Form 8-K ARENA PHARMACEUTICALS For: Jan 06
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 6, 2017
Arena Pharmaceuticals, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
Delaware |
000-31161 |
23-2908305 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
|
|
6154 Nancy Ridge Drive, San Diego, CA |
|
92121 |
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (858) 453-7200
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
In this report, “Arena Pharmaceuticals,” “Arena,” “Company,” “we,” “us” and “our” refer to Arena Pharmaceuticals, Inc., and/or one or more of our wholly owned subsidiaries, unless the context otherwise provides. Arena Pharmaceuticals® and Arena® are registered service marks of Arena Pharmaceuticals, Inc.
Item 2.02 Results of Operations and Financial Condition.
Commencing on January 8, 2017, we expect to disclose the following information in discussions to be held in connection with the Annual J.P. Morgan Healthcare Conference: As of December 31, 2016, Arena Pharmaceuticals, Inc. (the “Company”) had approximately $90 million of cash and cash equivalents. See additional information in the presentation furnished as an Exhibit to Item 7.01.
Item 7.01 Regulation FD Disclosure.
Included as Exhibit 99.1 to this Form 8-K is a presentation titled “Corporate Presentation – JP Morgan Healthcare Conference”, dated January 2017, which is incorporated herein by reference. We intend to utilize this presentation in various meetings with securities analysts, investors and others in connection with the Annual J.P. Morgan Healthcare Conference, commencing on January 8, 2017.
The information contained in Exhibit 99.1 hereto is being “furnished” and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended, is not subject to the liabilities of that section and is not deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except as shall be expressly set forth by specific reference in such a filing.
Item 8.01 Other Events.
We are providing the following information to summarize and update certain aspects of our publicly disclosed description of our clinical programs.
Etrasimod
Etrasimod, an orally available modulator of the S1P receptor, is our internally discovered investigational drug candidate intended for the potential treatment of a number of autoimmune diseases. S1P receptor modulators have been demonstrated to be involved in several biological responses, including lymphocyte trafficking from lymph nodes to the peripheral blood. By isolating lymphocytes in lymph nodes, fewer immune cells are available in the circulating blood to effect tissue damage. Drugs in this class have been associated with certain side effects, including cardiovascular effects, respiratory effects, infection, macular edema and elevations in liver enzymes. We have optimized etrasimod as a potent and selective small molecule S1P receptor modulator that reduces the severity of disease in preclinical autoimmune-disease models.
We are currently developing etrasimod for ulcerative colitis. In July 2015, we initiated patient dosing in a 12-week, randomized, double-blind and placebo-controlled Phase 2 clinical trial of etrasimod for ulcerative colitis. We are working diligently to obtain data from this trial by the end of 2017. To achieve our goal of obtaining data from this trial by the end of 2017 and lower costs, we are assessing potential changes to the trial protocol that we believe would improve data readout and enhance overall efficiency of the trial, while maintaining study conduct, data integrity, patient safety and the overall study objectives.
In addition, to further enhance the overall profile of our etrasimod program, we intend to explore development in additional indications, including
|
|
• |
Dermatological Extra-Intestinal Manifestations (EIM) in Inflammatory Bowel Disease (IBD) |
|
|
• |
Pyoderma Gangrenosum (PG) |
|
|
• |
Primary Biliary Cirrhosis (PBC) |
We are designing exploratory Phase II clinical trials for each of these additional indications. We intend to initiate these Phase II trials during 2017.
Ralinepag
Ralinepag, an oral, selective IP receptor agonist targeting the prostacyclin pathway, is our internally discovered investigational drug candidate intended for the treatment of pulmonary arterial hypertension, or PAH. In September 2014, ralinepag was granted orphan drug status for the treatment of PAH by the FDA.
In January 2015, we initiated patient dosing in a 22-week, randomized, double-blind and placebo-controlled Phase 2 clinical trial of ralinepag. We completed enrollment in the trial in December 2016 and expect to obtain data from this trial by the end of the third quarter of 2017.
APD371
APD371, an orally available, peripherally restricted, highly selective, full agonist of the cannabinoid-2 (CB2) receptor, is an internally discovered investigational drug candidate we are exploring for the treatment of pain.
In October 2015, we initiated a Phase 1 multiple-ascending dose trial of APD371. In the first half of 2016, we completed this trial with favorable results. In the first quarter of 2017, we intend to commence a Phase 2 clinical trial of APD371 for pain associated with Crohn’s disease and expect to obtain data from this trial by the end of 2017.
Temanogrel
Temanogrel, an orally available inverse agonist of the serotonin 2A receptor, is an internally discovered investigational drug candidate intended for the treatment of thrombotic diseases. We believe temanogrel has the potential to inhibit serotonin-mediated platelet aggregation and vasoconstriction. We believe temanogrel’s dual mechanism may be therapeutically useful for the treatment or prevention of thrombotic diseases.
We previously completed a Phase 1a clinical evaluation of temanogrel, and provided Ildong Pharmaceuticals Co., Ltd., the development and commercialization rights to temanogrel in South Korea (while we retained all other rights worldwide). Ildong is not currently advancing temanogrel in clinical trials for South Korea. We are continuing to evaluate options for this program.
Item 9.01 Financial Statements and Exhibits.
|
Exhibit Number |
|
Description |
|
99.1 |
|
Corporate Presentation – JP Morgan Healthcare Conference |
|
|
|
|
Forward-Looking Statements
Statements in this report on Form 8-K that are not statements of historical fact are forward-looking statements, which involve a number of risks and uncertainties. Such forward-looking statements include, without limitation, statements about the timing of obtaining data from clinical trials, changes in clinical protocol, and planned or intended clinical trials and indications. Words such as “believe,” “plan,” “expect,” “intend,” “will,” “would,” “may,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from our expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, the risk that we may not be able to recruit patients for certain trials as anticipated, and the risk that we may not successfully implement anticipated changes in clinical protocol, as well as risks related to: research and development; regulatory approval; commercialization; competition; intellectual property; and our financial and other resources. Additional factors that could cause actual results to differ materially from those stated or implied by Arena’s forward-looking statements are disclosed in our filings with the Securities and Exchange Commission. These forward-looking statements represent our judgment as of the time of this report on Form 8-K. We disclaim any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
Date: January 6, 2017 |
Arena Pharmaceuticals, Inc. |
||
|
|
|
|
|
|
|
By: |
|
/s/ Amit Munshi |
|
|
|
|
Amit Munshi |
|
|
|
|
President and Chief Executive Officer |
|
Exhibit Number |
|
Description |
|
99.1 |
|
Corporate Presentation – JP Morgan Healthcare Conference |
|
|
|
|

Non-Confidential January 2017 Corporate Presentation JP Morgan Healthcare Conference Exhibit 99.1
Forward-Looking Statements This presentation includes forward-looking statements that involve a number of risks and uncertainties, including statements about our focus, goals, strategy, plans, timelines and guidance; research and development (R&D) programs and BELVIQ, including with respect to potential, safety, efficacy, indication, significance of related data, differentiation, status, timing, regulatory activities, value and commercialization; partnered programs; and other statements that are not historical facts, including statements that may include words such as “may,” “will,” “intend,” “plan,” “expect,” “potential” or other similar words. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from expectations, and you are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the time they were made. Factors that could cause actual results to differ materially from such statements include, without limitation, the timing, success and cost of R&D and related strategy and decisions; trial and study results are subject to different interpretations and may not be predictive of future results; trials and studies may not proceed at the time or in the manner expected or at all; unexpected or unfavorable new data; nonclinical and clinical data is voluminous and detailed, and regulatory agencies may interpret or weigh data differently and reach different conclusions than we or others, request additional information, have additional recommendations or change their guidance or requirements; information related to R&D may not meet regulatory requirements or otherwise be sufficient for (or we or a partner may not pursue) further R&D, regulatory review or approval or continued marketing; competition; risks related to commercializing drugs, including regulatory, manufacturing, supply and marketing issues and their availability and use; having adequate funds and other assets and their effective use; reimbursement and pricing decisions; risks related to relying on partners and other third parties; the entry into or modification or termination of partnering arrangements; our and third parties’ intellectual property rights; and satisfactory resolution of litigation or other disagreements. Additional factors that could cause actual results to differ materially from those stated or implied by our forward-looking statements are disclosed in our SEC filings. We disclaim any intent or obligation to update these forward-looking statements, other than as may be required under applicable law.
