Form 8-K AMICUS THERAPEUTICS INC For: Jan 11
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT PURSUANT TO
SECTION 13 OR 15(D) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): January 11, 2016
AMICUS THERAPEUTICS, INC.
(Exact Name of Registrant as Specified in Its Charter)
Delaware
(State or Other Jurisdiction of
Incorporation)
|
001-33497 |
|
71-0869350 |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
1 Cedar Brook Drive, Cranbury, NJ |
|
08512 |
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (609) 662-2000
(Former Name or Former Address, if Changed Since Last Report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 2.02. Results of Operations and Financial Condition.
On January 11, 2016, Amicus Therapeutics, Inc. (the “Company”) issued a press release (the “Press Release”) regarding its financial condition for the year ended December 31, 2015. A copy of the Press Release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 7.01. Regulation FD Disclosure
Press Release
The Press Release also includes information regarding the Company’s 2015 accomplishments and its strategic outlook and financial guidance for the year ending December 31, 2016.
A copy of the Press Release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Corporate Presentations
The Company has updated its corporate presentation as of January 11, 2016. The slides from this presentation are attached hereto as Exhibit 99.2. The attached materials will be posted on the Company’s website at www.amicusrx.com. The Company does not undertake to update this presentation.
The Company presented a corporate presentation at the 3rd Annual Dermatology Summit: SD-101 for Epidermolysis Bullosa on Sunday, January 10, 2016. The slides from this presentation are attached hereto as Exhibit 99.3. The attached materials will be posted on the Company’s website at www.amicusrx.com. The Company does not undertake to update this presentation.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits:
|
Exhibit No. |
|
Description |
|
99.1 |
|
Press Release dated January 11, 2016 |
|
99.2 |
|
Corporate Presentation |
|
99.3 |
|
Corporate Presentation |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
Amicus Therapeutics, Inc. | |
|
|
| |
|
|
|
|
|
Date: January 11, 2016 |
By: |
/s/ William D. Baird III |
|
|
|
William D. Baird III |
|
|
|
Chief Financial Officer |
EXHIBIT INDEX
|
Exhibit No. |
|
Description |
|
99.1 |
|
Press Release dated January 11, 2016 |
|
99.2 |
|
Corporate Presentation |
|
99.3 |
|
Corporate Presentation |
Exhibit 99.1

Amicus Therapeutics Provides Full-Year 2016 Strategic Outlook and Financial Guidance
Migalastat for Fabry Disease Moving Toward CHMP Opinion in European Union
Pompe Disease Clinical Study Initiated to Investigate Novel Enzyme Replacement Therapy
Epidermolysis Bullosa (EB) Phase 3 Study Advances
CRANBURY, NJ, January 11, 2016 — Amicus Therapeutics (Nasdaq: FOLD), a biopharmaceutical company at the forefront of therapies for rare and orphan diseases, today provided its full-year 2016 strategic outlook and financial guidance.
John F. Crowley, Chairman and Chief Executive Officer of Amicus Therapeutics, Inc., stated, “During 2015 we established a global infrastructure with a leading commercial team, acquired a Phase 3 rare disease program in EB, submitted our first marketing application in Fabry in the EU, and advanced our first biologic medicine into the clinic in Pompe. These achievements have transformed Amicus and strongly position us to build one of the world’s leading biotechnology companies focused on rare and devastating diseases. In 2016 we will continue to focus on advancing our three lead programs in Fabry, Pompe and EB. The vision for Amicus continues to be to advance our pipeline and to acquire capabilities, programs and technologies that deliver on our mission for patients and that create substantial shareholder value.”
Key 2015 Accomplishments
· MAA Submission - submitted marketing authorization application (MAA) to European Medicines Agency (EMA) for approval of migalastat in Fabry patients with amenable genetic mutations
· Global Commercial Capabilities - established global commercial infrastructure with key leadership in place for EU migalastat launch
· Late-Stage Product Acquisition - strengthened pipeline with the acquisition of SD-101, a Phase 3 program for Epidermoylsis Bullosa (EB)
· Biologics Manufacturing - completed successful manufacturing scale up to supply clinical study of ATB200 — a novel ERT for Pompe Disease
· Pompe Clinical Study Initiation - initiated clinical study of ATB200 and oral chaperone (AT2221) in Pompe patients
Mr. Crowley will discuss Amicus’ corporate objectives and key milestones in a presentation at the 34th Annual J.P. Morgan Healthcare Conference on Tuesday, January 12, 2016 at 9:00 a.m. PT (12:00 p.m. ET). A live webcast of the presentation can be accessed through the Investors section of the Amicus Therapeutics corporate web site at http://ir.amicustherapeutics.com/events.cfm, and will be archived for 90 days.
Program Highlights
Migalastat for Fabry Disease
Migalastat is an oral personalized medicine intended to treat Fabry disease in patients who have amenable genetic mutations. Amicus has built a commercial organization in the EU that is prepared to launch migalastat upon approval. The EMA’s review of the MAA for migalastat is currently in progress. Amicus continues to expect an opinion from the Committee for Medicinal Products for Human Use (CHMP) in early 2016. As previously reported, the timing of a new drug
application (NDA) submission in the U.S. will be based on the determination of the optimal regulatory pathway. The Company expects to provide an update on the U.S. strategy for migalastat in the first quarter of 2016.
Anticipated 2016 Fabry Program Milestones
· CHMP Opinion in Europe (early 2016)
· Oral presentations and posters at the 12th Annual WORLDSymposium™ 2016 (February 29 - March 4, 2016 in San Diego, CA). Oral presentations will include:
· Podocyte globotriaosylceramide (GL-3) content in male adult patients with Fabry disease reduces following 6-12 months of treatment with migalastat
· Comparison of integrated white blood cell alpha-galactosidase A activity exposure between every-other-day orally administered migalastat and biweekly infusions of agalsidase beta or agalsidase alfa
· U.S. regulatory update (1Q16)
· Phase 3 data publications
Novel ERT for Pompe Disease (ATB200 + Chaperone)
Amicus has initiated the clinical study (ATB200-02) in Pompe patients with a novel treatment paradigm that consists of ATB200, a uniquely engineered recombinant human acid alpha-glucosidase (rhGAA) enzyme with an optimized carbohydrate structure to enhance uptake, administered with a pharmacological chaperone (AT2221) to improve activity and stability.
Amicus completed good manufacturing practice (GMP) production runs of ATB200 during 2015, and has sufficient supply for this clinical study in Pompe patients. Amicus finalized the design of the ongoing study following in-person meetings with regulatory authorities in both the U.S. and EU.
Anticipated Pompe Program Milestones
· Dosing of first patient in clinical study (early 2016)
· Oral presentations and posters at WORLDSymposium™. Oral presentation will include:
· Co-administration of the pharmacological chaperone AT2221 with a proprietary recombinant human acid alfa-glucosidase leads to greater plasma exposure and substrate reduction compared to alglucosidase alfa
· Data from clinical study ATB200-02
SD-101 for Epidermolysis Bullosa (EB)
SD-101 is a novel, late-stage, proprietary topical treatment and potential first-to-market therapy for EB. This investigational product was granted FDA breakthrough therapy designation in 2013 based on results from a Phase 2a study for the treatment of lesions in patients suffering with EB. SD-101 is the first-ever treatment in EB clinical studies to show improvements in wound closure across all major EB subtypes.
SD-101 is currently being investigated in a Phase 3 study (SD-005) to support global regulatory submissions. Amicus continues to open additional clinical sites for this Phase 3 study and expects enrollment to be complete by mid-year with data in the second half of 2016. Amicus Chief Business Officer Dipal Doshi has assumed the additional responsibilities as General Manager of the Scioderm Division of Amicus.
Anticipated EB Program Milestones
· Completion of enrollment in Phase 3 study (mid-2016)
· Top-line Phase 3 data (2H16)
2016 Financial Guidance
Cash, cash equivalents, and marketable securities totaled $214.0 million at December 31, 2015 compared to $169.1 million at December 31, 2014. The Company’s balance sheet was strengthened during 2015 with a $258 million public offering. Amicus expects full-year 2016 net cash spend between $135 million and $155 million. The current cash position is projected to fund operations into mid-2017.
About Amicus Therapeutics
Amicus Therapeutics (Nasdaq: FOLD) is a biotechnology company at the forefront of therapies for rare and orphan diseases. The Company has a robust pipeline of advanced therapies for a broad range of human genetic diseases. Amicus’ lead programs in development include the small molecule pharmacological chaperone migalastat as a monotherapy for Fabry disease, SD-101 for Epidermolysis Bullosa (EB), as well as novel enzyme replacement therapy (ERT) products for Fabry disease, Pompe disease, and other lysosomal storage disorders.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of Amicus’ candidate drug products, the timing and reporting of results from preclinical studies and clinical trials evaluating Amicus’ candidate drug products and the projected cash position for the Company. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “potential,” “plan,” “targets,” “likely,” “may,” “will,” “would,” “should” and “could,” and similar expressions or words identify forward-looking statements. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions and uncertainties. The inclusion of forward-looking statements should not be regarded as a representation by Amicus that any of its plans will be achieved. Any or all of the forward-looking statements in this press release may turn out to be wrong. They can be affected by inaccurate assumptions Amicus might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing and outcomes of discussions with regulatory authorities and the potential goals, progress, timing and results of preclinical studies and clinical trials, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in the business of Amicus, including, without limitation: the potential that results of clinical or pre-clinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities may not grant or may delay approval for our product candidates; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; the potential that we will need additional funding to complete all of our studies and, our dependence on third parties in the conduct of our clinical studies. Further, the results of earlier preclinical studies and/or clinical trials may not be predictive of future results. With respect to statements regarding projections of the Company’s cash position, actual results may differ based on market factors and the Company’s ability to execute its operational and budget plans. In addition, all forward looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the year ended December 31, 2014 and Form 10-Q for the quarter ended June 30, 2015. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this news release to reflect events or circumstances after the date hereof. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.
