Form 8-K DYAX CORP For: Mar 31
UNITED STATES
| SECURITIES AND EXCHANGE COMMISSION | ||
| WASHINGTON, D.C. 20549 | ||
| FORM 8-K | ||
| CURRENT REPORT | ||
|
PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
| Date of Report (Date of earliest event reported): March 31, 2015 | ||
| DYAX CORP. | ||
| (Exact Name of Registrant as Specified in Charter) | ||
| Delaware | 000-24537 | 04-3053198 |
| (State or Other Jurisdiction of Incorporation) | (Commission File Number) | (IRS Employer Identification No.) |
| 55 Network Drive | ||
| Burlington, MA 01803 | ||
| (Address of Principal Executive Offices) (Zip Code) | ||
| (617) 225-2500 | ||
| (Registrant's telephone number, including area code) | ||
| Not Applicable | ||
| (Former name or former address, if changed since last report) | ||
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| o | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| o | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| o | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| o | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 7.01. | Regulation FD Disclosure. |
On Tuesday, March 31, 2015 at 5:00 p.m. Eastern Time, Dyax Corp. (“Dyax”) will host a conference call and live webcast to provide a presentation on the results of Dyax’s Phase 1b clinical trial of DX-2930 (the “Clinical Trial”). The slides that will be used for the presentation will be posted on Dyax’s website (www.dyax.com) at the time of the conference call. A copy of the slides is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The slides are being furnished pursuant to Item 7.01 and the information contained therein shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities under that Section. Furthermore, the information in Exhibit 99.1 shall not be deemed to be incorporated by reference into Dyax’s filings under the Securities Act of 1933, as amended.
| Item 8.01. | Other Events. |
On Tuesday, March 31, 2015, Dyax issued a press release announcing the results of the Clinical Trial. A copy of the press release is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated by reference herein.
| Item 9.01. | Financial Statements and Exhibits. |
| (d) | Exhibits. |
| 99.1 | Presentation slides of Dyax Corp. dated March 31, 2015. |
| 99.2 | Press release of Dyax Corp. dated March 31, 2015 announcing results from Phase 1b clinical trial of DX-2930. |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| DYAX CORP. | |||
| Dated: March 31, 2015 | By: | /s/ Gustav Christensen | |
| Gustav Christensen | |||
| Chief Executive Officer and President | |||
EXHIBIT INDEX
Exhibit
| No. | Description |
| 99.1 | Presentation slides of Dyax Corp. dated March 31, 2015. |
| 99.2 | Press release of Dyax Corp. dated March 31, 2015 announcing results from Phase 1b clinical trial of DX-2930. |
Exhibit 99.1

DX - 2930 Phase 1b Data Review MARCH 31, 2015

This presentation contains forward - looking statements, including statements regarding the prospects for therapeutic benefits and treatment advantages of an investigational product, DX - 2930, being developed by Dyax for hereditary angioedema (HAE). Statements that are not historical facts are based on our current expectations, beliefs, assumptions, estimates, forecasts an d projections about the industry and markets in which Dyax competes. The statements contained in this presentation are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results to differ materia lly from what is expressed in such forward - looking statements. We caution investors not to place undue reliance on such forward - looking statements. There are many factors that could cause actual results to differ materially from those in these forward - looking statements. Thes e factors include the following: • The results from our Phase 1b study may not be predictive of the results or success of future clinical trials that will be re qui red to permit application for regulatory approval of DX - 2930; • even if DX - 2930 progresses through clinical trials and gains regulatory approval, it may not gain market acceptance; • others may develop technologies or products superior to DX - 2930 or that reach the market before DX - 2930; • Dyax is dependent on the expertise, effort, priorities and contractual obligations of third parties in the manufacture, quali ty control, storage and clinical development of DX - 2930; • t he costs of prosecuting, maintaining, defending and enforcing our patents and other intellectual property rights; • t he overall condition of the financial markets; and • a variety of other risks common to our industry. Additional factors that could cause actual results to differ materially from those projected or suggested in any forward - looking statements are contained in our recent annual and quarterly reports filed with the Securities and Exchange Commission, includ ing those factors discussed under the caption "Risk Factors" in such filings, which are incorporated in this presentation by this reference. Forward - looking statements speak only as of the date of this presentation, and we undertake no obligation to update o r revise them, except as may be required by law. Forward Looking Statements 2