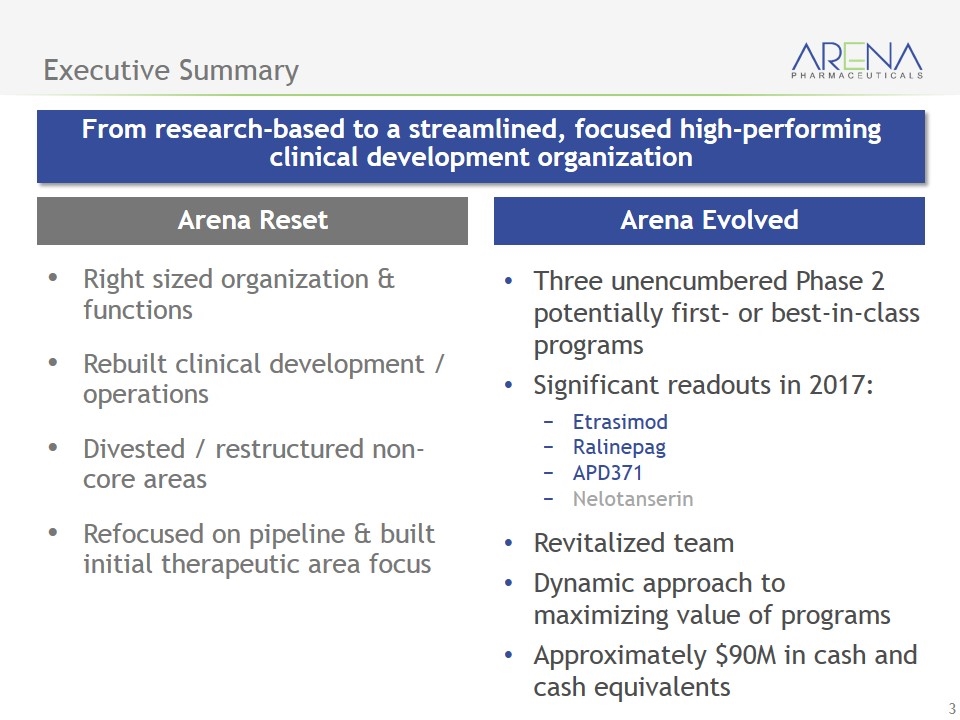
Arena Evolved Executive Summary From research-based to a streamlined, focused high-performing clinical development organization Three unencumbered Phase 2 potentially first- or best-in-class programs Significant readouts in 2017: Etrasimod Ralinepag APD371 Nelotanserin Revitalized team Dynamic approach to maximizing value of programs Approximately $90M in cash and cash equivalents Arena Reset Right sized organization & functions Rebuilt clinical development / operations Divested / restructured non-core areas Refocused on pipeline & built initial therapeutic area focus
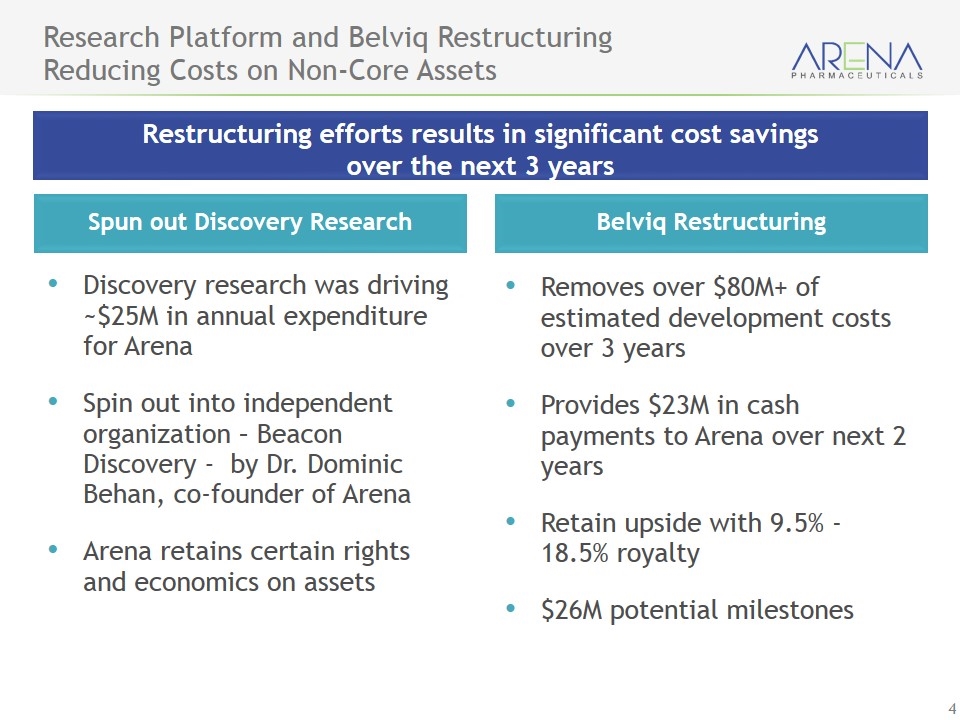
Research Platform and Belviq Restructuring Reducing Costs on Non-Core Assets Discovery research was driving ~$25M in annual expenditure for Arena Spin out into independent organization – Beacon Discovery - by Dr. Dominic Behan, co-founder of Arena Arena retains certain rights and economics on assets Spun out Discovery Research Removes over $80M+ of estimated development costs over 3 years Provides $23M in cash payments to Arena over next 2 years Retain upside with 9.5% - 18.5% royalty $26M potential milestones Belviq Restructuring Restructuring efforts results in significant cost savings over the next 3 years
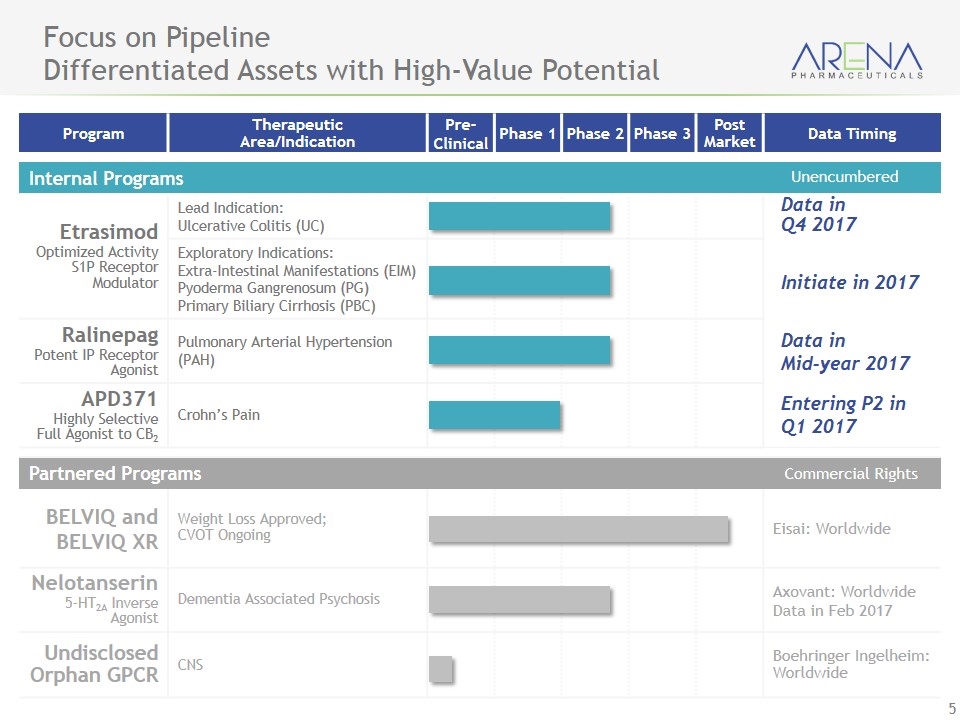
Program Therapeutic Area/Indication Pre- Clinical Phase 1 Phase 2 Phase 3 Post Market Data Timing Internal Programs Etrasimod Optimized Activity S1P Receptor Modulator Lead Indication: Ulcerative Colitis (UC) Exploratory Indications: Extra-Intestinal Manifestations (EIM) Pyoderma Gangrenosum (PG) Primary Biliary Cirrhosis (PBC) Ralinepag Potent IP Receptor Agonist Pulmonary Arterial Hypertension (PAH) APD371 Highly Selective Full Agonist to CB2 Crohn’s Pain Partnered Programs BELVIQ and BELVIQ XR Weight Loss Approved; CVOT Ongoing Eisai: Worldwide Nelotanserin 5-HT2A Inverse Agonist Dementia Associated Psychosis Axovant: Worldwide Data in Feb 2017 Undisclosed Orphan GPCR CNS Boehringer Ingelheim: Worldwide Focus on Pipeline Differentiated Assets with High-Value Potential Commercial Rights Data in Q4 2017 Initiate in 2017 Data in Mid-year 2017 Entering P2 in Q1 2017 Unencumbered

