Form 8-K MYRIAD GENETICS INC For: Jun 30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 30, 2016
MYRIAD GENETICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 0-26642 | 87-0494517 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
320 Wakara Way
Salt Lake City, Utah 84108
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (801) 584-3600
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| ITEM 7.01 | Regulation FD Disclosure. |
On June 30, 2016, Myriad Genetics, Inc. (“Myriad” or the “Company”) held a conference call in relation to Myriad’s myChoice HRD test successfully identifying patients that meet primary endpoint in TESARO’s pivotal phase 3 ovarian cancer study with Niraparib. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated herein by reference. The slide presentation will also be available under the “Investors –Events & Presentations” section of Myriad’s website at www.myriad.com.
FORWARD-LOOKING STATEMENTS
Exhibit 99.1 may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements relating to our business, goals, strategy and financial and operational outlook. These “forward-looking statements” are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by forward-looking statements. These risks and uncertainties include, but are not limited to: the risk that sales and profit margins of our existing molecular diagnostic tests and pharmaceutical and clinical services may decline or will not continue to increase at historical rates; risks related to our ability to transition from our existing product portfolio to our new tests; risks related to changes in the governmental or private insurers reimbursement levels for our tests or our ability to obtain reimbursement for our new tests at comparable levels to our existing tests; risks related to increased competition and the development of new competing tests and services; the risk that we may be unable to develop or achieve commercial success for additional molecular diagnostic tests and pharmaceutical and clinical services in a timely manner, or at all; the risk that we may not successfully develop new markets for our molecular diagnostic tests and pharmaceutical and clinical services, including our ability to successfully generate revenue outside the United States; the risk that licenses to the technology underlying our molecular diagnostic tests and pharmaceutical and clinical services tests and any future tests are terminated or cannot be maintained on satisfactory terms; risks related to delays or other problems with operating our laboratory testing facilities; risks related to public concern over our genetic testing in general or our tests in particular; risks related to regulatory requirements or enforcement in the United States and foreign countries and changes in the structure of the healthcare system or healthcare payment systems; risks related to our ability to obtain new corporate collaborations or licenses and acquire new technologies or businesses on satisfactory terms, if at all; risks related to our ability to successfully integrate and derive benefits from any technologies or businesses that we license or acquire; risks related to our projections about the potential market opportunity for our products; the risk that we or our licensors may be unable to protect or that third parties will infringe the proprietary technologies underlying our tests; the risk of patent-infringement claims or challenges to the validity of our patents; risks related to changes in intellectual property laws covering our molecular diagnostic tests and pharmaceutical and clinical services and patents or enforcement in the United States and foreign countries, such as the Supreme Court decision in the lawsuit brought against us by the Association for Molecular Pathology et al; risks of new, changing and competitive technologies and regulations in the United States and internationally; and other factors discussed under the heading “Risk Factors” contained in Item 1A of our most recent Annual Report on Form 10-K, which has been filed with the Securities and Exchange Commission, as well as any updates to those risk factors filed from time to time in our Quarterly Reports on Form 10-Q or Current Reports on Form 8-K. All information in the exhibits is as of the date of the exhibits, and Myriad undertakes no duty to update this information unless required by law.
Page 2
| ITEM 9.01 | Financial Statements and Exhibits. |
(d)
| Exhibit |
Description | |
| 99.1 | Slide Presentation dated June 30, 2016. | |
The exhibit(s) may contain hypertext links to information on our website or other parties’ websites. The information on our website and other parties’ websites is not incorporated by reference into this Current Report on Form 8-K and does not constitute a part of this Form 8-K.
In accordance with General Instruction B-2 of Form 8-K, the information set forth in Item 7.01 and in Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section, and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Page 3
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| MYRIAD GENETICS, INC. | ||||||
| Date: June 30, 2016 | By: | /s/ R. Bryan Riggsbee | ||||
| R. Bryan Riggsbee | ||||||
| Executive Vice President, Chief Financial Officer | ||||||
Page 4
EXHIBIT INDEX
| Exhibit |
Description | |
| 99.1 | Slide Presentation dated June 30, 2016. | |
Page 5

Myriad Genetics myChoice HRD® Update 06/30/2016 Exhibit 99.1

Forward Looking Statements Some of the information presented here today may contain projections or other forward-looking statements regarding future events or the future financial performance of the Company. These statements are based on management’s current expectations and the actual events or results may differ materially and adversely from these expectations. We refer you to the documents the Company files from time to time with the Securities and Exchange Commission, specifically, the Company’s annual reports on Form 10-K, its quarterly reports on Form 10-Q, and its current reports on Form 8-K. These documents identify important risk factors that could cause the actual results to differ materially from those contained in the Company’s projections or forward-looking statements.

