Form 8-K AVEO PHARMACEUTICALS For: Apr 01
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): April 1, 2016
AVEO Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in Charter)
| Delaware | 001-34655 | 04-3581650 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) | ||
| One Broadway, 14th Floor Cambridge, Massachusetts |
02142 | |||
| (Address of Principal Executive Offices) | (Zip Code) | |||
Registrant’s telephone number, including area code: (617) 588-1960
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS:
This Form 8-K and the exhibit attached hereto contain forward-looking statements of AVEO Pharmaceuticals, Inc. (“AVEO,” “we” or the “Company”) that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this Form 8-K and the attached exhibit are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “contemplate,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about: AVEO’s plans to leverage biomarkers and pursue strategic partnerships for certain of its assets; AVEO’s goals and business strategy; the timing, design and results of preclinical and clinical trials; the timing and outcome of meetings with and applications to regulatory authorities by AVEO and its partners; the competitive landscape for AVEO’s therapeutic candidates; AVEO’s ability to demonstrate tivozanib’s safety and efficacy as a combination therapy and AVEO’s estimates for its cash runway.
Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements AVEO makes due to a number of important factors, including substantial risks and uncertainties relating to: AVEO’s ability to successfully implement its strategic plans; AVEO’s ability to successfully develop, test and gain regulatory approval of its product candidates, including its companion diagnostics; developments, expenses and outcomes related to AVEO’s ongoing shareholder litigation, including the risk that the Company may be required to incur substantial costs to settle such litigation; AVEO’s ability to obtain necessary financing required to perform its clinical trials and achieve its other goals; AVEO’s ability to establish and maintain strategic partnerships; AVEO’s ability to obtain and maintain intellectual property rights; competition; AVEO’s dependence on its strategic partners and other third parties; adverse general economic and industry conditions; and those risk factors discussed in the “Risk Factors” and elsewhere in AVEO’s Annual Report on Form 10-K for the year ended December 31, 2015, and other periodic filings AVEO makes with the SEC. All forward-looking statements contained in this Form 8-K and the attached exhibit speak only as of the date of this presentation, and AVEO undertakes no obligation to update any of these statements, except as required by law. You should, therefore, not rely on these forward-looking statements as representing the Company’s views as of any date subsequent to the date of this Form 8-K.
| Item 7.01. | Regulation FD. |
From time to time, we conduct meetings with third parties in which we utilize a corporate slide presentation. A copy of our current corporate slide presentation, dated April 1, 2016, is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference. We may amend or update this information at any time and from time to time through another Current Report on Form 8-K, a later company filing, or other means.
The information in this Item 7.01 and Exhibit 99.1 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) The exhibit to this Current Report on Form 8-K is listed in the Exhibit Index attached hereto.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| AVEO Pharmaceuticals, Inc. | ||
| Date: April 1, 2016 | ||
| By: | /s/ Michael Bailey | |
| Michael Bailey | ||
| President and Chief Executive Officer | ||
EXHIBIT INDEX
| Exhibit No. |
Description | |
| 99.1 | Corporate Presentation Slide Deck, dated April 1, 2016 | |