DX - 2930 Phase 1b Data Review

DX - 2930 phase 1b data review Presenters Burt Adelman, Executive Vice President of R&D and Chief Medical Officer Ryan Iarrobino, Senior Director Clinical Development Dan Sexton, Distinguished Scientist Yung Chyung, Vice President Medical Research 4

5 DX - 2930 phase 1b study Key analyses: Safety & tolerability Pharmacokinetic profile Pharmacodynamic bioactivity Efficacy in preventing HAE attacks M ulti - center , randomized, placebo - controlled, multiple ascending dose trial in subjects with HAE

6 DX - 2930 phase 1b study design 3 dosing cohorts originally planned: 30 mg, 100 mg, 300 mg Subjects randomized to active drug vs placebo in 2:1 ratio (each subject receives 2 doses separated by 14 days ) Follow - up visits occur through 15 weeks following second dose Protocol allows for expansion of additional subjects and additional dose levels

7 Expansion and data analysis 16 additional subjects randomized to 400 mg DX - 2930 or placebo (overall total of 37 treated subjects) Data analysis was conducted after all subjects in the 400 mg dose group completed Day 50 follow - up visit Analyses include safety, pharmacokinetics, pharmacodynamics, and efficacy Study is ongoing until all subjects in 400 mg dose group reach Day 120
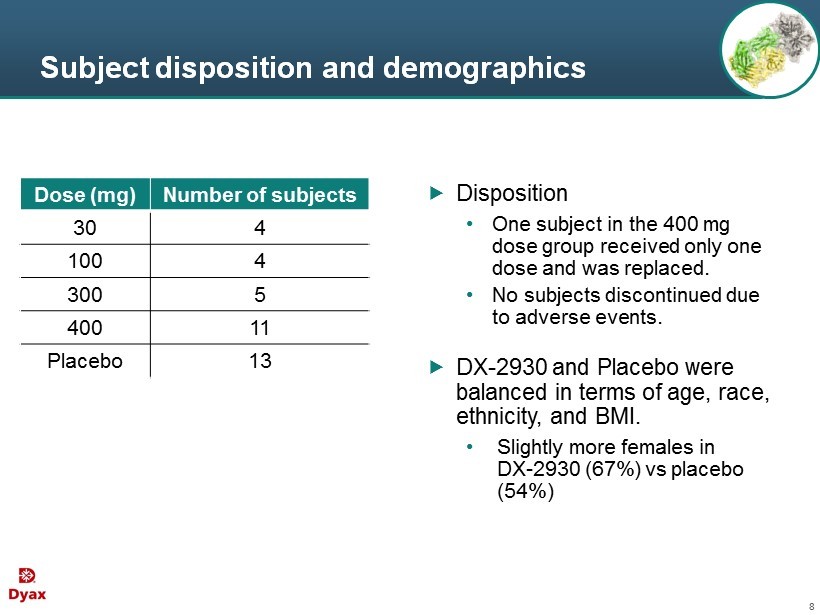
8 Subject disposition and demographics Disposition • One subject in the 400 mg dose group received only one dose and was replaced. • No subjects discontinued due to adverse events . DX - 2930 and Placebo were balanced in terms of age, race , ethnicity, and BMI. • Slightly more females in DX - 2930 (67%) vs placebo (54 %) Dose (mg) Number of subjects 30 4 100 4 300 5 400 11 Placebo 13

Phase 1b Safety

10 Summary of adverse events DX - 2930 30 mg (N = 4) DX - 2930 100 mg (N = 4) DX - 2930 300 mg (N = 5) DX - 2930 400 mg (N = 11) DX - 2930 total (N = 24) Placebo (N = 13) Adverse events (AEs)* 1 3 2 8 14 (58%) 10 (77%) Deaths or discontinuations due to AEs 0 0 0 0 0 0 Serious adverse events 0 0 0 0 0 1 (8%) Severe AEs 1 0 2 2 5 (21%) 5 (39%) Related AEs** 1 2 0 4 7 (29%) 5 (39%) * AEs in this table refer to treatment emergent adverse events (TEAE). An AE is treatment emergent if the onset time is after administration of study drug through the Day 120 post dose final follow - up visit, or in the event that onset time precedes study drug administration, th e AE increases in severity during the 120 day post dose follow - up period . ** Treatment - related AEs: Relatedness of AEs to study drug was assessed by a blinded investigator.