CONTACTS:
Investors/Media:
Amicus Therapeutics
Sara Pellegrino
Director, Investor Relations
(609) 662-5044
Media:
Pure Communications
Dan Budwick
(973) 271-6085
FOLD—G
Exhibit 99.2
34th Annual J.P. Morgan Healthcare Conference John F. Crowley, Chairman and Chief Executive Officer January 12, 2016

Safe Harbor This presentation will contain, “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of Amicus’ candidate drug products, the timing and reporting of results from preclinical studies and clinical trials evaluating Amicus’ candidate drug products, financing plans, and the projected cash position for the Company. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “potential,” “plan,” “targets,” “likely,” “may,” “will,” “would,” “should” and “could,” and similar expressions or words identify forward-looking statements. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions and uncertainties. The inclusion of forward-looking statements should not be regarded as a representation by Amicus that any of its plans will be achieved. Any or all of the forward-looking statements in this press release may turn out to be wrong. They can be affected by inaccurate assumptions Amicus might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing and outcomes of discussions with regulatory authorities, and in particular the timing of an NDA submission for migalastat monotherapy, and the potential goals, progress, timing and results of preclinical studies and clinical trials, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in the business of Amicus, including, without limitation: the potential that results of clinical or pre-clinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities may not grant or may delay approval for our product candidates; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; the potential that we will need additional funding to complete all of our studies and, our dependence on third parties in the conduct of our clinical studies. Further, the results of earlier preclinical studies and/or clinical trials may not be predictive of future results. With respect to statements regarding projections of the Company’s cash position, actual results may differ based on market factors and the Company’s ability to execute its operational and budget plans. In addition, all forward looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the year ended December 31, 2014 and Form 10-Q for the quarter ended June 30, 2015. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this news release to reflect events or circumstances after the date hereof. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995. 2 Introduction

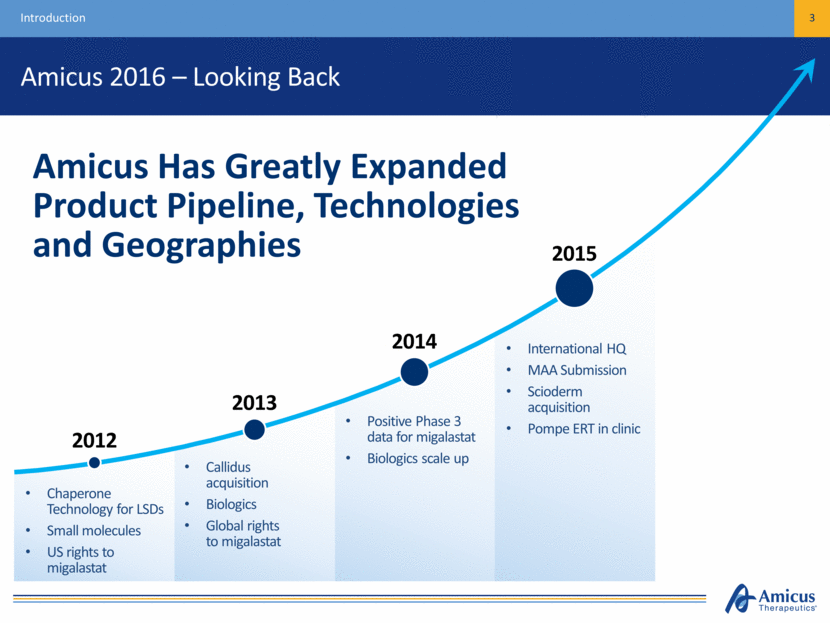
Amicus 2016 – Looking Back 3 Introduction Chaperone Technology for LSDs Small molecules US rights to migalastat Callidus acquisition Biologics Global rights to migalastat Positive Phase 3 data for migalastat Biologics scale up International HQ MAA Submission Scioderm acquisition Pompe ERT in clinic Amicus Has Greatly Expanded Product Pipeline, Technologies and Geographies 2012 2013 2014 2015
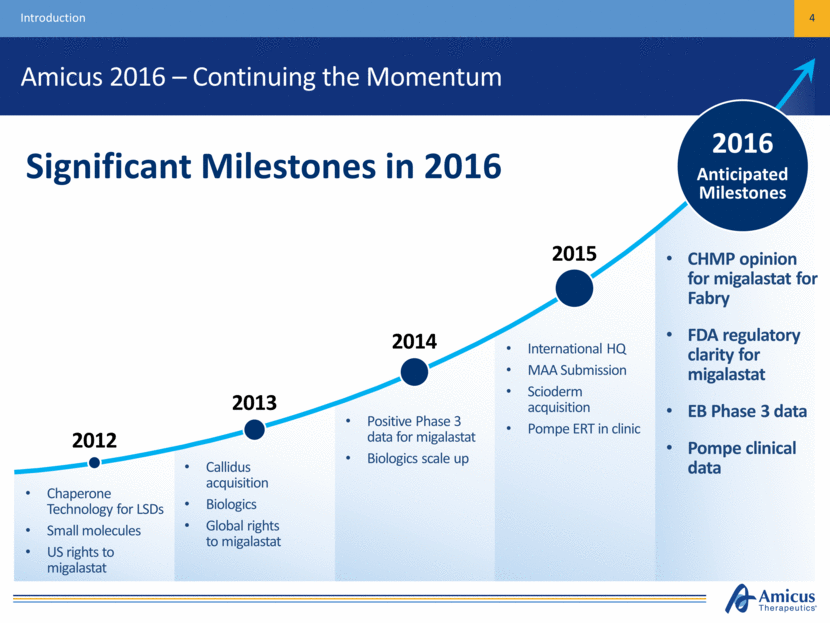
Amicus 2016 – Continuing the Momentum 4 Introduction CHMP opinion for migalastat for Fabry FDA regulatory clarity for migalastat EB Phase 3 data Pompe clinical data 2016 Anticipated Milestones Chaperone Technology for LSDs Small molecules US rights to migalastat Callidus acquisition Biologics Global rights to migalastat Positive Phase 3 data for migalastat Biologics scale up International HQ MAA Submission Scioderm acquisition Pompe ERT in clinic Significant Milestones in 2016 2012 2013 2014 2015
Amicus Vision Introduction 5 Amicus Therapeutics is a global biotechnology company at the forefront of developing advanced therapies to treat a range of devastating rare and orphan diseases Potential First-in-Class / Best-in-Class Meaningful benefits for patients Rare & Devastating Diseases
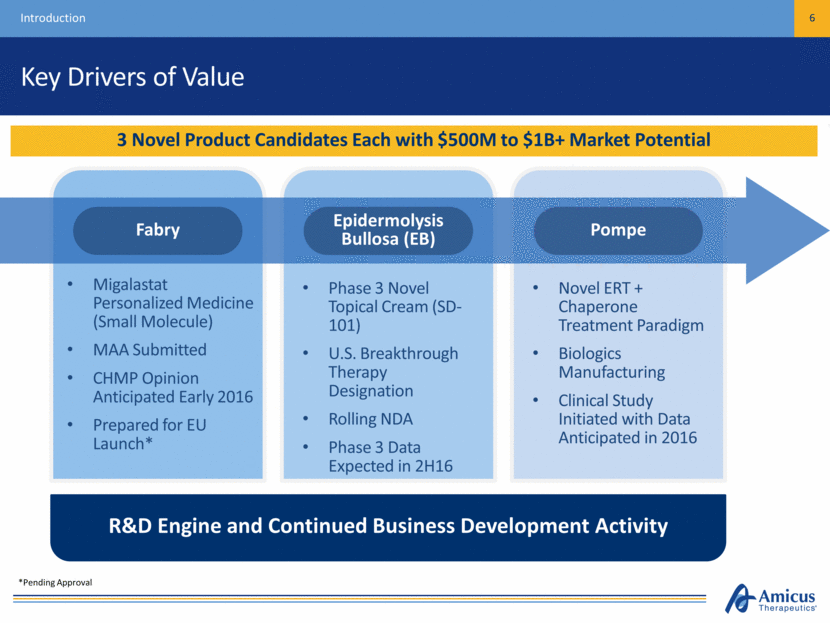
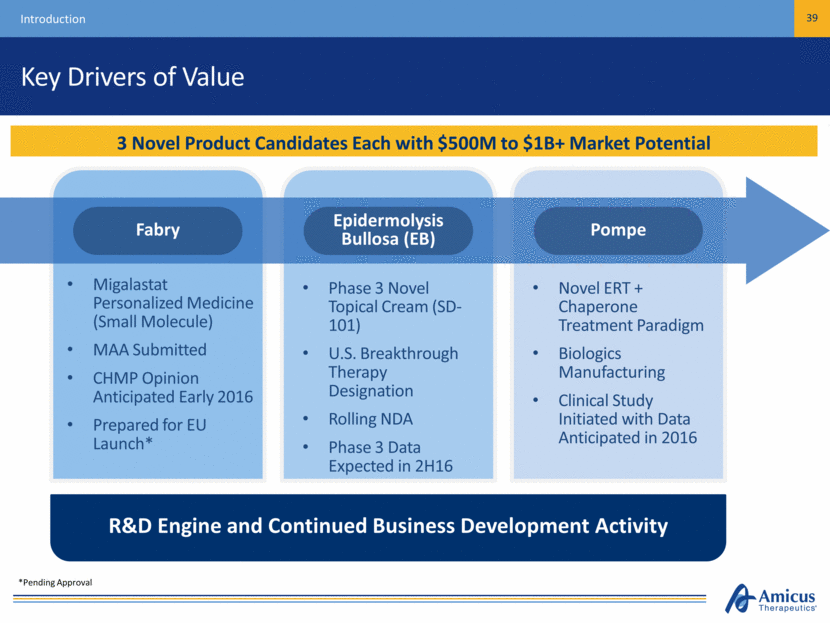
Key Drivers of Value Introduction 6 3 Novel Product Candidates Each with $500M to $1B+ Market Potential Migalastat Personalized Medicine (Small Molecule) MAA Submitted CHMP Opinion Anticipated Early 2016 Prepared for EU Launch* Phase 3 Novel Topical Cream (SD-101) U.S. Breakthrough Therapy Designation Rolling NDA Phase 3 Data Expected in 2H16 Novel ERT + Chaperone Treatment Paradigm Biologics Manufacturing Clinical Study Initiated with Data Anticipated in 2016 R&D Engine and Continued Business Development Activity Pompe Epidermolysis Bullosa (EB) Fabry *Pending Approval
Migalastat Personalized Medicine for Fabry Disease