Clinical Development Focus and Externally-Driven Thinking Supported by Revamped Operational Team Amit Munshi, President and CEO CEO, Epirus and Percivia (sold to JNJ) Founder, CBO, Kythera (sold to Allergan) Kevin Lind, Chief Financial Officer TSSP / TPG Pharma Partners Lehman Brothers Cheryl Lassen BSc., MBBCh, VP, Clinical Development Senior development roles at Novartis, BMS, Abbott and Pharmacia / Pfizer Vincent Aurentz, Chief Business Officer President, HemoShear Therapeutics EVP, R&D, Portfolio and Corporate Development Merck Serono Anna Crivici, PhD, VP, Program Management VP Program Management, NantkWest, Amylin Global Project Leader, Pfizer Maurice Mezzino, SVP Corporate Development Business Development executive at BMS, Biogen Idec, BeiGene Chartered Financial Analyst (CFA) Steven Spector, General Counsel Partner Morgan Lewis Board member, Association of Corporate Counsel
Oral, Next Generation, S1P Receptor Modulator Optimized Activity Being Evaluated for Multiple Autoimmune Diseases Etrasimod
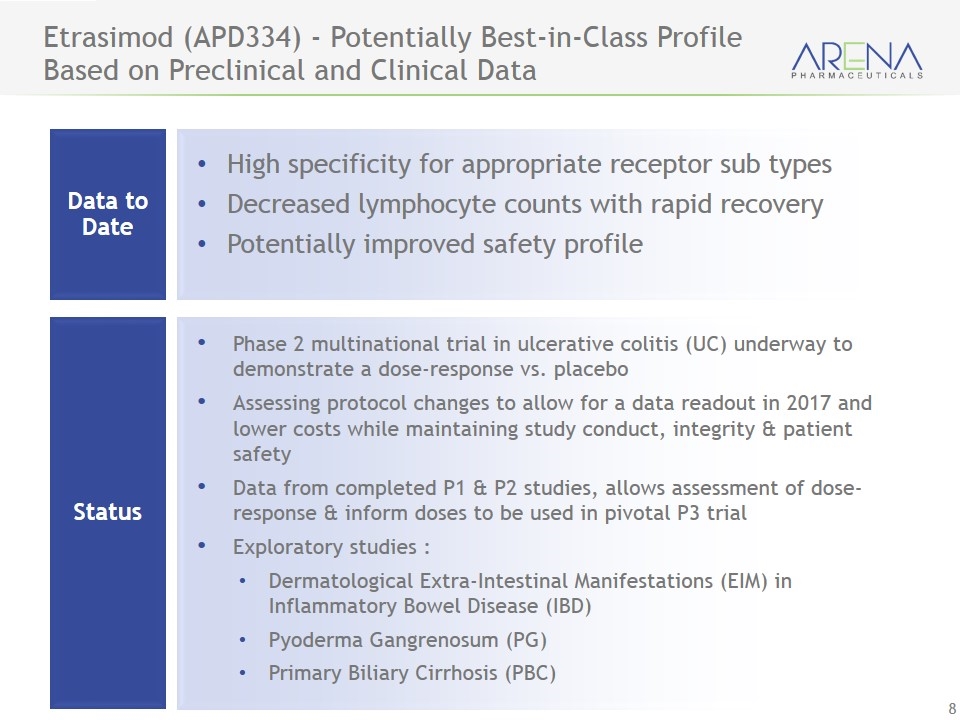
Etrasimod (APD334) - Potentially Best-in-Class Profile Based on Preclinical and Clinical Data Status High specificity for appropriate receptor sub types Decreased lymphocyte counts with rapid recovery Potentially improved safety profile Data to Date Phase 2 multinational trial in ulcerative colitis (UC) underway to demonstrate a dose-response vs. placebo Assessing protocol changes to allow for a data readout in 2017 and lower costs while maintaining study conduct, integrity & patient safety Data from completed P1 & P2 studies, allows assessment of dose-response & inform doses to be used in pivotal P3 trial Exploratory studies : Dermatological Extra-Intestinal Manifestations (EIM) in Inflammatory Bowel Disease (IBD) Pyoderma Gangrenosum (PG) Primary Biliary Cirrhosis (PBC)
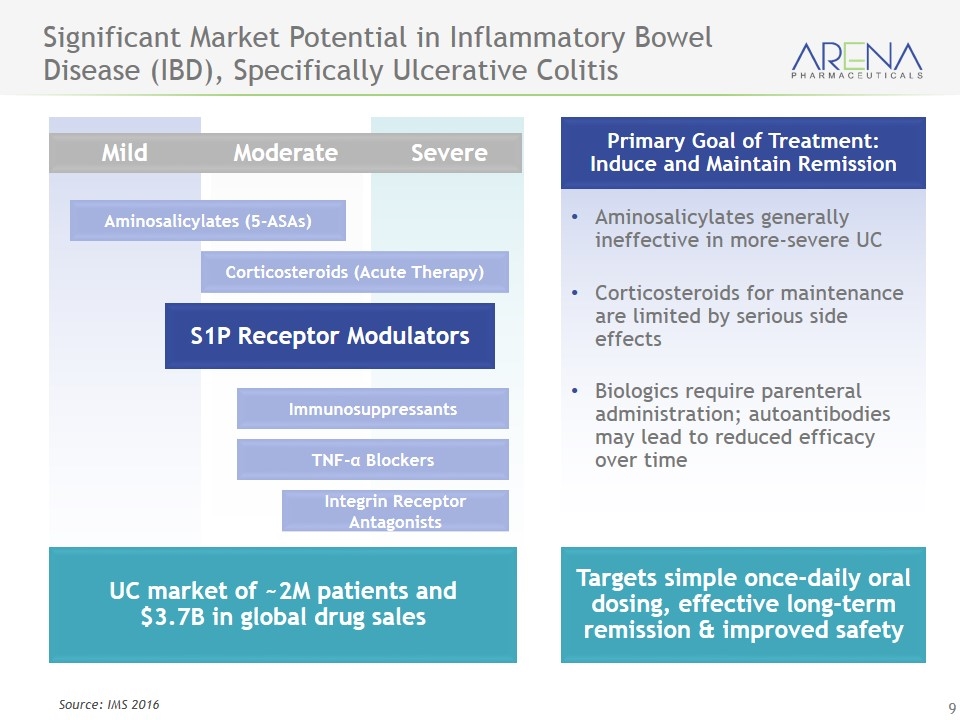
Aminosalicylates (5-ASAs) Corticosteroids (Acute Therapy) Immunosuppressants TNF-α Blockers Integrin Receptor Antagonists S1P Receptor Modulators Significant Market Potential in Inflammatory Bowel Disease (IBD), Specifically Ulcerative Colitis UC market of ~2M patients and $3.7B in global drug sales Primary Goal of Treatment: Induce and Maintain Remission Aminosalicylates generally ineffective in more-severe UC Corticosteroids for maintenance are limited by serious side effects Biologics require parenteral administration; autoantibodies may lead to reduced efficacy over time Targets simple once-daily oral dosing, effective long-term remission & improved safety Source: IMS 2016 Mild Moderate Severe
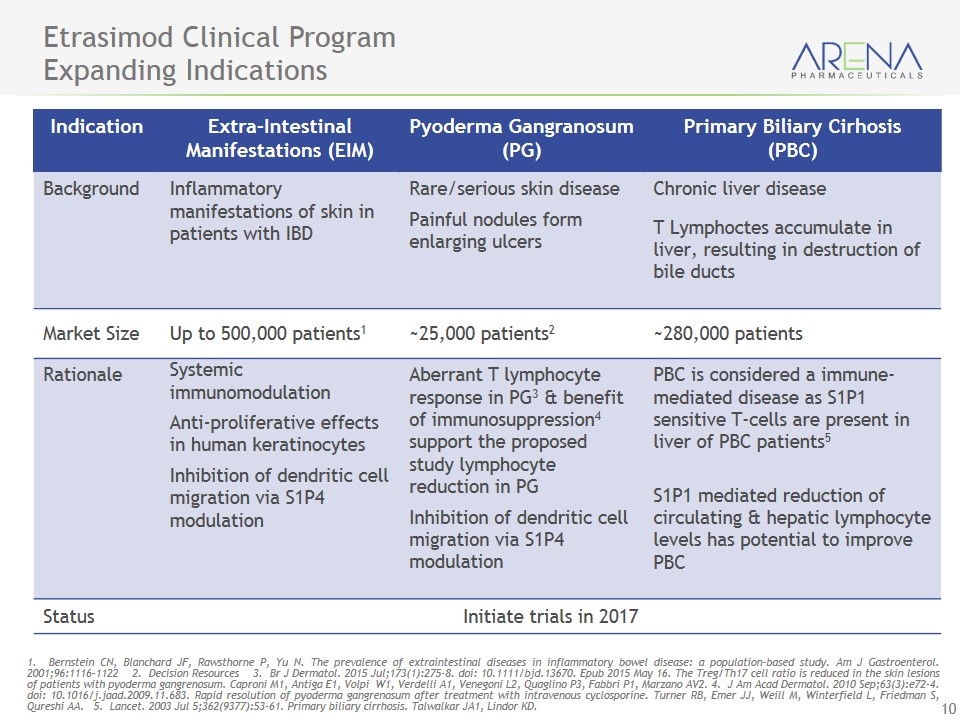