11 Adverse events Most common AEs were HAE attacks, injection site pain, and headache; r ates not appreciably higher in DX - 2930 2 subjects with 3 related severe TEAEs • One DX - 2930 subject (30 mg) with injection site pain lasting 1 minute • One DX - 2930 subject (400 mg) with worsening headache lasting 1 minute and night sweats

12 Summary of safety assessments No safety signals identified for ● Vital signs ● P hysical examinations ● Clinical laboratory tests ● Electrocardiogram (ECG)

13 Summary of safety R esults suggest DX - 2930 was well tolerated in this study N o evidence of dose - limiting toxicity at doses up to 400 mg

Phase 1b Pharmacokinetics and Pharmacodynamics

15 Mean DX - 2930 concentration following SC administration to HAE subjects Pharmacokinetics Note: 400 mg PK analysis excludes one patient who received only 1 dose of DX - 2930. C max ( ng /ml) T max (day) t ½ (day) 30 mg 4063.2 20 14.3 100 mg 7728.1 21 14.7 300 mg 31275.7 19.6 13.8 400 mg pending pending pending 0 10000 20000 30000 40000 50000 0 20 40 60 80 100 120 DX - 2930 ( ng /mL ) Days 30 mg (n=4) 100 mg (n=4) 300 mg (n=5) 400 mg (n=10)
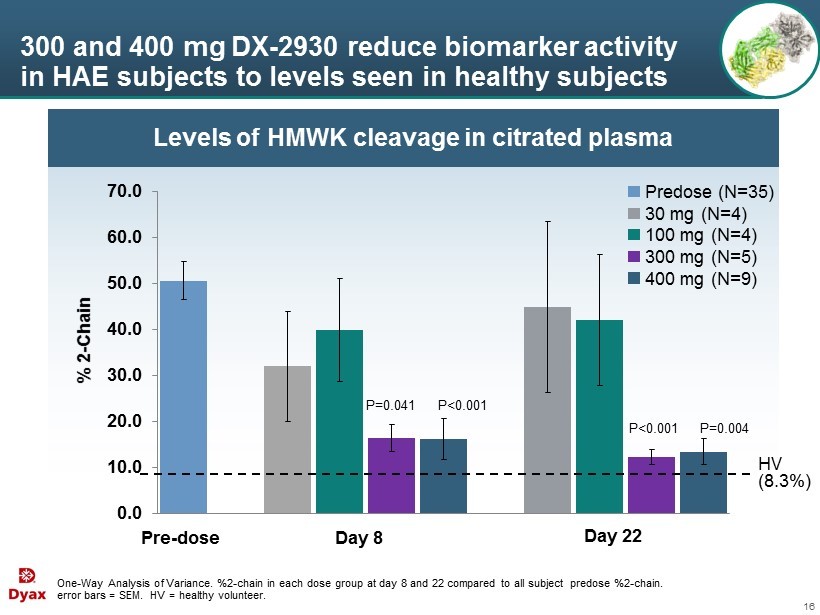
16 Levels of HMWK cleavage in citrated plasma 300 and 400 mg DX - 2930 reduce biomarker activity in HAE subjects to levels seen in healthy subjects One - Way Analysis of Variance. %2 - chain in each dose group at day 8 and 22 compared to all subject predose %2 - chain. error bars = SEM. HV = healthy volunteer. 0.0 10.0 20.0 30.0 40.0 50.0 60.0 70.0 Pre - dose Day 8 Day 22 P=0.041 P<0.001 P<0.001 P=0.004 HV (8.3%) % 2 - Chain Predose (N=35) 30 mg (N=4) 100 mg (N=4) 300 mg (N=5) 400 mg (N=9)