Fabry Disease Overview – For Meetings Migalastat: Potential Personalized Medicine for Fabry Disease 8 Fabry Disease is a Fatal Genetic Disorder that Affects Multiple Organ Systems Leading Causes of Death Life-Limiting Symptoms TRANSIENT ISCHEMIC ATTACK (TIA) & STROKE1 KIDNEY DISEASE3 Protein in the urine Decreased kidney function Kidney failure HEART DISEASE2 Irregular heartbeat (fast or slow) Heart attack or heart failure Enlarged heart GASTROINTESTINAL3 Nausea, vomiting, cramping, and diarrhea Pain/bloating after eating, feeling full Constipation Difficulty managing weight Deficiency of α-Gal A enzyme leading to GL-3 accumulation >800 known mutations 5-10K diagnosed WW (51% female/49% male4) Newborn screening studies suggest prevalence of ~1:1000 to ~1:4000 Key Facts 1. Desnick R, et al. Ann Intern Med. 2003 2. Yousef Z, et al. Eur Heart J. 2013 3. Germain D. Orphanet J Rare Dis. 2010 4. Fabry Registry 2011
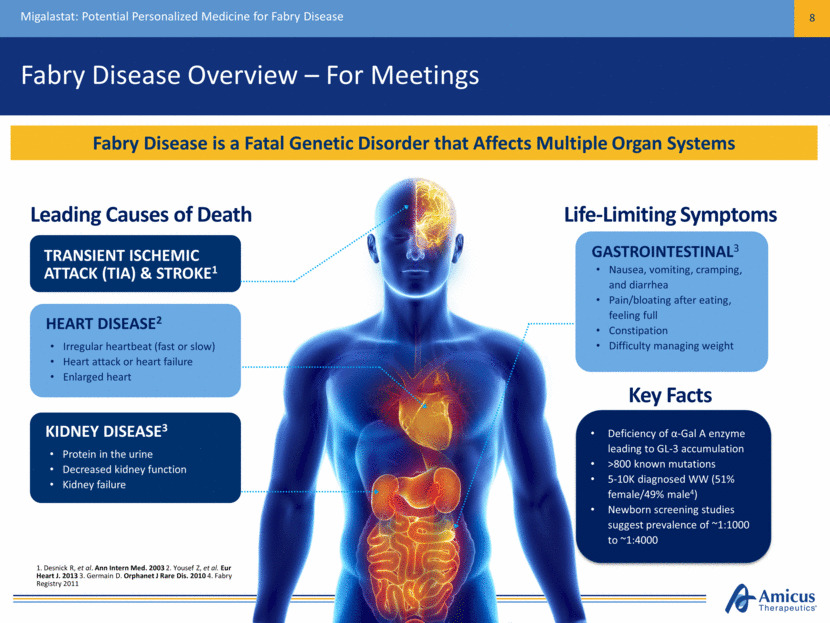
Fabry Disease Overview – For Podium Migalastat: Potential Personalized Medicine for Fabry Disease 9 Fabry Disease is a Fatal Genetic Disorder that Affects Multiple Organ Systems Leading Causes of Death Life-Limiting Symptoms TRANSIENT ISCHEMIC ATTACK (TIA) & STROKE1 KIDNEY DISEASE3 HEART DISEASE2 GASTROINTESTINAL3 1. Desnick R, et al. Ann Intern Med. 2003 2. Yousef Z, et al. Eur Heart J. 2013 3. Germain D. Orphanet J Rare Dis. 2010 4. Fabry Registry 2011 Deficiency of α-Gal A enzyme leading to GL-3 accumulation >800 known mutations 5-10K diagnosed WW (51% female/49% male4) Newborn screening studies suggest prevalence of ~1:1000 to ~1:4000 Key Facts
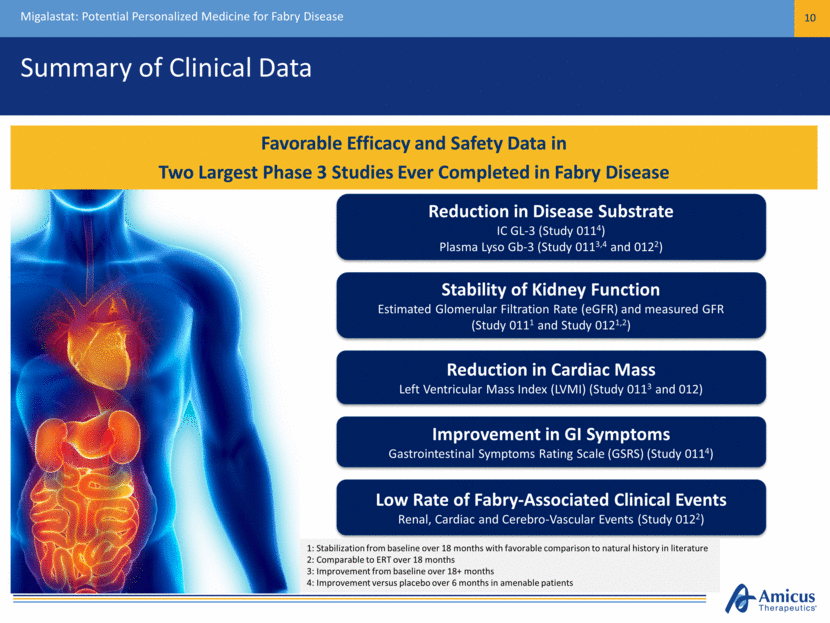
Summary of Clinical Data Migalastat: Potential Personalized Medicine for Fabry Disease 10 Favorable Efficacy and Safety Data in Two Largest Phase 3 Studies Ever Completed in Fabry Disease 1: Stabilization from baseline over 18 months with favorable comparison to natural history in literature 2: Comparable to ERT over 18 months 3: Improvement from baseline over 18+ months 4: Improvement versus placebo over 6 months in amenable patients Reduction in Cardiac Mass Left Ventricular Mass Index (LVMI) (Study 0113 and 012) Improvement in GI Symptoms Gastrointestinal Symptoms Rating Scale (GSRS) (Study 0114) Reduction in Disease Substrate IC GL-3 (Study 0114) Plasma Lyso Gb-3 (Study 0113,4 and 0122) Low Rate of Fabry-Associated Clinical Events Renal, Cardiac and Cerebro-Vascular Events (Study 0122) Stability of Kidney Function Estimated Glomerular Filtration Rate (eGFR) and measured GFR (Study 0111 and Study 0121,2)
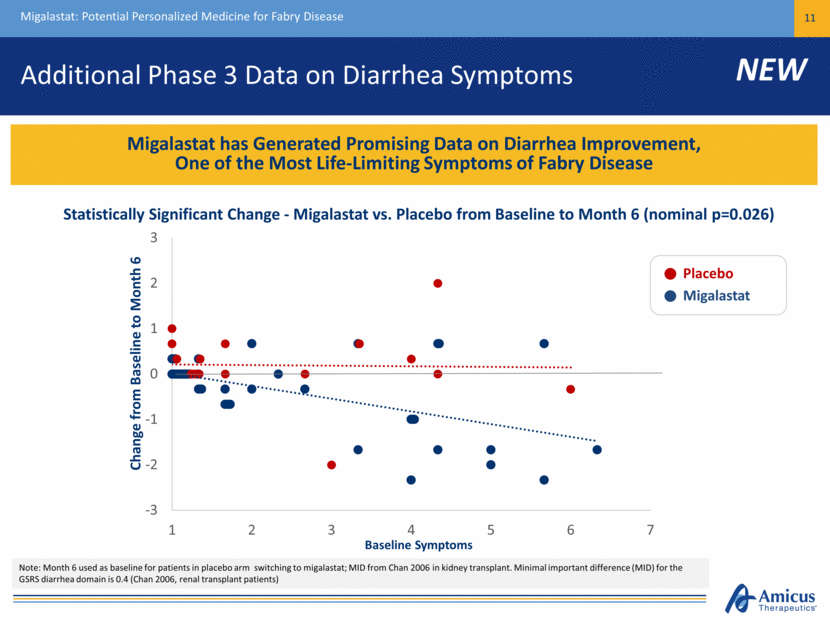
Additional Phase 3 Data on Diarrhea Symptoms Migalastat: Potential Personalized Medicine for Fabry Disease 11 Migalastat has Generated Promising Data on Diarrhea Improvement, One of the Most Life-Limiting Symptoms of Fabry Disease Note: Month 6 used as baseline for patients in placebo arm switching to migalastat; MID from Chan 2006 in kidney transplant. Minimal important difference (MID) for the GSRS diarrhea domain is 0.4 (Chan 2006, renal transplant patients) Baseline Symptoms Change from Baseline to Month 6 Placebo Migalastat Statistically Significant Change - Migalastat vs. Placebo from Baseline to Month 6 (nominal p=0.026) NEW -3 -2 -1 0 1 2 3 1 2 3 4 5 6 7
Fabry Patient Perspective Migalastat: Potential Personalized Medicine for Fabry Disease 12 Case Report from Long-Term Treatment with Migalastat Shows Improvement in Pain and Return to Everyday Activities1 1. Schiffmann, et al. Nine-year follow-up in a patient receiving migalastat pharmacological chaperone therapy for Fabry disease. SSIEM 2015. Enzyme activity increased Cardiac and renal functions remained normal Phase 2 Study + OLE for 4.5 Years Phase 3 OLE for 4.5 Years (Still Ongoing) Paresthesias and sensation of stiffness improved Feels well, goes to gym and works Discontinued pain medication Patient Journey to Diagnosis Chronic pain Weakness and fatigue Pain medication

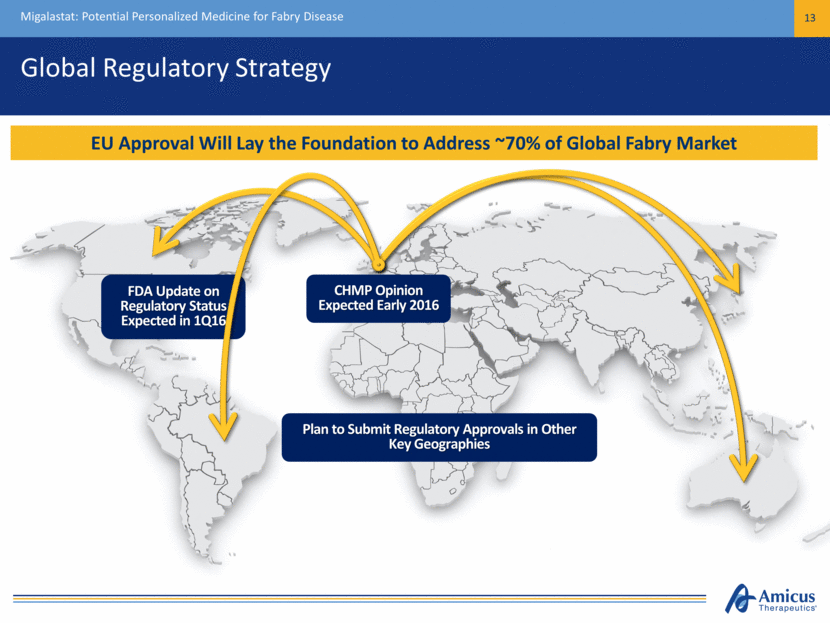
Global Regulatory Strategy Migalastat: Potential Personalized Medicine for Fabry Disease 13 FDA Update on Regulatory Status Expected in 1Q16 CHMP Opinion Expected Early 2016 Plan to Submit Regulatory Approvals in Other Key Geographies EU Approval Will Lay the Foundation to Address ~70% of Global Fabry Market
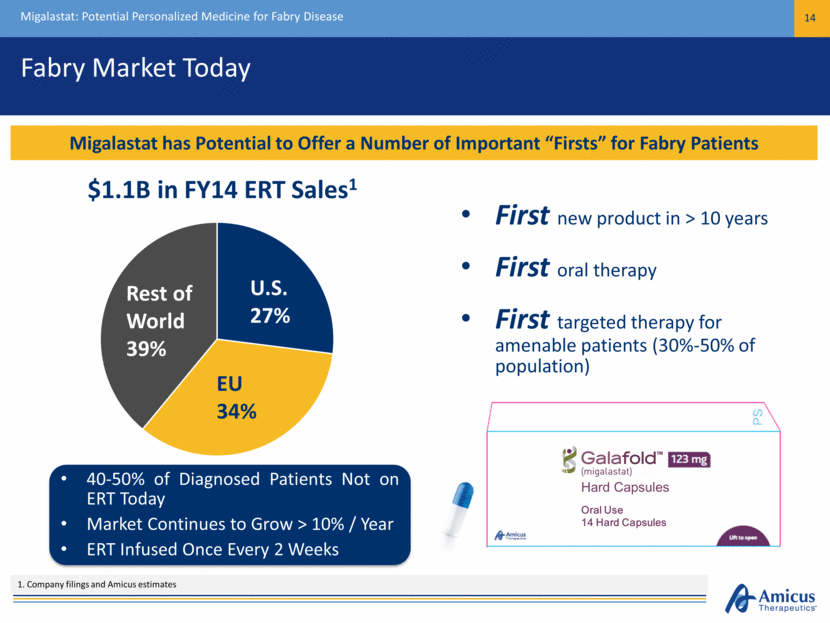