A Pioneering Discovery: myChoice HRD
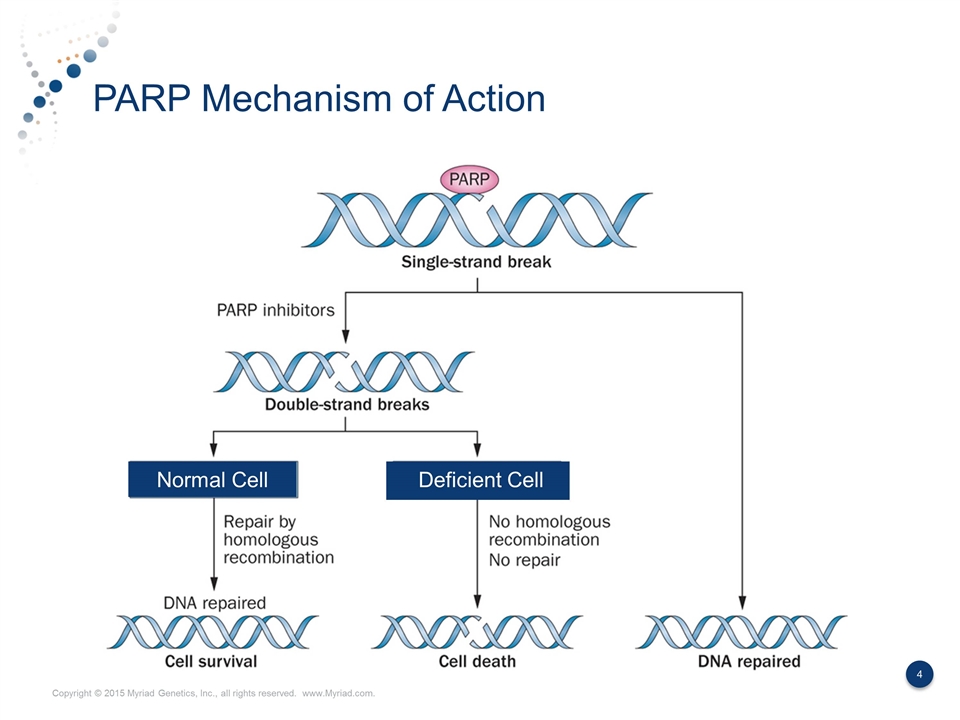
PARP Mechanism of Action Normal Cell Deficient Cell
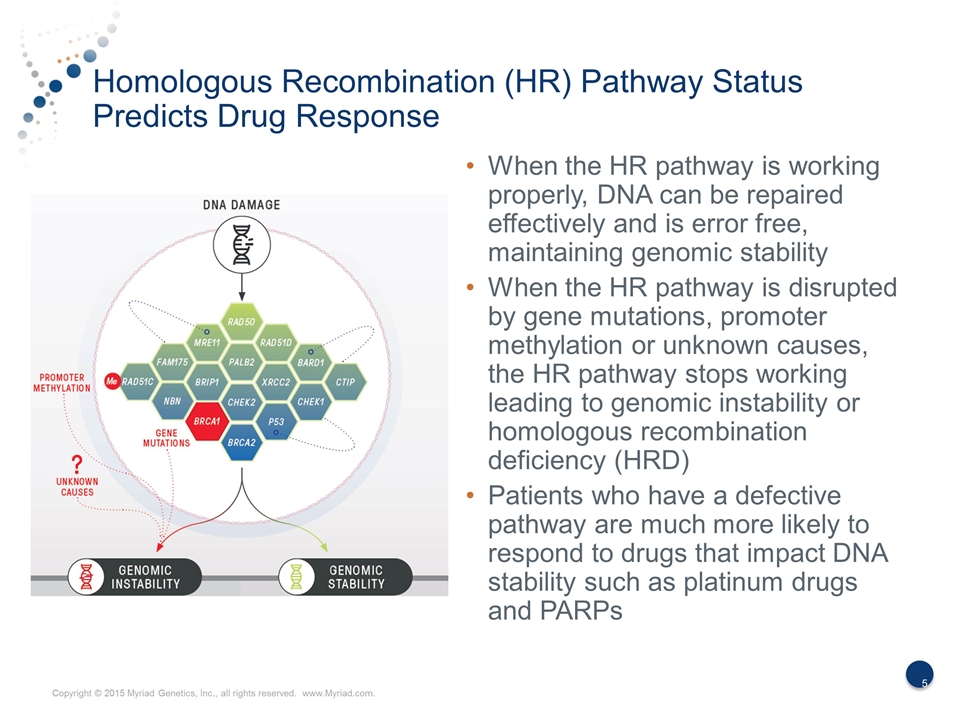
Homologous Recombination (HR) Pathway Status Predicts Drug Response When the HR pathway is working properly, DNA can be repaired effectively and is error free, maintaining genomic stability When the HR pathway is disrupted by gene mutations, promoter methylation or unknown causes, the HR pathway stops working leading to genomic instability or homologous recombination deficiency (HRD) Patients who have a defective pathway are much more likely to respond to drugs that impact DNA stability such as platinum drugs and PARPs
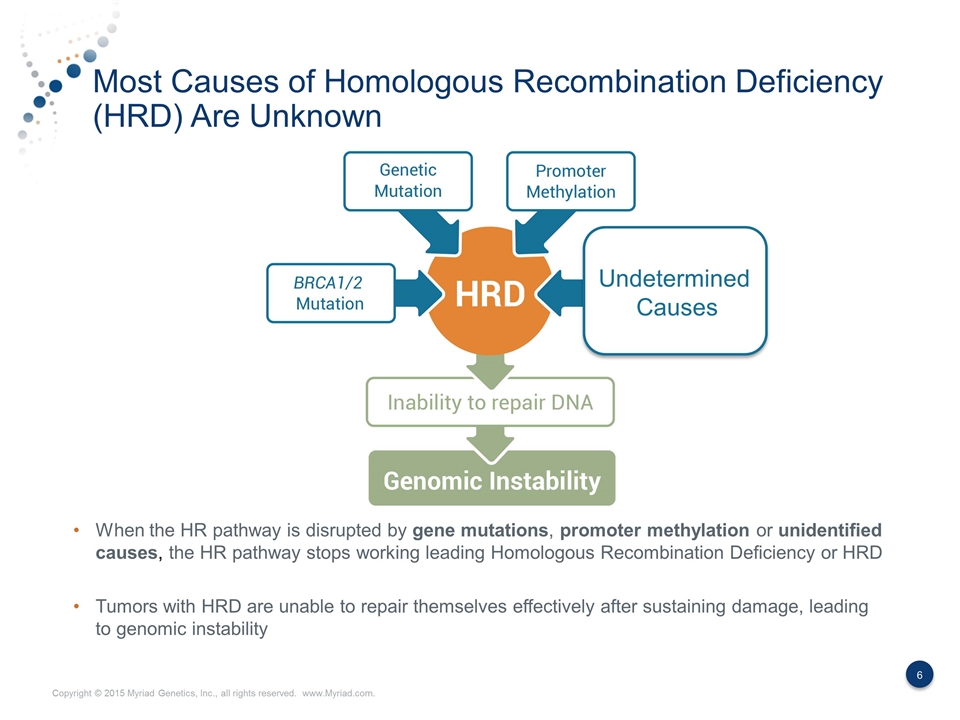
Most Causes of Homologous Recombination Deficiency (HRD) Are Unknown When the HR pathway is disrupted by gene mutations, promoter methylation or unidentified causes, the HR pathway stops working leading Homologous Recombination Deficiency or HRD Tumors with HRD are unable to repair themselves effectively after sustaining damage, leading to genomic instability Undetermined Causes

The Cause? Lane closure? Traffic light failure? Overturned truck? Fender bender? Presidential motorcade? Who cares…. Pioneering Discovery: Measure the Effects vs. the Causes The EFFECT…..