AVEO Overview April 2016 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “contemplate,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about: AVEO’s plans to leverage biomarkers and pursue strategic partnerships for certain of its assets; AVEO’s goals and business strategy; the timing, design and results of preclinical and clinical trials; the timing and outcome of meetings with and applications to regulatory authorities by AVEO and its partners; the competitive landscape for AVEO’s therapeutic candidates; AVEO’s ability to demonstrate tivozanib’s safety and efficacy as a combination therapy and AVEO’s estimates for its cash runway. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements AVEO makes due to a number of important factors, including substantial risks and uncertainties relating to: AVEO’s ability to successfully implement its strategic plans; AVEO’s ability to successfully develop, test and gain regulatory approval of its product candidates, including its companion diagnostics; developments, expenses and outcomes related to AVEO’s ongoing shareholder litigation; AVEO’s ability to obtain necessary financing required to perform its clinical trials and achieve its other goals; AVEO’s ability to establish and maintain strategic partnerships; AVEO’s ability to obtain and maintain intellectual property rights; competition; AVEO’s dependence on its strategic partners and other third parties; adverse general economic and industry conditions; and those risk factors discussed in the “Risk Factors” and elsewhere in AVEO’s Annual Report on Form 10-K for the year ended December 31, 2015, and other periodic filings AVEO makes with the SEC. All forward-looking statements contained in this presentation speak only as of the date of this presentation, and AVEO undertakes no obligation to update any of these statements, except as required by law.

AVEO Oncology Highlights Significant N.A. rights for all oncology therapeutic assets Retained North American tivozanib oncology rights TIVO-3 pivotal refractory RCC study planned to support both 1st & 3rd line indications in U.S. Exploring immunotherapy combination in RCC and CRC biomarker Ficlatuzumab and AV-203 partner-funded through POC studies Potential value creation through advancing portfolio through partnerships Tivozanib under regulatory review in EU and Russia Partnered Ex-N.A. (EUSA, Pharmstandard) and ocular (Ophthotech) rights Novartis with AV-380; Biodesix with ficlatuzumab; CANbridge with AV-203 $34.1M in cash and securities at year end 2015 Potential for >$40M in additional partnership payments through mid-2017 including ~$10M in operational, not clinical milestones (IND filings, option exercises) Lean organization, experienced management team and Board
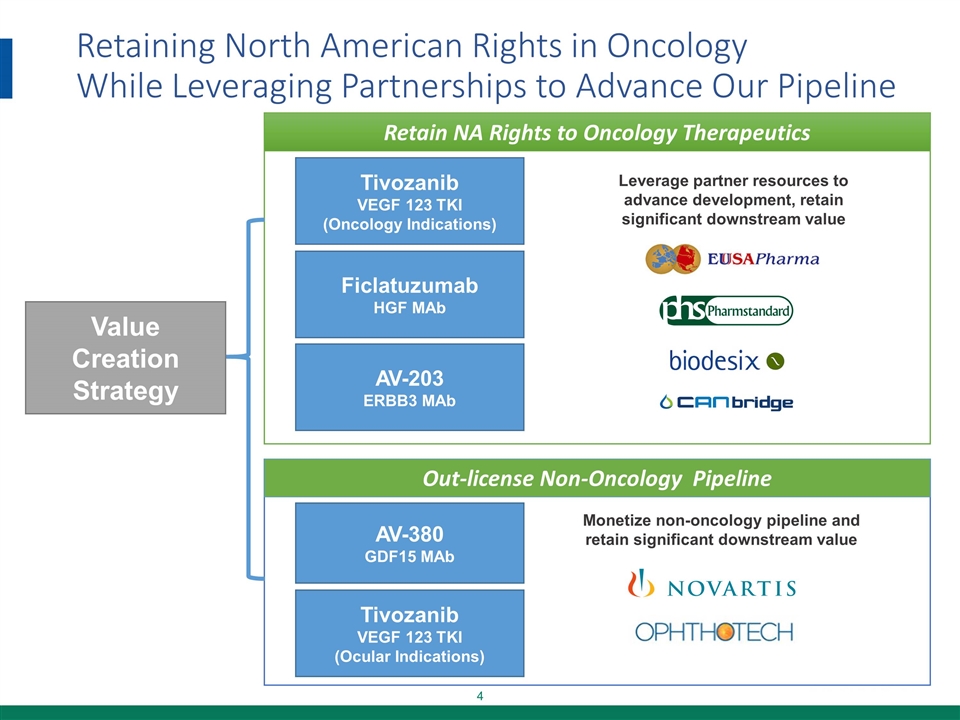
Retaining North American Rights in Oncology While Leveraging Partnerships to Advance Our Pipeline AV-380 GDF15 MAb Tivozanib VEGF 123 TKI (Oncology Indications) AV-203 ERBB3 MAb Ficlatuzumab HGF MAb Value Creation Strategy Leverage partner resources to advance development, retain significant downstream value Retain NA Rights to Oncology Therapeutics Out-license Non-Oncology Pipeline Tivozanib VEGF 123 TKI (Ocular Indications) Monetize non-oncology pipeline and retain significant downstream value
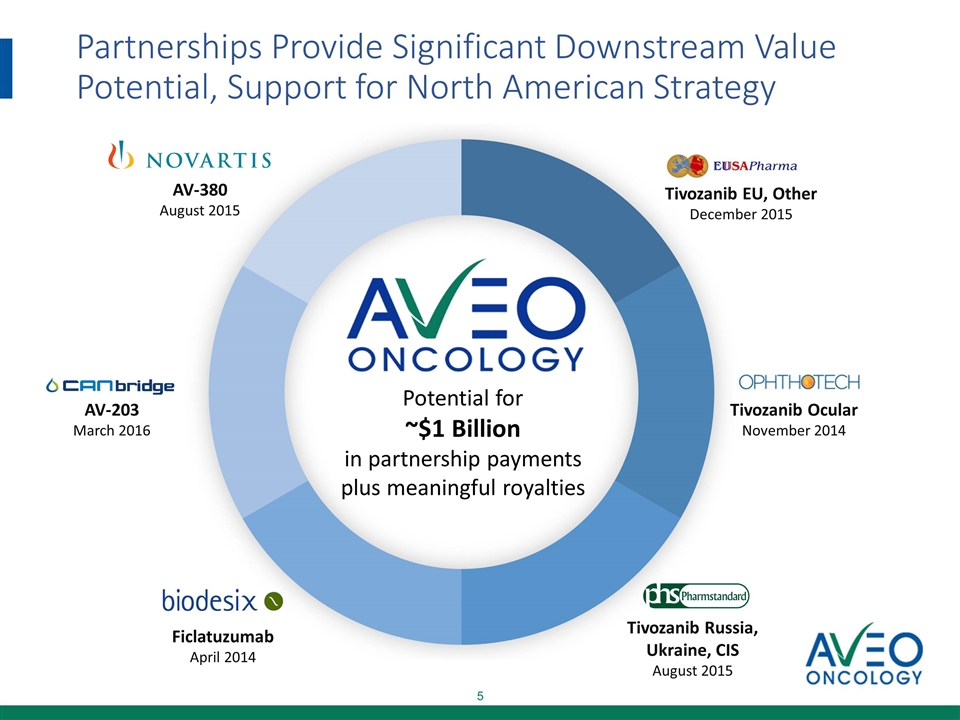
Partnerships Provide Significant Downstream Value Potential, Support for North American Strategy Tivozanib EU, Other December 2015 Tivozanib Ocular November 2014 Tivozanib Russia, Ukraine, CIS August 2015 AV-380 August 2015 Ficlatuzumab April 2014 Potential for ~$1 Billion in partnership payments plus meaningful royalties AV-203 March 2016
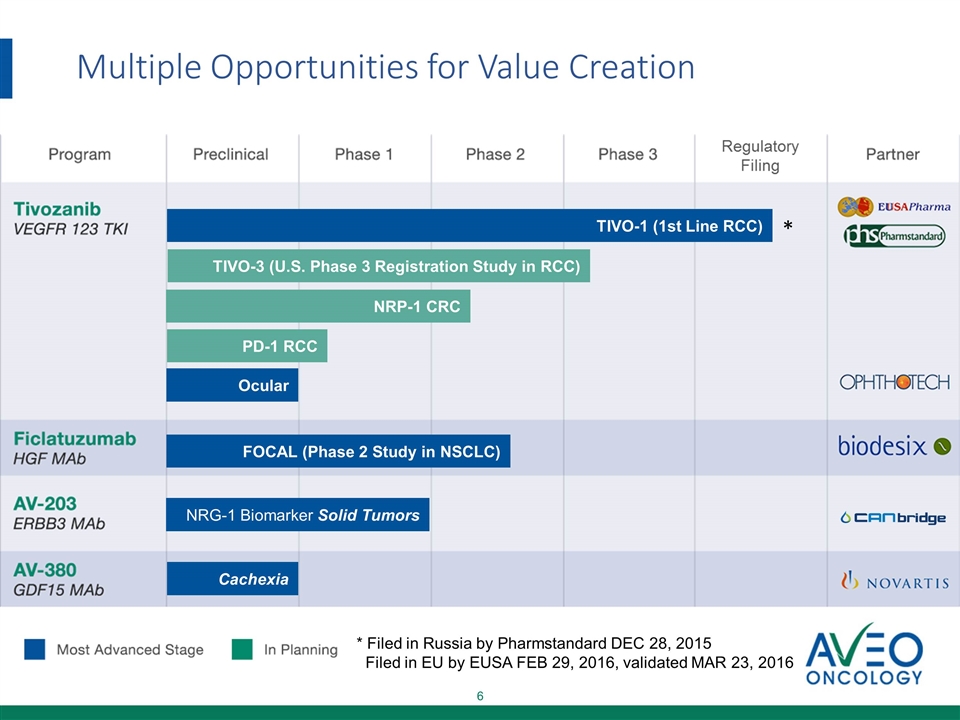
TIVO-1 (1st Line RCC) * Ocular FOCAL (Phase 2 Study in NSCLC) NRG-1 Biomarker Solid Tumors Cachexia TIVO-3 (U.S. Phase 3 Registration Study in RCC) NRP-1 CRC PD-1 RCC Multiple Opportunities for Value Creation * Filed in Russia by Pharmstandard DEC 28, 2015 Filed in EU by EUSA FEB 29, 2016, validated MAR 23, 2016

