Form 8-K CTI BIOPHARMA CORP For: Jan 09
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 9, 2017
CTI BIOPHARMA CORP.
(Exact name of registrant as specified in its charter)
Washington | 001-12465 | 91-1533912 | ||
(State or other jurisdiction of incorporation or organization) | (Commission File Number) | (I.R.S. Employer Identification Number) | ||
3101 Western Avenue, Suite 600
Seattle, Washington 98121
(Address of principal executive offices)
Registrant’s telephone number, including area code: (206) 282-7100
Not applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Item 2.02. Results of Operations and Financial Condition.
Commencing on or after January 9, 2017, members of management at CTI BioPharma Corp. (the “Company”) will be providing a corporate update, including preliminary, unaudited estimates of its cash and cash equivalents balance and capital structure as of December 31, 2016, to analysts and investors through a series of one-on-one meetings.
The information in this Current Report on Form 8-K, including the slides to be used in these presentations attached as Exhibit 99.1 hereto, are being furnished and not filed pursuant to Item 2.02 of Form 8-K. Such information shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference into any of the Company’s filings under the Securities Act of 1933, as amended (the "Securities Act"), or the Exchange Act whether made before or after the date hereof and regardless of any general incorporation language in such filings, except to the extent expressly set forth by specific reference in such a filing.
Item 7.01. Regulation FD Disclosure.
The information set forth under Item 2.02 is incorporated by reference into this Item 7.01.
The slides to be used in these presentations are attached as Exhibit 99.1 hereto and are being furnished and not filed pursuant to Item 7.01 of Form 8-K. Such information shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference into any of the Company’s filings under the Securities Act or the Exchange Act whether made before or after the date hereof and regardless of any general incorporation language in such filings, except to the extent expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
Exhibit No. | Description | Location | ||
99.1 | CTI BioPharma Corp. Presentation Slides. | Furnished herewith. | ||
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
CTI BIOPHARMA CORP. | ||||
Date: January 9, 2017 | By: | /s/ Richard L. Love | ||
Richard L. Love | ||||
Interim President and | ||||
Chief Executive Officer | ||||
EXHIBIT INDEX
Exhibit No. | Description | Location | ||
99.1 | CTI BioPharma Corp. Presentation Slides. | Furnished herewith. | ||

Conquering cancer. That’s in our blood.
CORPORATE PRESENTATION
San Francisco – January 9, 2017
© Copyright 2017 CTI BioPharma Corp. All rights reserved.

Forward Looking Statement
This presentation includes forward-looking statements within the meaning of the Safe Harbor provisions of the Private Securities Litigation
Reform Act of 1995. Such statements are subject to a number of risks and uncertainties, the outcome of which could materially and/or adversely
affect actual future results and the trading price of CTI BioPharma's securities. Such statements include, but are not limited to, statements
regarding CTI BioPharma’s expectations with respect to the development of CTI BioPharma and its product and product candidate portfolio,
expectations with respect to the timing and planned enrollment of PAC203 and our ability to be able to interpret clinical trial data and results
and expectations with respect to the potential therapeutic utility of pacritinib, including pacritinib's potential to achieve treatment goals across
patients with myelofibrosis, regardless of baseline characteristics, such as starting platelet count and in particular, its potential to reduce spleen
volume and symptom burden and improve HRQoL, the development and achievement of a registrational strategy and development program for
tosedostat, expected outcome of the PIX306 post-marketing commitment study, the initiation of additional investigation-sponsored PIXUVRI
combination studies, and its ability to increase the addressable market and potential to provide basis for U.S. NDA, liquidity, revenue and
expense projections, the anticipated achievement of partner-related milestones and product and geographic sales, initiation of investigator-
sponsored trials, the ability to obtain additional partnerships, and the execution of CTI BioPharma’s strategy to commercialize globally. Risks
that contribute to the uncertain nature of the forward-looking statements include, among others, risks associated with the biopharmaceutical
industry in general and with CTI BioPharma and its product and product candidate portfolio in particular including, among others, risks
associated with the following: that CTI BioPharma cannot predict or guarantee the pace or geography of enrollment of its clinical trials, that CTI
BioPharma cannot predict or guarantee the outcome of preclinical and clinical studies, that CTI BioPharma may not obtain favorable
determinations by other regulatory, patent and administrative governmental authorities, that CTI BioPharma may experience delays in the
commencement of preclinical and clinical studies, risks related to the costs of developing pacritinib and CTI BioPharma’s other product
candidates, and other risks, including, without limitation, competitive factors, technological developments, that CTI BioPharma may not be able
to sustain its current cost controls, further reduce its operating expenses or achieve its anticipated liquidity, revenue and expense projections,
that CTI BioPharma may not achieve previously announced goals and objectives as or when projected, that CTI BioPharma’s average net
operating burn rate may increase, that CTI BioPharma will continue to need to raise capital to fund its operating expenses, but may not be able
to raise sufficient amounts to fund its continued operation, as well as other risks listed or described from time to time in CTI's most recent filings
with the Securities and Exchange Commission on Forms 10-K, 10-Q and 8-K. Except as required by law, CTI does not intend to update any of
the statements in this presentation upon further developments.
2