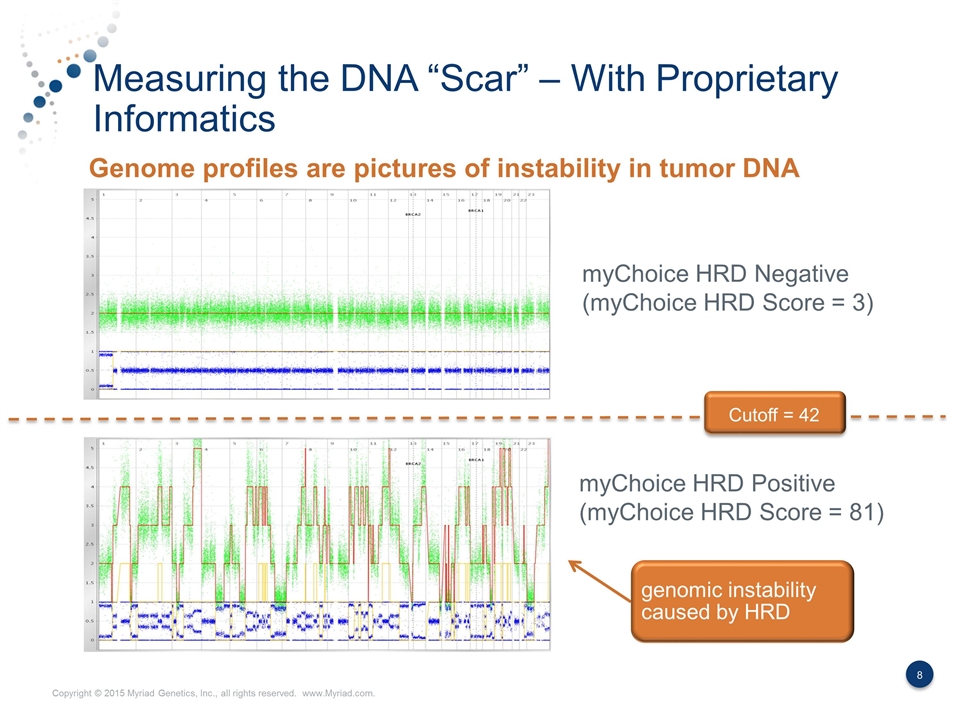
Measuring the DNA “Scar” – With Proprietary Informatics myChoice HRD Negative (myChoice HRD Score = 3) myChoice HRD Positive (myChoice HRD Score = 81) Genome profiles are pictures of instability in tumor DNA Cutoff = 42 genomic instability caused by HRD
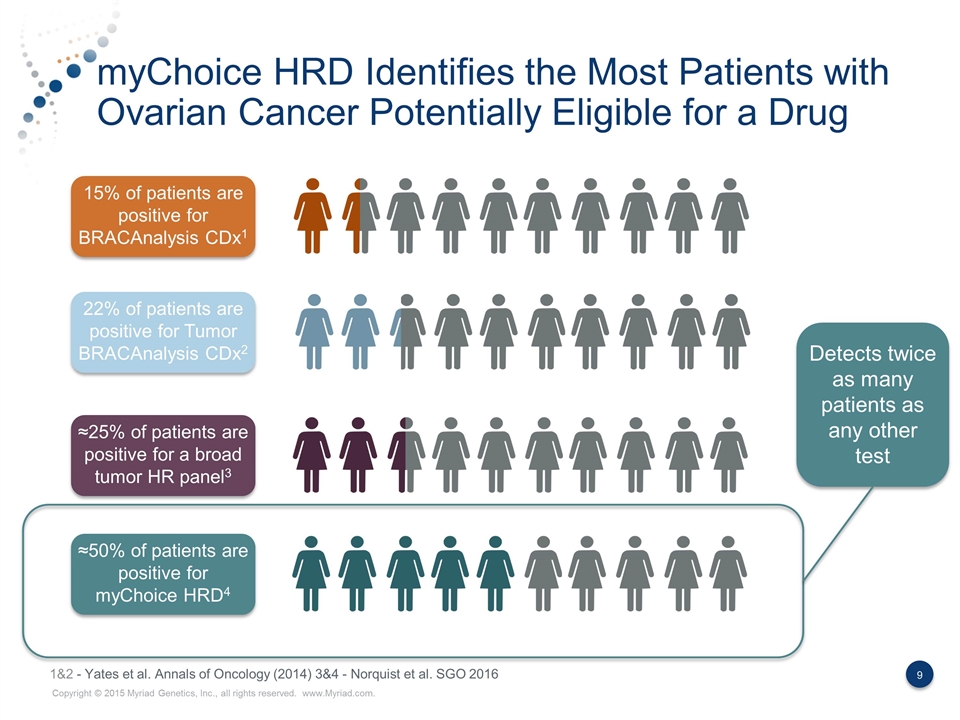
myChoice HRD Identifies the Most Patients with Ovarian Cancer Potentially Eligible for a Drug 15% of patients are positive for BRACAnalysis CDx1 22% of patients are positive for Tumor BRACAnalysis CDx2 ≈25% of patients are positive for a broad tumor HR panel3 ≈50% of patients are positive for myChoice HRD4 Detects twice as many patients as any other test 1&2 - Yates et al. Annals of Oncology (2014) 3&4 - Norquist et al. SGO 2016
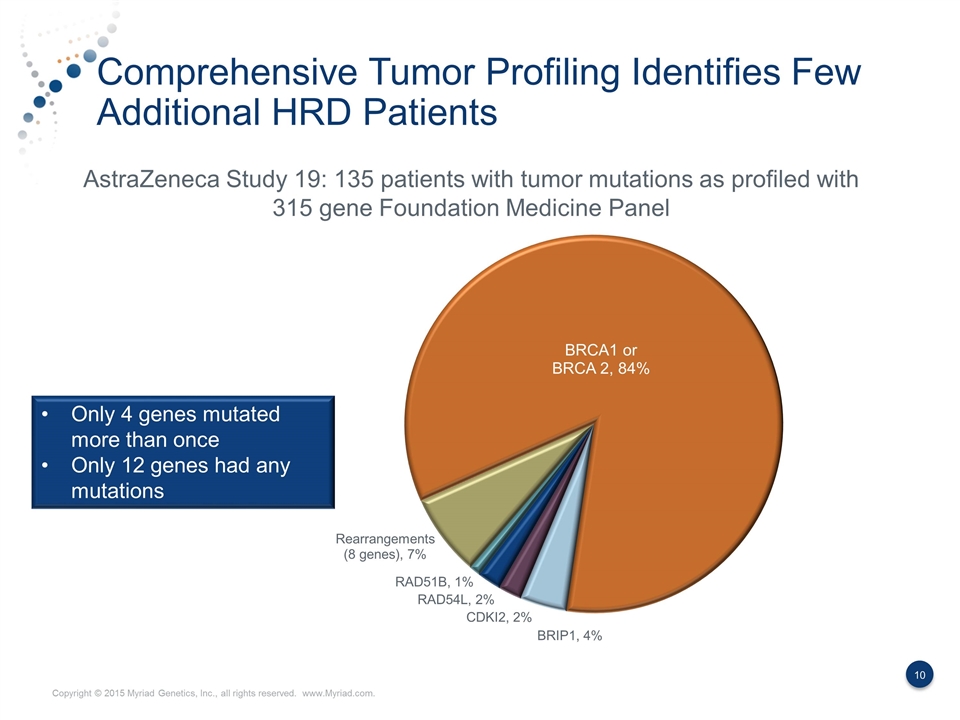