Tivozanib A potent, selective, long half-life inhibitor of vascular endothelial growth factor (VEGF) 1, 2 and 3 receptors
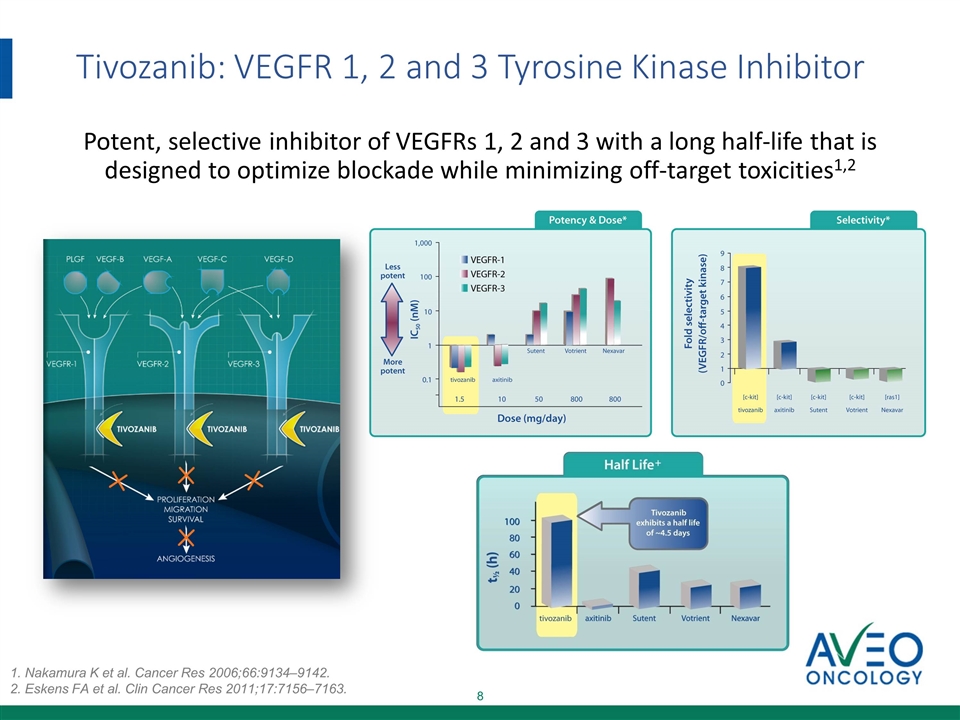
Tivozanib: VEGFR 1, 2 and 3 Tyrosine Kinase Inhibitor Potent, selective inhibitor of VEGFRs 1, 2 and 3 with a long half-life that is designed to optimize blockade while minimizing off-target toxicities1,2 1. Nakamura K et al. Cancer Res 2006;66:9134–9142. 2. Eskens FA et al. Clin Cancer Res 2011;17:7156–7163.
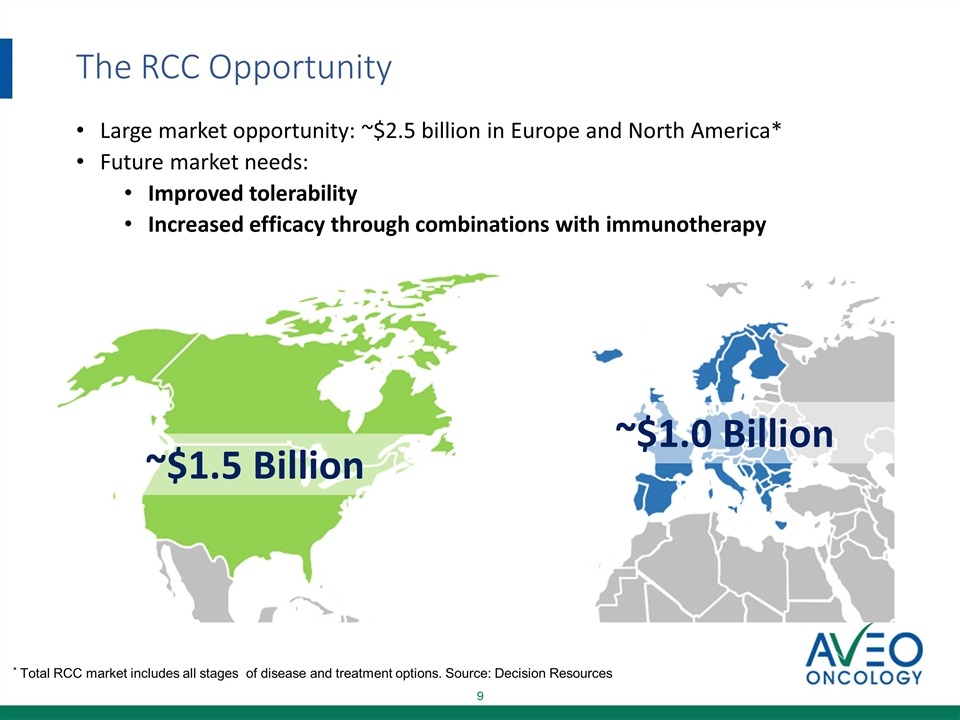
The RCC Opportunity ~$1.5 Billion ~$1.0 Billion Large market opportunity: ~$2.5 billion in Europe and North America* Future market needs: Improved tolerability Increased efficacy through combinations with immunotherapy * Total RCC market includes all stages of disease and treatment options. Source: Decision Resources
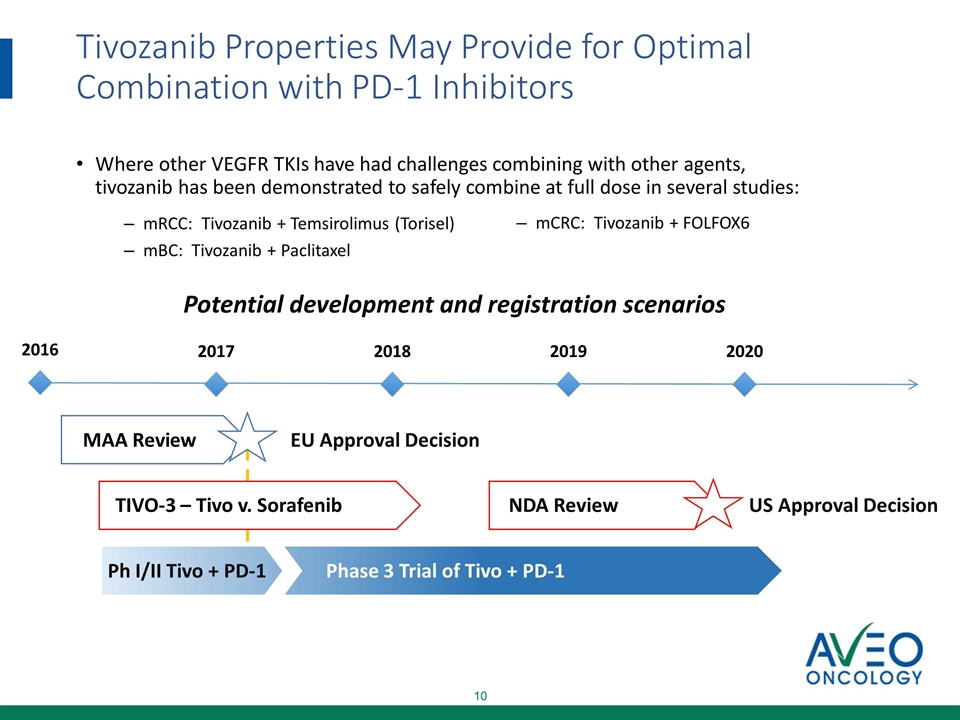
Tivozanib Properties May Provide for Optimal Combination with PD-1 Inhibitors Where other VEGFR TKIs have had challenges combining with other agents, tivozanib has been demonstrated to safely combine at full dose in several studies: mRCC: Tivozanib + Temsirolimus (Torisel) mBC: Tivozanib + Paclitaxel mCRC: Tivozanib + FOLFOX6 MAA Review TIVO-3 – Tivo v. Sorafenib US Approval Decision EU Approval Decision NDA Review 2016 2017 2018 2019 2020 Ph I/II Tivo + PD-1 Phase 3 Trial of Tivo + PD-1 Potential development and registration scenarios

Tivozanib RCC Front-line Setting
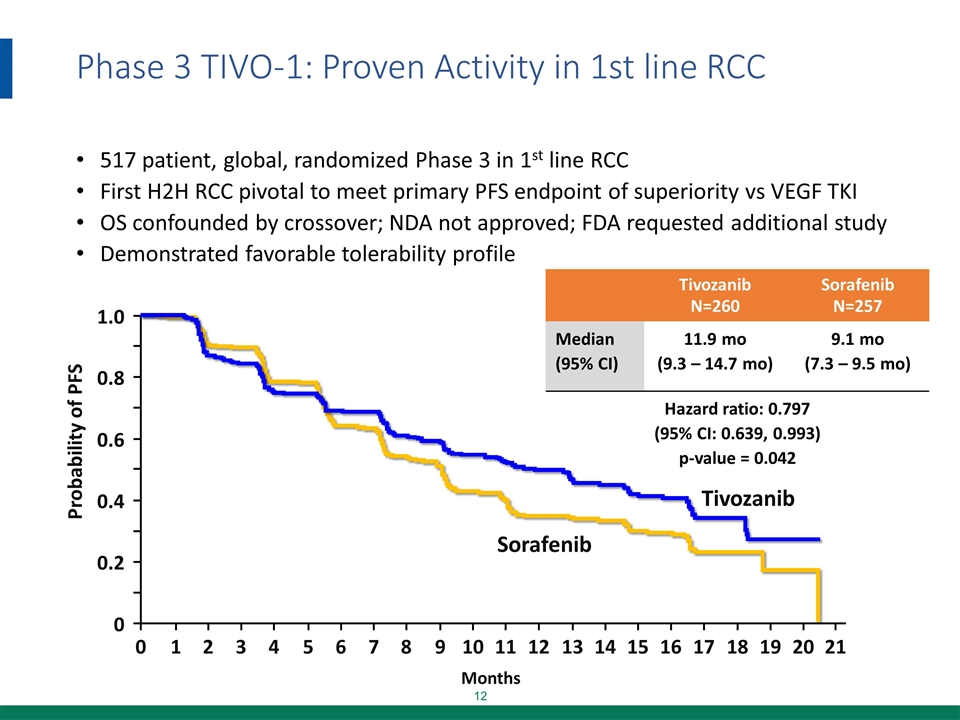
Phase 3 TIVO-1: Proven Activity in 1st line RCC 517 patient, global, randomized Phase 3 in 1st line RCC First H2H RCC pivotal to meet primary PFS endpoint of superiority vs VEGF TKI OS confounded by crossover; NDA not approved; FDA requested additional study Demonstrated favorable tolerability profile Months Probability of PFS 0 Sorafenib 1.0 0.8 0.6 0.4 0.2 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Tivozanib Tivozanib N=260 Sorafenib N=257 Median (95% CI) 11.9 mo (9.3 – 14.7 mo) 9.1 mo (7.3 – 9.5 mo) Hazard ratio: 0.797 (95% CI: 0.639, 0.993) p-value = 0.042
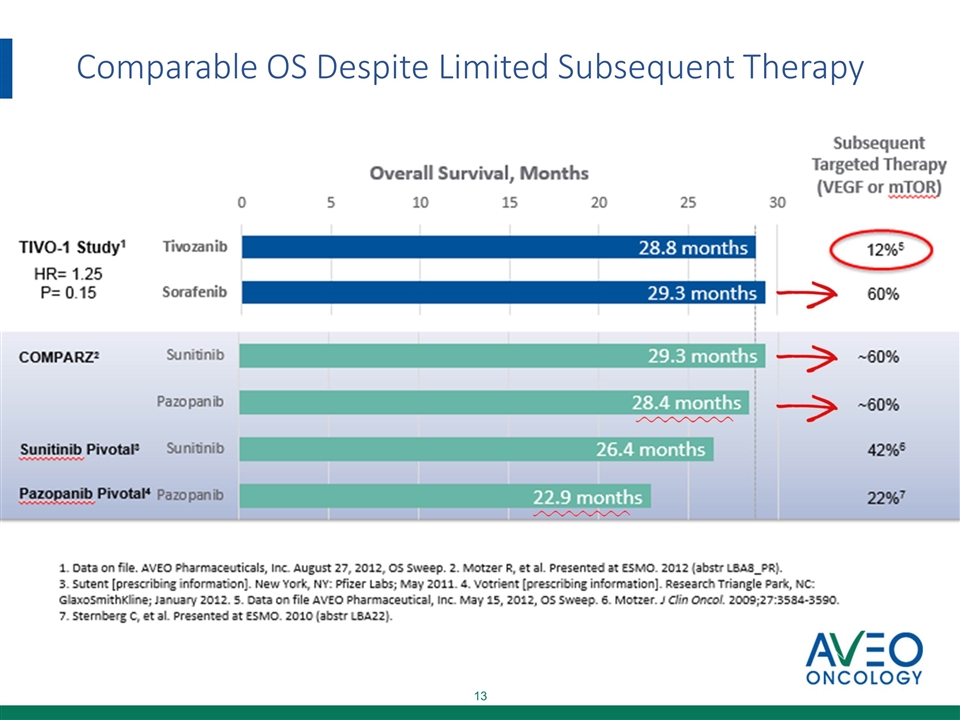
Comparable OS Despite Limited Subsequent Therapy
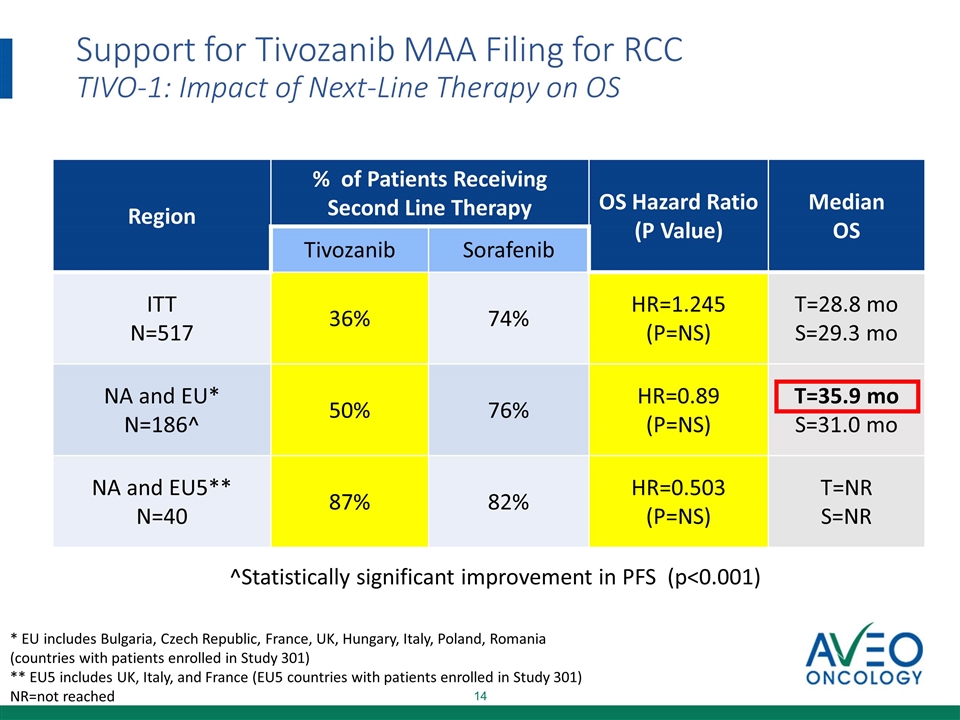
Support for Tivozanib MAA Filing for RCC TIVO-1: Impact of Next-Line Therapy on OS * EU includes Bulgaria, Czech Republic, France, UK, Hungary, Italy, Poland, Romania (countries with patients enrolled in Study 301) ** EU5 includes UK, Italy, and France (EU5 countries with patients enrolled in Study 301) NR=not reached ^Statistically significant improvement in PFS (p<0.001) Region % of Patients Receiving Second Line Therapy OS Hazard Ratio (P Value) Median OS Tivozanib Sorafenib ITT N=517 36% 74% HR=1.245 (P=NS) T=28.8 mo S=29.3 mo NA and EU* N=186^ 50% 76% HR=0.89 (P=NS) T=35.9 mo S=31.0 mo NA and EU5** N=40 87% 82% HR=0.503 (P=NS) T=NR S=NR
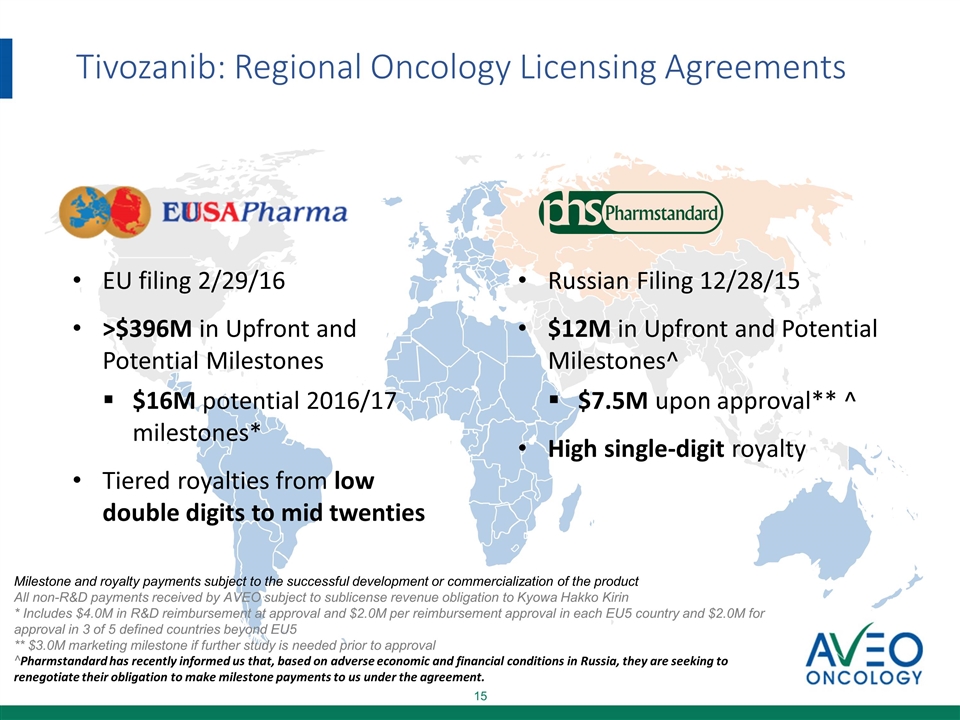
Tivozanib: Regional Oncology Licensing Agreements EU filing 2/29/16 >$396M in Upfront and Potential Milestones $16M potential 2016/17 milestones* Tiered royalties from low double digits to mid twenties Russian Filing 12/28/15 $12M in Upfront and Potential Milestones^ $7.5M upon approval** ^ High single-digit royalty Milestone and royalty payments subject to the successful development or commercialization of the product All non-R&D payments received by AVEO subject to sublicense revenue obligation to Kyowa Hakko Kirin * Includes $4.0M in R&D reimbursement at approval and $2.0M per reimbursement approval in each EU5 country and $2.0M for approval in 3 of 5 defined countries beyond EU5 ** $3.0M marketing milestone if further study is needed prior to approval ^Pharmstandard has recently informed us that, based on adverse economic and financial conditions in Russia, they are seeking to renegotiate their obligation to make milestone payments to us under the agreement.