Phase 1b Efficacy

18 Primary efficacy assessment period 300 mg DX - 2930 400 mg DX - 2930 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day Notable drug exposure from day 8 to day 50
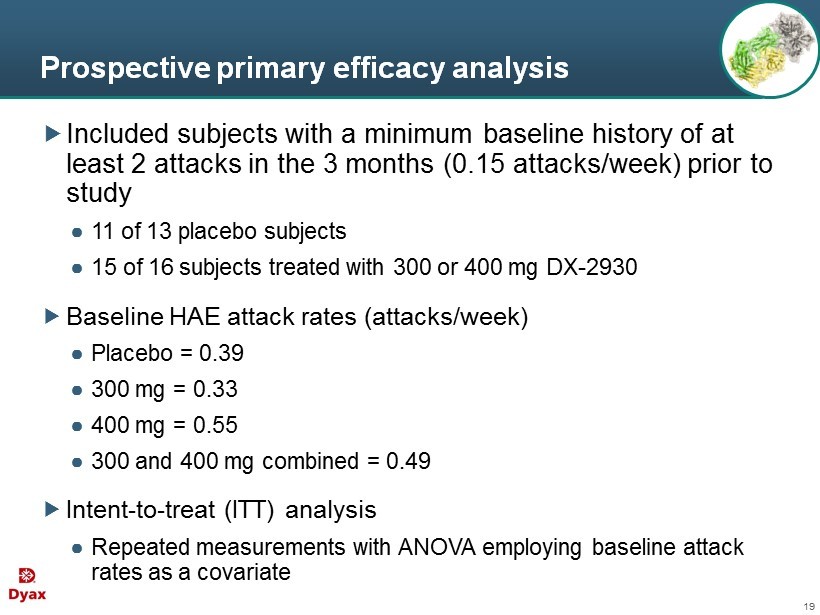
19 Prospective primary efficacy analysis Included subjects with a minimum baseline history of at least 2 attacks in the 3 months (0.15 attacks/week) prior to study ● 11 of 13 placebo subjects ● 15 of 16 subjects treated with 300 or 400 mg DX - 2930 Baseline HAE attack rates (attacks/week) ● Placebo = 0.39 ● 300 mg = 0.33 ● 400 mg = 0.55 ● 300 and 400 mg combined = 0.49 Intent - to - treat (ITT) analysis ● Repeated measurements with ANOVA employing baseline attack rates as a covariate

20 Primary efficacy analysis (Day 8 to 50) DX - 2930 300 mg (N = 4) DX - 2930 400 mg (N = 11) DX - 2930 Combined 300 and 400 mg (N = 15) % Reduction vs placebo 100 88 91 P - value vs placebo * <0.0001 0.005 0.0012 Note: Only subjects who have a baseline attack rate of at least 2 attacks in the last 3 months are included. * Mixed Model Repeated Measurements with Analysis of Variance (baseline attack frequency as covariate) and assuming Poisson distribution.
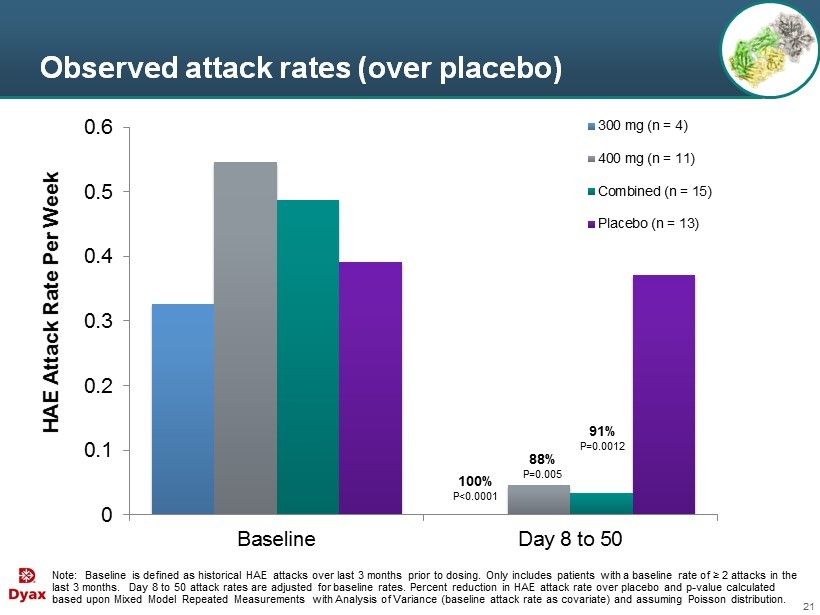
21 Observed attack rates (over placebo) Note: Baseline is defined as historical HAE attacks over last 3 months prior to dosing. Only includes patients with a baseline rate of ≥ 2 attacks in the last 3 months. Day 8 to 50 a ttack rates are adjusted for baseline rates. Percent reduction in HAE attack rate over placebo and p - value calculated based upon Mixed Model Repeated Measurements with Analysis of Variance (baseline attack rate as covariate) and assuming Poiss on distribution. 0 0.1 0.2 0.3 0.4 0.5 0.6 Baseline Day 8 to 50 HAE Attack Rate Per Week 300 mg (n = 4) 400 mg (n = 11) Combined (n = 15) Placebo (n = 13) 88% P=0.005 100% P<0.0001 91% P=0.0012