Fabry Market Today Migalastat: Potential Personalized Medicine for Fabry Disease 14 Migalastat has Potential to Offer a Number of Important “Firsts” for Fabry Patients $1.1B in FY14 ERT Sales1 1. Company filings and Amicus estimates 40-50% of Diagnosed Patients Not on ERT Today Market Continues to Grow > 10% / Year ERT Infused Once Every 2 Weeks First new product in > 10 years First oral therapy First targeted therapy for amenable patients (30%-50% of population) U.S. 27% EU 34% Rest of World 39% Hard Capsules Oral Use 14 Hard Capsules
Global Infrastructure and International Team Migalastat: Potential Personalized Medicine for Fabry Disease 15 World-Class Global Commercial Team to Support Migalastat Launch Upon Regulatory Approvals with Further Expansion in 2016 Global HQ Germany UK/Intl. HQ France Netherlands
Global Infrastructure and International Team Migalastat: Potential Personalized Medicine for Fabry Disease 16 World-Class Global Commercial Team to Support Migalastat Launch Upon Regulatory Approvals with Further Expansion in 2016 Global HQ Germany UK/Intl. HQ France Netherlands
Commercial Launch Preparation Activities 17 Migalastat: Potential Personalized Medicine for Fabry Disease Experienced commercial leadership team with established international operations International distribution system Medical education and patient advocacy ongoing on behalf of Fabry patients Global value dossier complete and local submissions in development Amicus is Preparing for Potential Launches in 2016 Patient and physician mapping
SD-101 for Epidermolysis Bullosa (EB) Poised to deliver pivotal data for a devastating rare disease in 2016

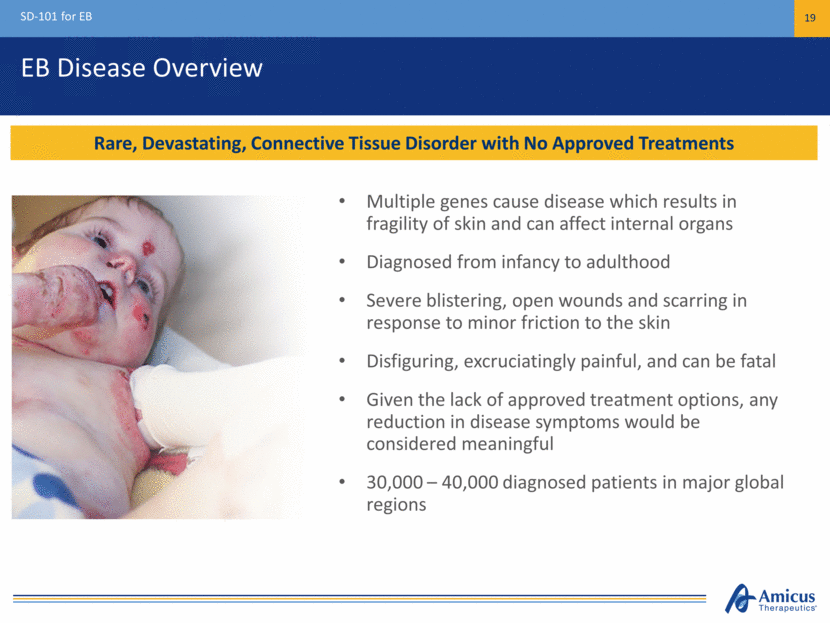
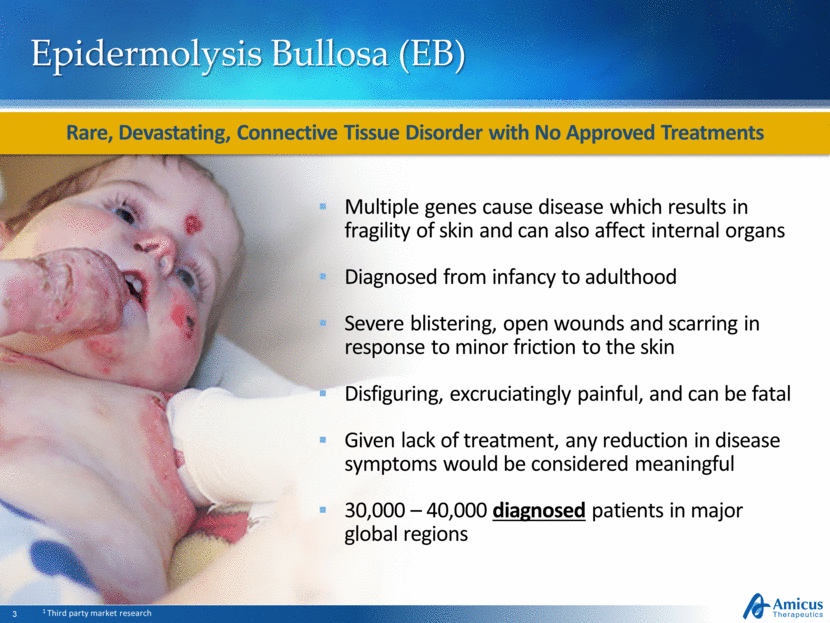
EB Disease Overview SD-101 for EB 19 Rare, Devastating, Connective Tissue Disorder with No Approved Treatments Multiple genes cause disease which results in fragility of skin and can affect internal organs Diagnosed from infancy to adulthood Severe blistering, open wounds and scarring in response to minor friction to the skin Disfiguring, excruciatingly painful, and can be fatal Given the lack of approved treatment options, any reduction in disease symptoms would be considered meaningful 30,000 – 40,000 diagnosed patients in major global regions
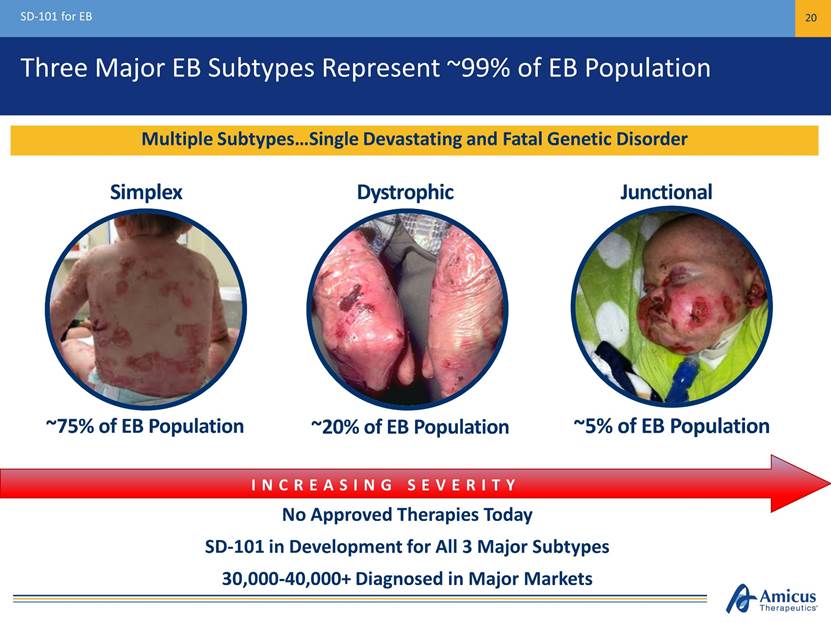
Three Major EB Subtypes Represent ~99% of EB Population SD-101 for EB 20 No Approved Therapies Today SD-101 in Development for All 3 Major Subtypes 30,000-40,000+ Diagnosed in Major Markets INCREASING SEVERITY Multiple Subtypes Single Devastating and Fatal Genetic Disorder Simplex Dystrophic Junctional ~75% of EB Population ~20% of EB Population ~5% of EB Population
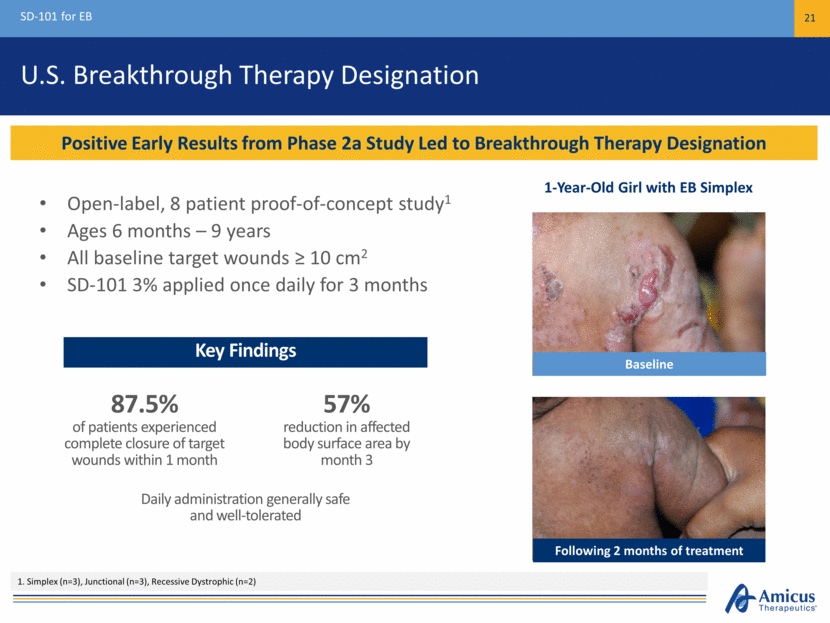
U.S. Breakthrough Therapy Designation Open-label, 8 patient proof-of-concept study1 Ages 6 months – 9 years All baseline target wounds ≥ 10 cm2 SD-101 3% applied once daily for 3 months SD-101 for EB 21 Positive Early Results from Phase 2a Study Led to Breakthrough Therapy Designation 1. Simplex (n=3), Junctional (n=3), Recessive Dystrophic (n=2) Key Findings 1-Year-Old Girl with EB Simplex Baseline Following 2 months of treatment 87.5% of patients experienced complete closure of target wounds within 1 month Daily administration generally safe and well-tolerated 57% reduction in affected body surface area by month 3
Phase 2b Design (Study 003) SD-101 for EB 22 Placebo (n=17) SD-101 6% (n=15) Open-Label Zorblisa (6%) 3-Month Double-Blind Treatment Period1 Primary Efficacy Endpoint: Target Wound Healing at Month 1 Baseline wound: Chronic (≥ 21 days), size 5-50 cm2 SD-101 3% (n=16) Secondary Efficacy Endpoints Include: Time to target wound closure Change in Body Surface Area (BSA) of lesional skin 42/44 Patients entered extension study $400K FDA Grant for Extension Study 48 EB patients (age ≥ 6 months)1 - 1:1:1 Randomization - Daily Topical Application 1. Assessments: 0, 14, 30, 60, 90 Days. 2. Initial Disease Severity: Mean target lesion size (cm2) 14.0 (range 5-39); mean lesional BSA: 19.4% (range 0.4-48%); mean wound age (days): 182 (range 21-1,639). EB Subtypes enrolled: Simplex (n=11), Recessive Dystrophic (n=29), and Junctional (n=8) Optional Extension (SD-004)
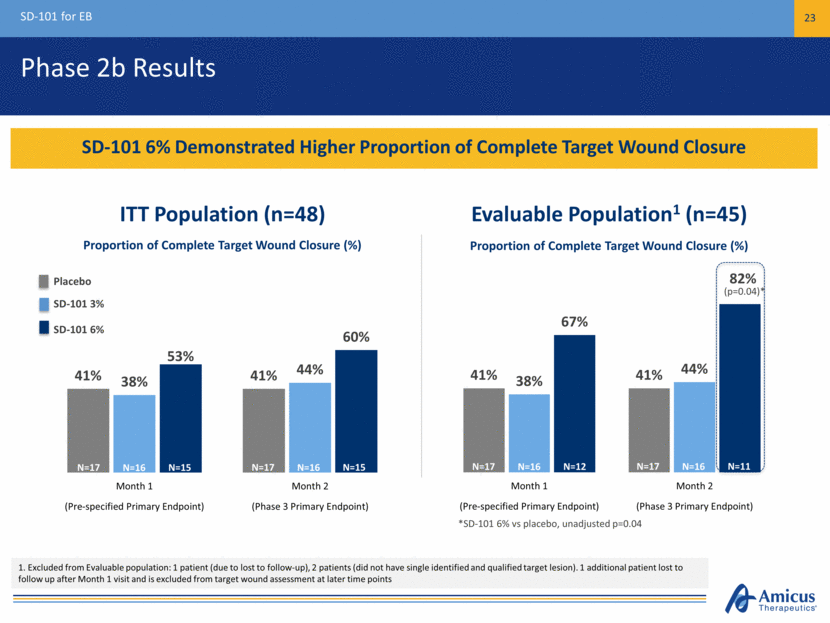
Phase 2b Results SD-101 for EB 23 SD-101 6% Demonstrated Higher Proportion of Complete Target Wound Closure *SD-101 6% vs placebo, unadjusted p=0.04 ITT Population (n=48) Evaluable Population1 (n=45) (p=0.04)* Proportion of Complete Target Wound Closure (%) Proportion of Complete Target Wound Closure (%) 1. Excluded from Evaluable population: 1 patient (due to lost to follow-up), 2 patients (did not have single identified and qualified target lesion). 1 additional patient lost to follow up after Month 1 visit and is excluded from target wound assessment at later time points N=17 N=15 N=16 N=17 N=15 N=16 N=17 N=12 N=16 N=17 N=11 N=16 Placebo SD-101 3% SD-101 6% 41% 41% 38% 44% 67% 82% Month 1 Month 2 (Pre-specified Primary Endpoint) (Phase 3 Primary Endpoint) 41% 41% 38% 44% 53% 60% Month 1 Month 2 (Pre-specified Primary Endpoint) (Phase 3 Primary Endpoint)