Comprehensive Tumor Profiling Identifies Few Additional HRD Patients AstraZeneca Study 19: 135 patients with tumor mutations as profiled with 315 gene Foundation Medicine Panel Only 4 genes mutated more than once Only 12 genes had any mutations
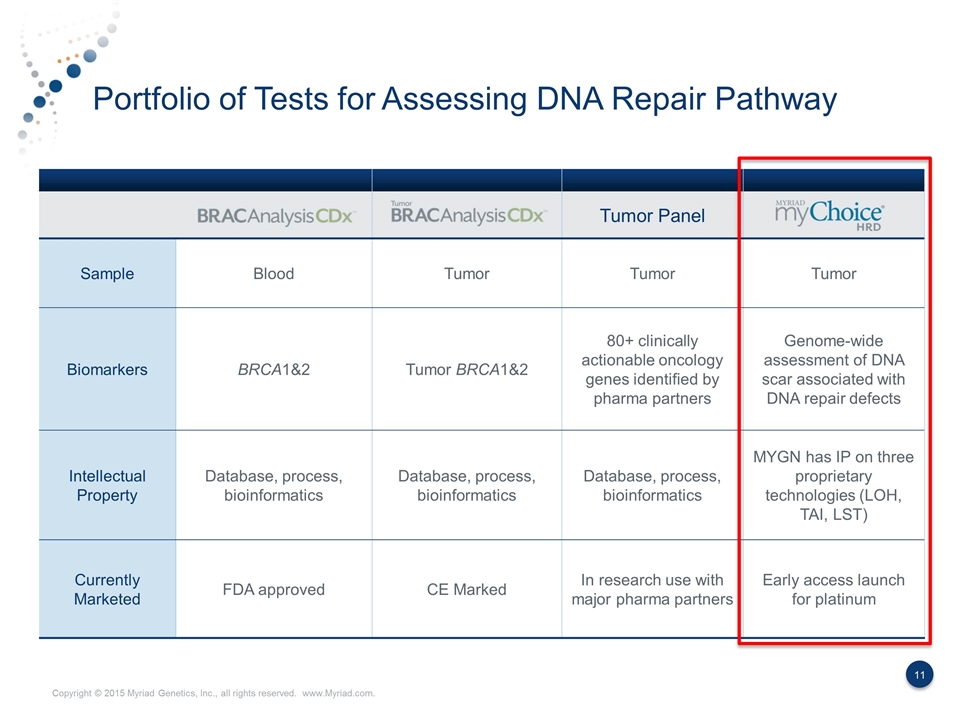
Portfolio of Tests for Assessing DNA Repair Pathway Tumor Panel Sample Blood Tumor Tumor Tumor Biomarkers BRCA1&2 Tumor BRCA1&2 80+ clinically actionable oncology genes identified by pharma partners Genome-wide assessment of DNA scar associated with DNA repair defects Intellectual Property Database, process, bioinformatics Database, process, bioinformatics Database, process, bioinformatics MYGN has IP on three proprietary technologies (LOH, TAI, LST) Currently Marketed FDA approved CE Marked In research use with major pharma partners Early access launch for platinum
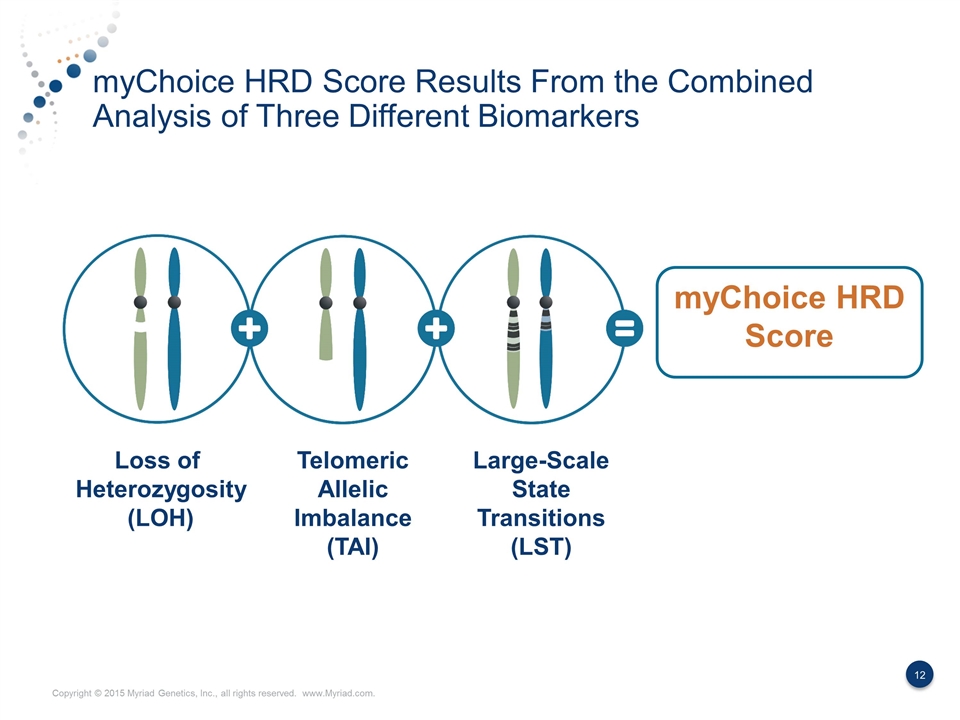