Etrasimod Clinical Program Expanding Indications 1. Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–1122 2. Decision Resources 3. Br J Dermatol. 2015 Jul;173(1):275-8. doi: 10.1111/bjd.13670. Epub 2015 May 16. The Treg/Th17 cell ratio is reduced in the skin lesions of patients with pyoderma gangrenosum. Caproni M1, Antiga E1, Volpi W1, Verdelli A1, Venegoni L2, Quaglino P3, Fabbri P1, Marzano AV2. 4. J Am Acad Dermatol. 2010 Sep;63(3):e72-4. doi: 10.1016/j.jaad.2009.11.683. Rapid resolution of pyoderma gangrenosum after treatment with intravenous cyclosporine. Turner RB, Emer JJ, Weill M, Winterfield L, Friedman S, Qureshi AA. 5. Lancet. 2003 Jul 5;362(9377):53-61. Primary biliary cirrhosis. Talwalkar JA1, Lindor KD. Indication Extra-Intestinal Manifestations (EIM) Pyoderma Gangranosum (PG) Primary Biliary Cirhosis (PBC) Background Inflammatory manifestations of skin in patients with IBD Rare/serious skin disease Painful nodules form enlarging ulcers Chronic liver disease T Lymphoctes accumulate in liver, resulting in destruction of bile ducts Market Size Up to 500,000 patients1 ~25,000 patients2 ~280,000 patients Rationale Systemic immunomodulation Anti-proliferative effects in human keratinocytes Inhibition of dendritic cell migration via S1P4 modulation Aberrant T lymphocyte response in PG3 & benefit of immunosuppression4 support the proposed study lymphocyte reduction in PG Inhibition of dendritic cell migration via S1P4 modulation PBC is considered a immune-mediated disease as S1P1 sensitive T-cells are present in liver of PBC patients5 S1P1 mediated reduction of circulating & hepatic lymphocyte levels has potential to improve PBC Status Initiate trials in 2017
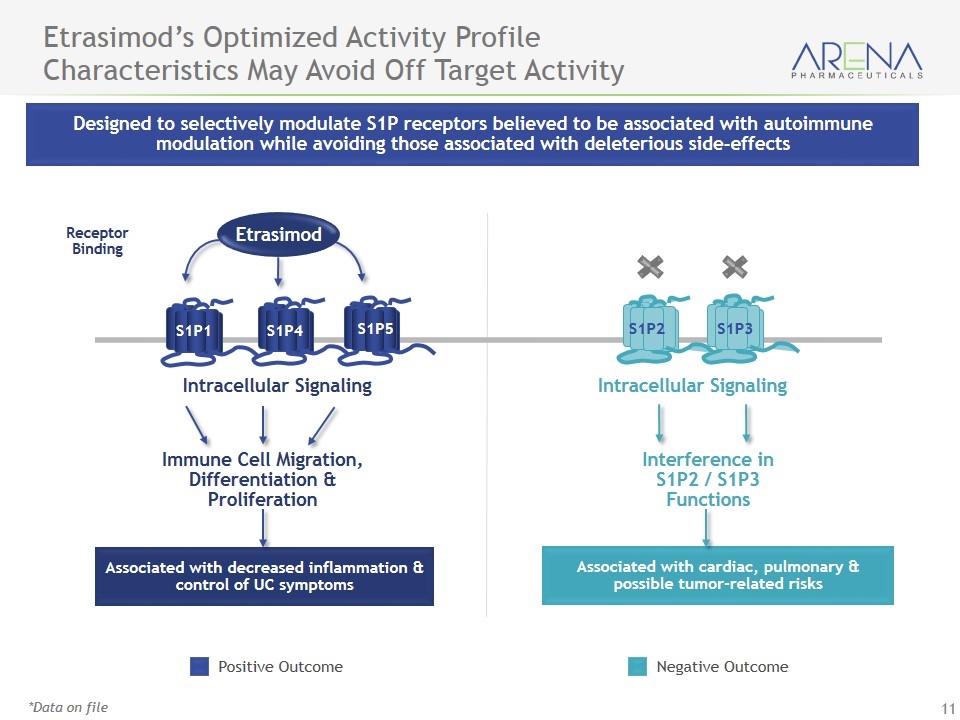
Designed to selectively modulate S1P receptors believed to be associated with autoimmune modulation while avoiding those associated with deleterious side-effects Etrasimod’s Optimized Activity Profile Characteristics May Avoid Off Target Activity *Data on file Associated with cardiac, pulmonary & possible tumor-related risks Associated with decreased inflammation & control of UC symptoms Immune Cell Migration, Differentiation & Proliferation Interference in S1P2 / S1P3 Functions Receptor Binding Intracellular Signaling Intracellular Signaling S1P2 S1P3 S1P1 S1P4 S1P5 Etrasimod Positive Outcome Negative Outcome
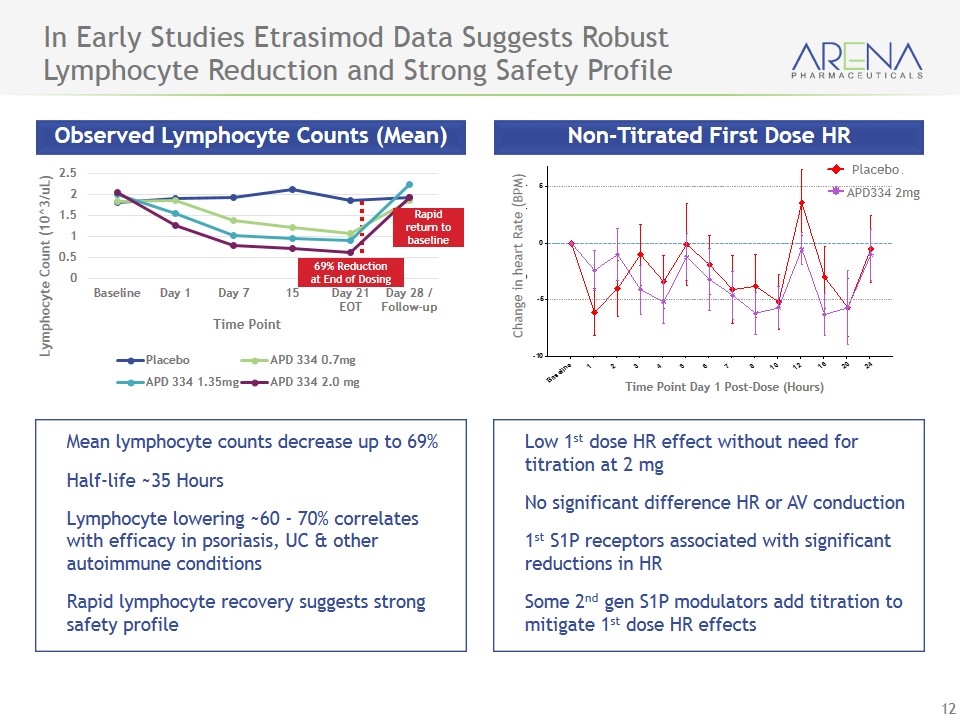