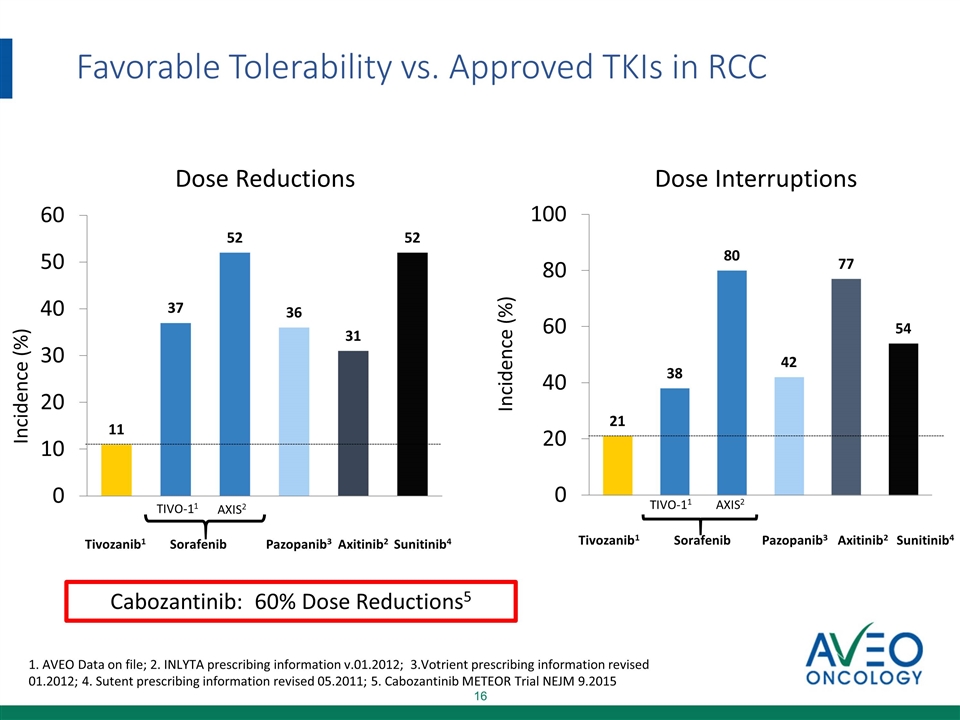
Favorable Tolerability vs. Approved TKIs in RCC 1. AVEO Data on file; 2. INLYTA prescribing information v.01.2012; 3.Votrient prescribing information revised 01.2012; 4. Sutent prescribing information revised 05.2011; 5. Cabozantinib METEOR Trial NEJM 9.2015 Cabozantinib: 60% Dose Reductions5 Incidence (%) Incidence (%) Tivozanib1 Sorafenib Pazopanib3 Axitinib2 TIVO-11 AXIS2 Sunitinib4 Dose Reductions Dose Interruptions Tivozanib1 Sorafenib Pazopanib3 Axitinib2 TIVO-11 AXIS2 Sunitinib4
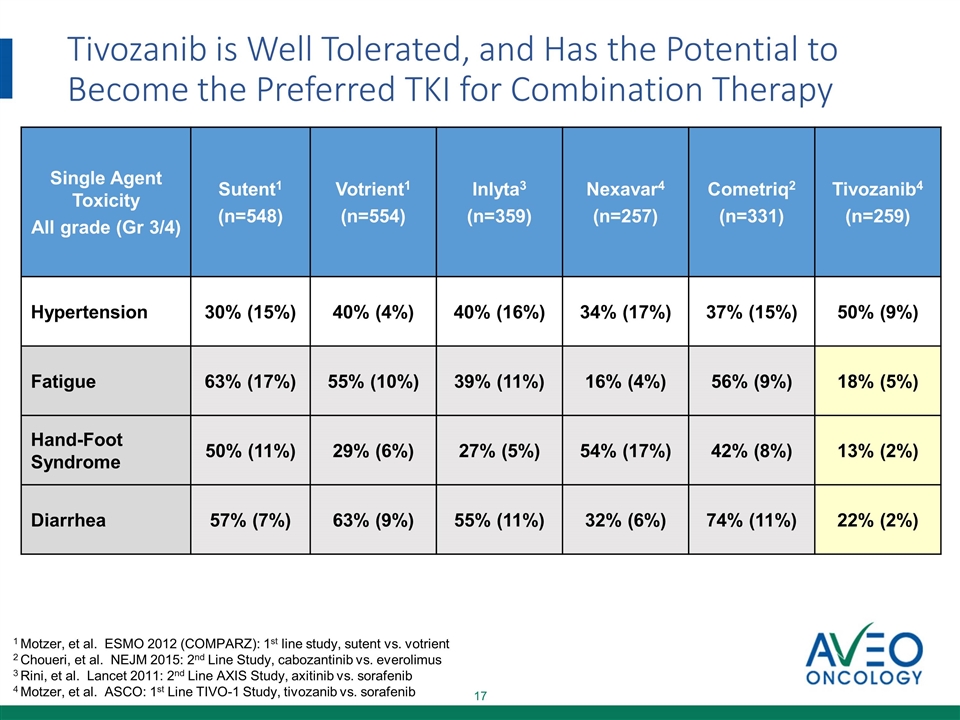
Tivozanib is Well Tolerated, and Has the Potential to Become the Preferred TKI for Combination Therapy Single Agent Toxicity All grade (Gr 3/4) Sutent1 (n=548) Votrient1 (n=554) Inlyta3 (n=359) Nexavar4 (n=257) Cometriq2 (n=331) Tivozanib4 (n=259) Hypertension 30% (15%) 40% (4%) 40% (16%) 34% (17%) 37% (15%) 50% (9%) Fatigue 63% (17%) 55% (10%) 39% (11%) 16% (4%) 56% (9%) 18% (5%) Hand-Foot Syndrome 50% (11%) 29% (6%) 27% (5%) 54% (17%) 42% (8%) 13% (2%) Diarrhea 57% (7%) 63% (9%) 55% (11%) 32% (6%) 74% (11%) 22% (2%) 1 Motzer, et al. ESMO 2012 (COMPARZ): 1st line study, sutent vs. votrient 2 Choueri, et al. NEJM 2015: 2nd Line Study, cabozantinib vs. everolimus 3 Rini, et al. Lancet 2011: 2nd Line AXIS Study, axitinib vs. sorafenib 4 Motzer, et al. ASCO: 1st Line TIVO-1 Study, tivozanib vs. sorafenib
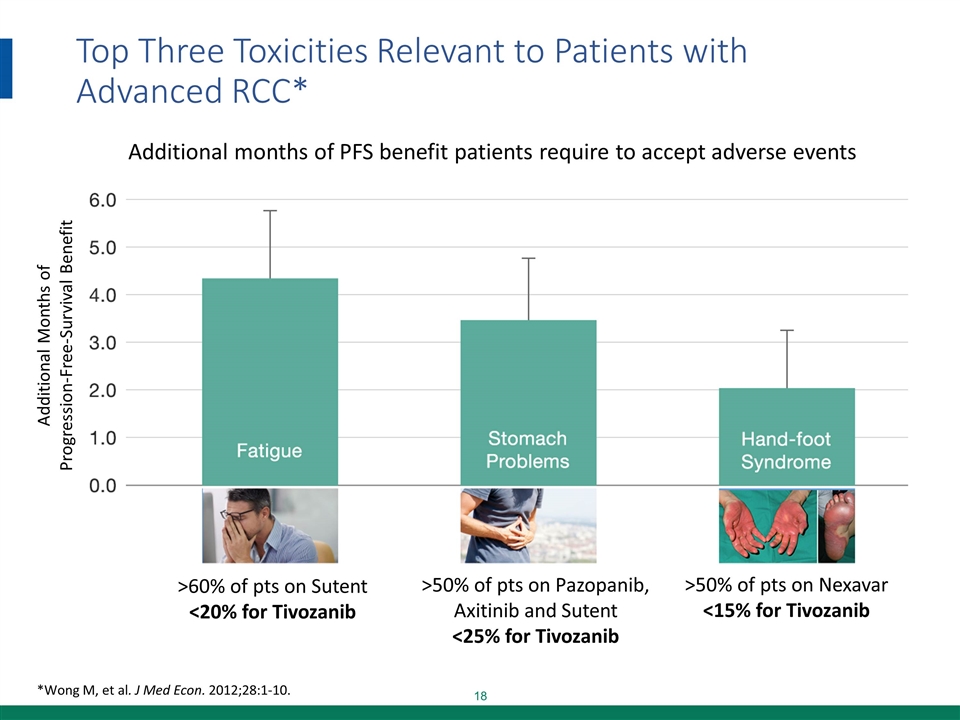
Top Three Toxicities Relevant to Patients with Advanced RCC* *Wong M, et al. J Med Econ. 2012;28:1-10. Additional months of PFS benefit patients require to accept adverse events >50% of pts on Nexavar <15% for Tivozanib >60% of pts on Sutent <20% for Tivozanib >50% of pts on Pazopanib, Axitinib and Sutent <25% for Tivozanib Additional Months of Progression-Free-Survival Benefit

Tivozanib North American Registration Strategy
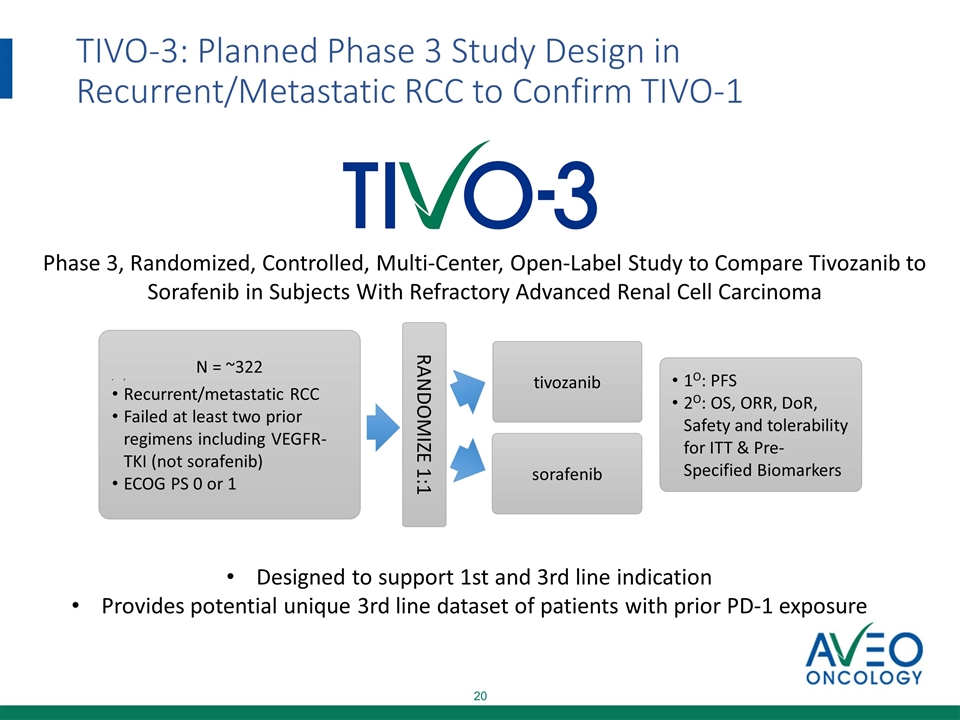
TIVO-3: Planned Phase 3 Study Design in Recurrent/Metastatic RCC to Confirm TIVO-1 N = ~322 F Recurrent/metastatic RCC Failed at least two prior regimens including VEGFR-TKI (not sorafenib) ECOG PS 0 or 1 RANDOMIZE 1:1 tivozanib sorafenib 1O: PFS 2O: OS, ORR, DoR, Safety and tolerability for ITT & Pre-Specified Biomarkers Phase 3, Randomized, Controlled, Multi-Center, Open-Label Study to Compare Tivozanib to Sorafenib in Subjects With Refractory Advanced Renal Cell Carcinoma Designed to support 1st and 3rd line indication Provides potential unique 3rd line dataset of patients with prior PD-1 exposure