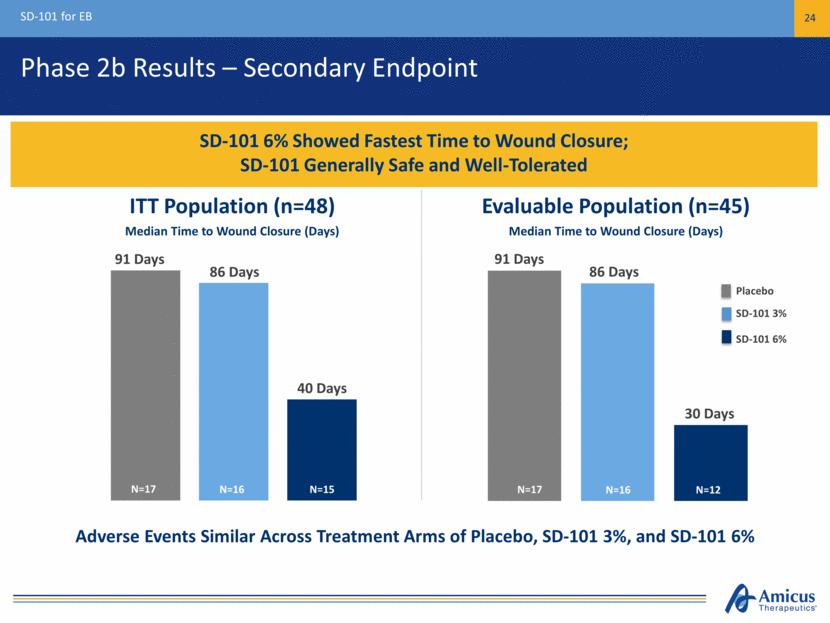
Phase 2b Results – Secondary Endpoint SD-101 for EB 24 SD-101 6% Showed Fastest Time to Wound Closure; SD-101 Generally Safe and Well-Tolerated Median Time to Wound Closure (Days) Median Time to Wound Closure (Days) 91 Days 86 Days 40 Days ITT Population (n=48) Evaluable Population (n=45) Adverse Events Similar Across Treatment Arms of Placebo, SD-101 3%, and SD-101 6% Placebo SD-101 3% SD-101 6% 91 Days 86 Days 30 Days N=17 N=15 N=16 N=17 N=12 N=16
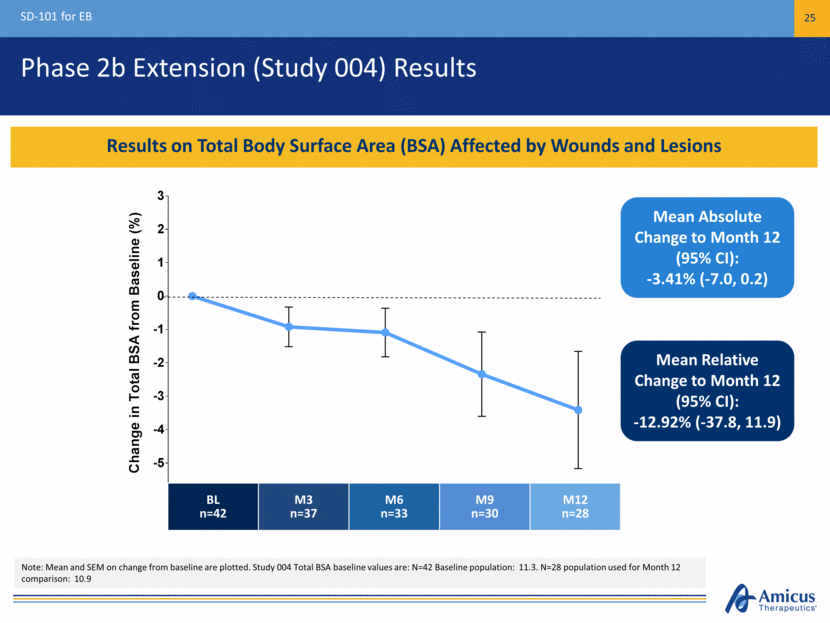
Phase 2b Extension (Study 004) Results SD-101 for EB 25 Results on Total Body Surface Area (BSA) Affected by Wounds and Lesions Mean Absolute Change to Month 12 (95% CI): -3.41% (-7.0, 0.2) BL n=42 M3 n=37 M6 n=33 M9 n=30 M12 n=28 Note: Mean and SEM on change from baseline are plotted. Study 004 Total BSA baseline values are: N=42 Baseline population: 11.3. N=28 population used for Month 12 comparison: 10.9 Mean Relative Change to Month 12 (95% CI): -12.92% (-37.8, 11.9) 3 6 9 12 -5 -4 -3 -2 -1 0 1 2 3 Baseline Time, Months C h a n g e i n T o t a l B S A f r o m B a s e l i n e ( % )
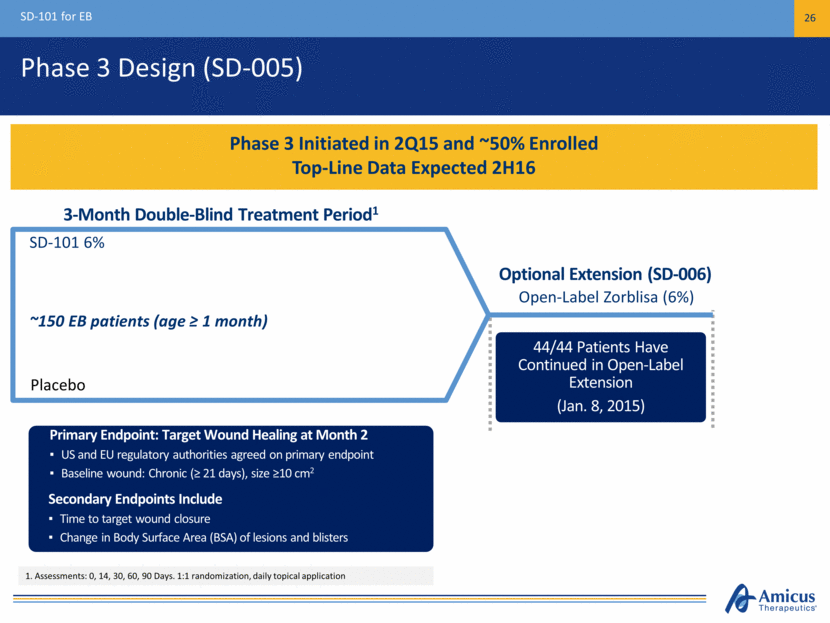
Phase 3 Design (SD-005) SD-101 for EB 26 Phase 3 Initiated in 2Q15 and ~50% Enrolled Top-Line Data Expected 2H16 ~150 EB patients (age ≥ 1 month) Placebo SD-101 6% Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (≥ 21 days), size ≥10 cm2 44/44 Patients Have Continued in Open-Label Extension (Jan. 8, 2015) Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (≥ 21 days), size ≥10 cm2 Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (≥ 21 days), size ≥10 cm2 Secondary Endpoints Include Time to target wound closure Change in Body Surface Area (BSA) of lesions and blisters 3-Month Double-Blind Treatment Period1 Open-Label Zorblisa (6%) Optional Extension (SD-006) 1. Assessments: 0, 14, 30, 60, 90 Days. 1:1 randomization, daily topical application
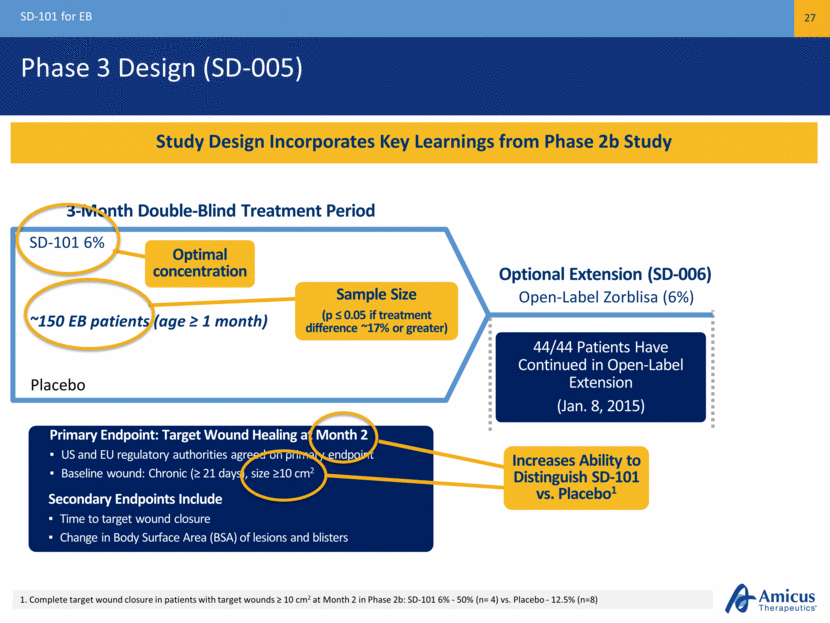
Phase 3 Design (SD-005) SD-101 for EB 27 Study Design Incorporates Key Learnings from Phase 2b Study ~150 EB patients (age ≥ 1 month) Placebo SD-101 6% Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (≥ 21 days), size ≥10 cm2 44/44 Patients Have Continued in Open-Label Extension (Jan. 8, 2015) Sample Size (p ≤ 0.05 if treatment difference ~17% or greater) Optimal concentration 1. Complete target wound closure in patients with target wounds ≥ 10 cm2 at Month 2 in Phase 2b: SD-101 6% - 50% (n= 4) vs. Placebo - 12.5% (n=8) Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (≥ 21 days), size ≥10 cm2 Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (≥ 21 days), size ≥10 cm2 Secondary Endpoints Include Time to target wound closure Change in Body Surface Area (BSA) of lesions and blisters Increases Ability to Distinguish SD-101 vs. Placebo1 3-Month Double-Blind Treatment Period Open-Label Zorblisa (6%) Optional Extension (SD-006)
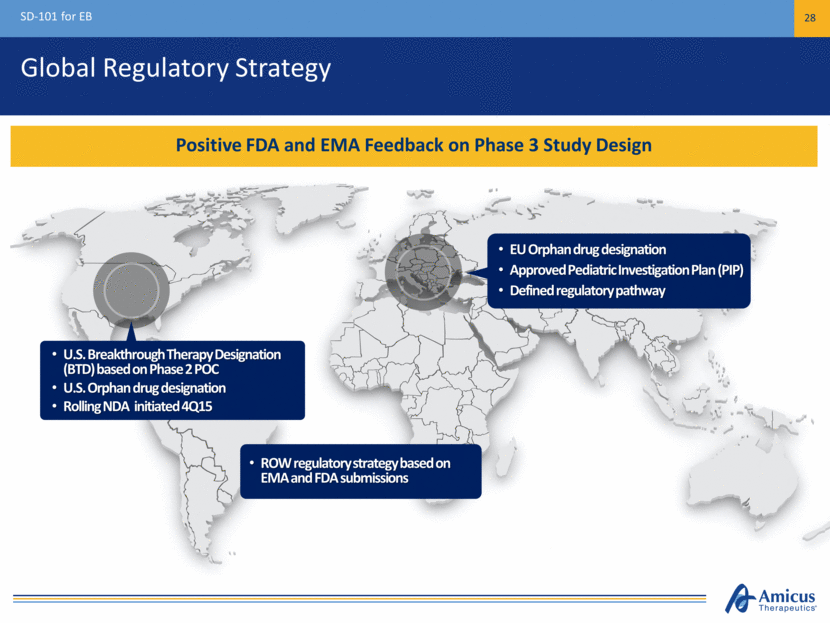
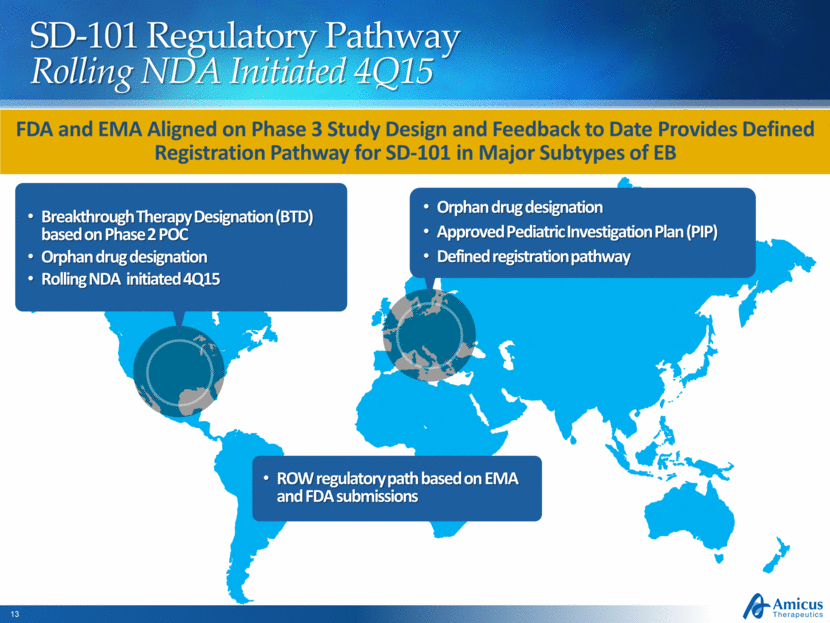
Global Regulatory Strategy SD-101 for EB 28 Positive FDA and EMA Feedback on Phase 3 Study Design U.S. Breakthrough Therapy Designation (BTD) based on Phase 2 POC U.S. Orphan drug designation Rolling NDA initiated 4Q15 EU Orphan drug designation Approved Pediatric Investigation Plan (PIP) Defined regulatory pathway ROW regulatory strategy based on EMA and FDA submissions
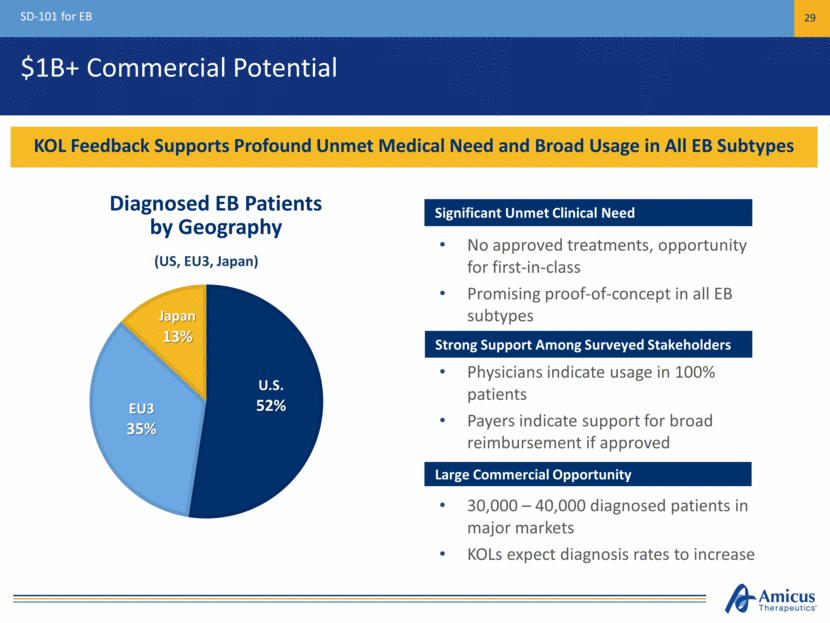
$1B+ Commercial Potential SD-101 for EB 29 KOL Feedback Supports Profound Unmet Medical Need and Broad Usage in All EB Subtypes Diagnosed EB Patients by Geography Significant Unmet Medical Need Significant Unmet Clinical Need No approved treatments, opportunity for first-in-class Promising proof-of-concept in all EB subtypes Strong Support Among Surveyed Stakeholders Physicians indicate usage in 100% patients Payers indicate support for broad reimbursement if approved Large Commercial Opportunity 30,000 – 40,000 diagnosed patients in major markets KOLs expect diagnosis rates to increase (US, EU3, Japan) U.S. 52% EU3 35% Japan 13%
ATB200 Novel ERT for Pompe Disease A Proprietary, Clinical-Stage Biologics Program