In Early Studies Etrasimod Data Suggests Robust Lymphocyte Reduction and Strong Safety Profile Observed Lymphocyte Counts (Mean) Non-Titrated First Dose HR Mean lymphocyte counts decrease up to 69% Half-life ~35 Hours Lymphocyte lowering ~60 - 70% correlates with efficacy in psoriasis, UC & other autoimmune conditions Rapid lymphocyte recovery suggests strong safety profile Low 1st dose HR effect without need for titration at 2 mg No significant difference HR or AV conduction 1st S1P receptors associated with significant reductions in HR Some 2nd gen S1P modulators add titration to mitigate 1st dose HR effects Rapid return to baseline 69% Reduction at End of Dosing Change in heart Rate (BPM) Placebo APD334 2mg Time Point Day 1 Post-Dose (Hours)

No symptomatic bradycardia No heart block (therapeutic dose) Based on Early Clinical Data Etrasimod Emerging as a Potential Best-in-Class S1P Modulator Excellent Lymphocyte Modulation 60-70% reduction in lymphocyte counts Recovery to baseline within one week Encouraging CV Safety Profile Clean Hepatotoxicity Safety Profile Favorable Pulmonary and Ocular Profile Promising PK and Adverse Event (AE) Profile Optimized Activity for the S1P Receptors No elevations in liver enzyme tests >1.3x in any dose group No clinically significant changes in pulmonary function tests No macular edema No titration; no discontinuations due to AEs No serious AEs; AEs not dose responsive No S1P2 internalization limits potential safety concerns

An Oral, Selective IP Receptor Agonist Targeting the Prostacyclin Pathway for the Treatment of Pulmonary Arterial Hypertension (PAH) Ralinepag

Ralinepag (APD811) - Potentially Best-in-Class Profile - Unique Receptor Activation & Pharmacokinetics Status Ralinepag demonstrated superior in-vitro performance, compared to selexipag in: Functional G-protein activation (cAMP) Potency for inhibition of vascular remodeling & vasodilation Potency for inhibition of platelet aggregation “IV-like” pharmacokinetic profile Data to Date Enrollment completed on Phase 2 multinational trial in PAH Data expected in mid-year 2017
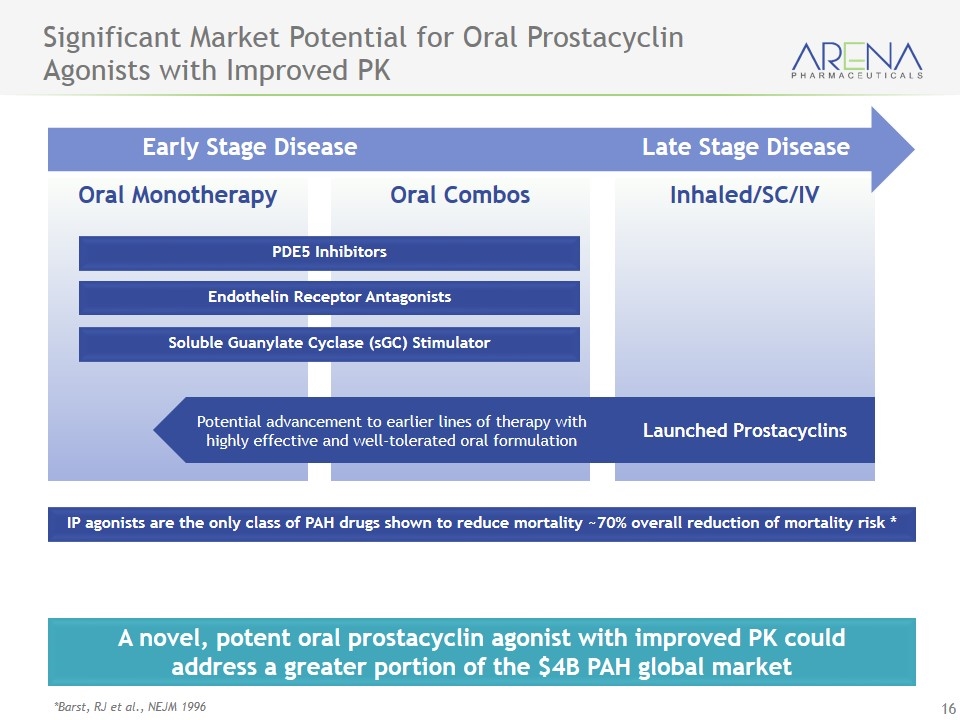