22 Proportion of subjects who were attack - free (Day 8 to 50) Note: Only subjects who have a baseline attack rate of at least 2 attacks in the last 3 months are included . Fisher exact test for each treatment arm vs. placebo. DX - 2930 300 mg (N = 4) DX - 2930 400 mg (N = 11) DX - 2930 Combined 300 and 400 mg (N = 15) Placebo (N = 11) Attack - free subjects (Day 8 to 50) 4/4 (100%) p = 0.026 9/11 (82%) p = 0.030 13/15 (87%) p = 0.004 3/11 (27%)

23 Characteristics of HAE attacks (Day 8 to 50) DX - 2930 300 mg (N = 4) DX - 2930 400 mg (N = 11) Placebo (N = 11) Attacks 0 3 24 Primary Attack Location Peripheral 0 3 10 Abdominal 0 0 13 Laryngeal 0 0 1 Attack Severity Mild 0 1 8 Moderate 0 1 6 Severe 0 1 10 Acute attacks requiring treatment 0 2 22
24 Modified intent - to - treat ( M ITT) analysis Goal: Evaluate clinical effects in the context of HAE patients receiving the dose regimen as specified Post - hoc analysis Same as ITT population except 2 subjects excluded ● 1 subject received only 1 administration of DX - 2930 (subject discontinued for reasons unrelated to treatment) – Subject had 2 peripheral attacks within day 8 to 50 ● 1 subject did not have HAE type I or II – No attacks during day 8 to 50 Note: Only subjects who have a baseline attack rate of at least 2 attacks in the last 3 months are included .
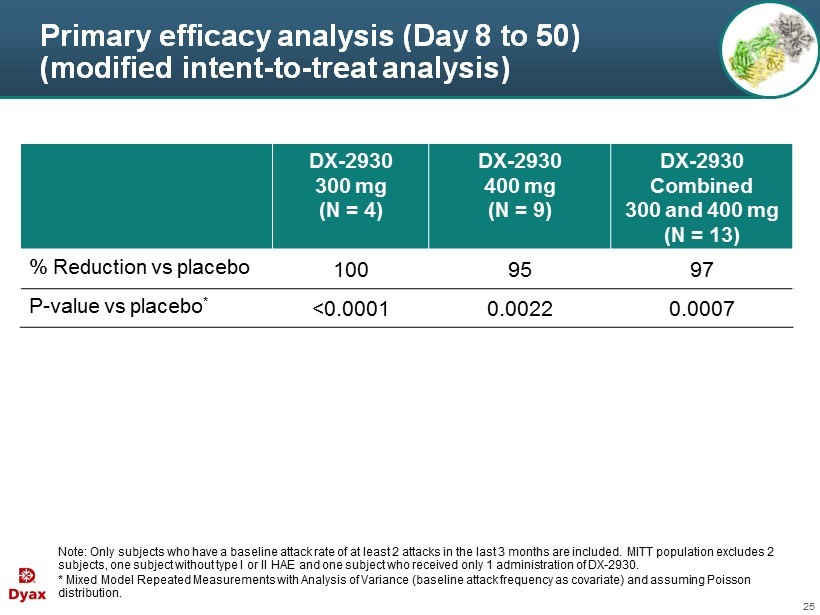
25 Primary efficacy analysis (Day 8 to 50) (modified intent - to - treat analysis) Note: Only subjects who have a baseline attack rate of at least 2 attacks in the last 3 months are included . M ITT population excludes 2 subjects, one subject without type I or II HAE and one subject who received only 1 administration of DX - 2930. * Mixed Model Repeated Measurements with Analysis of Variance (baseline attack frequency as covariate) and assuming Poisson distribution. DX - 2930 300 mg (N = 4) DX - 2930 400 mg (N = 9) DX - 2930 Combined 300 and 400 mg (N = 13) % Reduction vs placebo 100 95 97 P - value vs placebo * <0.0001 0.0022 0.0007

