Form 8-K Biodel Inc For: Mar 16
UNITED STATES
SECURITIES AND EXCHANGE
COMMISSION
WASHINGTON, DC 20549
______________
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or
15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): March 16, 2015
BIODEL
INC.
(Exact name of
registrant as specified in its charter)
Commission File Number 001-33451
| Delaware | 90-0136863 |
| (State or other jurisdiction of incorporation or organization) | (IRS Employer Identification Number) |
| 100 Saw Mill Road | |
| Danbury, Connecticut | 06810 |
| (Address of principal executive offices) | (Zip code) |
(203)
796-5000
(Registrant's
telephone number, including area code)
| Not Applicable |
| (Former Name or Former Address, if Changed Since Last Report) |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
[ ] |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
[ ] |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
[ ] |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
[ ] |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Item 7.01 Regulation FD Disclosure.
Biodel Inc. (the “Company”) has attached hereto as Exhibit 99.1 a slide presentation, which it intends to present and/or distribute to the investment community beginning on March 16, 2015. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
In accordance with General Instruction B.2 of Form 8-K, the information in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1, shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Exchange Act for the Securities Act of 1933, as amended, except as shall be expressly set forth by reference in such filing.
Forward Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements represent Biodel’s management's judgment regarding future events. All statements, other than statements of historical facts, including statements regarding Biodel’s strategy, future operations, future clinical trial results, future financial position, future revenues, projected costs, prospects, plans and objectives of management are forward-looking statements. The words "anticipates," "believes," "could," "estimates," "expects," "intends," "may," "plans," "potential," "predicts," "projects," "should," "will," "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Biodel’s forward-looking statements are subject to a number of known and unknown risks and uncertainties that could cause actual results, performance or achievements to differ materially from those described or implied in the forward-looking statements, including those risks and uncertainties identified in Biodel’s most recent report on Form 10-K for the fiscal year ended September 30, 2014 and other subsequent filings with the Securities and Exchange Commission.
Item 8.01 Other Events.
On March 16, 2015, the Company issued a press release reporting preliminary results from a Phase 1 clinical trial of its product candidate BIOD-961, a glucagon formulation designed for use in the Company’s proposed Glucagon Emergency Management (GEM) auto-reconstitution device. The full text of the press release is attached as Exhibit 99.2 to this Current Report on Form 8-K.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
| 99.1 | Slide Presentation of Biodel Inc. | ||
| 99.2 | Press Release issued by the Company on March 16, 2015. |
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: March 16, 2015 | BIODEL INC. | ||
| By: | /s/ Paul S. Bavier | ||
| Paul S. Bavier, General Counsel and Secretary | |||
3
EXHIBIT INDEX
| Exhibit No. | Description | ||