Our Pipeline – Deep Hematology Expertise
3
Program Indication Phase 1 Phase 2 Phase 3 Approved Partner
Pacritinib
PERSIST-1:
Myelofibrosis
(all platelet counts)
Unpartnered
PERSIST-2:
Myelofibrosis (platelets
≤100K/µL)
PAC203: Dose
exploration study
PIXUVRI®
(pixantrone)
Aggressive B-cell NHL,
3rd-4th line
PIX306:
aNHL, 2nd-4th line
(combination w/
rituximab)1
Tosedostat
Frontline AML
(+ LDAC or HMA or
“7+3”) - multiple Phase
2 ISTs
Unpartnered
1. E.U. confirmatory Phase 3 trial. PIXUVRI® has conditional approval in the E.U. The benefit of PIXUVRI treatment has not
been established in patients when used as fifth-line or greater chemotherapy in patients who are refractory to last therapy.
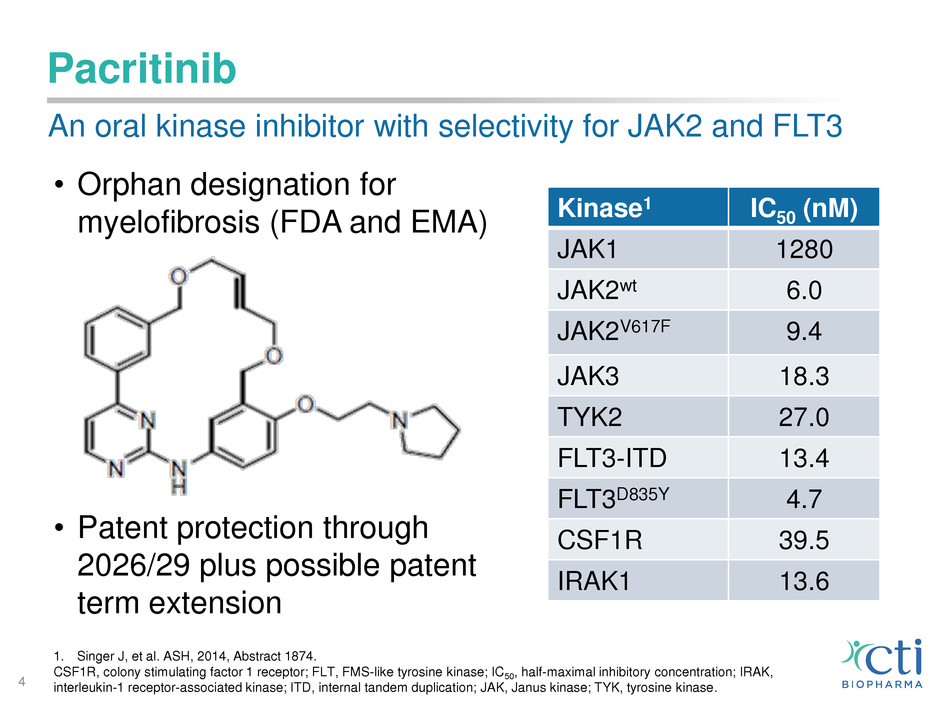
Pacritinib
• Orphan designation for
myelofibrosis (FDA and EMA)
• Patent protection through
2026/29 plus possible patent
term extension
An oral kinase inhibitor with selectivity for JAK2 and FLT3
4
1. Singer J, et al. ASH, 2014, Abstract 1874.
CSF1R, colony stimulating factor 1 receptor; FLT, FMS-like tyrosine kinase; IC50, half-maximal inhibitory concentration; IRAK,
interleukin-1 receptor-associated kinase; ITD, internal tandem duplication; JAK, Janus kinase; TYK, tyrosine kinase.
Kinase1 IC50 (nM)
JAK1 1280
JAK2wt 6.0
JAK2V617F 9.4
JAK3 18.3
TYK2 27.0
FLT3-ITD 13.4
FLT3D835Y 4.7
CSF1R 39.5
IRAK1 13.6

Myelofibrosis: A Chronic Disease
• Malignant bone marrow disorder
• Activation of JAK2 pathway triggers an inflammatory
response, bone marrow scarring
• Poor QOL: enlargement of the spleen, anemia,
thrombocytopenia, extreme fatigue, pain, severe itching,
drenching night sweats and GI side effects
• Median survival after diagnosis is 7-9 years
• Incidence: ~1 per 100K; Prevalence: ~6-10 per 100K
(~18K US, ~24K in Western EU)
• Only approved therapy: ruxolitinib (JAK1/JAK2)
5

Thrombocytopenia and MF
1. Platelet distribution for patients in Europe is similar to US.
2. Platelet distribution based on Visani et al. 1990 Br J Haematol; Caramazza et al. 2011 Leukemia; Tam et al. 2009 JCO.
3. Based on Mehta, et al. 2014 Leukemia & Lymphoma.
“When you combine disease-related
and treatment-emergent
thrombocytopenia, the percentage
of MF patients with platelet counts
<100k exceeds 50%.”
-Dr. Reuben Mesa, Mayo Clinic,
ASCO 2015
6
<50K
50K – 100K
100K – 150K
>150K
10%
11%
16%
63%
US MF Patients
by Platelet Count1,2 (n=18,0003)
~26% of Rx-naïve patients have platelets <100K/µL

Pacritinib Targets Major Unmet Needs
7
• Despite baseline platelets
≤100K/µL excluded in COMFORT
trials1, dose reductions (52%) at
≤12 weeks of therapy due to
anemia and/or thrombocytopenia2
• Most patients (85%) who
discontinue frontline rux received
no further Rx, 7 month median OS3
1. Verstovsek, et al. 2013 NEJM.
2. Verstovsek, et al. 2013 Haematologica.
3. Mehra, et al , ASH 2016 poster, Abstract 4769
Ruxolitinib Intolerance
MF Patients with Low Platelets
Ruxolitinib Failures
• Ruxolitinib not indicated for patients with platelets < 50 K/µL

