actual results to differ materially from those contained in the Company’s projections or forward-looking statements.

Myriad Genetics Acquisition of Sividon Diagnostics Consideration For Sividon Shareholders Acquired Sividon for €35M upfront and €15M in performance based milestones FY17 EndoPredict revenues estimated to be $8M (not including U.S. revenue) Funding deal using cash on hand Closed May 31, 2016 Myriad Financial Considerations Neutral to FY17 sales and EPS (excludes potential impact of U.S. revenue) Accretive revenue and adjusted EPS in FY18 and beyond Expected to be one of seven products generating >$50M in sales by FY20 Long term gross margin profile expected to be >80% Strategic Rationale Synergistic product within 4in6 strategy Substantial market opportunity Best in class product Broadens comprehensive product offering in oncology European kit manufacturer of high value diagnostics Core product is EndoPredict a breast cancer prognostic test Brings to Myriad substantial kit manufacturing and development expertise Myriad is the current exclusive distributor of EndoPredict in all countries except the U.S. and China
Strategic Rationale Substantial market opportunity Broadens comprehensive product offering in oncology Best in class product Synergistic product within strategy
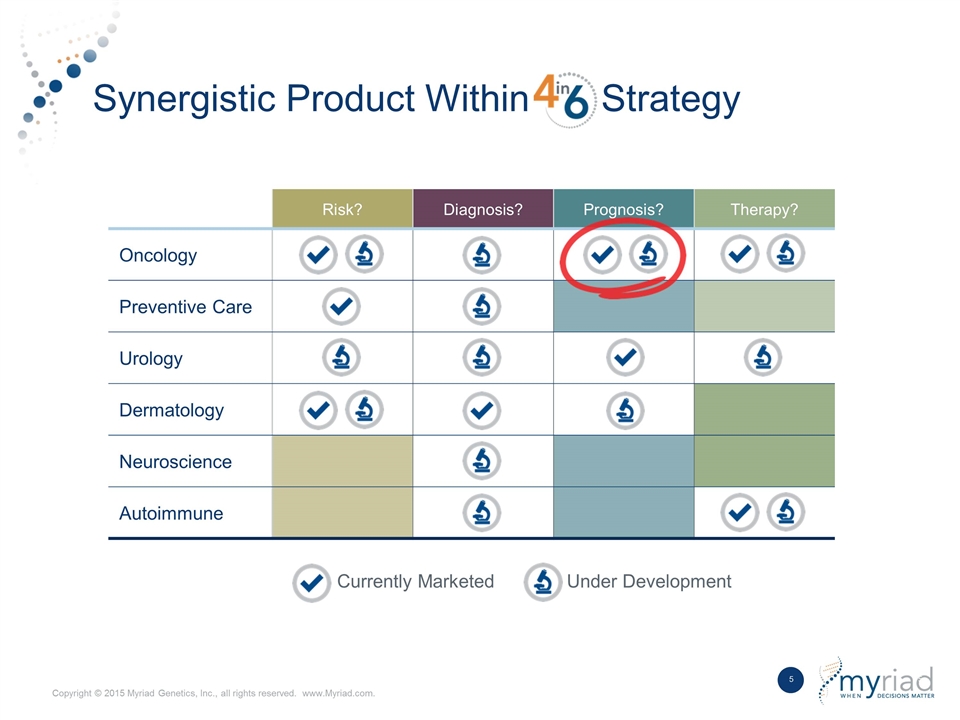
Synergistic Product Within Strategy Currently Marketed Under Development Risk? Diagnosis? Prognosis? Therapy? Oncology Preventive Care Urology Dermatology Neuroscience Autoimmune
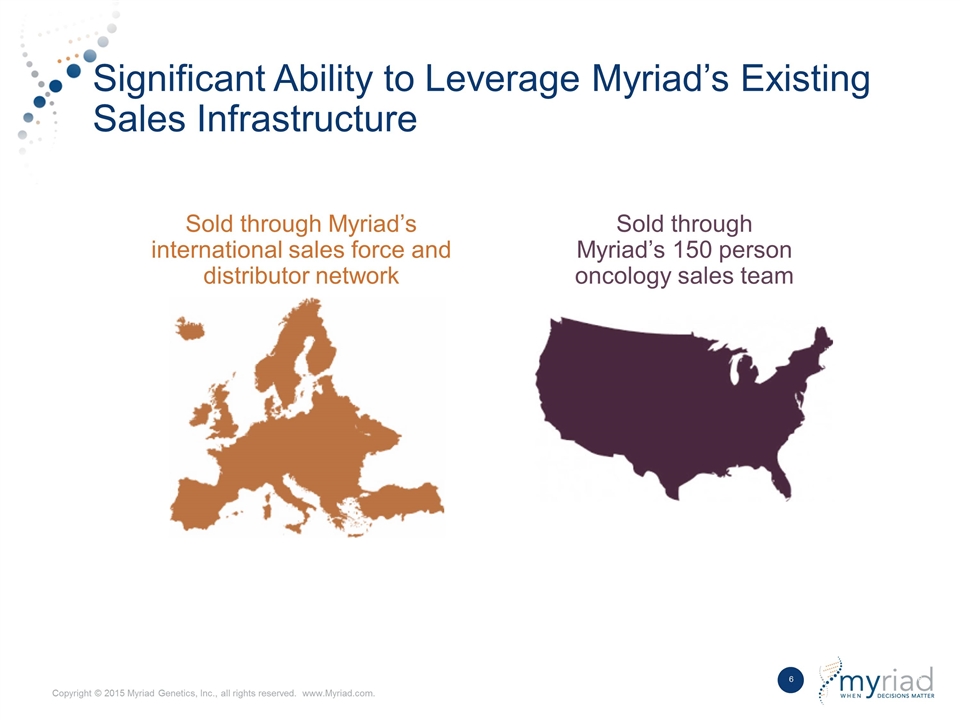
Significant Ability to Leverage Myriad’s Existing Sales Infrastructure Sold through Myriad’s 150 person oncology sales team Sold through Myriad’s international sales force and distributor network
Strategic Rationale Substantial market opportunity Broadens comprehensive product offering in oncology Best in class product Synergistic product within strategy
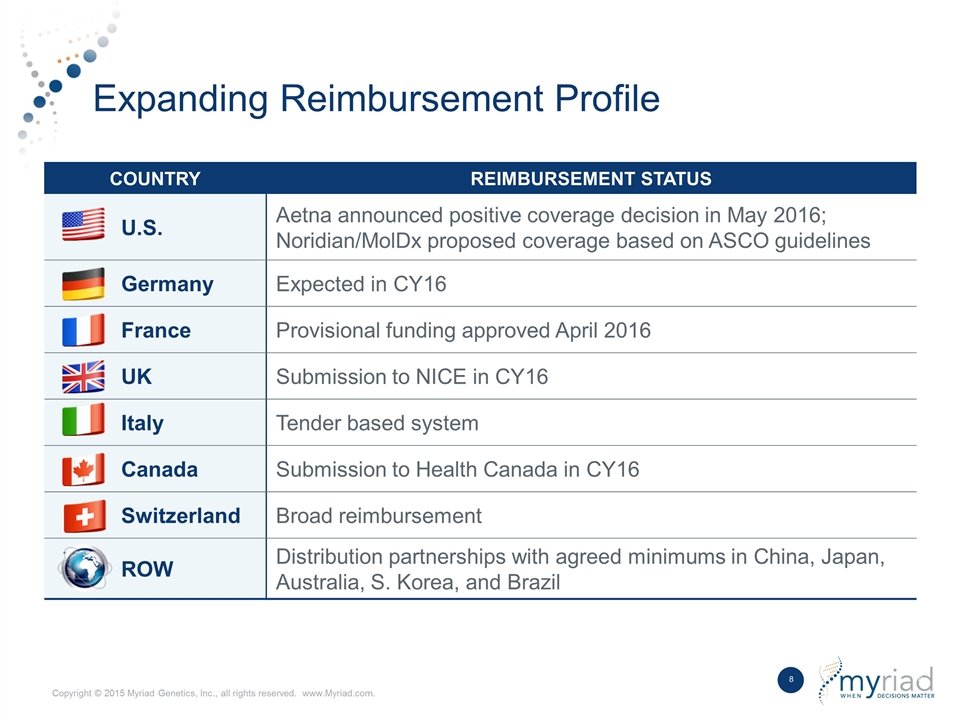
Expanding Reimbursement Profile COUNTRY REIMBURSEMENT STATUS U.S. Aetna announced positive coverage decision in May 2016; Noridian/MolDx proposed coverage based on ASCO guidelines Germany Expected in CY16 France Provisional funding approved April 2016 UK Submission to NICE in CY16 Italy Tender based system Canada Submission to Health Canada in CY16 Switzerland Broad reimbursement ROW Distribution partnerships with agreed minimums in China, Japan, Australia, S. Korea, and Brazil