| 99.1 | Slide Presentation of Biodel Inc. | ||
| 99.2 | Press Release issued by the Company on March 16, 2015. | ||
4










BIOD-961 STUDY 6-101 RESULTS
Biodel’s Glucagon
Formulation for Use in a Proprietary Auto-Reconstitution Device for the
Treatment of
Severe Hypoglycemia Meets Primary Efficacy Endpoint in Phase 1
Clinical Trial
BIOD-961 Administration
Results in Glucagon Exposure and Glucose Response that Meets Standard
Bioequivalence
Criteria When Compared to Marketed Glucagon
Conference Call and Webcast Will be Held on Monday, March 16, 2015 at 8:30 a.m. Eastern Daylight Time
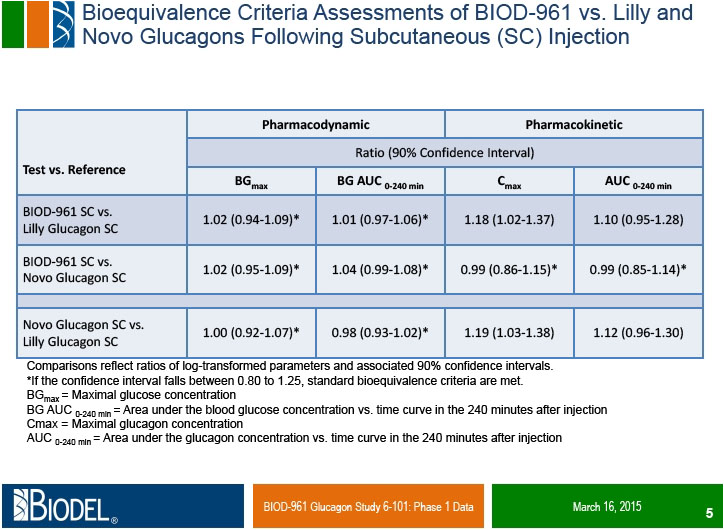
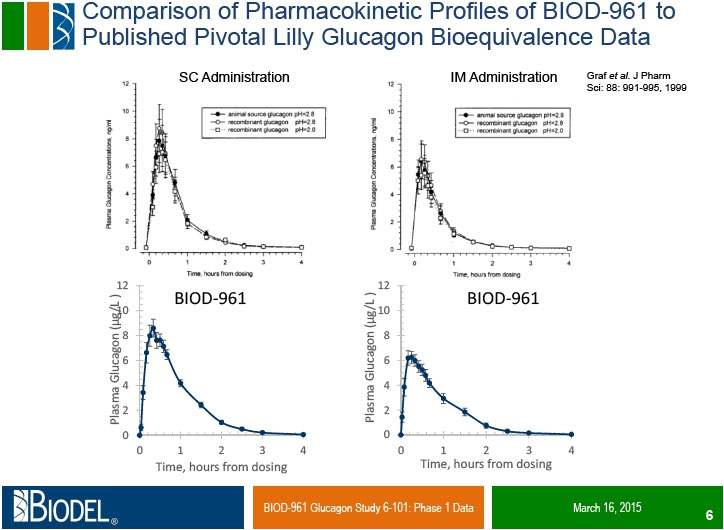
DANBURY, Conn., _____- Biodel Inc. (Nasdaq: BIOD) announced positive preliminary results from Study 6-101, a Phase 1 clinical trial comparing Biodel’s lyophilized glucagon formulation BIOD-961, designed for use in a proprietary Glucagon Emergency Management (GEM) auto-reconstitution device, to Eli Lilly’s Glucagon Emergency Rescue Kit and Novo Nordisk’s GlucaGen® HypoKitTM, which are marketed for the treatment of severe hypoglycemia. Study 6-101 was a randomized, single-center, double blind, six-period cross over study in 15 healthy volunteers who received each glucagon administered subcutaneously (SC) and intramuscularly (IM) in a randomized treatment sequence. The objectives of the study were to compare the pharmacokinetic (PK) profiles, the pharmacodynamic (PD, glucose) responses, and the PK/PD relationships of IM and SC dosing, as well as to assess safety profiles of the three test glucagons.
Data Highlights
Biodel formulated BIOD-961 for use in a user-friendly injection device, referred to as the Glucagon Emergency Management (GEM) system. The GEM device is a customized version of an auto-reconstitution syringe developed by Unilife Corporation. The device is specifically designed to address and expand an underserved market currently wrestling with cumbersome glucagon kits that are especially difficult to use and operate during an emergency for the treatment of severe hypoglycemia.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release
BIOD-961 STUDY 6-101 RESULTS
Dr. Alan Krasner, chief medical officer of Biodel, stated “We are very pleased that in this exploratory trial BIOD-961 shows the ability to meet pharmacokinetic and pharmacodynamic criteria for bioequivalence to a marketed glucagon formulation. The FDA requires PK and PD bioequivalence as co-primary objectives of our pivotal trial for approval of this product. We look forward to sharing these data with the FDA and discussing the components of the remaining development program, including the design of the pivotal bioequivalence trial.”
Dr. Errol De Souza, president and chief executive officer of Biodel, stated: “The results of this study exceeded expectations. This is the first glucagon formulation in development for the treatment of severe hypoglycemia which has demonstrated comparable pharmacokinetic and pharmacodynamic profiles to marketed glucagon formulations. In addition to the pivotal clinical trial, a human factors study is planned for the first half 2015 to compare the usability of the GEM device to the existing manually reconstituted commercial glucagon kits in the hands of representative caregivers, with the final pivotal human factors study expected to be completed by the end of 2015. We plan to request an end-of-Phase 2 meeting with the FDA in the second calendar quarter of 2015 and submit the NDA in mid-2016”.