Study Centers: Europe, Australia, New Zealand, Russia and U.S.
Enrolled: 327 patients
Results: Topline March 2015; oral presentations at ASCO and EHA 2015
Principal Investigators: Ruben Mesa, M.D., Mayo Clinic, Arizona
Claire Harrison, M.D., Guy’s Hospital, London
*Cross-over from BAT allowed after progression or assessment of the primary endpoint.
Eligibility Criteria
No exclusion for
platelet levels,
stratified for platelet
counts of 100,000/µL
and 50,000/µL
No prior treatment
with JAK2 inhibitors
2:1
Randomization*
n = 327
Primary Endpoint
% of patients achieving
≥35% reduction in
spleen size from
baseline to Week 24
Best Available
Therapy (BAT)
excl. ruxolitinib
Pacritinib
(400 mg QD)
PERSIST-1 Phase 3
8

PERSIST-1 Phase 3 Results: Highlights
Primary Endpoint:
• Significantly more patients achieved ≥35% spleen volume
reduction (SVR), regardless of baseline platelet count
(p=0.0003, ITT population)
Low Platelet Subset:
• Significant SVR also observed in highest-risk predefined
subset (baseline platelets <50,000/μL (p=0.045, ITT
population)
TSS Reduction:
• Significantly more patients achieved >50% reduction in TSS
(p<0.0001,ITT population)
Improved Platelet Counts
• Patients with baseline platelets <50,000/μL had significant
increase in platelet counts of 35% by Week 24 (p=0.003)
Mesa RA, et al., ASCO 2015. Abstract LBA7006. 9
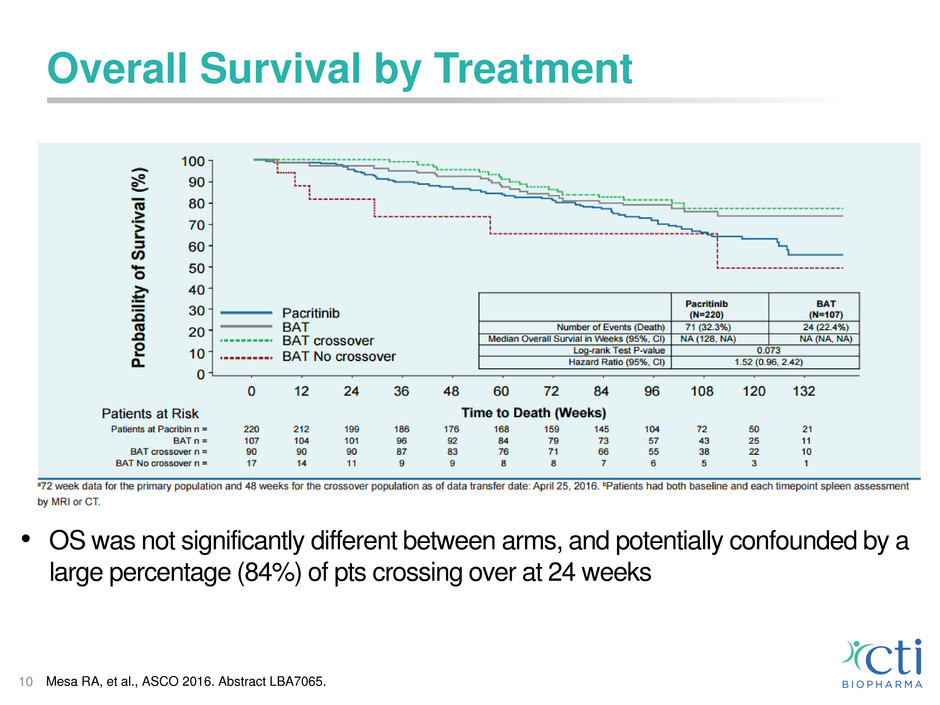
Overall Survival by Treatment
10
• OS was not significantly different between arms, and potentially confounded by a
large percentage (84%) of pts crossing over at 24 weeks
Mesa RA, et al., ASCO 2016. Abstract LBA7065.

PERSIST-1: Most Common Adverse Events
11
AE, adverse event; BAT, best available therapy, excluding ruxolitinib; PAC, pacritinib.
All Grades Grade 3 Grade 4
Adverse Event, n (%)
PAC
(n=220)
BAT
(n=106)
PAC
(n=220)
BAT
(n=106)
PAC
(n=22)
BAT
(n=106)
Non-hematologic (>10%)
Diarrhea
117
(53.2)
13 (12.3) 11 (5.0) 0 0 0
Nausea 59 (26.8) 7 (6.6) 2 (0.9) 0 0 0
Vomiting 35 (15.9) 6 (5.7) 2 (0.9) 0 0 0
Peripheral edema 16 (7.3) 12 (11.3) 1 (0.5) 1 (0.9) 0 0
Pyrexia 11 (5.0) 11 (10.4) 4 (1.8) 1 (0.9) 0 0
Hematologic (>2%)
Anemia 49 (22.3) 21 (19.8) 32 (14.5) 13 (12.3) 5 (2.3) 3 (2.8)
Thrombocytopenia 37 (16.8) 14 (13.2) 12 (5.5) 7 (6.6) 14 (6.4) 3 (2.8)
Neutropenia 8 (3.6) 2 (1.9) 1 (0.5) 1 (0.9) 4 (1.8) 1 (0.9)
• 10% of PAC patients had dose reductions due to AE (3% diarrhea; 2% anemia)
• Estimated pacritinib dose intensity ~99%
Within 24 weeks by investigator
Mesa RA, et al., ASCO 2015. Abstract LBA7006.