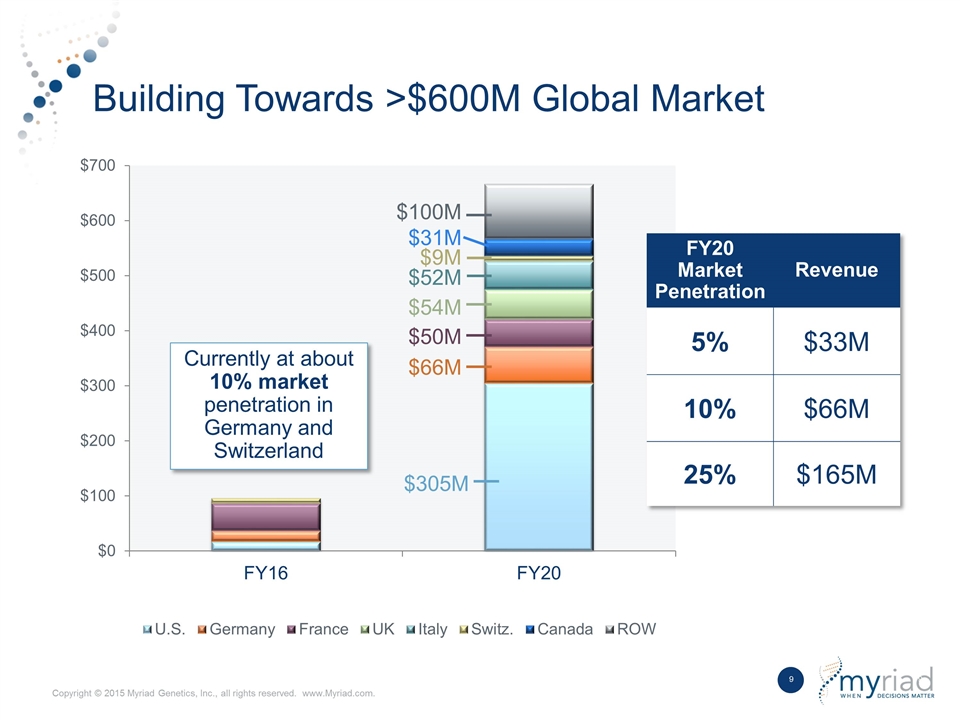
Building Towards >$600M Global Market $31M $9M $52M $54M $50M $66M $305M FY20 Market Penetration Revenue 5% $33M 10% $66M 25% $165M Currently at about 10% market penetration in Germany and Switzerland $100M
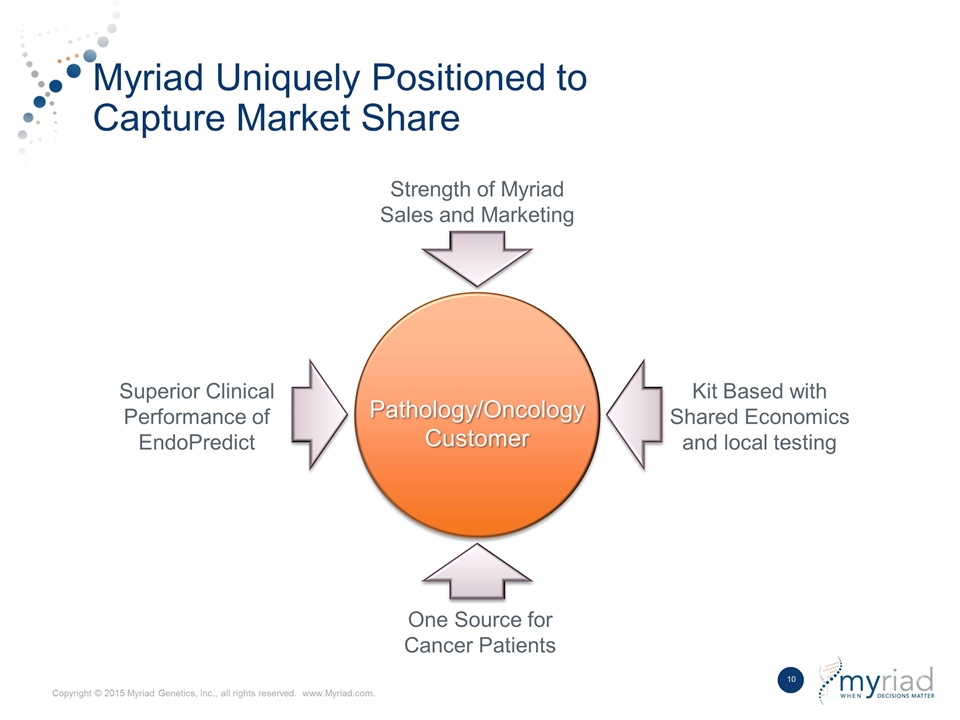
Myriad Uniquely Positioned to Capture Market Share Pathology/Oncology Customer One Source for Cancer Patients Kit Based with Shared Economics and local testing Superior Clinical Performance of EndoPredict Strength of Myriad Sales and Marketing
Strategic Rationale Substantial market opportunity Broadens comprehensive product offering in oncology Best in class product Synergistic product within strategy
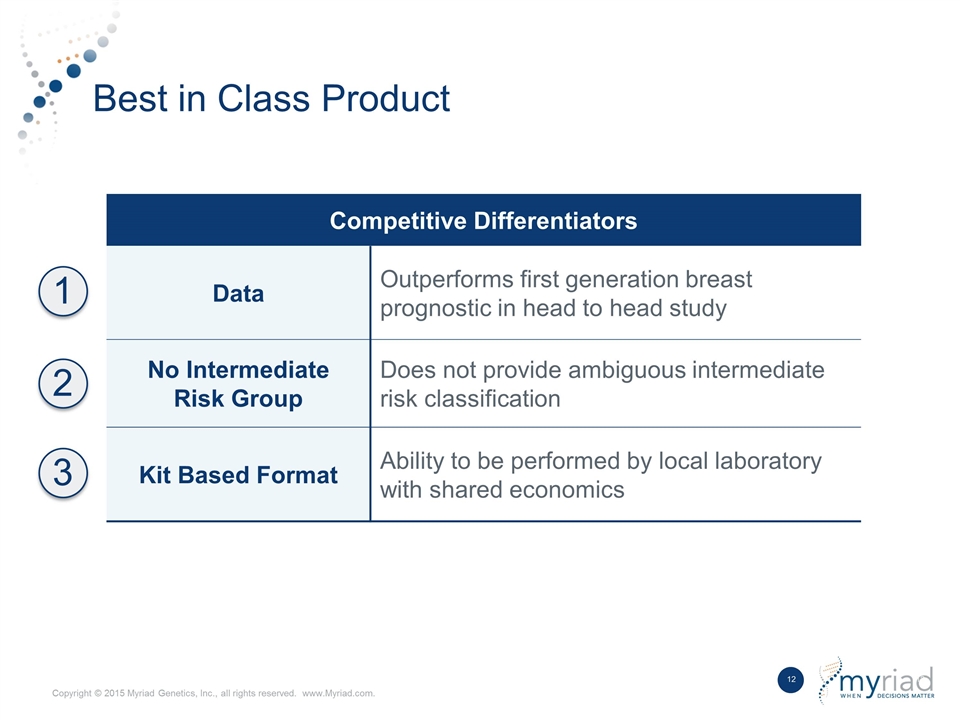
Best in Class Product Competitive Differentiators Data Outperforms first generation breast prognostic in head to head study No Intermediate Risk Group Does not provide ambiguous intermediate risk classification Kit Based Format Ability to be performed by local laboratory with shared economics 1 2 3