Oral Monotherapy Oral Combos Inhaled/SC/IV Significant Market Potential for Oral Prostacyclin Agonists with Improved PK A novel, potent oral prostacyclin agonist with improved PK could address a greater portion of the $4B PAH global market IP agonists are the only class of PAH drugs shown to reduce mortality ~70% overall reduction of mortality risk * *Barst, RJ et al., NEJM 1996 Early Stage Disease Late Stage Disease PDE5 Inhibitors Endothelin Receptor Antagonists Soluble Guanylate Cyclase (sGC) Stimulator Launched Prostacyclins Potential advancement to earlier lines of therapy with highly effective and well-tolerated oral formulation
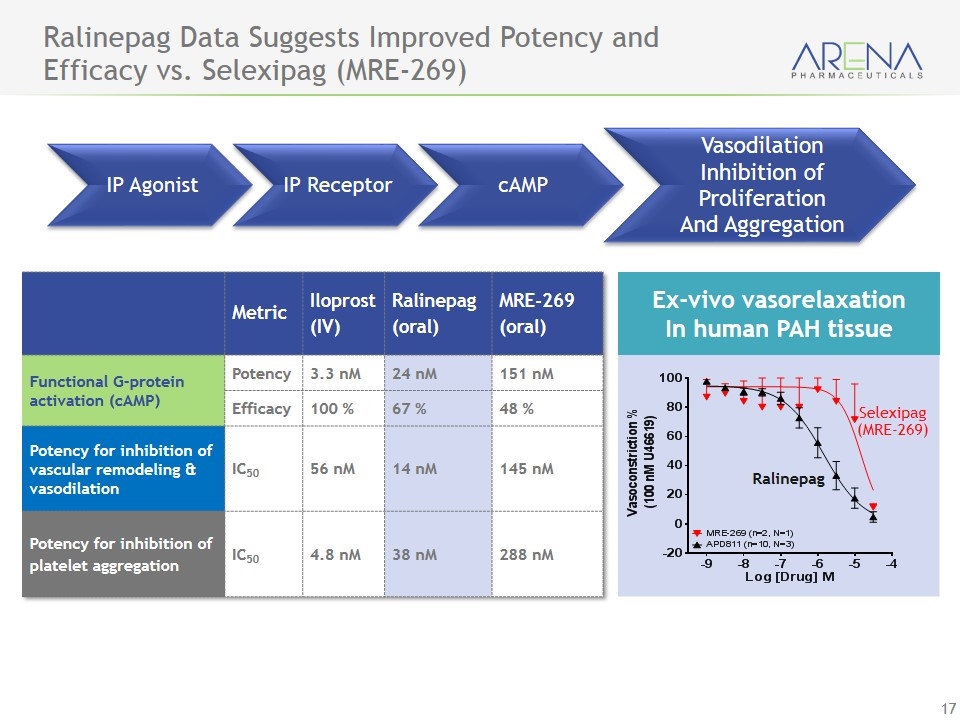
Ralinepag Data Suggests Improved Potency and Efficacy vs. Selexipag (MRE-269) Metric Iloprost (IV) Ralinepag (oral) MRE-269 (oral) Functional G-protein activation (cAMP) Potency 3.3 nM 24 nM 151 nM Efficacy 100 % 67 % 48 % Potency for inhibition of vascular remodeling & vasodilation IC50 56 nM 14 nM 145 nM Potency for inhibition of platelet aggregation IC50 4.8 nM 38 nM 288 nM Ex-vivo vasorelaxation In human PAH tissue Selexipag (MRE-269) Ralinepag IP Agonist cAMP Vasodilation Inhibition of Proliferation And Aggregation IP Receptor
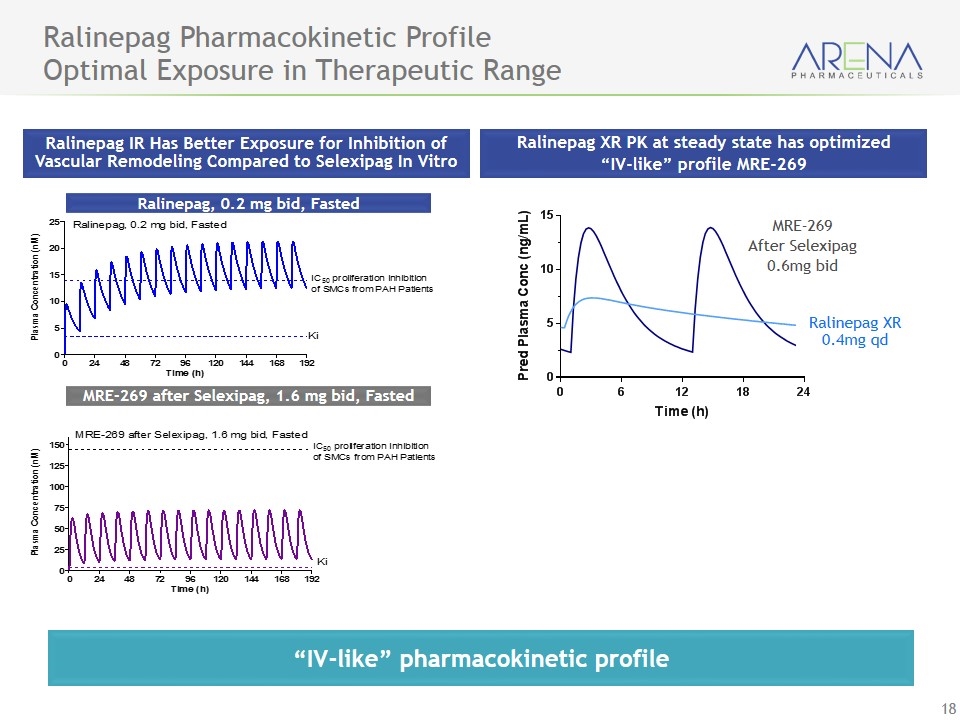
Ralinepag Pharmacokinetic Profile Optimal Exposure in Therapeutic Range Ralinepag IR Has Better Exposure for Inhibition of Vascular Remodeling Compared to Selexipag In Vitro MRE-269 after Selexipag, 1.6 mg bid, Fasted Ralinepag, 0.2 mg bid, Fasted Ralinepag XR PK at steady state has optimized “IV-like” profile MRE-269 MRE-269 After Selexipag 0.6mg bid “IV-like” pharmacokinetic profile Ralinepag XR 0.4mg qd
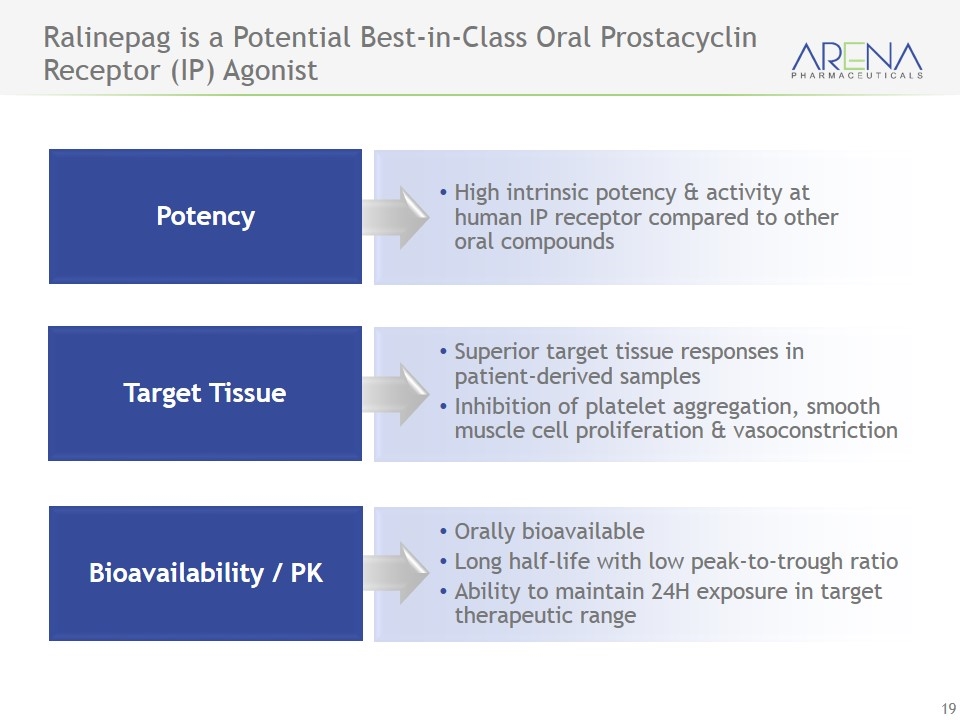
Ralinepag is a Potential Best-in-Class Oral Prostacyclin Receptor (IP) Agonist Potency High intrinsic potency & activity at human IP receptor compared to other oral compounds Superior target tissue responses in patient-derived samples Inhibition of platelet aggregation, smooth muscle cell proliferation & vasoconstriction Orally bioavailable Long half-life with low peak-to-trough ratio Ability to maintain 24H exposure in target therapeutic range Target Tissue Bioavailability / PK

APD371 Peripherally Restricted, Highly–Selective, Full Agonist to Cannabinoid 2 Receptor (CB2) Being Evaluated for Treatment of Pain Associated with Crohn’s Disease

APD371 - Potentially First-in-Class Profile Emerging Based on Preclinical and Clinical Data Phase 2 start early 2017 Data expected by year-end 2017 Superior pain option to opioids and other CB2 programs Highly selective, peripherally restricted avoids psychotropic activity Full CB2 receptor agonism prevents tachyphalaxis CB2 expressed in the target GI tissue and visceral afferent nerves Efficacy in preclinical models and strong safety profile from multiple Phase 1 studies Data to Date Status