Study Design
Study 6-101 was a Phase 1 randomized, single-center, double blind, six-period cross over study designed to evaluate the PK and PD profiles of BIOD-961 compared to marketed glucagon formulations manufactured by Eli Lilly and Novo Nordisk. BIOD-961 is a lyophilized glucagon formulation designed for use in the GEM auto-reconstitution device. On six separate dosing days, each subject received 1 mg of one of the test glucagons delivered either SC or IM. Fifteen normal, healthy subjects were randomized into the study and ten completed all dosings. The objectives of the study were to compare the PK profiles, the PD (glucose) responses, and the PK/PD relationships of IM and SC dosing, as well as to assess safety profiles of the three test glucagons. These data will facilitate selection of an appropriate marketed glucagon to use as a comparator in the planned pivotal study, in which the primary efficacy endpoint for approval is to demonstrate PK and PD bioequivalence of BIOD-961 to one of the marketed comparators.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release
BIOD-961 STUDY 6-101 RESULTS
About Glucagon Emergency Management (GEM) Device for the Treatment of Severe Hypoglycemia
Biodel's Glucagon Emergency Management (GEM) system is a lyophilized glucagon formulation provided in a proprietary auto-reconstitution device intended for the rescue treatment of patients with severe hypoglycemia. GEM is expected to be available in separate presentations for the delivery of pediatric (0.5 mg) and adult (1.0 mg) dosages of glucagon. The GEM device provides automatic needle retraction after dose delivery.
There is currently an unmet medical need for a more user-friendly glucagon emergency injection device since the complexity of existing kits increases caregiver training requirements, the likelihood of dosing errors (especially dosing only with diluent) and the time required to deliver the rescue therapy. It is believed that these factors lead to relatively low prescription, fill, refill and usage rates of existing glucagon emergency kits. The annual U.S. glucagon market for the treatment of severe hypoglycemia is approximately $160 million.
The GEM device has been designed to require little to no training for use and is intended to be a more user-friendly glucagon presentation compared to emergency kits currently marketed by Eli Lilly and Novo Nordisk. The automatic reconstitution of the glucagon formulation eliminates the possibility of dosing only with diluent. The automatic needle retraction after dose delivery reduces the risk of accidental needle stick injuries. Dating and storage of the GEM device is anticipated to be comparable to existing glucagon kits.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release
BIOD-961 STUDY 6-101 RESULTS
|
Pharmacokinetic (PK) and Pharmacodynamic (PD) Parameters: Biodel’s Glucagon Formulation (BIOD-961) vs. Eli Lilly’s Glucagon Emergency Rescue Kit (Lilly) vs. Novo Nordisk’s GlucaGen® HypoKitTM (Novo) | ||||||||
|
Parameter |
Treatment Groups | |||||||
|
Subcutaneous Injection (1 mg Glucagon) |
Intramuscular Injection (1 mg Glucagon) | |||||||
|
BIOD-961 |
Lilly |
Novo |
BIOD-961 |
Lilly |
Novo | |||
 |
Cmax (µg/L) |
9.01 ± 0.75 | 7.87 ± 0.79 | 9.16 ± 0.59 | 6.80 ± 0.52 | 6.75 ± 0.31 | 7.22 ± 0.44 | |
| AUC0-240 min (µg*min/L) |
559.5 ± 28.7 | 520.8 ± 29.8 | 570.5 ± 26.8 | 409.5 ± 39.2 | 402.6 ± 27.8 | 441.4 ± 42.7 | ||
| Tmax (min) |
26.7 ± 3.76 | 23.2 ± 1.82 | 20.8 ± 1.83 | 15.5 ± 2.07 | 17.7 ± 4.39 | 14.6 ± 2.64 | ||
 |
BGmax (mg/dL) |
178.2 ± 7.3 | 170.9 ± 5.3 | 175.3 ± 5.9 | 182.9 ± 6.5 | 171.1 ± 6.7 | 174.8 ± 5.5 | |
| BGAUC 0-240
min (mg*min/dL) |
26118 ± 630 | 25915 ± 685 | 25240 ± 424 | 26184 ± 607 | 24960 ± 568 | 25274 ± 485 | ||
| Time to BGmax (min) |
41.7 ± 4.1 | 43.6 ± 4.2 | 34.6 ± 3.2 | 40.5 ± 2.5 | 40.5 ± 3.4 | 44.6 ± 3.8 | ||
|
Data represent the
Mean ± Standard Error of the Mean | ||||||||
Conference Call and
Webcast Information
Biodel's senior management
will host a conference call on Monday, March 16, 2015 at 8:30 a.m. Eastern
Daylight Time to discuss these results. Live audio of the conference call will
be available to investors, members of the news media and the general public by
dialing +1 (877) 407-7181 (Toll-Free US & Canada) or +1 (201) 689-8047
(International). To access the call by live audio webcast, please log on to the
investor section of the company's website at http://www.biodel.com. An
archived version of the audio webcast will be available on Biodel's website
until January 14, 2015 or by dialing +1 (877) 660-6853 (Toll Free US &
Canada) or +1 (201) 612-7415 (International) and entering conference ID number
13588552.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release
BIOD-961 STUDY 6-101 RESULTS
About Biodel
Inc.