Sites: North America, Europe, Australia and New Zealand
PI: Serge Verstovsek, MD Anderson Cancer Center, Houston, TX
Patient Enrollment: Completed January 2016
Clinical Hold: Imposed February 2016, lifted January 2017
Results: Topline Aug. 2016; presented as ASH 2016 oral late breaker
by Lead investigator John Mascarenhas, M.D., Mt. Sinai
* Cross-over from BAT allowed after assessment of the primary endpoint at week 24, or after progression.
** BAT may include ruxolitinib at the approved dose per its label.
Pacritinib
200 mg BID
Eligibility Criteria
Patients with
baseline platelet
counts
≤100,000/µL,
prior/current JAK2
therapy allowed
1:1:1
Randomization*
n = 311
Co-Primary
Endpoints
% of patients achieving
≥35% reduction in
spleen volume from
baseline to Week 24
Patients achieving
≥50% reduction in total
symptom score (TSS)
from baseline to
Week 24
Best Available
Therapy (BAT)**
Pacritinib
400 mg QD
PERSIST-2 Phase 3
12

ITT Population and Efficacy Results
• ITT (n=311) – safety
- PAC 211 (QD 104 + BID 107); BAT 100
• ITT FAS1 (n=221) – efficacy
- PAC 149 (QD 75 + BID 74); BAT 72
13
Co-Primary:
Pac BID+QD
Secondary:
Pac BID
Secondary:
Pac QD
BAT
SVR 18% (p = 0.001) 22% (p = 0.001) 15% (p = 0.017) 3%
TSS 25% (p = 0.079) 32% (p = 0.011) 17% (p = 0.652) 14%
1. Full Analysis Set (FAS) is per ICH E9 guidelines, Section 5.2. Includes all ITT patients with a randomization date that
allowed the possibility to reach the 24 week endpoint.
Mascarenhas J, et al., ASH 2016. Abstract LBA-5.

BAT Choices and Crossover Data
• Patients could switch to a different BAT regiment during treatment
• Crossover allowed at Week 24, or with documented progression
- 50% of BAT patients crossed over to pacritinib, 86% at or after Week 24
14
BAT
Ruxolitinib 45%
Ruxolitinib only 33%
Watch and Wait 30%
Watch and Wait only 19%
Hydroxyurea 19%
Prednisone and other 35%
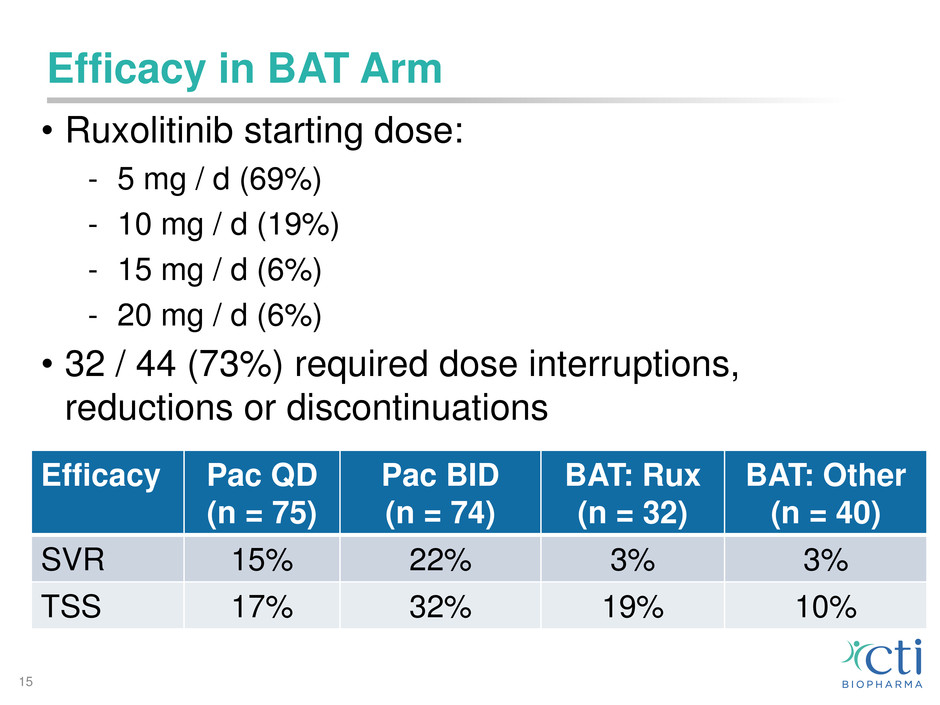
Efficacy in BAT Arm
• Ruxolitinib starting dose:
- 5 mg / d (69%)
- 10 mg / d (19%)
- 15 mg / d (6%)
- 20 mg / d (6%)
• 32 / 44 (73%) required dose interruptions,
reductions or discontinuations
15
Efficacy Pac QD
(n = 75)
Pac BID
(n = 74)
BAT: Rux
(n = 32)
BAT: Other
(n = 40)
SVR 15% 22% 3% 3%
TSS 17% 32% 19% 10%
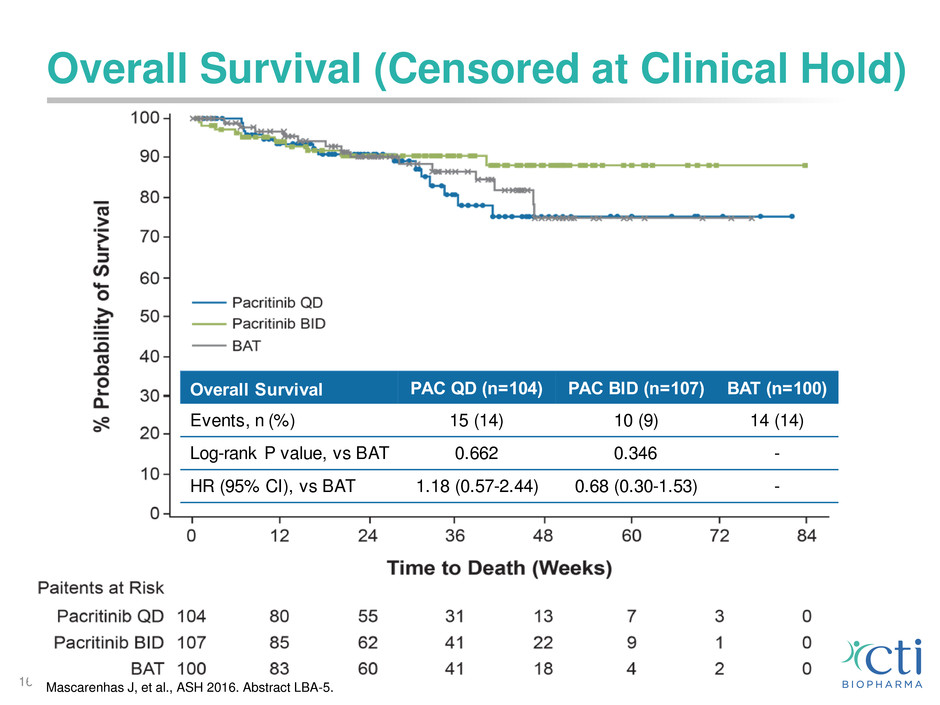
Overall Survival (Censored at Clinical Hold)
16
Overall Survival PAC QD (n=104) PAC BID (n=107) BAT (n=100)
Events, n (%) 15 (14) 10 (9) 14 (14)
Log-rank P value, vs BAT 0.662 0.346 -
HR (95% CI), vs BAT 1.18 (0.57-2.44) 0.68 (0.30-1.53) -
Mascarenhas J, et al., ASH 2016. Abstract LBA-5.