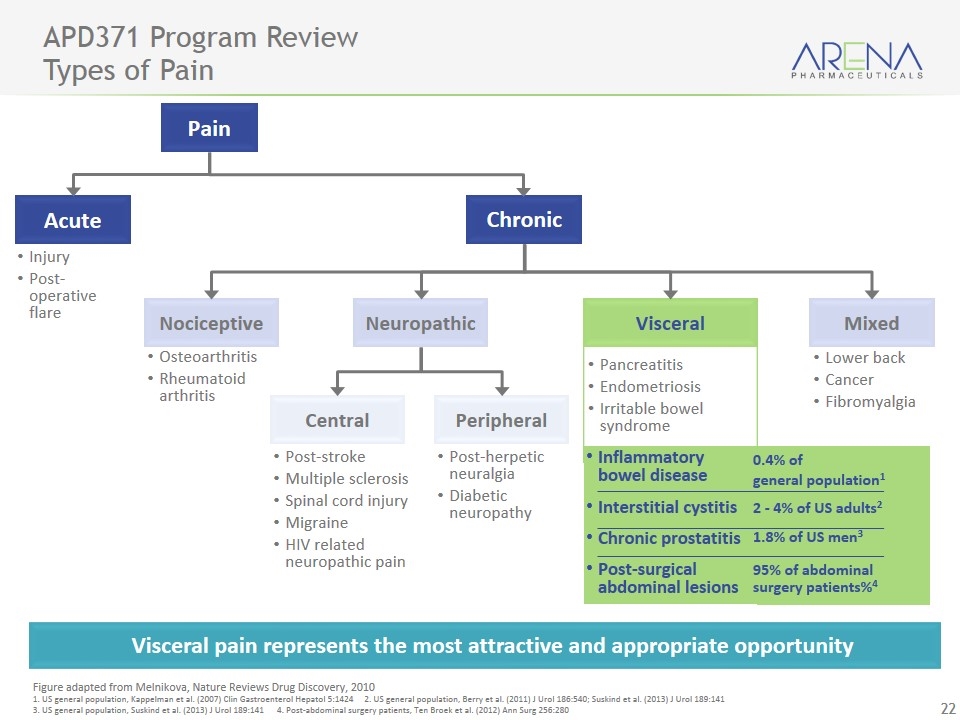
APD371 Program Review Types of Pain Visceral pain represents the most attractive and appropriate opportunity Figure adapted from Melnikova, Nature Reviews Drug Discovery, 2010 1. US general population, Kappelman et al. (2007) Clin Gastroenterol Hepatol 5:1424 2. US general population, Berry et al. (2011) J Urol 186:540; Suskind et al. (2013) J Urol 189:141 3. US general population, Suskind et al. (2013) J Urol 189:141 4. Post-abdominal surgery patients, Ten Broek et al. (2012) Ann Surg 256:280 Inflammatory bowel disease Interstitial cystitis Chronic prostatitis Post-surgical abdominal lesions Pain Acute Chronic Nociceptive Neuropathic Visceral Mixed Peripheral Central Lower back Cancer Fibromyalgia Post-herpetic neuralgia Diabetic neuropathy Post-stroke Multiple sclerosis Spinal cord injury Migraine HIV related neuropathic pain Osteoarthritis Rheumatoid arthritis Injury Post-operative flare 0.4% of general population1 2 - 4% of US adults2 95% of abdominal surgery patients%4 1.8% of US men3 Pancreatitis Endometriosis Irritable bowel syndrome
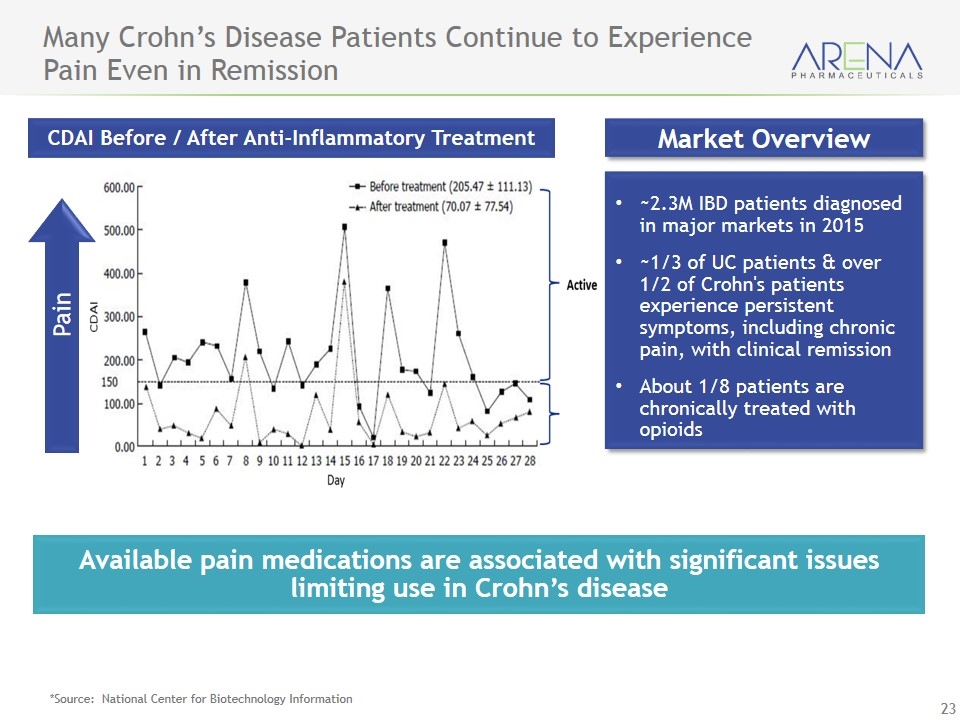
Many Crohn’s Disease Patients Continue to Experience Pain Even in Remission *Source: National Center for Biotechnology Information Available pain medications are associated with significant issues limiting use in Crohn’s disease Market Overview ~2.3M IBD patients diagnosed in major markets in 2015 ~1/3 of UC patients & over 1/2 of Crohn's patients experience persistent symptoms, including chronic pain, with clinical remission About 1/8 patients are chronically treated with opioids CDAI Before / After Anti-Inflammatory Treatment Pain
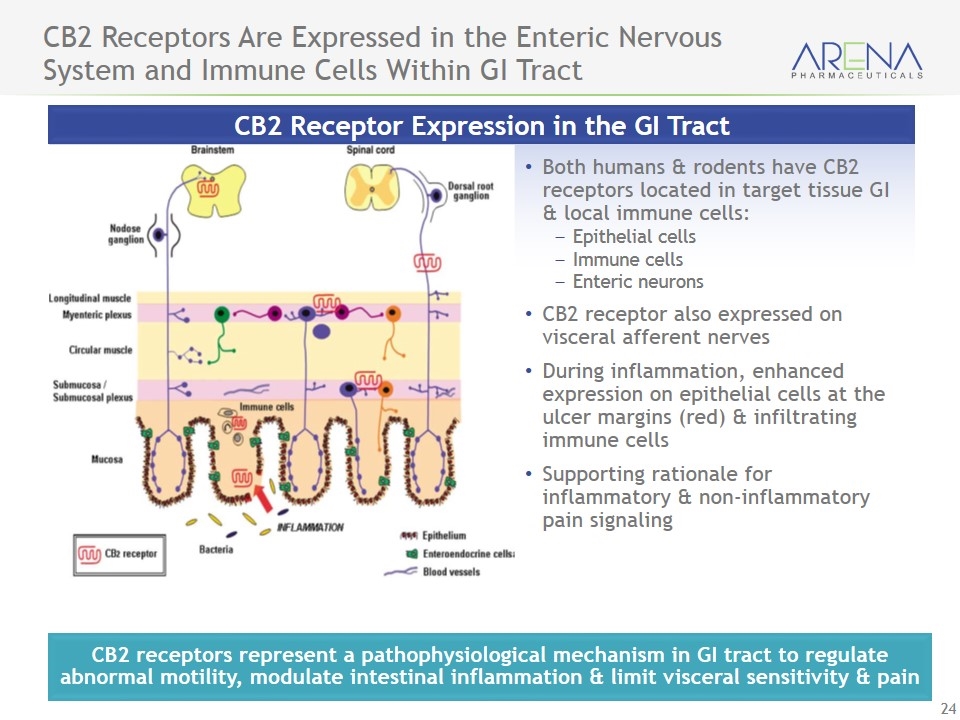
CB2 Receptors Are Expressed in the Enteric Nervous System and Immune Cells Within GI Tract Both humans & rodents have CB2 receptors located in target tissue GI & local immune cells: Epithelial cells Immune cells Enteric neurons CB2 receptor also expressed on visceral afferent nerves During inflammation, enhanced expression on epithelial cells at the ulcer margins (red) & infiltrating immune cells Supporting rationale for inflammatory & non-inflammatory pain signaling CB2 Receptor Expression in the GI Tract CB2 receptors represent a pathophysiological mechanism in GI tract to regulate abnormal motility, modulate intestinal inflammation & limit visceral sensitivity & pain