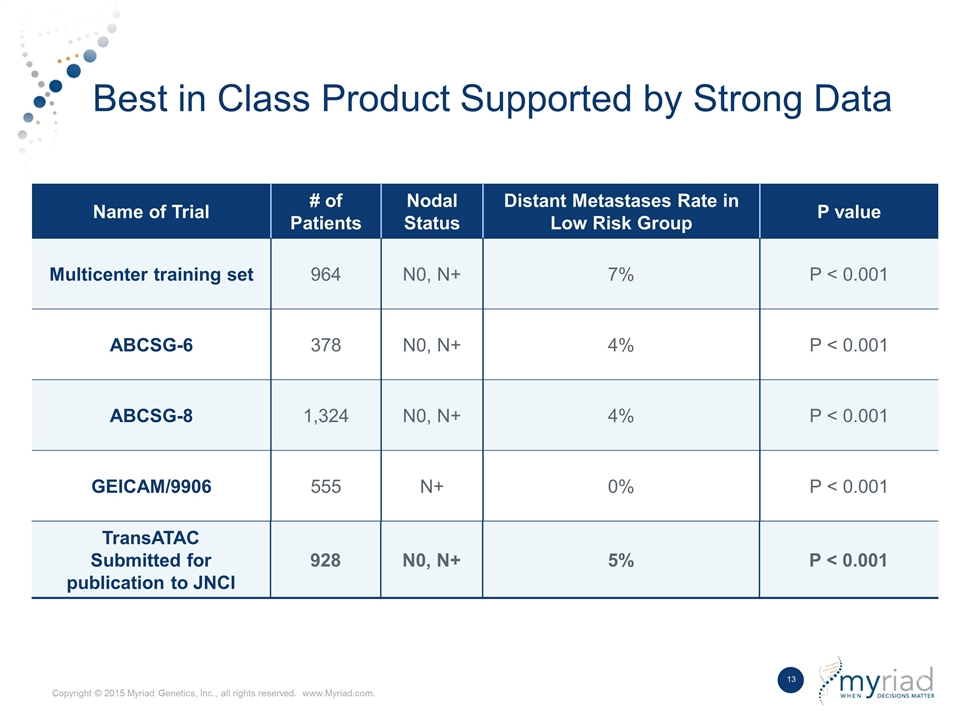
Best in Class Product Supported by Strong Data Name of Trial # of Patients Nodal Status Distant Metastases Rate in Low Risk Group P value Multicenter training set 964 N0, N+ 7% P < 0.001 ABCSG-6 378 N0, N+ 4% P < 0.001 ABCSG-8 1,324 N0, N+ 4% P < 0.001 GEICAM/9906 555 N+ 0% P < 0.001 TransATAC Submitted for publication to JNCI 928 N0, N+ 5% P < 0.001 TransATAC Submitted for publication to JNCI 928 N0, N+ 5% P < 0.001 Name of Trial # of Patients Nodal Status Distant Metastases Rate in Low Risk Group P value
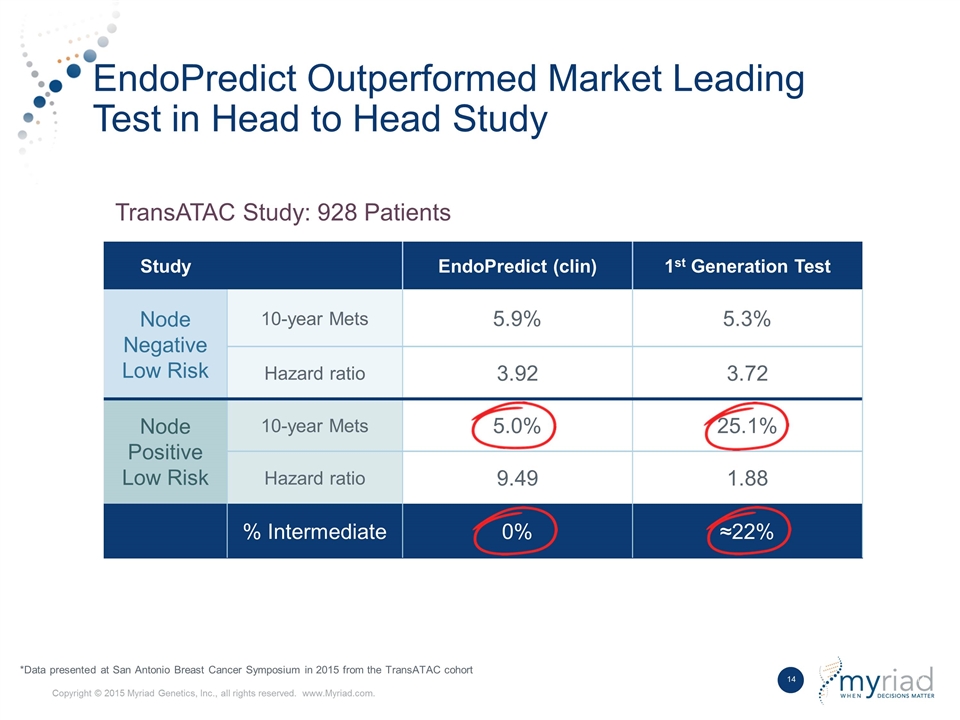
EndoPredict Outperformed Market Leading Test in Head to Head Study Study EndoPredict (clin) 1st Generation Test Node Negative Low Risk 10-year Mets 5.9% 5.3% Hazard ratio 3.92 3.72 Node Positive Low Risk 10-year Mets 5.0% 25.1% Hazard ratio 9.49 1.88 % Intermediate 0% ≈22% TransATAC Study: 928 Patients *Data presented at San Antonio Breast Cancer Symposium in 2015 from the TransATAC cohort
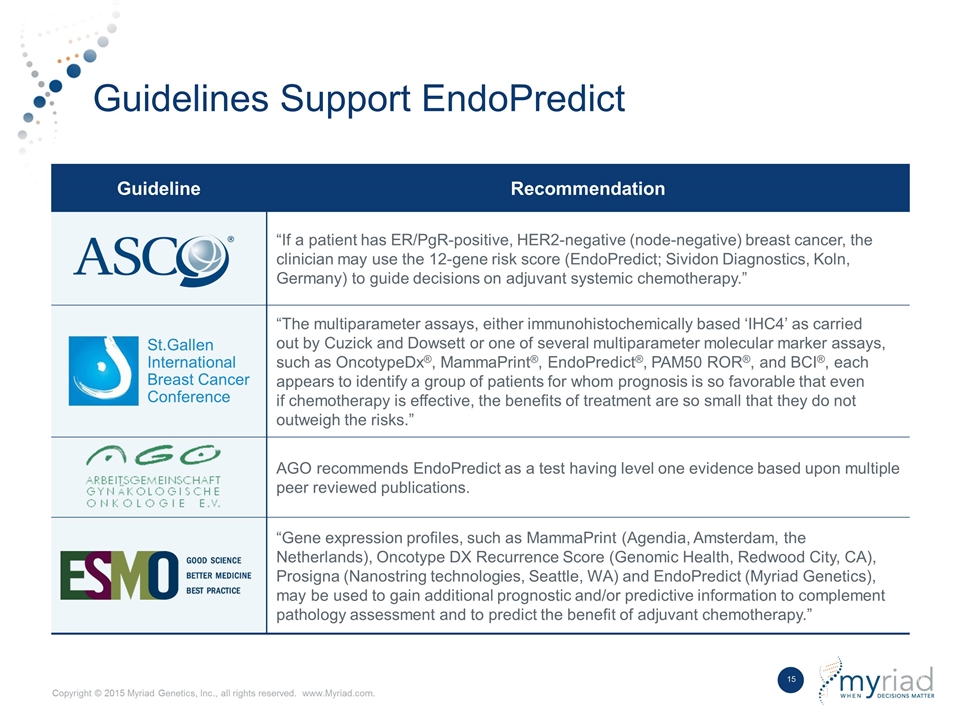
Guidelines Support EndoPredict Guideline Recommendation “If a patient has ER/PgR-positive, HER2-negative (node-negative) breast cancer, the clinician may use the 12-gene risk score (EndoPredict; Sividon Diagnostics, Koln, Germany) to guide decisions on adjuvant systemic chemotherapy.” St.Gallen International Breast Cancer Conference “The multiparameter assays, either immunohistochemically based ‘IHC4’ as carried out by Cuzick and Dowsett or one of several multiparameter molecular marker assays, such as OncotypeDx®, MammaPrint®, EndoPredict®, PAM50 ROR®, and BCI®, each appears to identify a group of patients for whom prognosis is so favorable that even if chemotherapy is effective, the benefits of treatment are so small that they do not outweigh the risks.” AGO recommends EndoPredict as a test having level one evidence based upon multiple peer reviewed publications. “Gene expression profiles, such as MammaPrint (Agendia, Amsterdam, the Netherlands), Oncotype DX Recurrence Score (Genomic Health, Redwood City, CA), Prosigna (Nanostring technologies, Seattle, WA) and EndoPredict (Myriad Genetics), may be used to gain additional prognostic and/or predictive information to complement pathology assessment and to predict the benefit of adjuvant chemotherapy.”