Pompe Disease Overview Novel ERT for Pompe Disease – ATB200 + Chaperone 31 Severe, Fatal Genetic Disorder with Significant Unmet Medical Need Deficiency of GAA leading to glycogen accumulation Age of onset ranges from infancy to adulthood Symptoms include muscle weakness, respiratory failure and cardiomyopathy Respiratory and cardiac failure are leading causes of morbidity and mortality 5,000 – 10,000 patients diagnosed WW1 ~$700M+ Global Pompe ERT sales in FY142 1. National Institute of Neurological Disorders and Stroke (NIH). 2. Sanofi Press Release & 10-K

Pompe Patient Perspectives Novel ERT for Pompe Disease – ATB200 + Chaperone 32 Very Significant Unmet Need Despite Availability of Currently Marketed Therapy

Pompe ERT - 3 Challenges Novel ERT for Pompe Disease – ATB200 + Chaperone 33 Activity/ Stability Rapid denaturation of ERT in pH of blood1 Tolerability / Immunogenicity Infusion-associated reactions in >50% of late-onset patients3 Uptake/ Targeting Low M6P receptor uptake into skeletal muscle2 Antibody titers shown to affect treatment outcomes4,5 Vast majority of rhGAA not delivered to lysosomes2 Protein Aggregation 1Khanna et al., PLoS ONE, 2012; 2Zhu et al., Amer. Soc. Gene Therapy, 2009 June; 3Banati et al., Muscle Nerve, 2011 Dec.; 4Banugaria et al., Gen. Med., 2011 Aug.; 5de Vries et al., Mol Genet Metab., 2010 Dec. Amicus Technology Platforms with Potential to Address Challenges with Existing Pompe ERT Uniquely Engineered rhGAA Optimized M6P & Carbohydrates
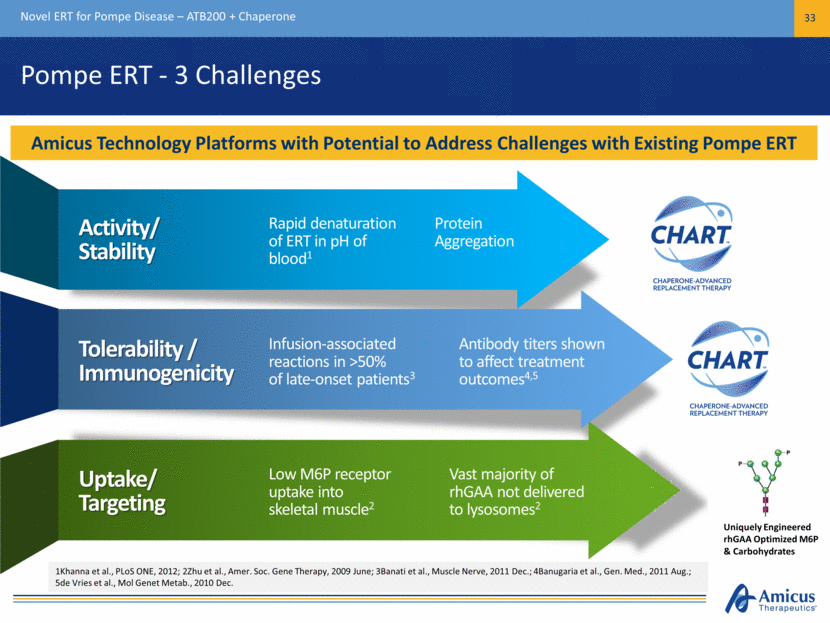
Preclinical Proof-of-Concept Novel ERT for Pompe Disease – ATB200 + Chaperone 34 ATB200 + Chaperone Results in Improved Substrate Clearance in Preclinical Models1 Untreated Alglucosidase Alfa ATB200 + AT2221 Wild Type PAS (20x) Untreated Alglucosidase Alfa ATB200 +AT2221 Wild Type LAMP1 (40x) 1. Following 2 doses of 20mg/kg alglucosidase alfa or ATB200 + AT2221 in Gaa KO mice, skeletal muscles evaluated for glycogen clearance and proliferated lysosomes. Treatment with alglucosidase alfa modestly reduced glycogen or proliferated lysosomes while ATB200, co-administered with AT2221 significantly decreased the muscle pathology associated with Pompe disease PAS-glycogen staining in Quadriceps LAMP1 Immunohistochemical staining in Soleus
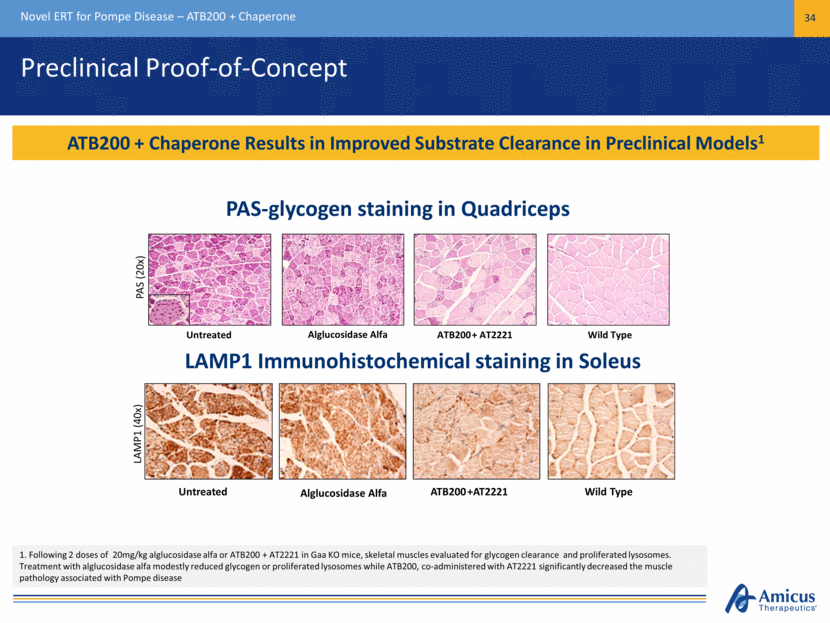
Biologics Manufacturing Capabilities Novel ERT for Pompe Disease – ATB200 + Chaperone 35 Optimized Glycosylation and Key Quality Attributes Maintained Through Scale Up rhGAA lacks M6P; cannot be targeted to lysosomes rhGAA contains M6P; targeted to lysosomes Lyophilized Vial of ATB200 GAA Activity (nmol/mL/hr) GAA Activity (nmol/mL/hr) GAA Activity (nmol/mL/hr) M6P [mM] M6P [mM] M6P [mM] CI-MPR Receptor Chromatography GAA Activity M6P Bench Scale 5L Scale 250L Scale Proof of Concept Studies 0 20 40 60 80 0 1 2 3 4 5 8% 92% G A A A c t i v i t y ( n m o l / m L / h r ) M 6 P [ m M ] 0 15 30 45 60 0 1 2 3 4 5 5L Bioreactor Run G A A A c t i v i t y ( n m o l / m L / h r ) M 6 P [ m M ] GAA Activity M6P (mM) 11% 89% 0 20 40 60 80 0 1 2 3 4 5 Engineering Run 2 G A A A c t i v i t y ( n m o l / m L / h r ) M 6 P [ m M ] 9% 91%
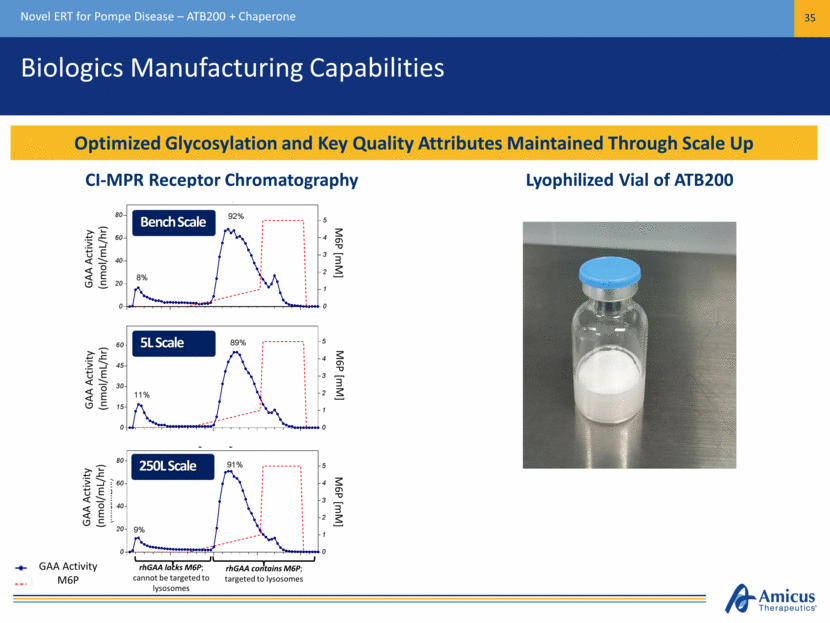
Clinical Study in Pompe Patients Novel ERT for Pompe Disease – ATB200 + Chaperone 36 Study Design Supported by US and EU Regulators Stage 2 (Multiple Ascending Dose) ATB200 20mg/kg + AT2221 (Low Dose) 3 doses ATB200 20mg/kg + AT2221 (High Dose) 3 doses ATB200 5 mg/kg Stage 1 (Single Ascending Dose) Single Dose ATB200 Every Other Week Fixed Dose ATB200 + Chaperone (AT2221) Every Other Week Long-Term Open Label Extension Fixed Dose ATB200 + Chaperone (AT2221) Every Other Week Assessments: Plasma PK (Enzyme Activity & Total protein) Safety/Tolerability Antibodies Infusion-Associated Reactions Pharmacodynamics (OLE) ATB200 20 mg/kg ATB200 10 mg/kg
Financial Summary Strong Balance Sheet to Invest in Rare Disease Pipeline

Strong Balance Sheet Financial Summary 38 Financial Position December 31, 2015 Current Cash: $214M Current Debt $50M FY16 Net Cash Spend Guidance: $135M-$155M Cash Runway Mid-2017 Capitalization Shares Outstanding 125,027,034 Cash Position Provides Runway Under Current Operating Plan into 1H17
Key Drivers of Value Introduction 39 3 Novel Product Candidates Each with $500M to $1B+ Market Potential Migalastat Personalized Medicine (Small Molecule) MAA Submitted CHMP Opinion Anticipated Early 2016 Prepared for EU Launch* Phase 3 Novel Topical Cream (SD-101) U.S. Breakthrough Therapy Designation Rolling NDA Phase 3 Data Expected in 2H16 Novel ERT + Chaperone Treatment Paradigm Biologics Manufacturing Clinical Study Initiated with Data Anticipated in 2016 R&D Engine and Continued Business Development Activity Pompe Epidermolysis Bullosa (EB) Fabry *Pending Approval
Thank You ©AMICUS THERAPEUTICS. CRANBURY, NJ. January 2016.