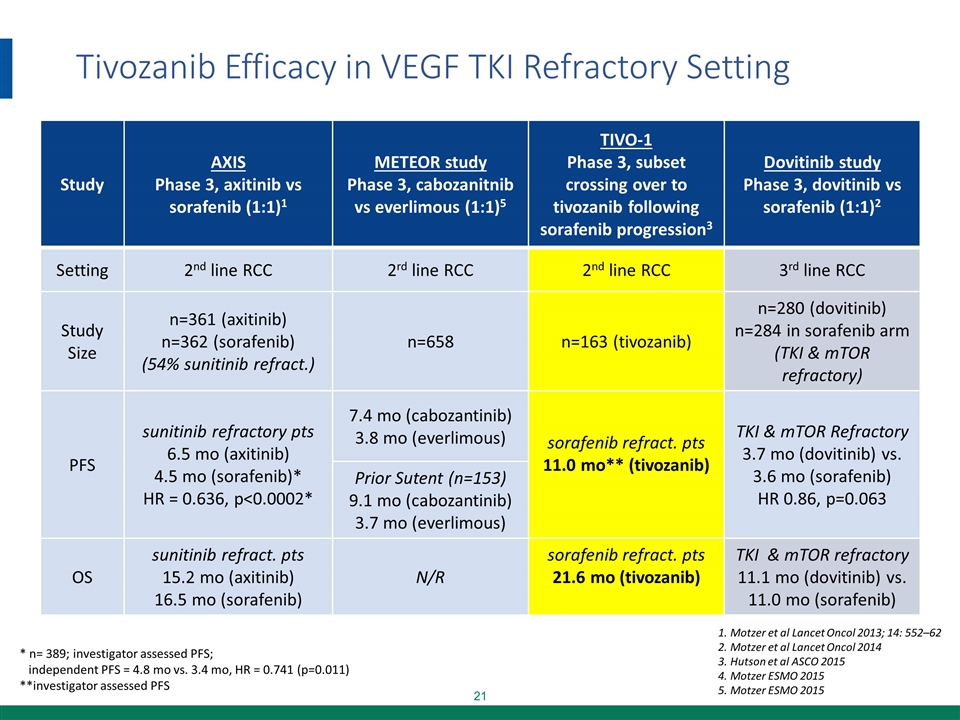
Tivozanib Efficacy in VEGF TKI Refractory Setting * n= 389; investigator assessed PFS; independent PFS = 4.8 mo vs. 3.4 mo, HR = 0.741 (p=0.011) **investigator assessed PFS Motzer et al Lancet Oncol 2013; 14: 552–62 Motzer et al Lancet Oncol 2014 Hutson et al ASCO 2015 Motzer ESMO 2015 Motzer ESMO 2015 Study AXIS Phase 3, axitinib vs sorafenib (1:1)1 METEOR study Phase 3, cabozanitnib vs everlimous (1:1)5 TIVO-1 Phase 3, subset crossing over to tivozanib following sorafenib progression3 Dovitinib study Phase 3, dovitinib vs sorafenib (1:1)2 Setting 2nd line RCC 2rd line RCC 2nd line RCC 3rd line RCC Study Size n=361 (axitinib) n=362 (sorafenib) (54% sunitinib refract.) n=658 n=163 (tivozanib) n=280 (dovitinib) n=284 in sorafenib arm (TKI & mTOR refractory) PFS sunitinib refractory pts 6.5 mo (axitinib) 4.5 mo (sorafenib)* HR = 0.636, p<0.0002* 7.4 mo (cabozantinib) 3.8 mo (everlimous) sorafenib refract. pts 11.0 mo** (tivozanib) TKI & mTOR Refractory 3.7 mo (dovitinib) vs. 3.6 mo (sorafenib) HR 0.86, p=0.063 Prior Sutent (n=153) 9.1 mo (cabozantinib) 3.7 mo (everlimous) OS sunitinib refract. pts 15.2 mo (axitinib) 16.5 mo (sorafenib) N/R sorafenib refract. pts 21.6 mo (tivozanib) TKI & mTOR refractory 11.1 mo (dovitinib) vs. 11.0 mo (sorafenib)
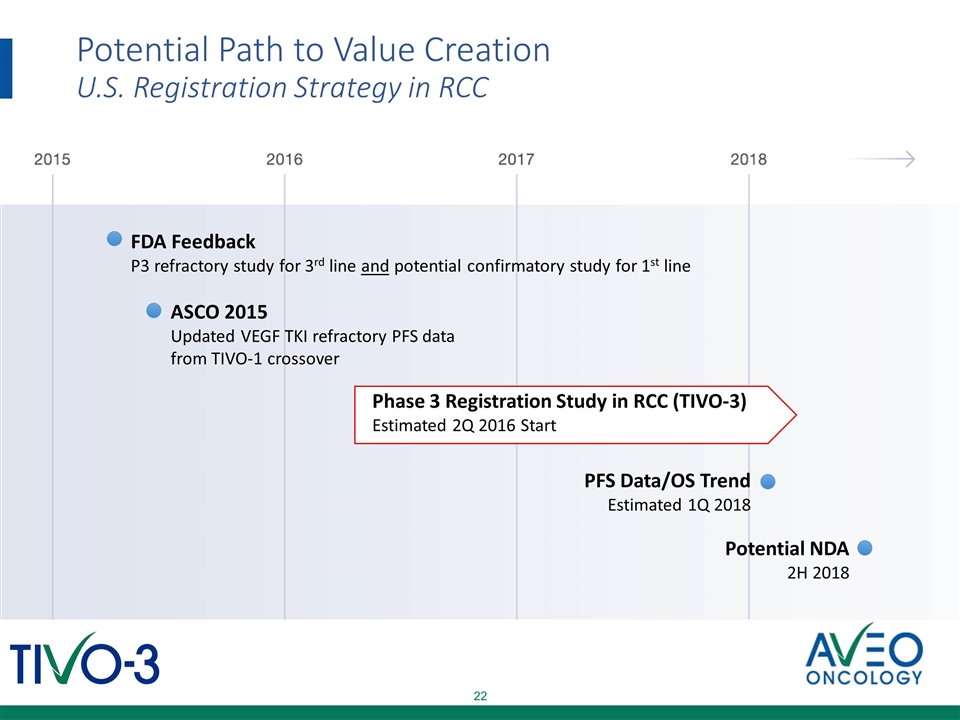
Potential Path to Value Creation U.S. Registration Strategy in RCC ASCO 2015 Updated VEGF TKI refractory PFS data from TIVO-1 crossover FDA Feedback P3 refractory study for 3rd line and potential confirmatory study for 1st line Phase 3 Registration Study in RCC (TIVO-3) Estimated 2Q 2016 Start Potential NDA 2H 2018 PFS Data/OS Trend Estimated 1Q 2018

Tivozanib PD-1 Combination Setting
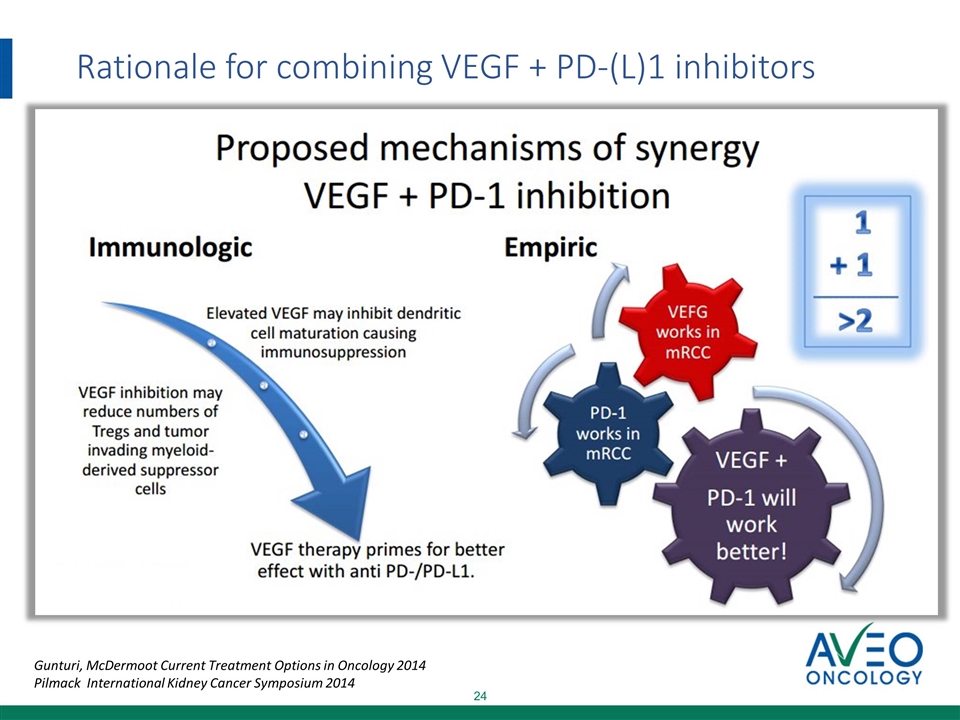
Rationale for combining VEGF + PD-(L)1 inhibitors Gunturi, McDermoot Current Treatment Options in Oncology 2014 Pilmack International Kidney Cancer Symposium 2014
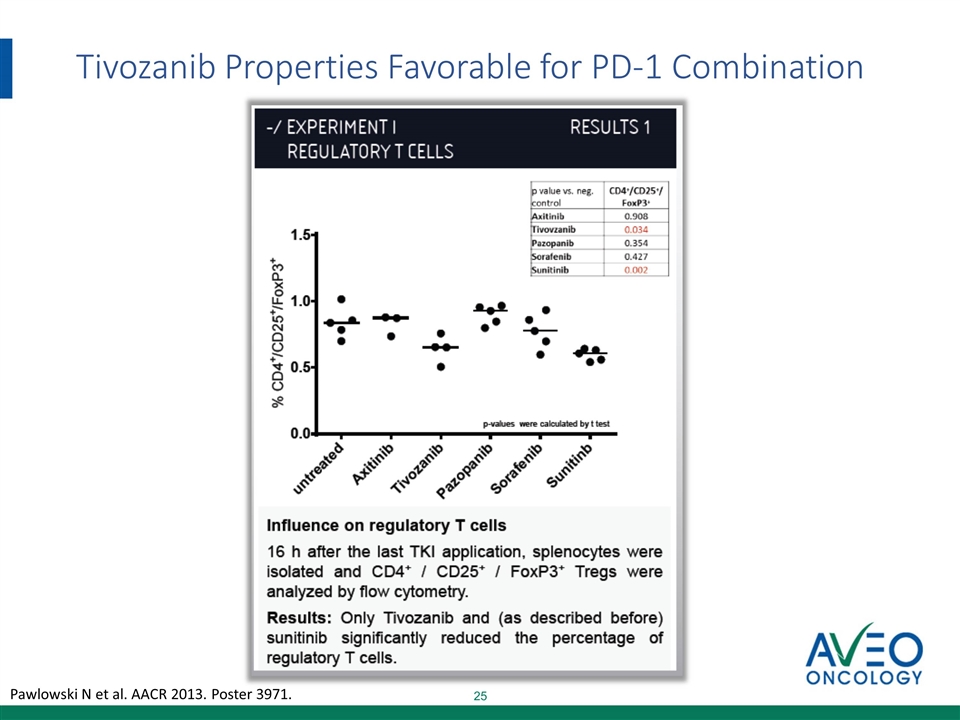
Tivozanib Properties Favorable for PD-1 Combination Pawlowski N et al. AACR 2013. Poster 3971.
