Form 8-K DYNASIL CORP OF AMERICA For: Nov 16
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
Current Report
Pursuant to section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 16, 2016
------------------
| Dynasil Corporation of America |
| (Exact name of registrant as specified in its charter) |
| Delaware | 000-27503 | 22-1734088 | ||
| (State or other | (Commission File Number) | (IRS Employer | ||
| jurisdiction of incorporation) | Identification No.) |
| 313 Washington Street, Suite 403, Newton, MA 02458 |
| (Address of principal executive offices) |
| (617)-668-6855 |
| (Registrant's telephone number, including area code) |
| Not Applicable |
| (Former name or former address, if changed since last report) |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Item 7.01 - Regulation FD Disclosure
Pursuant to Regulation FD, Dynasil Corporation of America (“Dynasil” or the “Company”) hereby furnishes slides that the Company will present to stockholders and investors via conference call and webcast on or after November 21, 2016. The slides are attached hereto as Exhibit 99.2. These slides will be available on Dynasil’s website at www.dynasil.com. The information furnished by the Company pursuant to this item, including Exhibit 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) or otherwise subject to the liability of that section, and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act.
Item 8.01 - Other Events.
On November 16, 2016, Xcede Technologies, Inc. (“Xcede”) a subsidiary of Dynasil Corporation of America (“Dynasil”) entered into a strategic in-kind loan arrangement with Cook Biotech Inc. (CBI) of West Lafayette, IN, with respect to the planned first-in-human (FIH) clinical trials of the Xcede Patch. Xcede and CBI already have existing manufacturing, licensing and supply agreements, pursuant to which, among other things, CBI supplies Xcede with its extracellular matrix technology, a component of the Patch.
Pursuant to this new arrangement, CBI has agreed to provide Xcede with preclinical and clinical services necessary to complete FIH trials, and will oversee and execute such work on behalf of Xcede. In exchange for the value of such services, Xcede will issue to CBI, at a various times, secured promissory notes of Xcede in the aggregate principal amount of $1.5 million, bearing interest at 2% per annum.
As part of this arrangement, the following other transactions occurred:
• Dynasil committed to invest $1.2 million in Xcede to fund the anticipated operating costs of Xcede through the anticipated completion of the FIH trials. These investments will be in the form of senior preferred stock.
• Peter Sulick, Dynasil Chairman and CEO, and members of his family also made an additional cash investment in Xcede of $450,000 in junior preferred stock.
With these arrangements, it is expected that Xcede will have sufficient funds to complete the FIH trials and related preclinical studies for the Patch. The preclinical studies are anticipated to begin in 2016 and the FIH trial is expected to begin in 2017.
A Special Committee of the Dynasil Board of Directors, consisting of independent directors responsible for overseeing Dynasil’s investments in Xcede, reviewed the foregoing and unanimously approved Dynasil’s investment. Immediately prior to these investments, the existing convertible notes of Xcede were restructured so that holders (including Dynasil) were converted into junior preferred securities. Once Dynasil’s investment is fully funded, the anticipated Xcede’s capitalization shall consist of the long-term secured debt owed to CBI and the following outstanding equity: approximately 945,000 shares of senior preferred stock (with Dynasil holding 100%); approximately 5,400,000 shares of junior preferred stock (with Dynasil holding 43%) and approximately 3,240,000 shares of common stock (with Dynasil holding 83%). On a fully converted common stock basis, the foregoing would equal a Dynasil interest of approximately 54%.
The Dynasil press release dated November 18, 2016 announcing titled “Dynasil Corporation and Cook Biotech Inc. Join Together to Advance Xcede Technologies Patch through First in Human Trials” is attached as Exhibit 99.1 and incorporated by reference herein.
Item 9.01 - Financial Statements and Exhibits.
(c) Exhibits
99.1 Dynasil Corporation of America press release dated November 18, 2016.
99.2 Slides presented by Dynasil Corporation via conference call and webcast on or after November 21, 2016.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| DYNASIL CORPORATION OF AMERICA | |
| (Registrant) | |
| Date: November 18, 2016 | By: /s/ Robert J. Bowdring |
| Robert J. Bowdring | |
| Chief Financial Officer |
EXHIBIT INDEX
| Exhibit No. | Description |
| 99.1 | Dynasil Corporation of America press release dated November 18, 2016. |
| 99.2 | Slides presented by Dynasil Corporation via conference call on or after November 21, 2016. |
Exhibit 99.1