Exhibit 99.3
3rd Annual Dermatology Summit SD-101 for Epidermolysis Bullosa (EB) Jay Barth, MD, Chief Medical Officer
Safe Harbor This presentation will contain, “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of Amicus’ candidate drug products, the timing and reporting of results from preclinical studies and clinical trials evaluating Amicus’ candidate drug products, financing plans, and the projected cash position for the Company. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “potential,” “plan,” “targets,” “likely,” “may,” “will,” “would,” “should” and “could,” and similar expressions or words identify forward-looking statements. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions and uncertainties. The inclusion of forward-looking statements should not be regarded as a representation by Amicus that any of its plans will be achieved. Any or all of the forward-looking statements in this press release may turn out to be wrong. They can be affected by inaccurate assumptions Amicus might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing and outcomes of discussions with regulatory authorities, and in particular the timing of an NDA submission for migalastat monotherapy, and the potential goals, progress, timing and results of preclinical studies and clinical trials, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in the business of Amicus, including, without limitation: the potential that results of clinical or pre-clinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities may not grant or may delay approval for our product candidates; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; the potential that we will need additional funding to complete all of our studies and, our dependence on third parties in the conduct of our clinical studies. Further, the results of earlier preclinical studies and/or clinical trials may not be predictive of future results. With respect to statements regarding projections of the Company’s cash position, actual results may differ based on market factors and the Company’s ability to execute its operational and budget plans. In addition, all forward looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the year ended December 31, 2014 and Form 10-Q for the quarter ended June 30, 2015. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this news release to reflect events or circumstances after the date hereof. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.

Epidermolysis Bullosa (EB) Multiple genes cause disease which results in fragility of skin and can also affect internal organs Diagnosed from infancy to adulthood Severe blistering, open wounds and scarring in response to minor friction to the skin Disfiguring, excruciatingly painful, and can be fatal Given lack of treatment, any reduction in disease symptoms would be considered meaningful 30,000 – 40,000 diagnosed patients in major global regions Rare, Devastating, Connective Tissue Disorder with No Approved Treatments 1 Third party market research
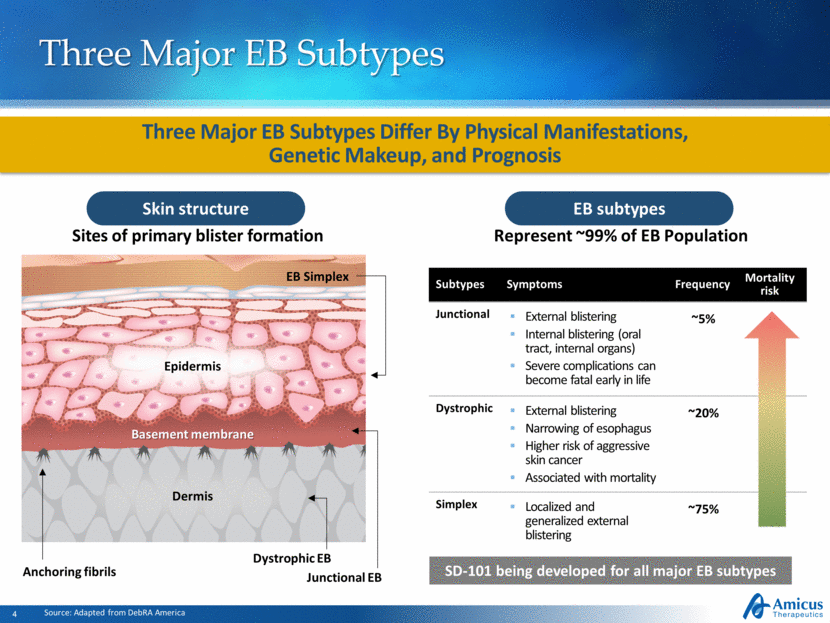
Subtypes Symptoms Frequency Mortality risk Junctional External blistering Internal blistering (oral tract, internal organs) Severe complications can become fatal early in life ~5% Dystrophic External blistering Narrowing of esophagus Higher risk of aggressive skin cancer Associated with mortality ~20% Simplex Localized and generalized external blistering ~75% Three Major EB Subtypes EB subtypes Source: Adapted from DebRA America Skin structure Sites of primary blister formation EB Simplex Junctional EB Dystrophic EB Basement membrane Anchoring fibrils Epidermis Dermis Three Major EB Subtypes Differ By Physical Manifestations, Genetic Makeup, and Prognosis Represent ~99% of EB Population SD-101 being developed for all major EB subtypes
SD-101 Overview Patented High Concentration Allantoin with Breakthrough Therapy Designation Novel, Proprietary Topical Cream Promotes Healing of Wounds in EB and is Differentiated by Applicability for All Major EB Subtypes *Margraf and Covey 1977; Meixell and Mecca 1966; Settle 1969; Flesch 1958; Fisher 1981; Cajkovac et al., 1992; Medda 1976 Active Ingredient & ROA Proposed Indication Development Phase Proposed MOA* Formulation Proprietary topical cream containing 6% allantoin, applied to entire body once daily All major EB subtypes (Simplex, Dystrophic, Junctional) Phase 3 registration study (SD-005) ongoing Aids inflammatory response, bactericidal effects, loosens protein bridges, promotes collagen Patented formulation to deliver high concentration in highly stable, soluble form

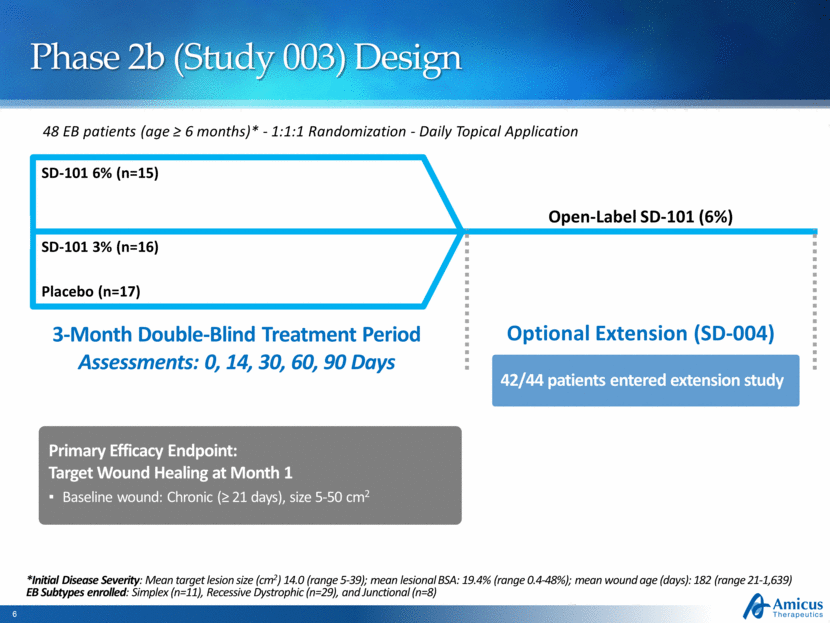
Phase 2b (Study 003) Design 48 EB patients (age ≥ 6 months)* - 1:1:1 Randomization - Daily Topical Application Placebo (n=17) SD-101 6% (n=15) Open-Label SD-101 (6%) 3-Month Double-Blind Treatment Period Assessments: 0, 14, 30, 60, 90 Days Optional Extension (SD-004) Primary Efficacy Endpoint: Target Wound Healing at Month 1 Baseline wound: Chronic (≥ 21 days), size 5-50 cm2 SD-101 3% (n=16) *Initial Disease Severity: Mean target lesion size (cm2) 14.0 (range 5-39); mean lesional BSA: 19.4% (range 0.4-48%); mean wound age (days): 182 (range 21-1,639) EB Subtypes enrolled: Simplex (n=11), Recessive Dystrophic (n=29), and Junctional (n=8) 42/44 patients entered extension study
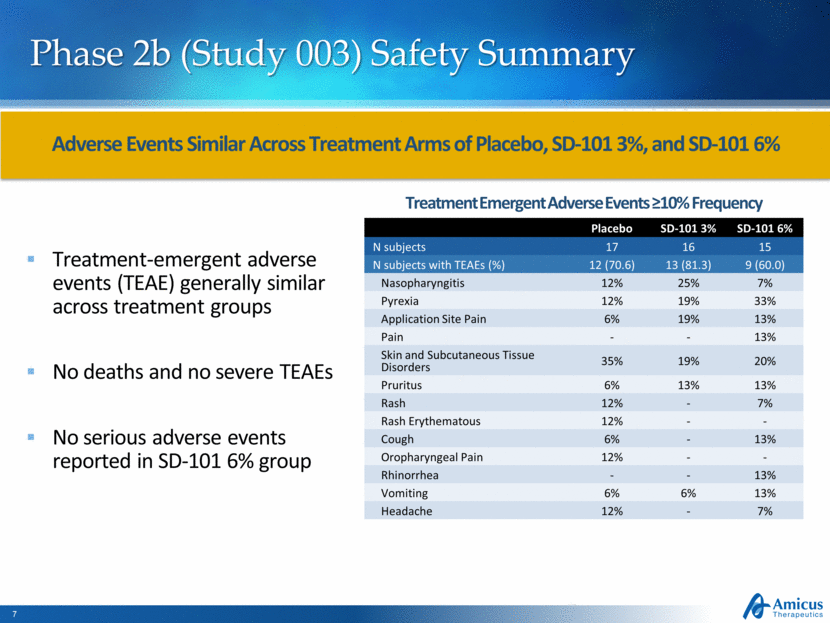