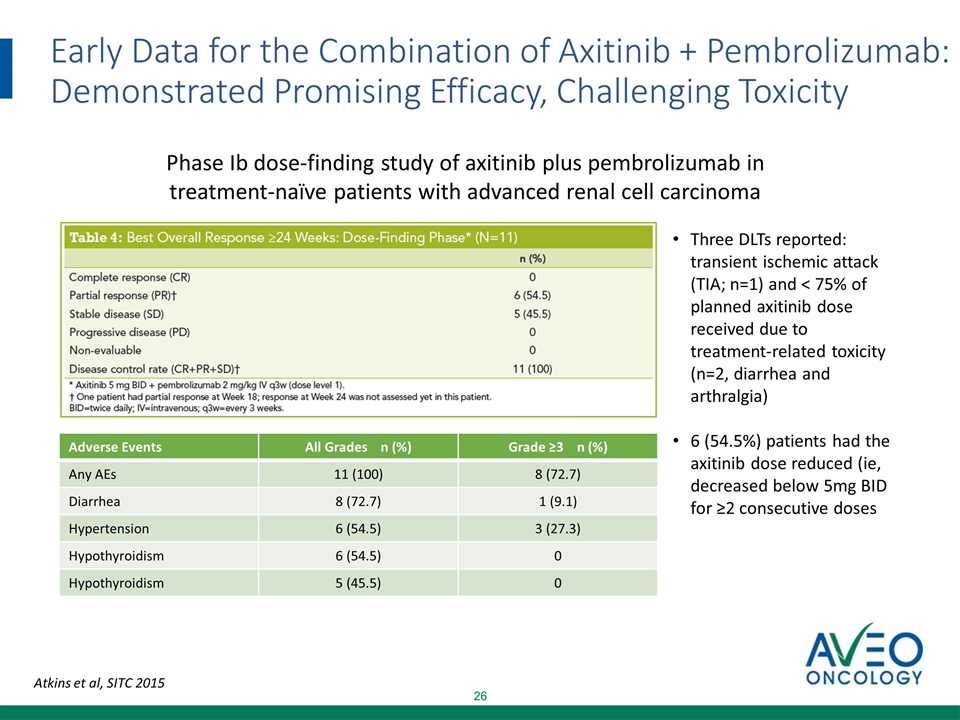
Early Data for the Combination of Axitinib + Pembrolizumab: Demonstrated Promising Efficacy, Challenging Toxicity Atkins et al, SITC 2015 Three DLTs reported: transient ischemic attack (TIA; n=1) and < 75% of planned axitinib dose received due to treatment-related toxicity (n=2, diarrhea and arthralgia) 6 (54.5%) patients had the axitinib dose reduced (ie, decreased below 5mg BID for ≥2 consecutive doses Phase Ib dose-finding study of axitinib plus pembrolizumab in treatment-naïve patients with advanced renal cell carcinoma Adverse Events All Grades n (%) Grade ≥3 n (%) Any AEs 11 (100) 8 (72.7) Diarrhea 8 (72.7) 1 (9.1) Hypertension 6 (54.5) 3 (27.3) Hypothyroidism 6 (54.5) 0 Hypothyroidism 5 (45.5) 0
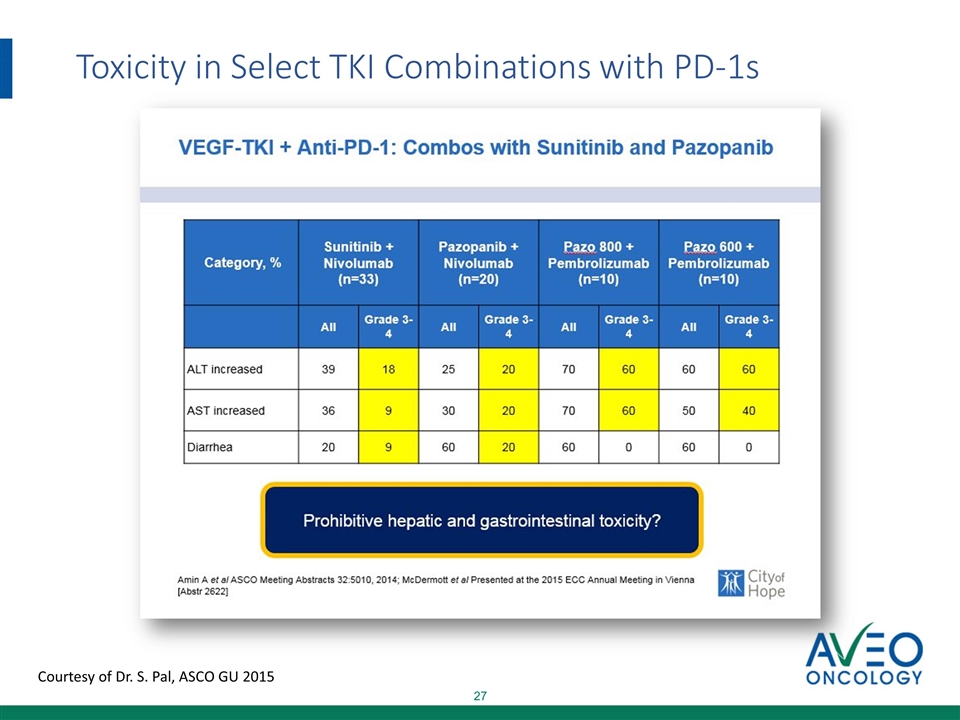
Toxicity in Select TKI Combinations with PD-1s Courtesy of Dr. S. Pal, ASCO GU 2015
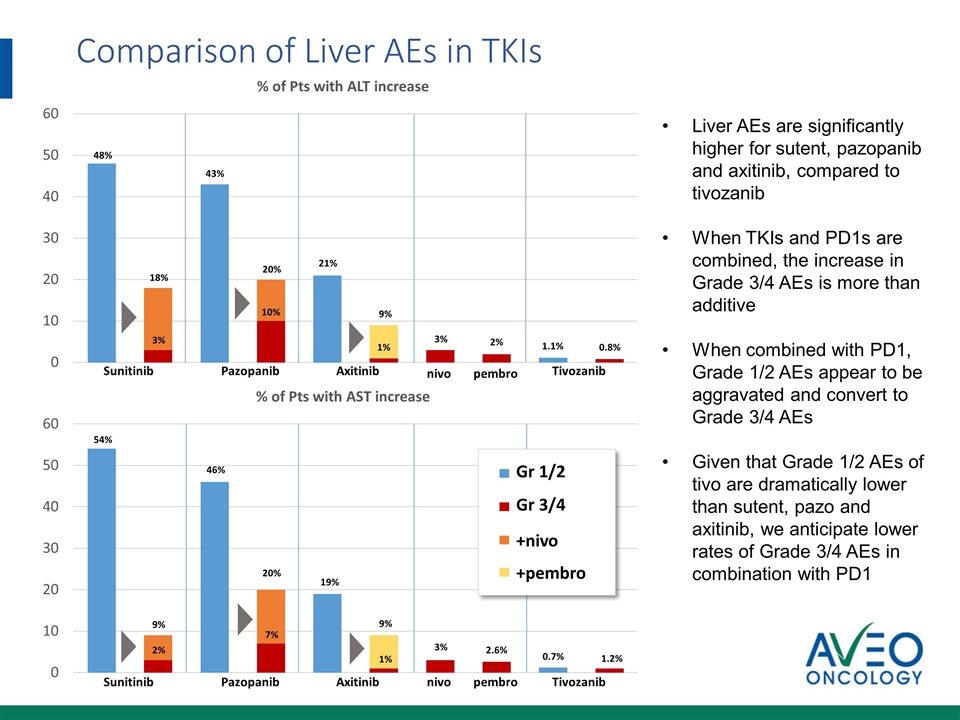
Axitinib Tivozanib Axitinib Tivozanib Comparison of Liver AEs in TKIs Liver AEs are significantly higher for sutent, pazopanib and axitinib, compared to tivozanib When TKIs and PD1s are combined, the increase in Grade 3/4 AEs is more than additive When combined with PD1, Grade 1/2 AEs appear to be aggravated and convert to Grade 3/4 AEs Given that Grade 1/2 AEs of tivo are dramatically lower than sutent, pazo and axitinib, we anticipate lower rates of Grade 3/4 AEs in combination with PD1 Sunitinib Pazopanib nivo 48% 18% 3% 43% 20% 10% 21% 9% 1% 1.1% 0.8% 54% 9% 2% 46% 20% 7% 19% 9% 1% 0.7% 1.2% Sunitinib Pazopanib pembro nivo pembro +nivo +pembro Gr 1/2 3% 2% Gr 3/4 3% 2.6%

Tivozanib Other Indications
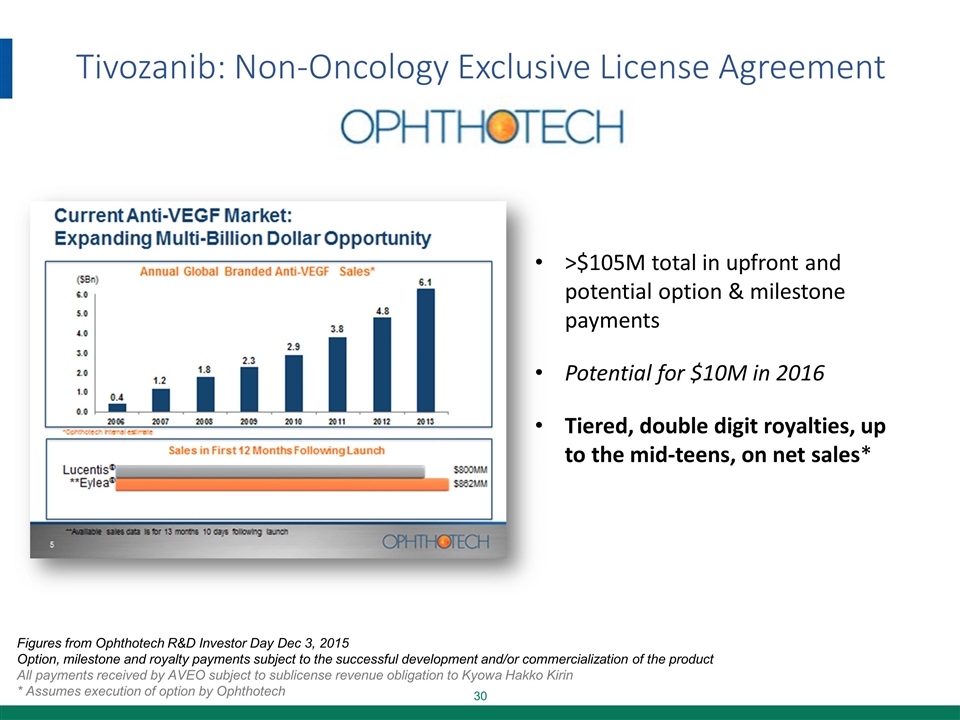
Tivozanib: Non-Oncology Exclusive License Agreement >$105M total in upfront and potential option & milestone payments Potential for $10M in 2016 Tiered, double digit royalties, up to the mid-teens, on net sales* Figures from Ophthotech R&D Investor Day Dec 3, 2015 Option, milestone and royalty payments subject to the successful development and/or commercialization of the product All payments received by AVEO subject to sublicense revenue obligation to Kyowa Hakko Kirin * Assumes execution of option by Ophthotech
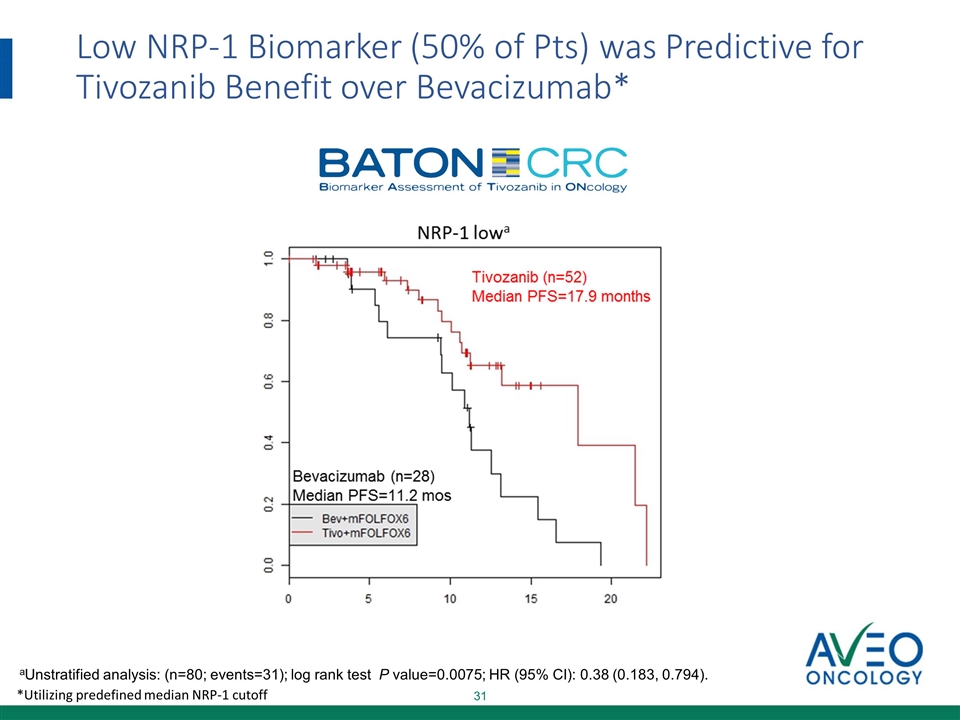