Most Common TEAEs
17
Characteristic
PAC QD
n=104
PAC BID
n=106
BAT
n=98
Pts with ≥1 TEAE 104 (100) 100 (94) 87 (89)
Diarrhea 70 (67) 51 (48) 15 (15)
Nausea 39 (38) 34 (32) 11 (11)
Thrombocytopenia 34 (33) 36 (34) 23 (23)
Anemia 29 (28) 25 (24) 15 (15)
Vomiting 22 (21) 20 (19) 5 (5)
Adverse Events were generally less frequent for BID vs. QD
Mascarenhas J, et al., ASH 2016. Abstract LBA-5.
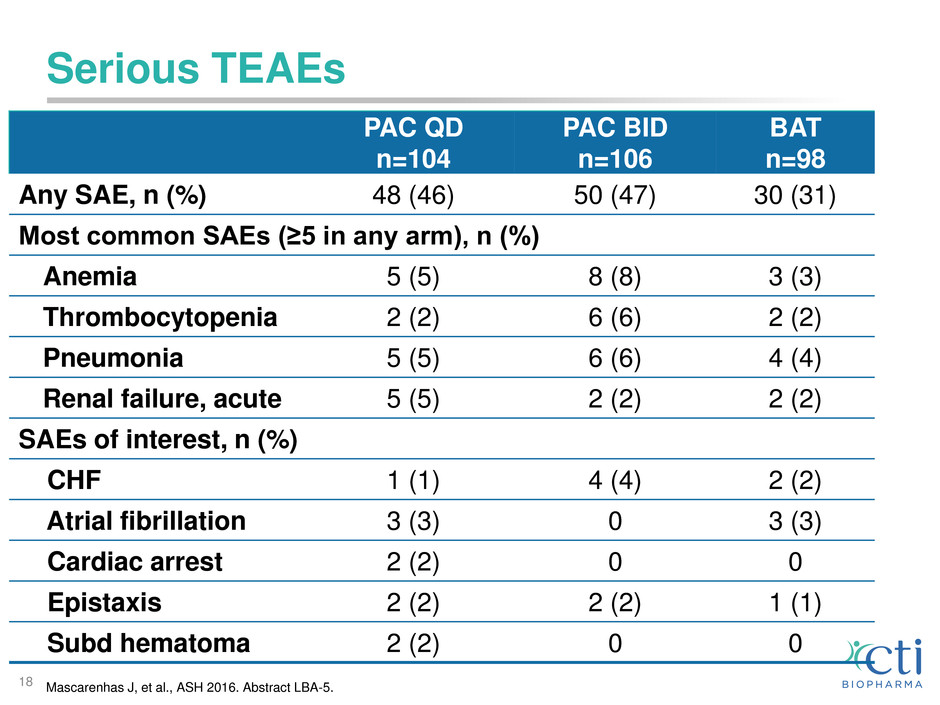
Serious TEAEs
18
PAC QD
n=104
PAC BID
n=106
BAT
n=98
Any SAE, n (%) 48 (46) 50 (47) 30 (31)
Most common SAEs (≥5 in any arm), n (%)
Anemia 5 (5) 8 (8) 3 (3)
Thrombocytopenia 2 (2) 6 (6) 2 (2)
Pneumonia 5 (5) 6 (6) 4 (4)
Renal failure, acute 5 (5) 2 (2) 2 (2)
SAEs of interest, n (%)
CHF 1 (1) 4 (4) 2 (2)
Atrial fibrillation 3 (3) 0 3 (3)
Cardiac arrest 2 (2) 0 0
Epistaxis 2 (2) 2 (2) 1 (1)
Subd hematoma 2 (2) 0 0
Mascarenhas J, et al., ASH 2016. Abstract LBA-5.