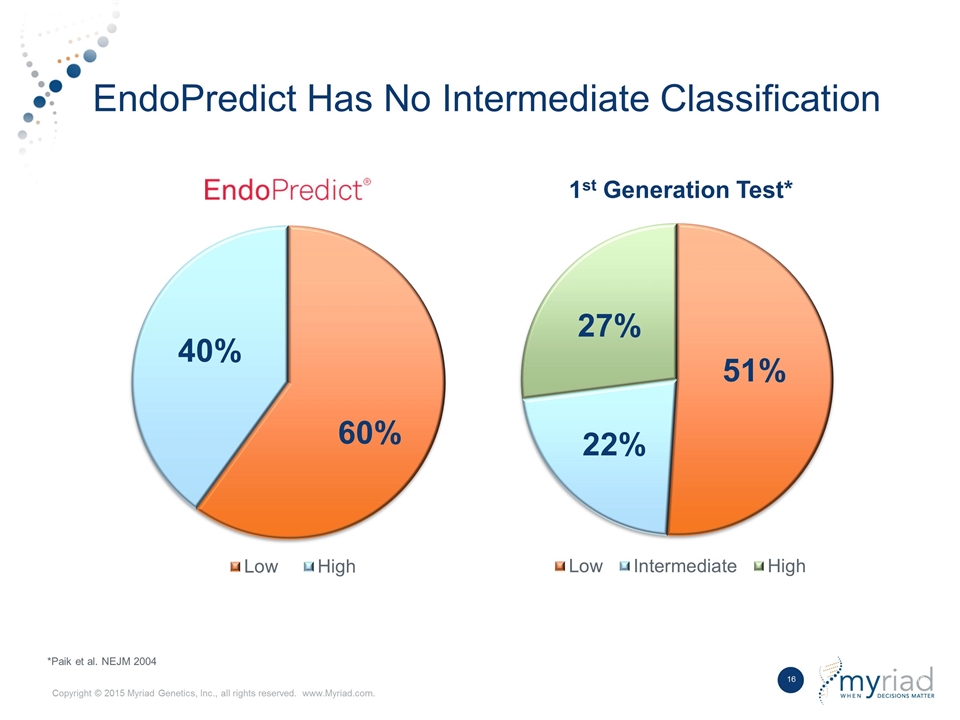
EndoPredict Has No Intermediate Classification *Paik et al. NEJM 2004 1st Generation Test*
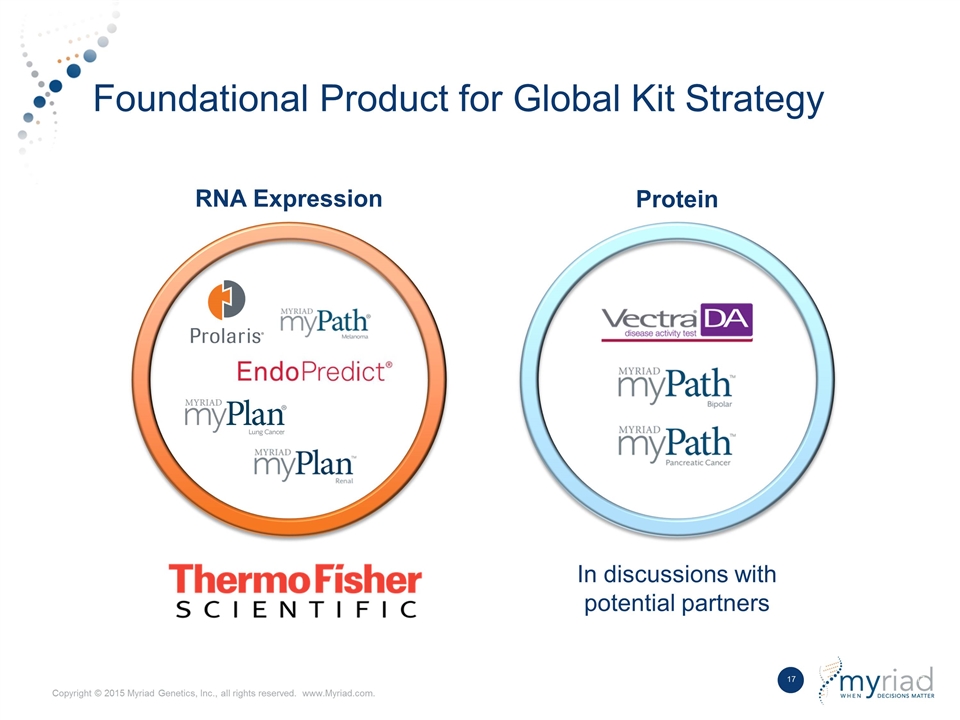
Foundational Product for Global Kit Strategy RNA Expression Protein In discussions with potential partners
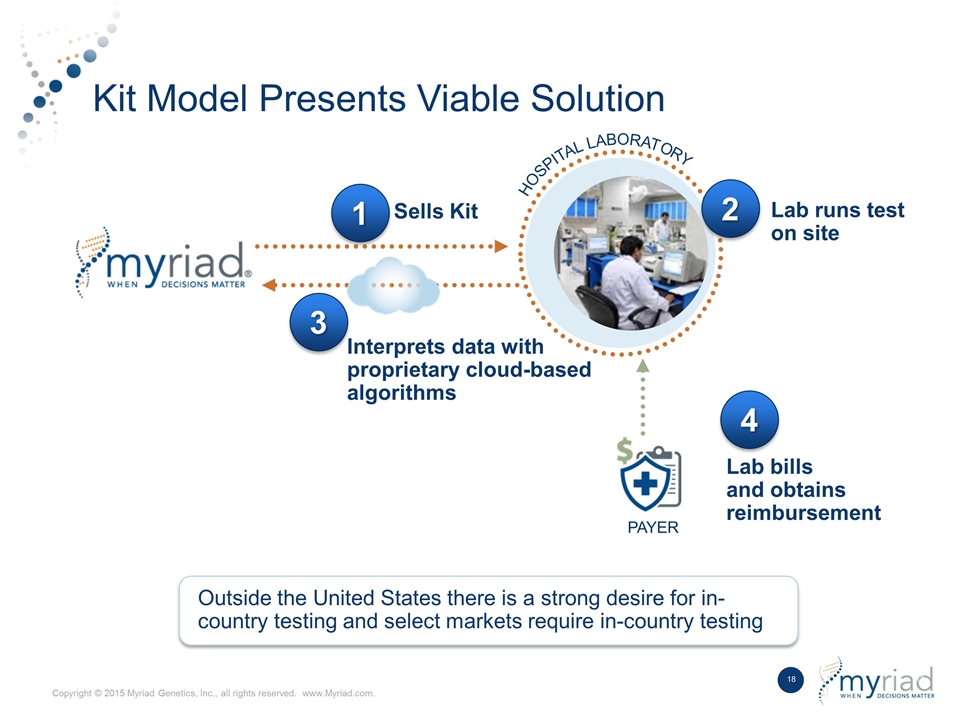
Kit Model Presents Viable Solution HOSPITAL LABORATORY Outside the United States there is a strong desire for in- country testing and select markets require in-country testing Sells Kit Lab runs test on site 3 Interprets data with proprietary cloud-based algorithms 1 2 4 Lab bills and obtains reimbursement PAYER
Strategic Rationale Substantial market opportunity Broadens comprehensive product offering in oncology Best in class product Synergistic product within strategy