myChoice HRD Score Results From the Combined Analysis of Three Different Biomarkers myChoice HRD Score Loss of Heterozygosity (LOH) Telomeric Allelic Imbalance (TAI) Large-Scale State Transitions (LST)
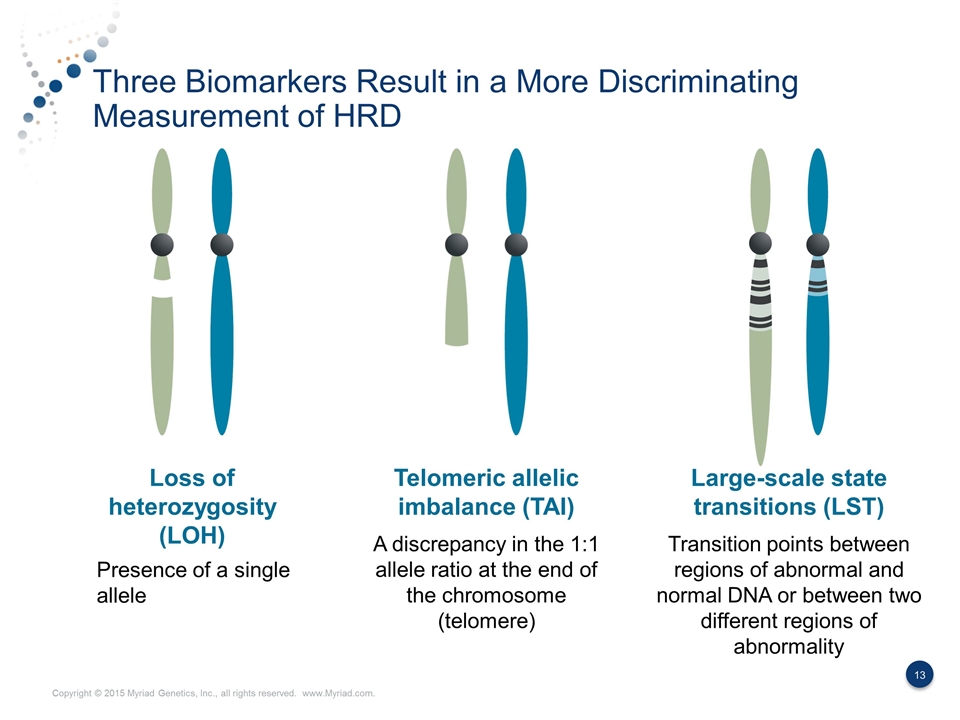
Three Biomarkers Result in a More Discriminating Measurement of HRD Loss of heterozygosity (LOH) Telomeric allelic imbalance (TAI) Large-scale state transitions (LST) Presence of a single allele A discrepancy in the 1:1 allele ratio at the end of the chromosome (telomere) Transition points between regions of abnormal and normal DNA or between two different regions of abnormality
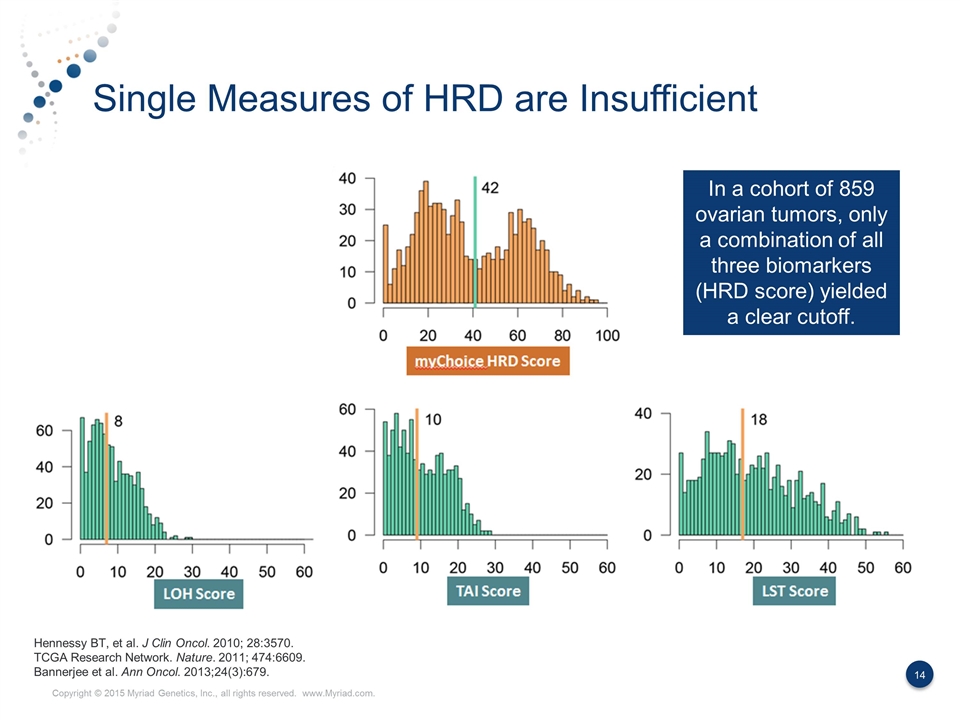
Single Measures of HRD are Insufficient Hennessy BT, et al. J Clin Oncol. 2010; 28:3570. TCGA Research Network. Nature. 2011; 474:6609. Bannerjee et al. Ann Oncol. 2013;24(3):679. In a cohort of 859 ovarian tumors, only a combination of all three biomarkers (HRD score) yielded a clear cutoff.