Low NRP-1 Biomarker (50% of Pts) was Predictive for Tivozanib Benefit over Bevacizumab* aUnstratified analysis: (n=80; events=31); log rank test P value=0.0075; HR (95% CI): 0.38 (0.183, 0.794). *Utilizing predefined median NRP-1 cutoff

Robust Pipeline Development Funded by Partnerships
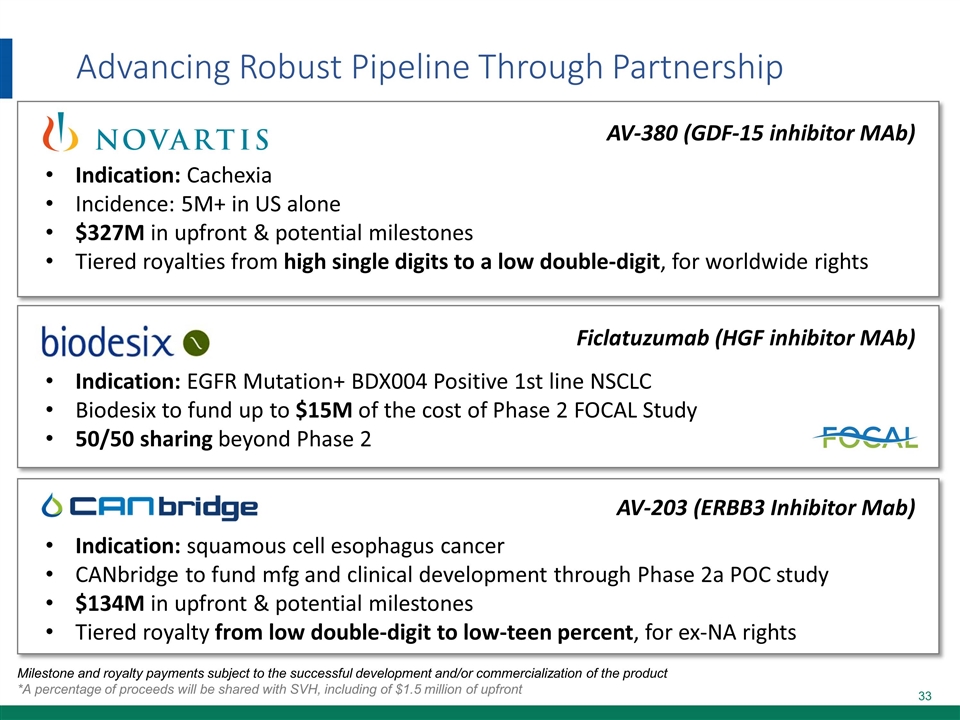
Advancing Robust Pipeline Through Partnership Milestone and royalty payments subject to the successful development and/or commercialization of the product *A percentage of proceeds will be shared with SVH, including of $1.5 million of upfront AV-380 (GDF-15 inhibitor MAb) Indication: Cachexia Incidence: 5M+ in US alone $327M in upfront & potential milestones Tiered royalties from high single digits to a low double-digit, for worldwide rights Ficlatuzumab (HGF inhibitor MAb) Indication: EGFR Mutation+ BDX004 Positive 1st line NSCLC Biodesix to fund up to $15M of the cost of Phase 2 FOCAL Study 50/50 sharing beyond Phase 2 AV-203 (ERBB3 Inhibitor Mab) Indication: squamous cell esophagus cancer CANbridge to fund mfg and clinical development through Phase 2a POC study $134M in upfront & potential milestones Tiered royalty from low double-digit to low-teen percent, for ex-NA rights

Financials & Summary

Financial Highlights $34.1M in cash and investments as of year end 2015 Excludes $3.5M received from Novartis, $4M paid for SEC settlement and $1M CANbridge upfront payment Sufficient to fund operations at least into the fourth quarter of 2017* >$40M in potential payments through mid-2017 including: ~$10M in operational, not clinical milestones (IND filings, option exercises) Ophthotech development milestones and option exercise Pharmstandard regulatory milestone^ EUSA R&D funding, as well as regulatory and reimbursement milestones Novartis development milestones Streamlined operations with a headcount of 20 Shares outstanding as of 2/29/16: ~58.2M *As of year end financial results; Does not include potential development expense or receipt of milestone payments associated with partnerships ^Pharmstandard has recently informed us that, based on adverse economic and financial conditions in Russia, they are seeking to renegotiate their obligation to make milestone payments to us under the agreement.
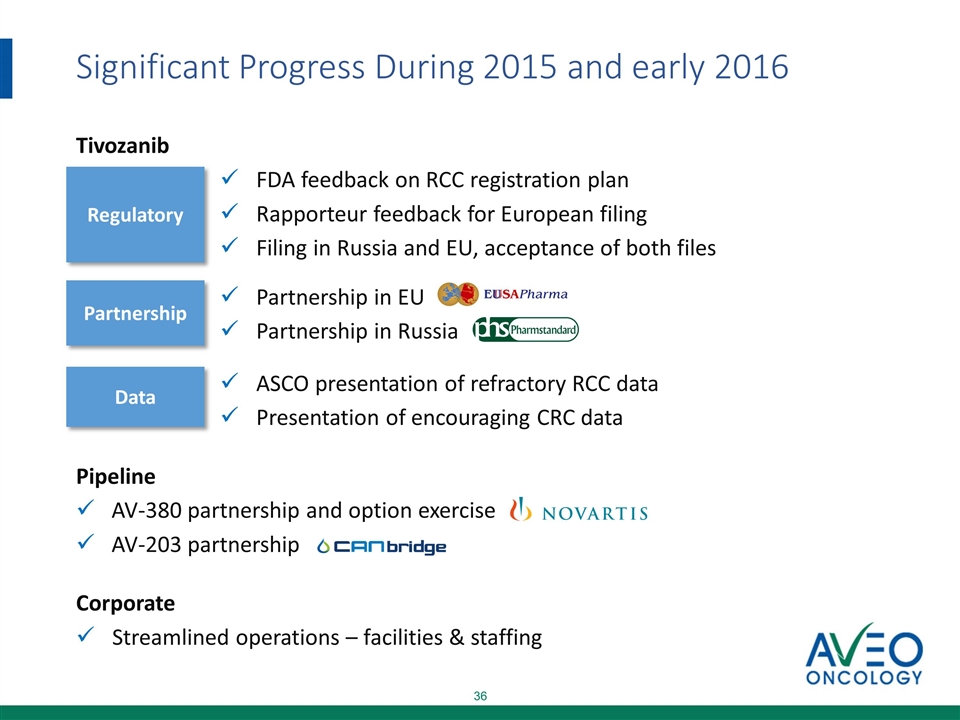