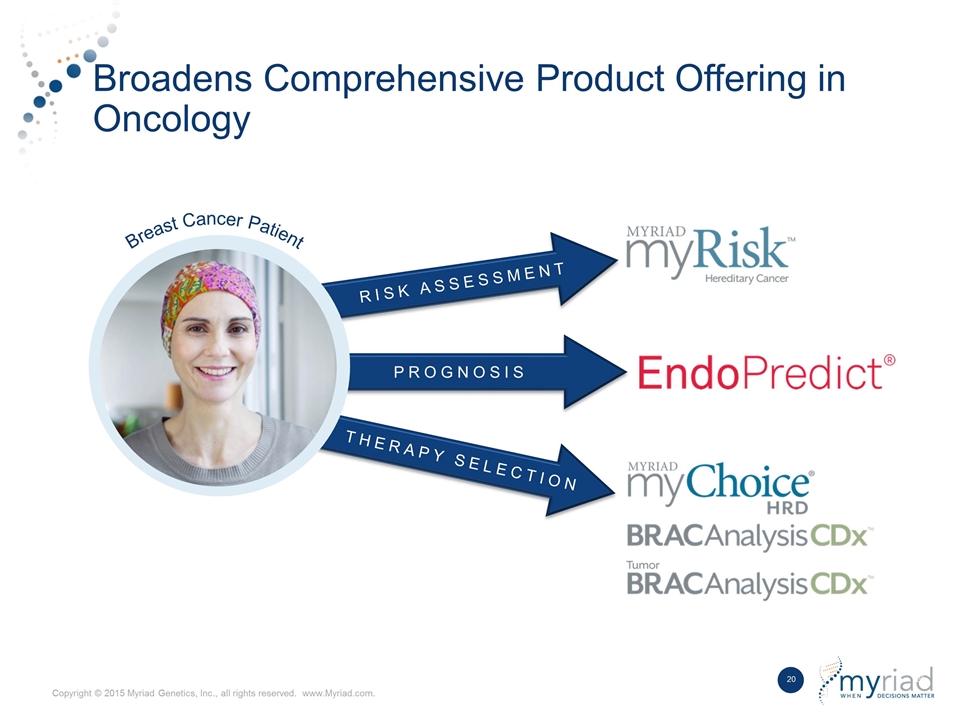
RISK ASSESSMENT Broadens Comprehensive Product Offering in Oncology PROGNOSIS THERAPY SELECTION Breast Cancer Patient
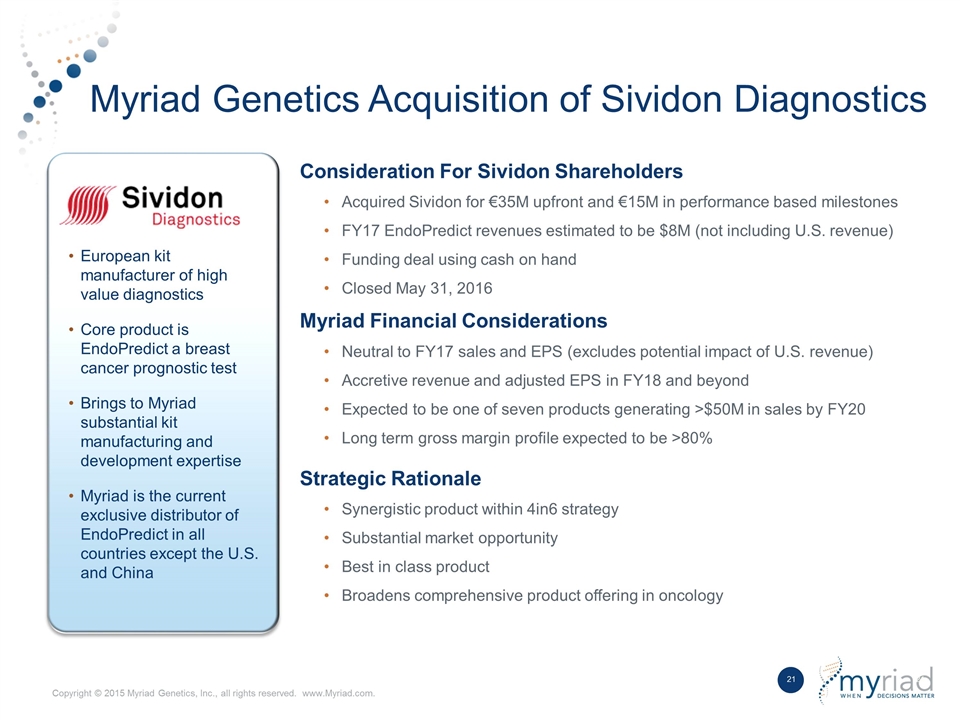
Myriad Genetics Acquisition of Sividon Diagnostics Consideration For Sividon Shareholders Acquired Sividon for €35M upfront and €15M in performance based milestones FY17 EndoPredict revenues estimated to be $8M (not including U.S. revenue) Funding deal using cash on hand Closed May 31, 2016 Myriad Financial Considerations Neutral to FY17 sales and EPS (excludes potential impact of U.S. revenue) Accretive revenue and adjusted EPS in FY18 and beyond Expected to be one of seven products generating >$50M in sales by FY20 Long term gross margin profile expected to be >80% Strategic Rationale Synergistic product within 4in6 strategy Substantial market opportunity Best in class product Broadens comprehensive product offering in oncology European kit manufacturer of high value diagnostics Core product is EndoPredict a breast cancer prognostic test Brings to Myriad substantial kit manufacturing and development expertise Myriad is the current exclusive distributor of EndoPredict in all countries except the U.S. and China
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- New Myriad Genetics Study Published in Prenatal Diagnosis Shows High Positive Predictive Value for 22q11.2 Microdeletion Syndrome Using Prequel® Prenatal Screen
- Emplifi Names Veteran Marketing Leader Susan Ganeshan as Its New CMO
- Jaime Aldama Named as New President of Momentus Securities
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share