NOVA Study and Data
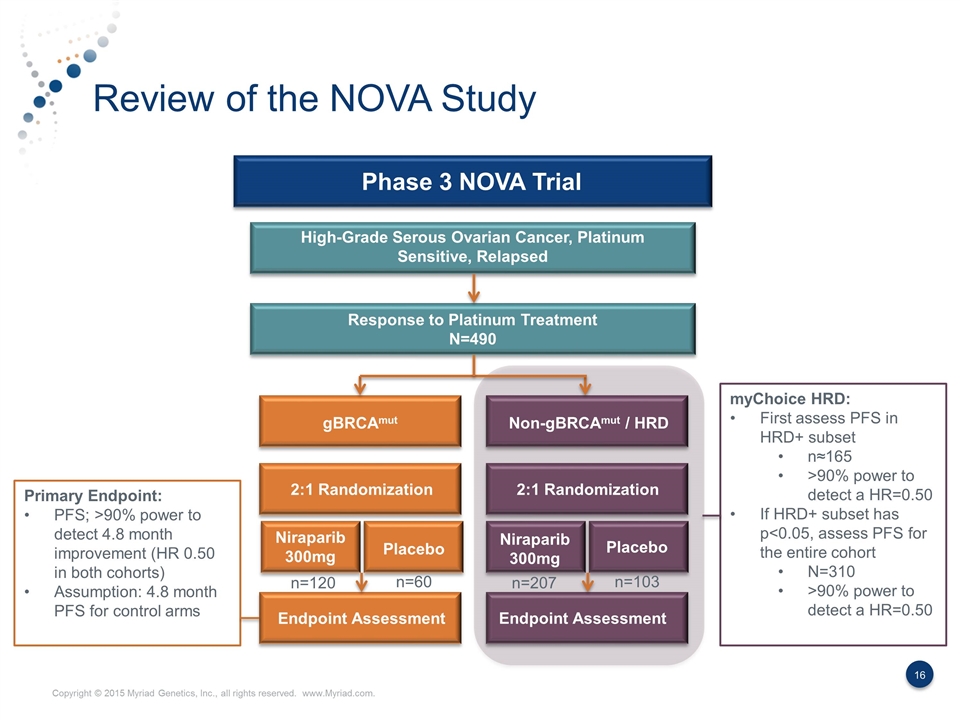
Review of the NOVA Study Phase 3 NOVA Trial High-Grade Serous Ovarian Cancer, Platinum Sensitive, Relapsed Response to Platinum Treatment N=490 gBRCAmut Non-gBRCAmut / HRD 2:1 Randomization 2:1 Randomization Niraparib 300mg Niraparib 300mg Placebo Placebo Endpoint Assessment Endpoint Assessment myChoice HRD: First assess PFS in HRD+ subset n≈165 >90% power to detect a HR=0.50 If HRD+ subset has p<0.05, assess PFS for the entire cohort N=310 >90% power to detect a HR=0.50 n=120 n=60 n=207 n=103 Primary Endpoint: PFS; >90% power to detect 4.8 month improvement (HR 0.50 in both cohorts) Assumption: 4.8 month PFS for control arms
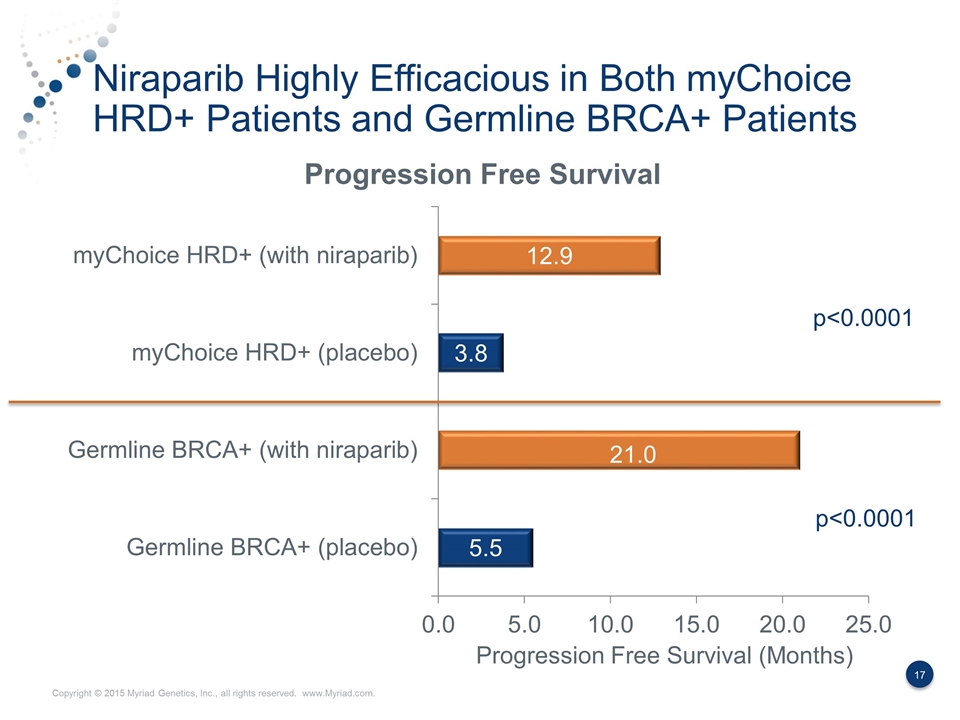
Niraparib Highly Efficacious in Both myChoice HRD+ Patients and Germline BRCA+ Patients p<0.0001 ? 21.0 Progression Free Survival (Months) p<0.0001
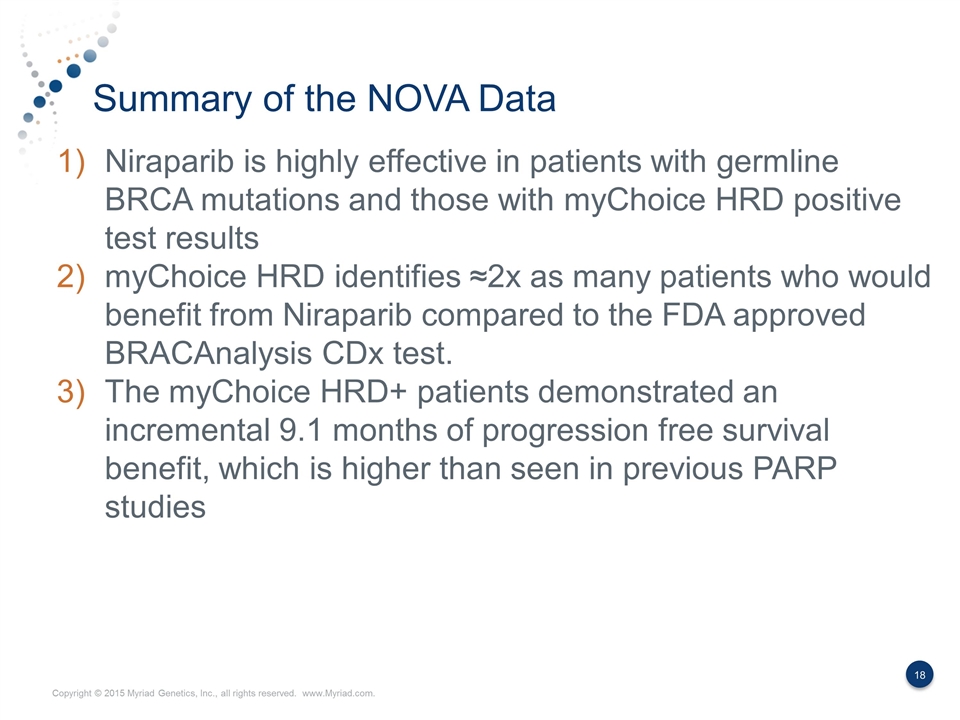
Summary of the NOVA Data Niraparib is highly effective in patients with germline BRCA mutations and those with myChoice HRD positive test results myChoice HRD identifies ≈2x as many patients who would benefit