26 Attack incidence plotted against mean PK curves Drug Concentration Time Attacks o bserved Few or n o attacks Attacks r e - emerge Hypothesis: Higher DX - 2930 drug levels should be associated with few or no attacks Note: Hypothetical data for illustrative purposes.
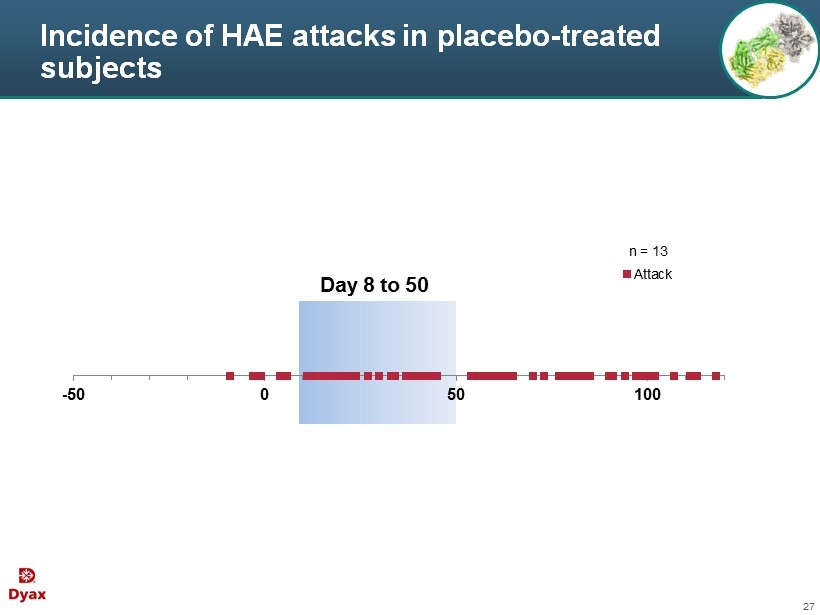
27 Incidence of HAE attacks in placebo - treated subjects -50 0 50 100 Attack Day 8 to 50 n = 13

28 Mean PK plots and HAE attack incidence following 30 and 100 mg DX - 2930 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 30 mg (n = 4) Attack 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 100 mg (n = 4) Attack Day 8 to 50 Day 8 to 50

29 Mean PK plots and HAE attack incidence following 300 mg DX - 2930 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 300 mg (n = 5) Attack Day 8 to 50
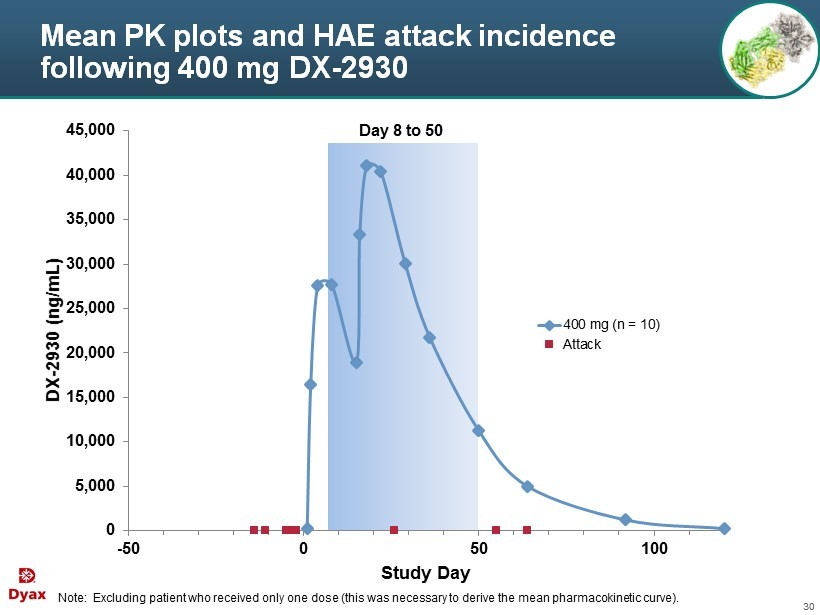
30 Mean PK plots and HAE attack incidence following 400 mg DX - 2930 Note: Excluding patient who received only one dose (this was necessary to derive the mean pharmacokinetic curve). 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 400 mg (n = 10) Attack Day 8 to 50

31 DX - 2930 concentration and HAE attacks in patients with historical attack rate ≥ 9 attacks in past 3 months -45 5 55 105 DX - 2930 (ng/mL) Study Day Placebo Historically: 35 attacks in last 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 300 mg Historically: 9 attacks in last 3 months attack DX - 2930

32 DX - 2930 concentration and HAE attacks in patients with historical attack rate ≥ 9 attacks in past 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 400 mg Historically: 9 attacks in last 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 400 mg Historically: 36 attacks in last 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 400 mg Historically: 9 attacks in last 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 400 mg Historically: 9 attacks in last 3 months attack DX - 2930

33 Summary of phase 1b No apparent safety signals have emerged to date for DX - 2930 PK profile is consistent with that of a monoclonal antibody, supporting dosing once every 2 weeks (or less frequently) Kininogen biomarker assays demonstrate DX - 2930 normalizes instability of HAE plasma A highly statistically significant result of 100% and 88% reduction in HAE attacks over placebo in the 300 and 400 mg DX - 2930 treatment groups, respectively

