Form 6-K NOVOGEN LTD For: Jan 10
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of January, 2017
Commission File Number
Novogen Limited
(Translation of registrant’s name into English)
Level 5, 20 George Street, Hornsby, NSW 2077, Australia
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☑ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
Indicate by check mark if the registrant by furnishing the information contained in this form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934. Yes ☐ No ☑
If “yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b)
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Novogen Limited (Registrant)
Kate Hill
Kate Hill
Interim Company Secretary
Date 10 January 2017



9th ANNUAL CONFERENCE
BIOTECH SHOWCASE TM NOVOGEN
Novogen Limited
Presentation to 9th Annual Biotech Showcase Conference
Dr James Garner
San Francisco, CA
Chief Executive Officer
9 January 2017
[email protected]
Version 1.2

Forward-Looking Statements
This presentation contains “forward-looking statements” within the meaning of the “safe-harbor” provisions of the Private Securities Litigation
Reform Act of 1995. Such statements involve known and unknown risks, uncertainties and other factors that could cause the actual results of the Company to differ materially from the results expressed or implied by such statements, including changes
from anticipated levels of customer acceptance of existing and new products and services and other factors. Accordingly, although the Company believes that the expectations reflected in such forward-looking statements are reasonable, there can be no
assurance that such expectations will prove to be correct. The Company has no obligation to sales, future international, national or regional economic and competitive conditions, changes in relationships with customers, access to capital,
difficulties in developing and marketing new products and services, marketing existing products and services update the forward-looking information contained in this presentation.
NOVOGEN
1 COMMERCIAL IN CONFIDENCE
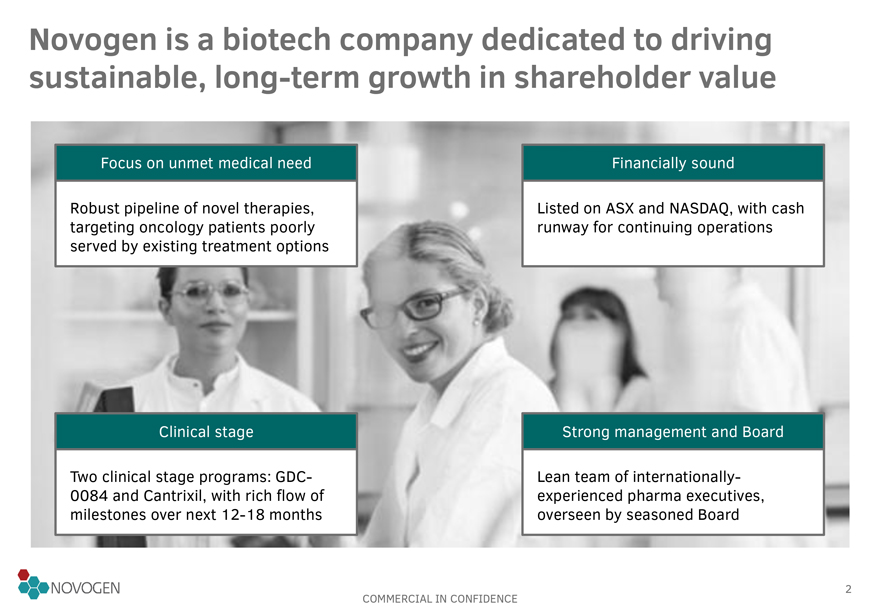
Novogen is a biotech company dedicated to driving sustainable, long-term growth in shareholder value
Focus on unmet medical need
Robust pipeline of novel therapies, targeting