Biodel Inc. is a specialty
biopharmaceutical company focused on the development and commercialization of
innovative treatments for diabetes that may be safer, more effective and more
convenient for patients. Biodel's product candidates are developed by applying
proprietary technologies to existing drugs in order to improve their therapeutic
profiles. More information about Biodel is available at www.biodel.com.
Safe-Harbor Statement
This press release contains
forward-looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995. Such forward-looking statements include
statements about future activities related to the clinical development plans for
Biodel's product candidates, potential timing, design and outcomes of clinical
trials and Biodel's ability to develop and commercialize its product candidates.
Forward-looking statements represent Biodel's management's judgment regarding
future events. All statements, other than statements of historical facts,
including statements regarding Biodel's strategy, future operations, future
clinical trial results, future financial position, future revenues, projected
costs, prospects, plans and objectives of management are forward-looking
statements. The words "anticipates," "believes," "could," "estimates,"
"expects," "intends," "may," "plans," "potential," "predicts," "projects,"
"should," "will," "would" and similar expressions are intended to identify
forward-looking statements, although not all forward-looking statements contain
these identifying words. Biodel's forward-looking statements are subject to a
number of known and unknown risks and uncertainties that could cause actual
results, performance or achievements to differ materially from those described
or implied in the forward-looking statements, including, but not limited to, the
progress, timing or success of Biodel's research and development and clinical
programs for Biodel's product candidates; Biodel's ability to conduct the
development work necessary to finalize the formulation and design of Biodel's
auto-reconstitution glucagon rescue product candidate, as well as the
preclinical studies, clinical trials and manufacturing activities necessary to
support the filing of a new drug application, or NDA, to the U.S. Food and Drug
Administration, or FDA, for that product candidate; Biodel's ability to engage a
strategic partner in the further development of Biodel's prandial
ultra-rapid-acting insulin formulations, including BIOD-531, which uses regular
human insulin, or RHI, as the active pharmaceutical ingredient, and Biodel's
insulin analog-based formulations; the success of Biodel's formulation
development work to improve the stability of Biodel's newer ultra-rapid-acting
insulin analog-based formulations while maintaining the pharmacokinetic and
injection site toleration characteristics associated with earlier formulations;
the results of Biodel's real-time stability programs for Biodel's RHI-, insulin
analog- and glucagon-based product candidates, including the reproducibility of
earlier, smaller scale, stability studies and Biodel's ability to accurately
project long term stability on the basis of accelerated testing; Biodel's
ability to accurately anticipate technical challenges that the company may face
in the development of Biodel's ultra-rapid-acting RHI- and insulin analog-based
product candidates or Biodel's glucagon rescue product candidates; Biodel's
ability to secure approval by the FDA for Biodel's product candidates under
Section 505(b)(2) of the Federal Food, Drug and Cosmetic Act; Biodel's ability
to enter into collaboration arrangements for the commercialization of Biodel's
product candidates and the success or failure of any such collaborations into
which the company enters, or Biodel's ability to commercialize its product
candidates on its own; Biodel's ability to enforce Biodel's patents for Biodel's
product candidates and Biodel's ability to secure additional patents for
Biodel's product candidates; and other factors identified in our most recent
annual report on Form 10-K for the fiscal year ended September 30, 2014. The
company disclaims any obligation to update any forward-looking statements as a
result of events occurring after the date of this press release.
BIOD-G
CONTACT: John Graziano (The
Trout Group), +1-646-378-2942
SOURCE Biodel Inc.
Confidential BIOD-961 Phase 1 Study 6-101 Data Press Release
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Report on Financial Results for the Year Ended December 31, 2023
- Bragar Eagel & Squire, P.C. Is Investigating Zoetis, and Morgan Stanley and Encourages Investors to Contact the Firm
- UK businesses must prioritise payment technology to build customer loyalty and stay competitive: New research from Lloyds Bank and FreedomPay
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share