Summary of Deaths
19
PAC QD
n=104
PAC BID
n=107
BAT*
n=100
ITT, censored at the time of full clinical hold
Deaths 15 (14) 10 (9) 14 (14)
Due to AEs
Cardiac AEs
Bleeding AEs
8 (8)
2 (2)
0
4 (4)
0
3 (3)
6 (6)
2 (2)
1 (1)
Due to PD 5 (5) 5 (5) 7 (7)
Other 2 (2) 1 (1) 1 (1)
After the full clinical hold
Deaths 7 (7) 10 (9) 6 (6)
Due to AEs
Cardiac AEs
Bleeding AEs
3 (3)
1 (1)
1 (1)
1 (1)
0
0
1 (1)
2 (2)
0
Due to PD 2 (2) 5 (5) 2 (2)
Other 2 (2) 4 (4) 3 (3)
*7 of 20 pts who died did so after crossover to PAC; 5 due to AEs (3 cardiac, 1 bleeding, 1 other)
Mascarenhas J, et al., ASH 2016. Abstract LBA-5.

Clinical Hold Lifted Jan. 2017
• PAC203 trial to evaluate safety and efficacy of 3 dose
regimens of pacritinib
- Patients will be randomized to 100 mg QD, 100 mg BID or
200 mg BID
- Treatment designed to continue for 24 weeks
- Expected to enroll up to approximately 105
- Study expected to initiate in 2Q2017
• Several pacritinib ISTs for non-MF indications were
already proceeding
20

Regulatory Strategy
• Pursue application for treatment of myelofibrosis patients
who are ineligible to receive, intolerant of or have
insufficient response to ruxolitinib
• MAA on file with EMA
- At time of filing only PERSIST-1 data was available
- Evaluating whether to update the current application with
additional data from PERSIST-2 or resubmit the MAA
• NDA
- Intend to discuss with FDA the future development of pacritinib
Serious unmet medical need in myelofibrosis
21

PIXUVRI PIXUVRI®

• Palliative care
(symptom control)
or clinical trials
• Pixuvri approved in
EU for 3rd-4th line
agg. B-cell NHL
PIXUVRI: Fulfilling An Unmet Clinical Need
23
1st Line 2nd Line 3rd Line+
®
( P I X A N T R O N E )
• Typically
anthracycline-
based treatment
R-CHOP
• Curative in 50% -
55% of patients1
• Cardiac toxicity
prevents re-use of
anthracyclines in
relapsed patients
• Intensive (toxic)
non-anthracycline
based salvage
therapy (R-DHAP),
+/- ASCT
• Also, ESHAP,
R-ICE are
commonly used2
• 95% of patients
will relapse after
2nd-line therapy3
1. Adult Non-Hodgkin Lymphoma Treatment (PDQ®), Aggressive DLBCL, National Cancer Institute. Available at
www.cancer.gov. Last accessed January 2015.
2. Papadatos-Pastos D, et al. Expert Rev. Hematol. 2013; 6(1), 25-33.
3. Hagemeister FB. Cancer Chemother Pharmacol 2002;49(suppl 1):S13-20.
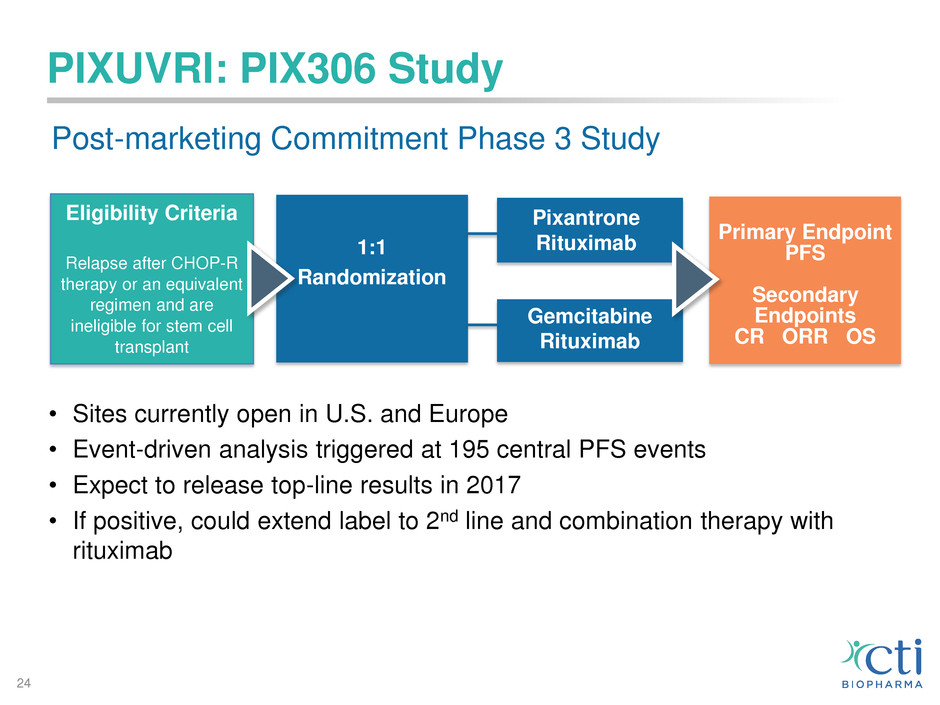
PIXUVRI: PIX306 Study
Eligibility Criteria
Relapse after CHOP-R
therapy or an equivalent
regimen and are
ineligible for stem cell
transplant
1:1
Randomization
Primary Endpoint
PFS
Secondary
Endpoints
CR ORR OS
Gemcitabine
Rituximab
Pixantrone
Rituximab
Post-marketing Commitment Phase 3 Study
• Sites currently open in U.S. and Europe
• Event-driven analysis triggered at 195 central PFS events
• Expect to release top-line results in 2017
• If positive, could extend label to 2nd line and combination therapy with
rituximab
24