Contacts:
Patty Kehe
Dynasil Corp of America
Phone: 617.668.6855
Dennis Abbott
Director, Global Business Development
Cook Biotech Inc.
1425 Innovation Place
West Lafayette, IN 47906
Phone: 765.807.1925
Dynasil Corporation and Cook Biotech Inc. Join Together to Advance
Xcede Technologies Patch through First in Human Trials
Rochester, Minn., Nov 18, 2016 – Dynasil Corporation of America (NASDAQ: DYSL) today announced that it has made a
commitment for a further $1.2 million investment in subsidiary Xcede Technologies, Inc. This investment comes alongside a commitment
from Cook Biotech Inc. (CBI) of West Lafayette, Ind., to manage the Xcede Patch first-in-human clinical study on behalf of Xcede
in exchange for secured loan considerations of up to $1.5 million. In addition, Peter Sulick, Dynasil Chairman and CEO, and members
of his family made an additional investment of $450,000. It is anticipated this $3.15 million funding will be sufficient for Xcede
to complete first-in-human clinical trials and related preclinical tests. The preclinical studies are anticipated to begin in 2016
and the trial in 2017.
The Xcede Patch is intended to be used during surgical procedures to stop bleeding (hemostasis). Preclinical testing to date indicates this product promotes hemostasis within 60 seconds, a time that is faster than currently approved hemostatic patches, which take approximately three minutes to stop bleeding. Worldwide, the topical hemostatic market is estimated to be in excess of $1 billion in revenue. In addition, Xcede believes that the ease of use and potential price point for this product will be compelling reasons for practitioners to adopt the patch once it is approved for use as part of their regular surgical practices. Because the patch promotes hemostasis quickly, it is also under development for severe, traumatic bleeding as a follow-on indication for use.
Xcede Technologies, a subsidiary of Dynasil Corporation of America (“DCA”), is based in Rochester, MN and Seattle, WA and began operations in October 2013 following a technology transfer from Dynasil Biomedical, a subsidiary of DCA. Xcede is committed to the development and manufacture of innovative hemostatic and sealant products to control bleeding in surgical applications based on its innovative adhesive technology. The Xcede Patch is expected to be the first of these ground breaking products. Following this latest round of financing, Dynasil will own approximately 54% of Xcede.
“We are excited to announce this investment in Xcede, which underpins our continued commitment to this technology,” said Peter Sulick, CEO of DYSL. “Those of you following the company closely know that this has been a lengthy and complicated process. We are very thankful for the ongoing support of our development partner, Cook Biotech Inc. CBI has agreed to provide yet another level of support for this extraordinary product, one component of which is CBI’s core technology. Once in commercial production, we believe the product will greatly improve operation room efficiencies and patient outcomes.” Linda Zuckerman, PhD, Xcede president and CEO concurs. “Dynasil’s investment combined with Cook’s extensive experience in medical device development and clinical trial execution will continue to move the Xcede Patch forward towards licensure. We are on a timeline to get the product in the marketplace within the next several years.”
Umesh Patel, PhD, Vice President & General Manager of CBI, states, “Cook's extracellular matrix technology combined with Xcede's adhesive technology provides an excellent foundation for Xcede’s first product. We look forward to helping move this technology into the clinical phase of development.”
Conference Call Information
Dynasil will host a conference call for investors and analysts at 2:00 p.m. on Monday, November 21, 2016. The call will be hosted by Peter Sulick, Dynasil’s Chairman, CEO and President and Linda Zuckerman, PhD, Xcede’s CEO and President. Those who wish to listen to the conference call can go to the event page at or visit the Investor Information section of the Company’s website at www.dynasil.com. The call also may be accessed by dialing (888) 346-2613 or (412) 902-4252. For interested individuals unable to join the live conference call, a webcast replay will be available on the Company’s website for one year.
About Dynasil
Dynasil Corporation of America (NASDAQ: DYSL) develops and manufactures detection and analysis technology, precision instruments and optical components for the homeland security, medical and industrial markets. Combining world-class technology with expertise in research and materials science, Dynasil is commercializing products including dual-mode radiation detection solutions for Homeland Security and commercial applications, probes for medical imaging and sensors for non-destructive testing. Dynasil has an impressive and growing portfolio of issued and pending U.S. patents. The Company is based in Watertown, Massachusetts, with additional operations in Mass., Minn., NY, NJ and the United Kingdom. More information about the Company is available at www.dynasil.com.
Forward Looking Statements
This news release may contain forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements regarding future events and our future results are based on current expectations, estimates, forecasts, and projections and the beliefs and assumptions of our management, including, without limitation, our expectations regarding results of operations, the commercialization of our technology, in particular the Xcede patch, the success of efforts to fund Xcede, positive outcomes of our pre-clinical and planned clinical trials, regulatory approvals and the strength of our intellectual property portfolio. These forward-looking statements may be identified by the use of words such as “plans”, “intends,” “may,” “could,” “expect,” “estimate,” “anticipate,” “continue” or similar terms, though not all forward-looking statements contain such words. The actual results of the future events described in such forward looking statements could differ materially from those stated in such forward looking statements due to a number of important factors. These factors that could cause actual results to differ from those anticipated or predicted include, without limitation, our ability to develop and commercialize the Xcede patch, including obtaining regulatory approvals, the size and growth of the potential markets for our products and our ability to serve those markets, the rate and degree of market acceptance of any of our products, general economic conditions, costs and availability of raw materials and management information systems, our ability to obtain and maintain intellectual property protection for our products, competition, the loss of key management and technical personnel, our ability to obtain timely payment of our invoices to governmental customers, litigation, the effect of governmental regulatory developments, the availability of financing sources, our ability to identify and execute on acquisition opportunities and integrate such acquisitions into our business, and seasonality, as well as the uncertainties set forth in Dynasil Corporation of America’s Annual Report on Form 10 K, as filed on December 17, 2015 and from time to time in the Company's other filings with the Securities and Exchange Commission. Dynasil Corporation of America disclaims any intention or obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. Dynasil Corporation of America disclaims any intention or obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
Exhibit 99.2