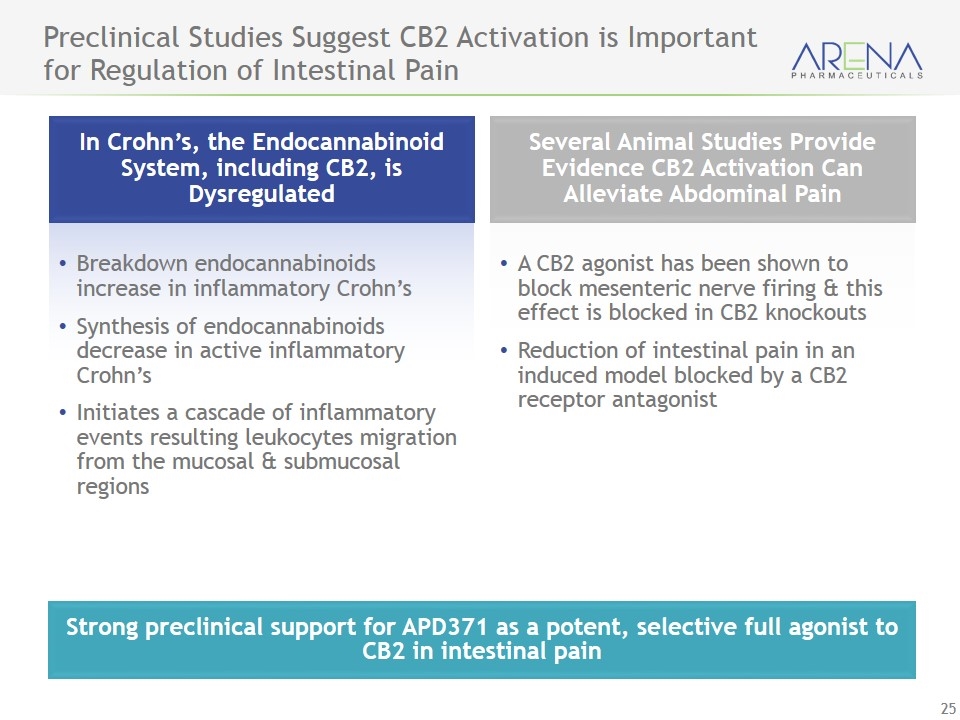
Strong preclinical support for APD371 as a potent, selective full agonist to CB2 in intestinal pain Preclinical Studies Suggest CB2 Activation is Important for Regulation of Intestinal Pain In Crohn’s, the Endocannabinoid System, including CB2, is Dysregulated Several Animal Studies Provide Evidence CB2 Activation Can Alleviate Abdominal Pain A CB2 agonist has been shown to block mesenteric nerve firing & this effect is blocked in CB2 knockouts Reduction of intestinal pain in an induced model blocked by a CB2 receptor antagonist Breakdown endocannabinoids increase in inflammatory Crohn’s Synthesis of endocannabinoids decrease in active inflammatory Crohn’s Initiates a cascade of inflammatory events resulting leukocytes migration from the mucosal & submucosal regions
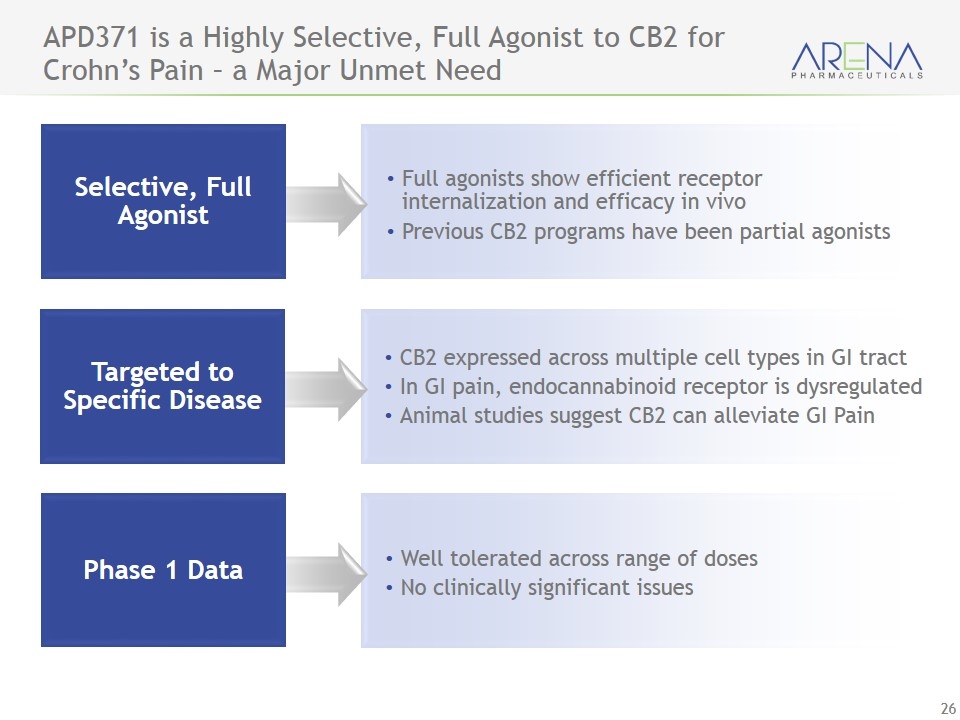
APD371 is a Highly Selective, Full Agonist to CB2 for Crohn’s Pain – a Major Unmet Need Selective, Full Agonist Full agonists show efficient receptor internalization and efficacy in vivo Previous CB2 programs have been partial agonists CB2 expressed across multiple cell types in GI tract In GI pain, endocannabinoid receptor is dysregulated Animal studies suggest CB2 can alleviate GI Pain Well tolerated across range of doses No clinically significant issues Targeted to Specific Disease Phase 1 Data
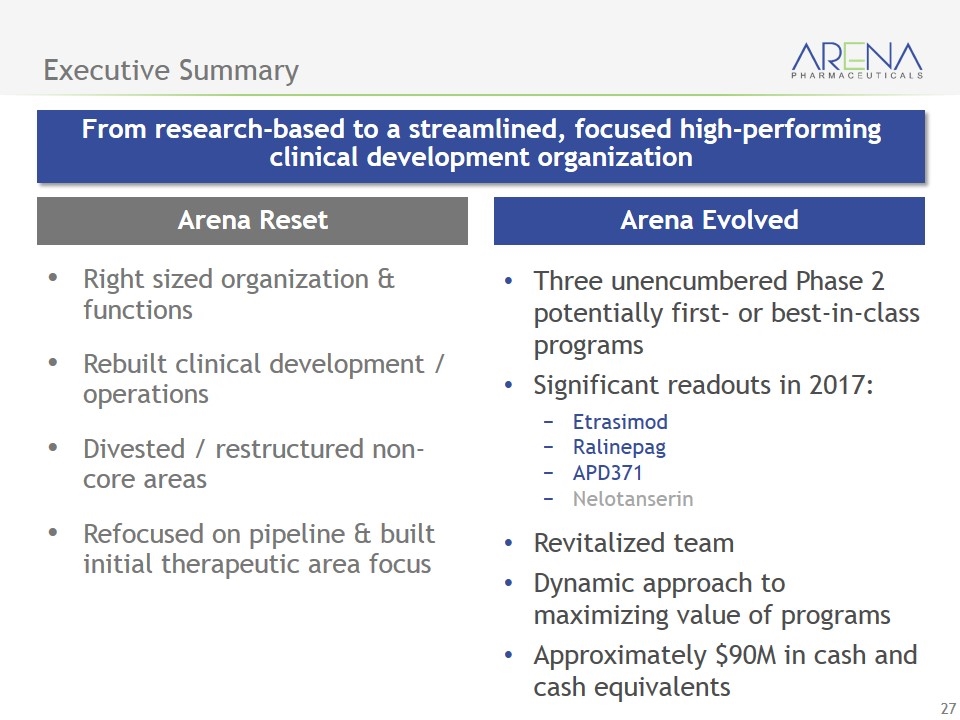
Arena Evolved Executive Summary From research-based to a streamlined, focused high-performing clinical development organization Three unencumbered Phase 2 potentially first- or best-in-class programs Significant readouts in 2017: Etrasimod Ralinepag APD371 Nelotanserin Revitalized team Dynamic approach to maximizing value of programs Approximately $90M in cash and cash equivalents Arena Reset Right sized organization & functions Rebuilt clinical development / operations Divested / restructured non-core areas Refocused on pipeline & built initial therapeutic area focus

NASDAQ: ARNA Thank you
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- WMTW, WGME-TV, WPFO, and WMEA-TV/WCBB Launch NextGen TV in Portland, Maine
- Gather attended the Dubai Web3 Summit and embarked on the path of globalization development
- Avery Dennison Increases Quarterly Dividend
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share