Tosedostat

Tosedostat
•Oral, once-daily,
aminopeptidase inhibitor
•Interferes with protein
recycling by preventing
breakdown of peptides into
amino acids necessary for
tumor cell survival
•Synergy with targeted agents
(HMA, proteosome inhibitors)
or chemotherapy
•Several Phase 2 ISTs in AML
or MDS underway
A Novel Treatment for MDS & AML
Lowenberg B, et al., 2010 JCO 28:4333-4338.
Ubiquitin
Cellular
Proteins
N C
Ubiquitylated
proteins
26S Proteasome
Amino Acid
Deprivation
Response
Inhibition of
mTOR
Amino Acids
N C
C-
terminally
truncated
proteins
TOSEDOSTAT
26
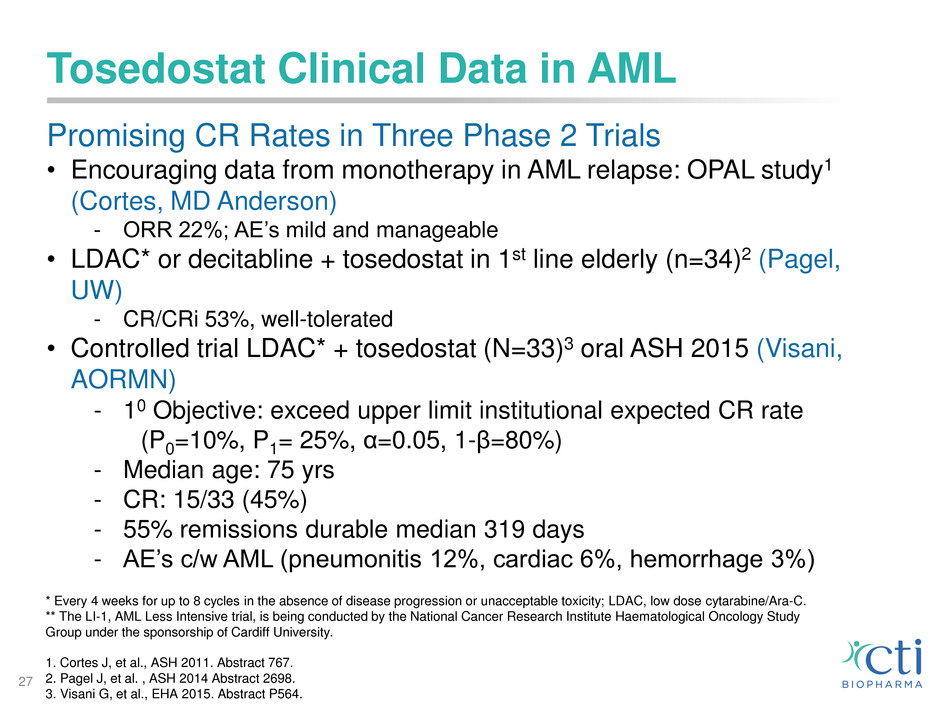
Tosedostat Clinical Data in AML
Promising CR Rates in Three Phase 2 Trials
• Encouraging data from monotherapy in AML relapse: OPAL study1
(Cortes, MD Anderson)
- ORR 22%; AE’s mild and manageable
• LDAC* or decitabline + tosedostat in 1st line elderly (n=34)2 (Pagel,
UW)
- CR/CRi 53%, well-tolerated
• Controlled trial LDAC* + tosedostat (N=33)3 oral ASH 2015 (Visani,
AORMN)
- 10 Objective: exceed upper limit institutional expected CR rate
(P0=10%, P1= 25%, α=0.05, 1-β=80%)
- Median age: 75 yrs
- CR: 15/33 (45%)
- 55% remissions durable median 319 days
- AE’s c/w AML (pneumonitis 12%, cardiac 6%, hemorrhage 3%)
27
* Every 4 weeks for up to 8 cycles in the absence of disease progression or unacceptable toxicity; LDAC, low dose cytarabine/Ara-C.
** The LI-1, AML Less Intensive trial, is being conducted by the National Cancer Research Institute Haematological Oncology Study
Group under the sponsorship of Cardiff University.
1. Cortes J, et al., ASH 2011. Abstract 767.
2. Pagel J, et al. , ASH 2014 Abstract 2698.
3. Visani G, et al., EHA 2015. Abstract P564.