Significant Progress During 2015 and early 2016 Tivozanib FDA feedback on RCC registration plan Rapporteur feedback for European filing Filing in Russia and EU, acceptance of both files Partnership in EU Partnership in Russia ASCO presentation of refractory RCC data Presentation of encouraging CRC data Pipeline AV-380 partnership and option exercise AV-203 partnership Corporate Streamlined operations – facilities & staffing Regulatory Partnership Data

Potential Corporate Milestones Tivozanib Initiation of 3rd line confirmatory RCC Phase 3 study (2Q16) Initiation of Phase 1 PD-1 combination study Initiation of Phase 2/Phase 3 CRC study (with CDx identification) Regulatory decision in Russia/Ukraine/CIS Regulatory decision in EU Proof of concept in AMD and option exercise TIVO-3 PFS data/OS trend (1Q18) Potential NDA submission (2H18) Pipeline Development of AV-380 Manufacturing transfer and re-initiation of AV-203 clinical dev. Data readout of FOCAL (ficlatuzumab) study
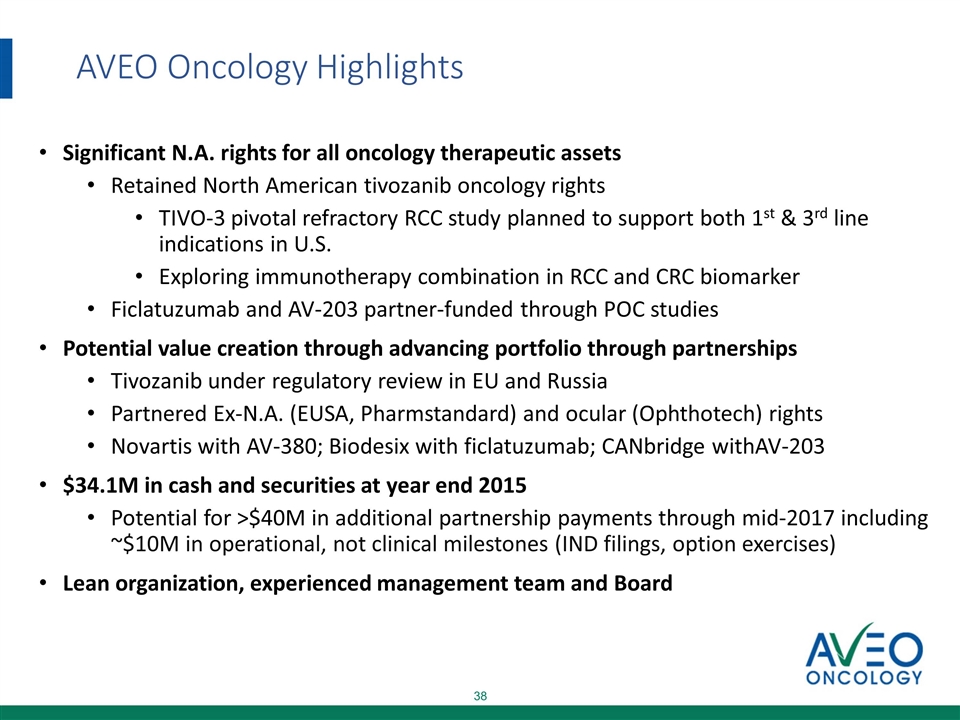
AVEO Oncology Highlights Significant N.A. rights for all oncology therapeutic assets Retained North American tivozanib oncology rights TIVO-3 pivotal refractory RCC study planned to support both 1st & 3rd line indications in U.S. Exploring immunotherapy combination in RCC and CRC biomarker Ficlatuzumab and AV-203 partner-funded through POC studies Potential value creation through advancing portfolio through partnerships Tivozanib under regulatory review in EU and Russia Partnered Ex-N.A. (EUSA, Pharmstandard) and ocular (Ophthotech) rights Novartis with AV-380; Biodesix with ficlatuzumab; CANbridge withAV-203 $34.1M in cash and securities at year end 2015 Potential for >$40M in additional partnership payments through mid-2017 including ~$10M in operational, not clinical milestones (IND filings, option exercises) Lean organization, experienced management team and Board

AVEO Overview April 2016
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Stockholm Exergi Selects Hansen to Automate and Optimise Power Trading Operations
- U.S. Bank CFO Survey: Corporate Finance Leaders Tighten Belts Amid Uncertainty
- Essential Utilities Donated $5.5 Million in 2023 to Strengthen Communities Across Service Territory
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share