Phase 2b (Study 003) Safety Summary Treatment-emergent adverse events (TEAE) generally similar across treatment groups No deaths and no severe TEAEs No serious adverse events reported in SD-101 6% group Adverse Events Similar Across Treatment Arms of Placebo, SD-101 3%, and SD-101 6% Placebo SD-101 3% SD-101 6% N subjects 17 16 15 N subjects with TEAEs (%) 12 (70.6) 13 (81.3) 9 (60.0) Nasopharyngitis 12% 25% 7% Pyrexia 12% 19% 33% Application Site Pain 6% 19% 13% Pain - - 13% Skin and Subcutaneous Tissue Disorders 35% 19% 20% Pruritus 6% 13% 13% Rash 12% - 7% Rash Erythematous 12% - - Cough 6% - 13% Oropharyngeal Pain 12% - - Rhinorrhea - - 13% Vomiting 6% 6% 13% Headache 12% - 7% Treatment Emergent Adverse Events ≥10% Frequency
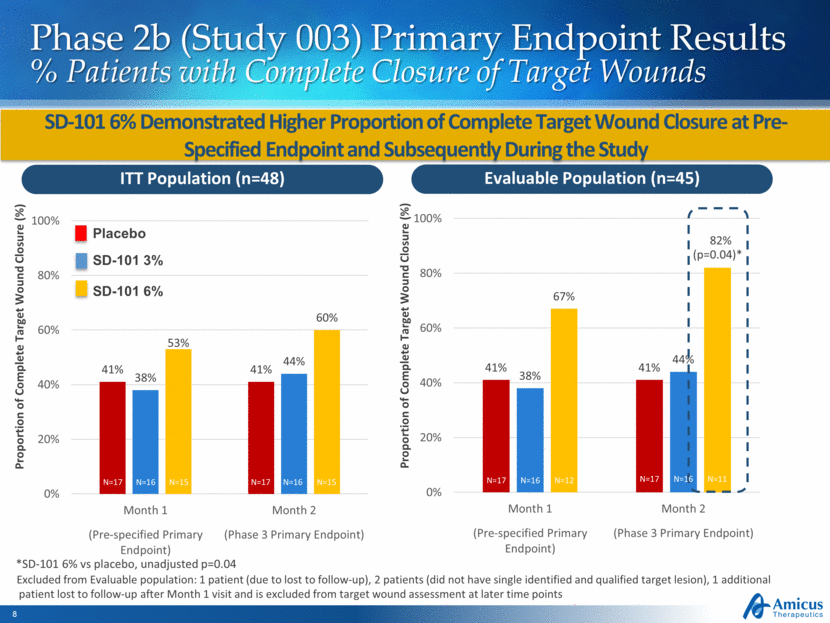
Phase 2b (Study 003) Primary Endpoint Results % Patients with Complete Closure of Target Wounds SD-101 6% Demonstrated Higher Proportion of Complete Target Wound Closure at Pre-Specified Endpoint and Subsequently During the Study *SD-101 6% vs placebo, unadjusted p=0.04 Excluded from Evaluable population: 1 patient (due to lost to follow-up), 2 patients (did not have single identified and qualified target lesion), 1 additional patient lost to follow-up after Month 1 visit and is excluded from target wound assessment at later time points ITT Population (n=48) Evaluable Population (n=45) (p=0.04)* Proportion of Complete Target Wound Closure (%) Proportion of Complete Target Wound Closure (%) N=17 N=16 N=15 N=17 N=16 N=15 N=17 N=16 N=12 N=17 N=16 N=11 Placebo SD-101 3% SD-101 6% 41% 41% 38% 44% 67% 82% 0% 20% 40% 60% 80% 100% Month 1 Month 2 (Pre-specified Primary Endpoint) (Phase 3 Primary Endpoint) 41% 41% 38% 44% 53% 60% 0% 20% 40% 60% 80% 100% Month 1 Month 2 (Pre-specified Primary Endpoint) (Phase 3 Primary Endpoint)
Phase 2b (Study 003) Secondary Endpoint Median Time to Wound Closure SD-101 6% Showed Fastest Time to Wound Closure in Both ITT and Evaluable Populations ITT Population (n=48) Evaluable Population (n=45) Median Time to Wound Closure (Days) Median Time to Wound Closure (Days) 91 Days 91 Days 86 Days 86 Days 40 Days 30 Days Excluded from Evaluable population: 1 patient (due to lost to follow-up), 2 patients (did not have single identified and qualified target lesion) Placebo SD-101 3% SD-101 6% N=17 N=16 N=15 N=17 N=16 N=12 0 10 20 30 40 50 60 70 80 90 100 0 10 20 30 40 50 60 70 80 90 100
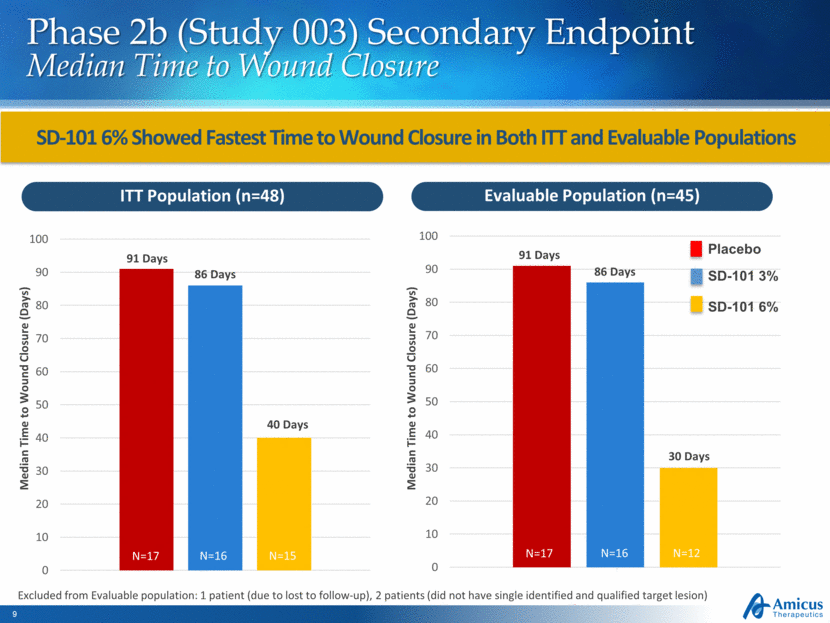
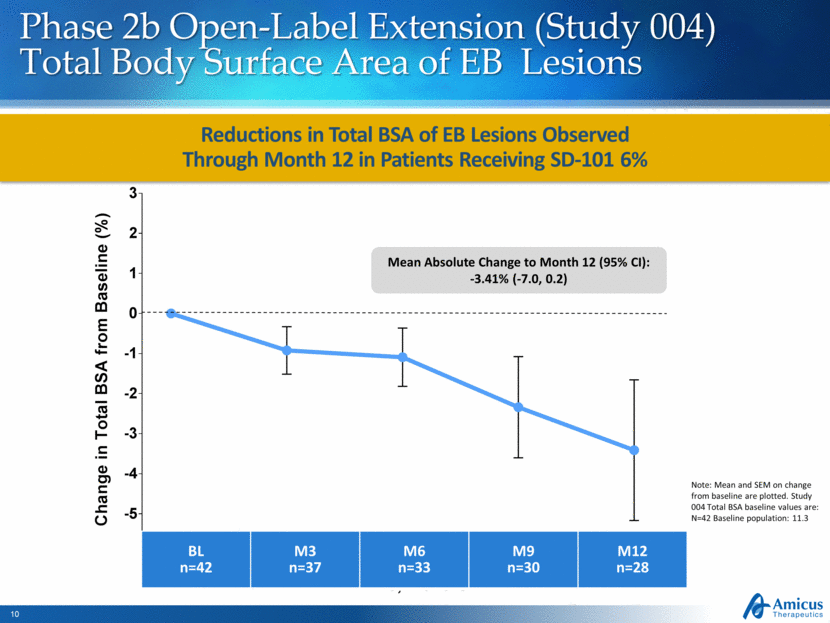
Reductions in Total BSA of EB Lesions Observed Through Month 12 in Patients Receiving SD-101 6% Phase 2b Open-Label Extension (Study 004) Total Body Surface Area of EB Lesions Note: Mean and SEM on change from baseline are plotted. Study 004 Total BSA baseline values are: N=42 Baseline population: 11.3 BL n=42 M3 n=37 M6 n=33 M9 n=30 M12 n=28 Mean Absolute Change to Month 12 (95% CI): -3.41% (-7.0, 0.2) 3 6 9 12 -5 -4 -3 -2 -1 0 1 2 3 Baseline Time, Months C h a n g e i n T o t a l B S A f r o m B a s e l i n e ( % )
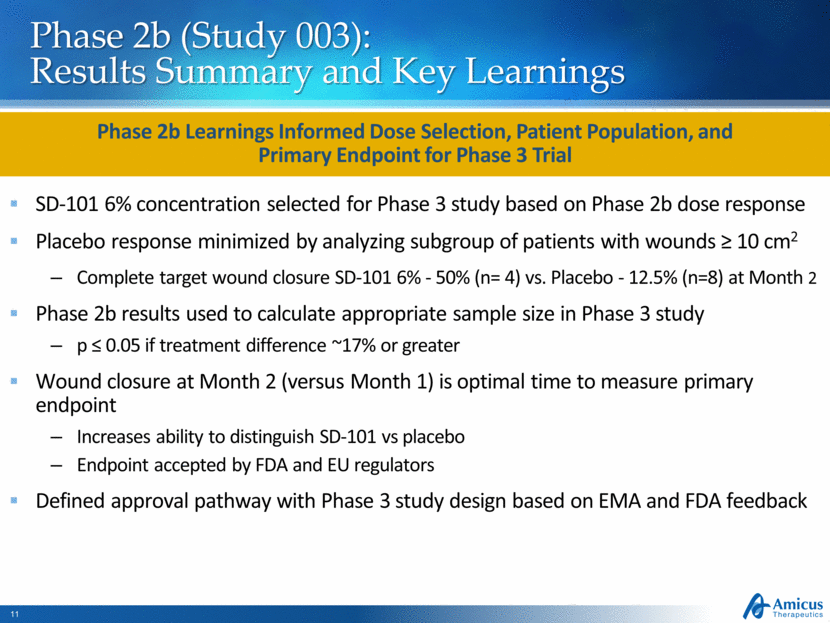
Phase 2b (Study 003): Results Summary and Key Learnings SD-101 6% concentration selected for Phase 3 study based on Phase 2b dose response Placebo response minimized by analyzing subgroup of patients with wounds ≥ 10 cm2 Complete target wound closure SD-101 6% - 50% (n= 4) vs. Placebo - 12.5% (n=8) at Month 2 Phase 2b results used to calculate appropriate sample size in Phase 3 study p ≤ 0.05 if treatment difference ~17% or greater Wound closure at Month 2 (versus Month 1) is optimal time to measure primary endpoint Increases ability to distinguish SD-101 vs placebo Endpoint accepted by FDA and EU regulators Defined approval pathway with Phase 3 study design based on EMA and FDA feedback Phase 2b Learnings Informed Dose Selection, Patient Population, and Primary Endpoint for Phase 3 Trial
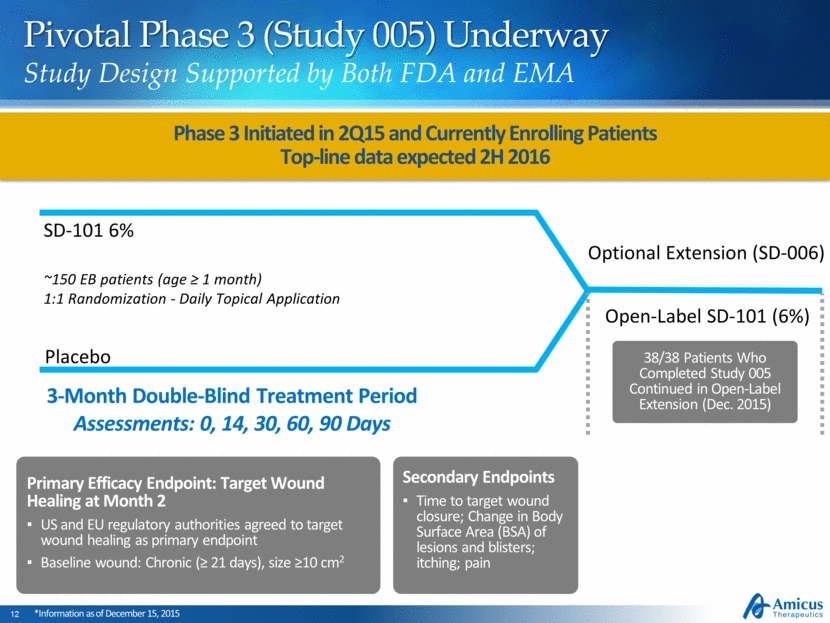
Pivotal Phase 3 (Study 005) Underway Study Design Supported by Both FDA and EMA ~150 EB patients (age ≥ 1 month) 1:1 Randomization - Daily Topical Application Placebo SD-101 6% Open-Label SD-101 (6%) 3-Month Double-Blind Treatment Period Assessments: 0, 14, 30, 60, 90 Days Optional Extension (SD-006) Primary Efficacy Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed to target wound healing as primary endpoint Baseline wound: Chronic (≥ 21 days), size ≥10 cm2 Phase 3 Initiated in 2Q15 and Currently Enrolling Patients Top-line data expected 2H 2016 *Information as of December 15, 2015 Secondary Endpoints Time to target wound closure; Change in Body Surface Area (BSA) of lesions and blisters; itching; pain 38/38 Patients Who Completed Study 005 Continued in Open-Label Extension (Dec. 2015)
SD-101 Regulatory Pathway Rolling NDA Initiated 4Q15 Breakthrough Therapy Designation (BTD) based on Phase 2 POC Orphan drug designation Rolling NDA initiated 4Q15 Orphan drug designation Approved Pediatric Investigation Plan (PIP) Defined registration pathway FDA and EMA Aligned on Phase 3 Study Design and Feedback to Date Provides Defined Registration Pathway for SD-101 in Major Subtypes of EB ROW regulatory path based on EMA and FDA submissions
3rd Annual Dermatology Summit SD-101 for Epidermolysis Bullosa (EB) Jay Barth, MD, Chief Medical Officer

Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Atlas Salt Inc. Announces Annual Filings and Company Update
- Helix Reports First Quarter 2024 Results
- Vext Announces Delay of Annual Filings and Postponement of Conference Call
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share