from Niraparib compared to the FDA approved BRACAnalysis CDx test. The myChoice HRD+ patients demonstrated an incremental 9.1 months of progression free survival benefit, which is higher than seen in previous PARP studies

Business Impact
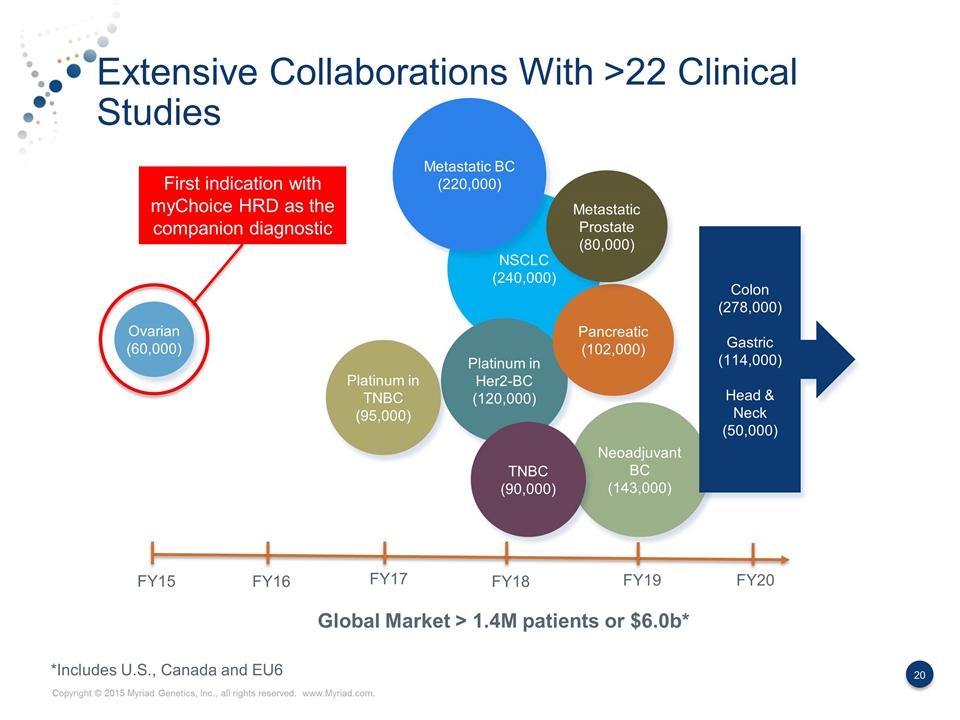
Extensive Collaborations With >22 Clinical Studies NSCLC (240,000) Global Market > 1.4M patients or $6.0b* Neoadjuvant BC (143,000) Ovarian (60,000) Platinum in Her2-BC (120,000) FY15 FY16 FY17 FY18 FY19 FY20 *Includes U.S., Canada and EU6 Platinum in TNBC (95,000) Pancreatic (102,000) Metastatic Prostate (80,000) Colon (278,000) Gastric (114,000) Head & Neck (50,000) Metastatic BC (220,000) TNBC (90,000) First indication with myChoice HRD as the companion diagnostic
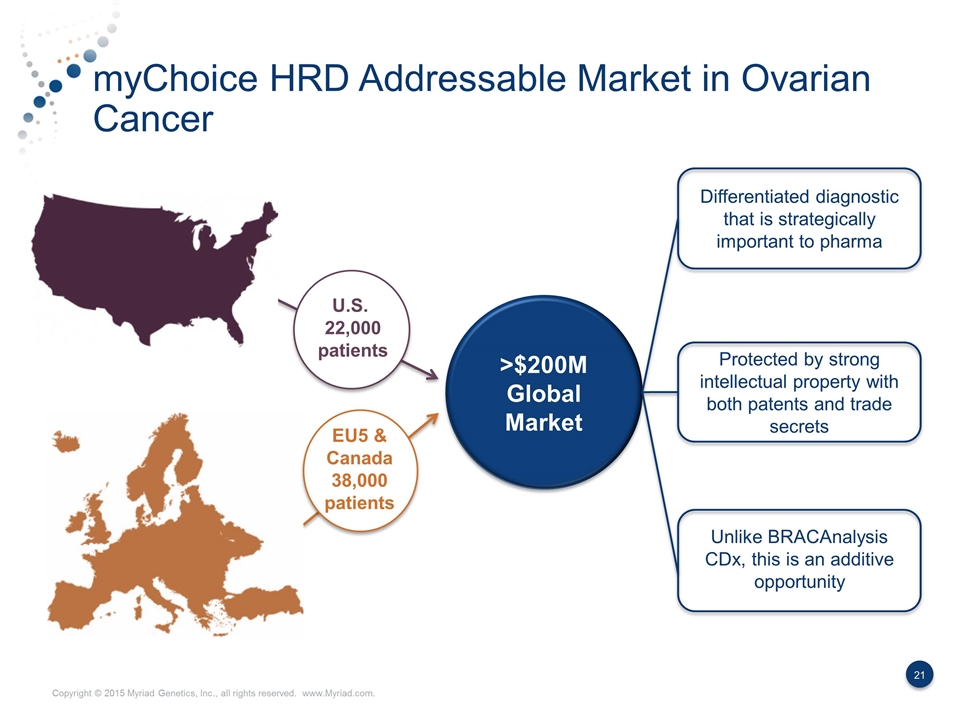
myChoice HRD Addressable Market in Ovarian Cancer U.S. 22,000 patients EU5 & Canada 38,000 patients Differentiated diagnostic that is strategically important to pharma Protected by strong intellectual property with both patents and trade secrets Unlike BRACAnalysis CDx, this is an additive opportunity >$200M Global Market