Dynasil Corporation of America Xcede Conference Call November 21, 2016

2 Forward - Looking Statements The statements made in this presentation which are not statements of historical fact are forward looking statements within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 . Forward - looking statements involve known and unknown risks, uncertainties and other factors . The words “potential,” “develop,” “promising,” “believe,” “will,” “would,” “expect,” “anticipate,” “intend,” “estimate,” “plan,” “may,” “likely,” “could,” and other expressions which are predictions of or indicate future events and trends and which do not constitute historical matters identify forward - looking statements, including without limitation management’s discussion of the company’s strategic plans . Future results of operations, projections, and expectations, which may relate to this release, involve certain risks and uncertainties that could cause actual results to differ materially from the forward - looking statements . Factors that would cause or contribute to such differences include, but are not limited to, our ability to develop and commercialize the Xcede patch, including obtaining regulatory approvals, the size and growth of the potential markets for our products and our ability to serve those markets, the rate and degree of market acceptance of any of our products, our ability to obtain and maintain intellectual property protection for our products, competition, the loss of key management and technical personnel, the availability of financing sources, as well as the factors detailed in the Company's Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q, as well as in the Company's other Securities and Exchange Commission filings .

Agenda • What is this Product? • Why is it Important? • How Much is the Financing? • When? – Timeline to Launch 3

What is this Product?
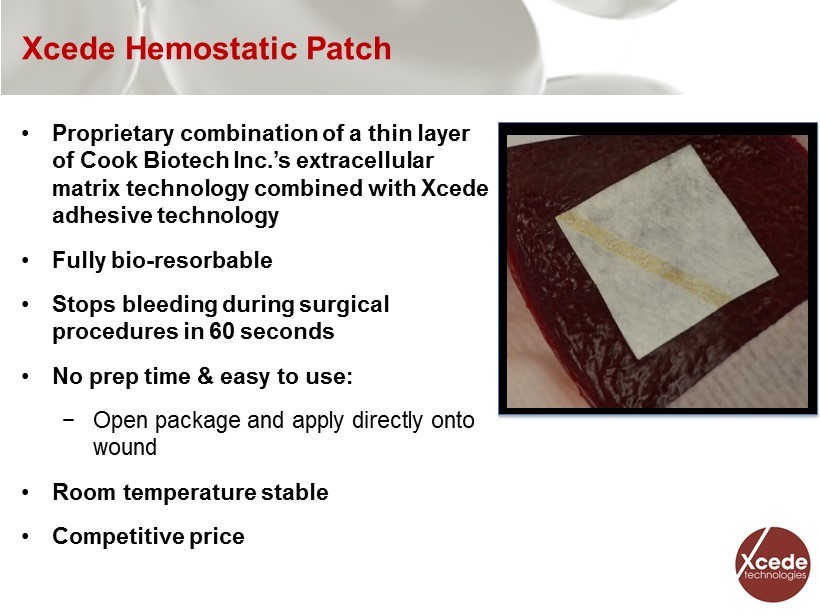
Xcede Hemostatic Patch • Proprietary combination of a thin layer of Cook Biotech Inc.’s extracellular matrix technology combined with Xcede adhesive technology • Fully bio - resorbable • Stops bleeding during surgical procedures in 60 seconds • No prep time & easy to use: − Open package and apply directly onto wound • Room temperature stable • Competitive price 5

Product Efficacy Anticipated Pharmacoeconomics Market Opportunity Xcede Patch Why is it Important?