Regulatory Update 34
Regulatory update • US Status • Orphan Drug designation • Fast Track designation • US Next Steps • Phase 1b data to inform the design and discussion of the Phase 3 pivotal trial design with the FDA • EU Next Steps • Meet with EU regulators for scientific advice regarding evidence required for approval 35

DX - 2930 Phase 1b Data Review MARCH 31, 2015 For additional information, please contact: Jennifer Robinson 617 - 250 - 5741 [email protected]
Exhibit 99.2
| Contact: |  |
| Jennifer Robinson | |
| Director, | |
| Investor Relations and Corporate Communications | |
| (617) 250-5741 | |
| [email protected] |
Dyax Announces Positive Results from Phase 1b Clinical Trial of DX-2930
Management to Host Conference Call with Detailed Data Presentation Today at 5:00 p.m. ET
BURLINGTON, MA, March 31, 2015 – Dyax Corp. (NASDAQ: DYAX) today announced positive safety, pharmacokinetic, biomarker, and efficacy results from the Phase 1b clinical study of their investigational product, DX-2930. Discovered by Dyax, DX-2930 is a fully human monoclonal antibody inhibitor of plasma kallikrein being developed for the prevention of hereditary angioedema (HAE) attacks.
The ongoing Phase 1b study is a multi-center, randomized, double-blind, placebo-controlled, multiple-ascending dose study designed to assess the safety, tolerability and pharmacokinetics of DX-2930 in HAE patients. An analysis of HAE attack rate was also conducted following a pre-specified statistical analysis plan. A total of 37 subjects were randomized to active drug or placebo in a 2:1 ratio across 4 dosing groups of 30, 100, 300, or 400 mg. Each subject received two doses of DX-2930 or placebo, separated by 14 days, and was followed for 15 weeks after the second dose.
DX-2930 was well tolerated at all dose levels. There were no deaths or subject discontinuations due to an adverse event. There were no serious adverse events in subjects treated with DX-2930 and no evidence of dose-limiting toxicity. There was no safety signal in treatment-emergent adverse events, clinical laboratory results, vital signs, or electrocardiograms. Subcutaneous injection was well tolerated.
Pharmacokinetic results demonstrated that DX-2930 has linear, dose-dependent exposure and a mean elimination half-life of approximately 14 days across all dose groups studied. Pharmacodynamic results from two different exploratory biomarker assays confirmed ex vivo plasma kallikrein inhibition in a dose- and time-dependent manner.
Primary proof-of-concept efficacy analyses were based on subjects in the 300 mg, 400 mg, and placebo dose groups who reported having at least 2 attacks in the 3 months prior to study entry. During the pre-specified, primary efficacy interval of 6 weeks (from days 8 to 50; corresponding to peak drug level), the HAE attack rate (adjusted for baseline attacks) was 0 in the 300 mg group and 0.045 attacks per week in the 400 mg group, compared to 0.37 attacks per week in the placebo group. This resulted in a 100% reduction for the 300 mg dose group as compared to placebo (P<0.0001), and an 88% reduction for the 400 mg dose group as compared to placebo (P=0.005). During this primary efficacy interval, 100% of subjects in the 300 mg group (P=0.026) and 82% of subjects in the 400 mg group (P=0.030) were attack-free compared with 27% of subjects in the placebo group. The study will be complete when all subjects in the 400 mg dose group finish the final safety assessments on study day 120.
Today Dyax also announced receipt of Fast Track designation from the U.S. Food and Drug Administration (FDA) for the investigation of DX-2930 for HAE.
“These data provide important clinical proof-of-concept, dose response and safety information in the target patient population,” said Burt Adelman, M.D., Executive Vice President of Research and Development and Chief Medical Officer at Dyax. “The study met all of its primary objectives, and notably, DX-2930 also demonstrated statistically significant reductions in attack rate compared to placebo, an important characteristic for a prophylactic treatment. We look forward to communicating these results to the FDA to ensure that our product development plan is supportive of drug approval. We plan to take full advantage of the opportunities that Fast Track designation allows in order to maximize the possibility of a more rapid path to approval.”
“The positive results from this trial are a significant milestone for Dyax and will be integral in guiding the future clinical development of DX-2930,” said Gustav Christensen, President and Chief Executive Officer of Dyax. “If approved, we believe that DX-2930, with its unique profile, is well positioned as a potential preventive treatment option for patients suffering from HAE.”
Conference Call & Webcast
| Date: | Tuesday, March 31, 2015 | |
| Time: | 5:00 p.m. ET | |
| Telephone Access: | Domestic callers, dial 877-674-2415, | |
| International callers, dial 708-290-1364, | ||
| Reference the Dyax conference call; | ||