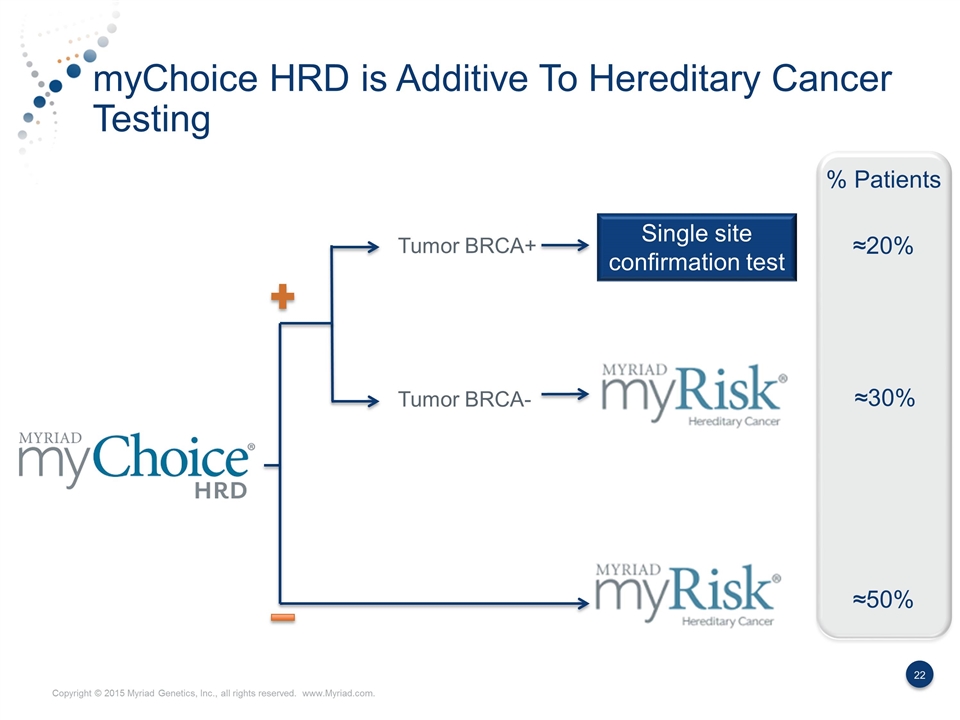
myChoice HRD is Additive To Hereditary Cancer Testing Tumor BRCA+ Single site confirmation test Tumor BRCA- % Patients ≈20% ≈30% ≈50%
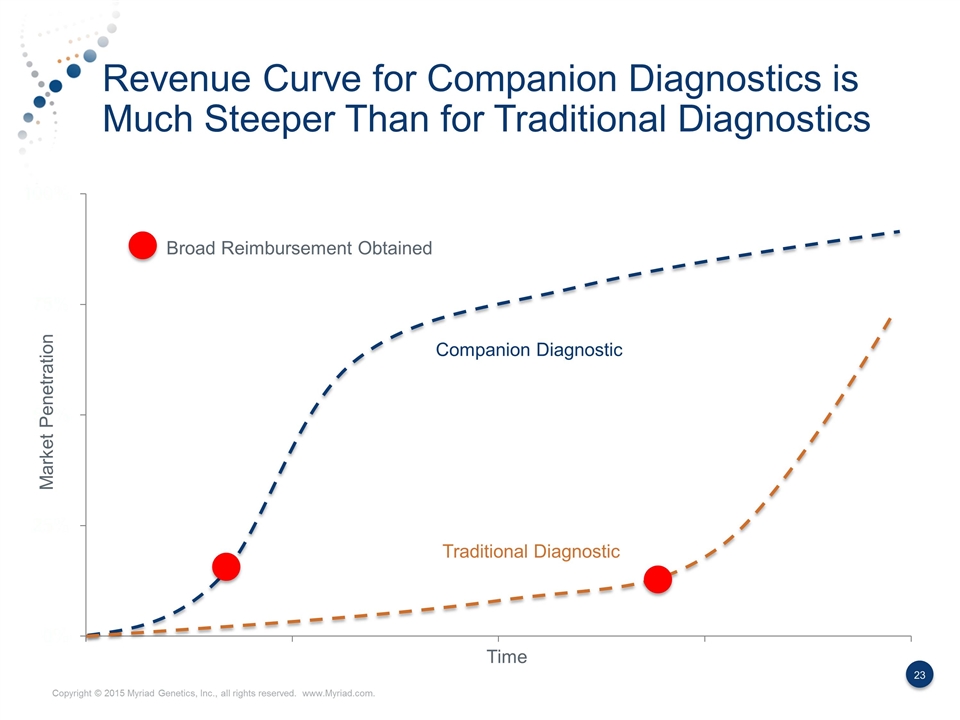
Revenue Curve for Companion Diagnostics is Much Steeper Than for Traditional Diagnostics Market Penetration Time Broad Reimbursement Obtained Companion Diagnostic Traditional Diagnostic

Other Strategic Considerations First prospective data and major validation of myChoice HRD – reduces risk for pharma partners and likely drives additional collaborations Growing core competency in FDA approved, high-value companion diagnostics Intellectual property around myChoice HRD is significant and provides global differentiation
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Biophytis Announces Transfer of ADSs to OTC Market
- The First Bancshares, Inc. Reports Results for First Quarter ended March 31, 2024
- Leading Industry Publication: Black & Veatch Remains Among Global Critical Infrastructure Leaders as Sustainability, Decarbonization Solutions Drive Growth
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share