Impressive Pre - Clinical Performance 7 Rapid time to hemostasis (stops bleeding) in preclinical studies have impressed surgeons: “ The Xcede Patch stops bleeding within 60 seconds in preclinical animal studies – an impressive time to hemostasis. It could be a significant new technology in the topical hemostatic space and I look forward to hearing about its development in the months to come.” - Thomas Biehl , MD, Chief of Dept. of General, Thoracic, and Vascular Surgery, Virginia Mason Medical Center, Seattle, WA. Deputy Chief of Surgery, Virginia Mason Medical Center “ The Xcede Patch is a new and exciting hemostatic technology that has the potential to initiate fast hemostasis.” - Grant V. Bochicchio, MD, MPH Chief, Acute and Critical Care Surgery; Edison Professor of Surgery Department of Surgery, Washington University School of Medicine

Beneficial for Patients and Hospital Payors 1. Achieving Hemostasis in 60 seconds may prevent significant blood loss and prevent related complications: – Reduced need for transfusions and blood products use – Reduced lack of blood to major organs (i.e., ischemia) – Unbalanced concentrations of coagulation and other blood factors (i.e., hemodynamic compromise) 2. Fast hemostasis could reduce hospital costs: – Operating room time – Length of hospital stay – ICU admissions 8 ANTICIPATED RESULT: BETTER OUTCOMES / LOWER COSTS

Significant Market Opportunities 9 $537 million $87 million $1.09 billion $465 million Untapped Severe Bleeding Market $42 million – Hospital $32 million – EMS/Law Enforcement $7.5 million – Military EU Topical Hemostatic Market US Topical Hemostatic Market MedMarket Diligence, LLC and other internal sources

How Much Funding and Dynasil Position 18 - Month Funding Plan CBI $1.5 M Loan DYSL $1.2 M Investment Revised Cap Table

Funding to 1 st Value Inflection Point • Xcede funding for next 18 months has been provided by: – P. Sulick $450,000 Bridge Investment – DYSL $1.2 million for G&A – Cook Biotech Inc. (CBI) commitment of $1.5 million to Xcede • Biocomp , toxicology & survivability studies • First - in - human clinical trial 11

CBI Funding of Xcede FIH: Commitment of $1.5M CBI funding (non dilutive): • CBI to perform clinical work through first - in - human (FIH) trials for up to $1.5 million • Structured as a secured promissory note, 2% interest with an outside maturity date of December 31, 2025 – Loan agreement ($1.5 million in 3 tranches of $500,000) – Services agreement – Statement of Work (document that summarizes scope of work) – Promissory Note – Security Agreement – CBI will not cover additional Xcede overhead or G&A 12

DYSL Funding of Xcede Key Terms: • Convertible Series B Preferred Stock • $1.2M funded in 6 tranches across 18 months – Funding to match Xcede projected expenses • Liquidation preference over Convertible Series A Preferred Stock • Conversion on a one for one basis into shares of common stock, subject to anti - dilution provisions • One vote per share on an “ as - if converted basis” • Standard anti - dilutions provisions 13

Revised Cap Table* 14 Shares Percentage Dynasil 5,983,000 54% Outside Investors 2,839,000 26% Mayo Foundation 756,000 7% Xcede Management (Options) 1,424,000 13% Total 11,002,000 100% * Fully diluted, as if converted

When? Timeline to Launch

Estimated Timeline to Approvals and Launch 16 Q4 2016 2017 2018 2019 2020 2021 Biocomp FIH Clinical CE Mark Filing CE Mark EU Launch US Pivotal Study PMA Filing PMA Approval US Launch Value inflection points

Questions?
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Komprise Receives Prestigious Stevie Gold Award for Data Platforms
- AM Best Downgrades Issuer Credit Rating of Members of Auto Club Enterprises Group; Removes From Under Review With Positive Implications, Upgrades Credit Ratings of Wawanesa General Insurance Company
- Hydrogen Fueling Station Market is Surge due to Efficiency, Durability & Cost Reduction | Global Size : US$ 1181.7 Mn by 2031 & CAGR of 15.37%
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share