Financial Overview
Capital Structure and Financial Statistics
Exchanges NASDAQ and MTA: CTIC
Market Capitalization* ~$125 mm
Shares Outstanding as of 12/31/16 ~28mm
Cash and Equivalents as of 12/31/16** ~$52mm
Debt as of 12/31/16 $19.5mm
*Based on stock price stock price of $4.46 per share as of January 5, 2017.
**Pro forma for receipt of €7.5mm milestone from Servier in January 2017.
28
Expected Cash Burn for 2017 is $65-75 mm; intend to meet cash requirements for
2017 with existing cash and by partnering one or more product assets during the
course of the year

2017 Goals
• Initiate FDA-required dose exploration study for pacritinib– Q2
• Obtain ex-U.S. partner for pacritinib and thereby obtain cash to
fund the company into 2018 – 2H
• Regulatory progress with FDA and EMA for pacritinib– YE
• Announce top-line PIX306 results - YE
29

Investment Opportunity
• Pacritinib: Potential opportunity to address major unmet
need in myelofibrosis; working towards meaningful
regulatory progress in 2017
• Pixuvri: Phase 3 readout in 2017; opportunity to expand
indication in Europe and potential for US approval
• Focus on shareholder value:
- Richard Love (Interim President and CEO) led ILEX Oncology
(Campath, Clofarabine) and Triton Biosciences (Betaseron®,
Fludarabine).
- Over $150mm cash from partnering activities since 2013
30

Thank You!
Investor Contact:
Ed Bell
Sr. Director, Investor Relations
[email protected]
206-272-4345
31

Supplemental Information

PERSIST-2 OS (Jan ‘16 cutoff)
33
Data cutoff: Jan 26, 2016
Unblinded to CTI in Aug 2016
0.5017

PERSIST Trials Patient Demographics
PERSIST: Aggressive disease, high symptom burden
34
PERSIST-1
(n=220)
PERSIST-2
(n=211)
Primary / Secondary1 65% / 35% 68% / 32%
Median time from Dx 1.0 y 2.3 y
Median platelets 169,500 65,000
Platelets <100K / µL 33% 100%
Hgb <100 g / L 38% 61%
1. Real world MF population is ~70% primary and 30% secondary to polycythema vera
(PV) or essential thrombocythemia (ET).

SVR and OS Correlation
SVR Pac BID+QD
OS HR (95% CI, n)
Pac BID
HR (95% CI, n)
Pac QD
HR (95% CI, n)
BAT
HR (95% CI, n)
≥ 35% 0.103 (0.014, 0.772)
(n=27)
0.000 (0.000, NA)
(n=16)
0.193 (0.025, 1.519)
(n=11)
1.555 (0.198, 12.195)
(n=2)
≥ 20 to <35% 0.091 (0.012, 0.681)
(n=31)
0.000 (0.000, NA)
(n=19)
0.203 (0.026, 1.586)
(n=12)
0.511 (0.066, 3.952)
(n=11)
≥10 to <20% 0.101 (0.014, 0.757)
(n=28)
0.000 (0.000, NA)
(n=14)
0.164 (0.021, 1.284)
(n=14)
0.000 (0.000, NA)
(n=6)
<10% Reference
(n=125)
Reference
(n=67)
Reference
(n=58)
Reference
(n=81)
35
• For patients in the combined pacritinib arms with ≥10%
SVR (41% of patients), highly favorable OS benefit
• No patient deaths on study for the patients in the pac BID
arm with ≥10% SVR
Data cutoff: February 8, 2016 (at time of full clinical hold)
SVR: Spleen volume response – change from baseline to week 24 by MRI or CT
OS: Overall survival
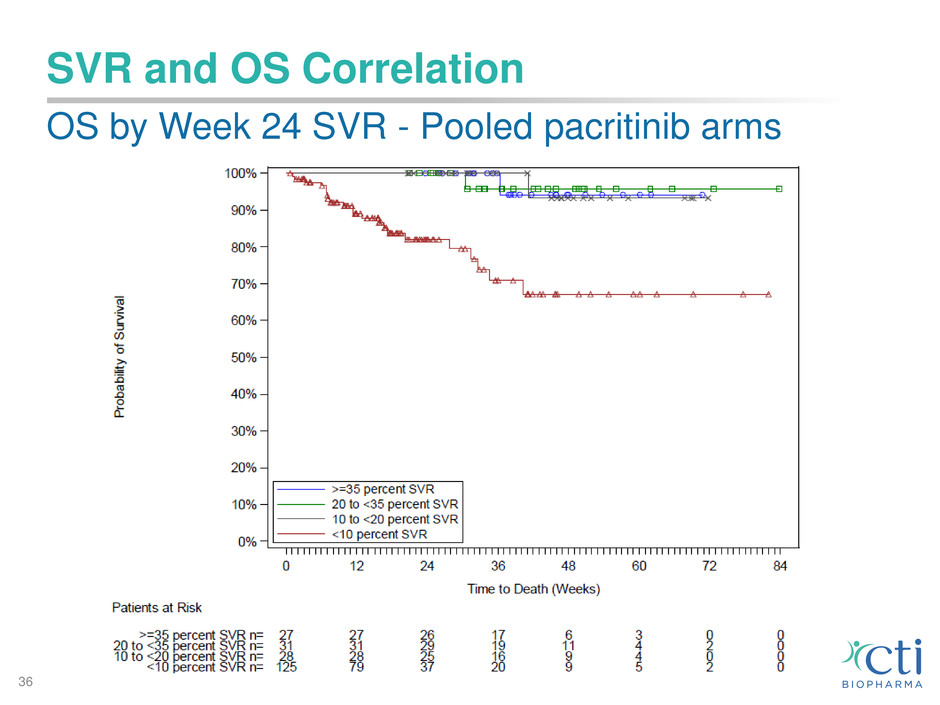
SVR and OS Correlation
OS by Week 24 SVR - Pooled pacritinib arms
36
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- U.S. Federal Magistrate Judge Recommends Invalidation of Jetaire Patents in Lawsuit Against AerSale
- Iovance Biotherapeutics Reports Inducement Grants under NASDAQ Listing Rule 5635(c)(4)
- Early Warning News Release
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share