| Online Access: | Go to the Investor Relations section of the Dyax website (http://investor.dyax.com/index.cfm) and follow instructions for accessing the live webcast. Please connect to the website at least 15 minutes prior to the start of the conference call to ensure adequate time for any software download that may be necessary. |
About DX-2930
DX-2930 is a novel, fully human monoclonal antibody inhibitor of plasma kallikrein (pKal) which is currently being developed as a subcutaneous injection for the prevention of HAE attacks. Uncontrolled pKal activity leads to excessive generation of bradykinin, a vasodilator thought to be responsible for the localized swelling, inflammation and pain characteristically associated with HAE.
About Hereditary Angioedema (HAE)
HAE is a rare acute inflammatory condition characterized by episodes of severe, often painful swelling affecting the extremities, gastrointestinal tract, genitalia, and larynx. HAE is caused by low or dysfunctional levels of C1 esterase inhibitor (C1-INH), a naturally occurring molecule that inhibits plasma kallikrein, a key mediator of inflammation, and other serine proteases in the blood. HAE is estimated to affect up to 1 in 50,000 individuals. Learn more at www.HAEHope.com.
About Dyax
Dyax is a fully integrated biopharmaceutical company focused on the development and commercialization of novel biotherapeutics for unmet medical needs. The Company currently markets KALBITOR® (ecallantide) for the treatment of acute attacks of HAE in patients 12 years of age and older. Dyax is also developing DX-2930, a fully human monoclonal antibody, for the potential prophylactic treatment of HAE.
Both KALBITOR and DX-2930 were identified using Dyax's proprietary phage display technology. Dyax has broadly licensed this technology under its Licensing and Funded Research Portfolio (LFRP). The current portfolio includes one FDA approved product, Eli Lilly and Company’s CYRAMZA® (ramucirumab), for which Dyax receives royalties, and multiple product candidates in various stages of clinical development for which the Company is eligible to receive future milestones and/or royalties.
For additional information about Dyax, please visit www.dyax.com.
For additional information about KALBITOR, including full prescribing information, please visit www.KALBITOR.com.
Disclaimer
The press release contains forward-looking statements, including statements regarding the prospects for therapeutic benefits and treatment advantages of an investigational product, DX-2930, being developed for HAE. Statements that are not historical facts are based on Dyax’s current expectations, beliefs, assumptions, estimates, forecasts and projections about the industry and markets in which Dyax competes. The statements contained in this press release are not guarantees of future performance and involve certain risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may differ materially from what is expressed in such forward-looking statements. There are many factors that could cause actual results to differ materially from those in these forward-looking statements. These factors include the following: the results from our Phase 1b study may not be predictive of the results or success of future clinical trials that will be required to permit application for regulatory approval of DX-2930; even if DX-2930 progresses through clinical trials and gains regulatory approval, it may not gain market acceptance; others may develop technologies or products superior to DX-2930 or that reach the market before DX-2930; Dyax is dependent on the expertise, effort, priorities and contractual obligations of third parties in the manufacture, quality control, storage and clinical development of DX-2930; the costs of prosecuting, maintaining, defending and enforcing our patents and other intellectual property rights; the overall condition of the financial markets; and a variety of other risks common to our industry; changing requirements and costs associated with Dyax's planned research and development activities; competition from new and existing treatments for HAE; the uncertainty of patent and intellectual property protection; and other risk factors described or referred to in Item 1A, “Risk Factors” in Dyax’s most recent Annual Report on Form 10-K and other periodic reports filed with the Securities and Exchange Commission. Dyax cautions investors not to place undue reliance on the forward-looking statements contained in this release. These statements speak only as of the date of this release, and Dyax undertakes no obligations to update or revise these statements, except as may be required by law.
Dyax, the Dyax logo and KALBITOR are registered trademarks of Dyax Corp.
CYRAMZA® is a registered trademark of Eli Lilly and Company.
###
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Danske Bank A/S, transactions by persons discharging managerial responsibilities
- Guidewire Launches DEVSummit
- Leicester NC Diesel Performance Parts For Improved Fuel Efficiency, Range Update
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share