oncology patients poorly served by existing treatment options
Financially sound
Listed on ASX and NASDAQ, with cash runway for continuing operations
Clinical
stage
Two clinical stage programs: GDC-0084 and Cantrixil, with rich flow of milestones over next 12-18 months
Strong management and Board
Lean team of internationally-experienced pharma executives,
overseen by seasoned Board
2 COMMERCIAL IN CONFIDENCE
NOVOGEN
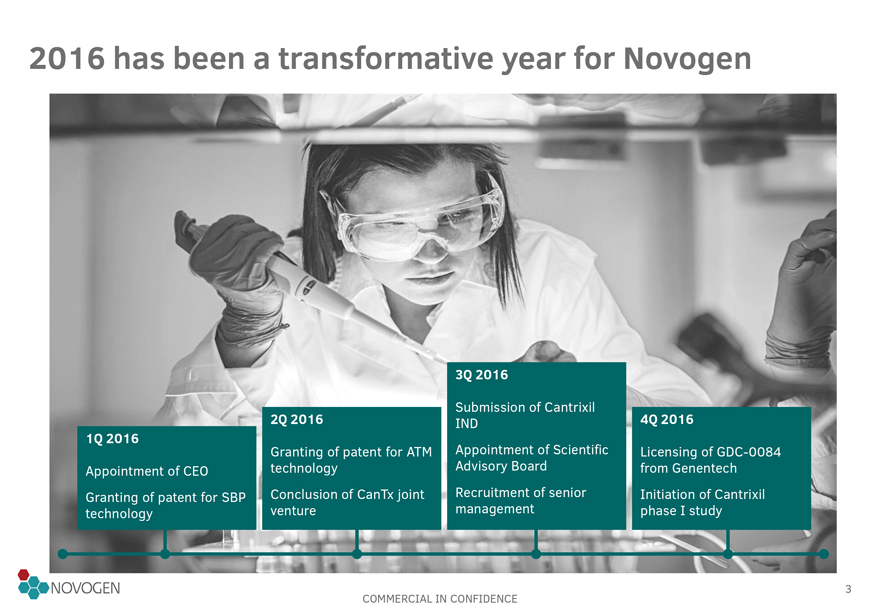
2016 has been a transformative year for Novogen
1Q 2016
Appointment of CEO
Granting of patent for SBP technology
2Q 2016
Granting of patent for ATM technology
Conclusion of CanTx joint venture
3Q 2016
Submission of Cantrixil IND
Appointment of Scientific Advisory Board
Recruitment of senior management
4Q 2016
Licensing of GDC-0084 from Genentech
Initiation of Cantrixil phase I study
NOVOGEN
COMMERCIAL IN CONFIDENCE
3
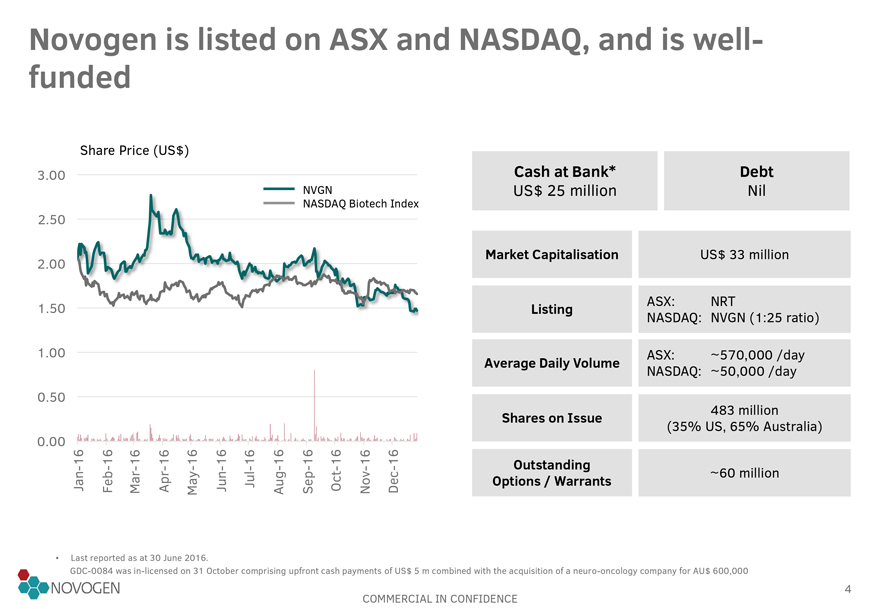
Novogen is listed on ASX and NASDAQ, and is well-funded
Share Price (US$)
3.00 Cash at Bank* Debt
NVGN US$ 25 million Nil
NASDAQ Biotech Index
2.50
2.00 Market Capitalisation US$ 33 million
1.50 Listing ASX: NRT
NASDAQ: NVGN (1:25 ratio)
1.00 ASX: ~570,000 /day
Average Daily Volume NASDAQ: ~50,000 /day
0.50
Shares on Issue 483 million
(35% US, 65% Australia)
0.00
-16 -16 -16 -16 -16 -16 -16 -16 -16 -16 -16 -16 Outstanding
Jan Feb Mar Apr May Jun Jul Aug
Sep Oct Nov Dec Options / Warrants ~60 million
Last reported as at 30 June 2016.
GDC-0084 was in-licensed on 31 October comprising upfront cash payments of US$ 5 m combined with the
acquisition of a neuro-oncology company for AU$ 600,000
NOVOGEN
COMMERCIAL IN
CONFIDENCE
4
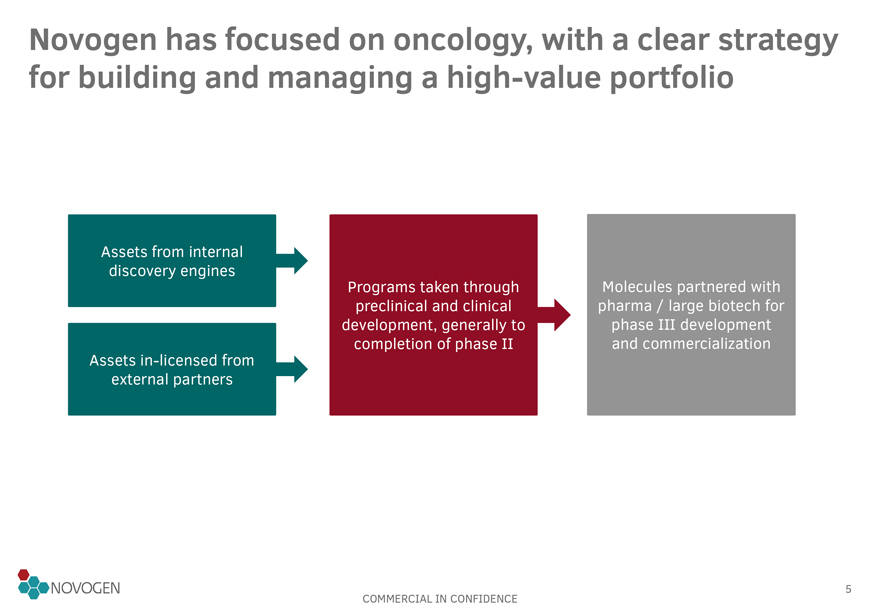
Novogen has focused on oncology, with a clear strategy for building and managing a high-value portfolio
Assets from internal discovery engines
Assets
in-licensed from external partners
Programs taken through preclinical and clinical development, generally to completion of
phase II
Molecules partnered with pharma / large biotech for phase III development and commercialization
COMMERCIAL IN CONFIDENCE
NOVOGEN
5

Novogen has built a strong management team with international experience in big pharma
Dr James Garner
Chief Executive Officer & Managing Director
Physician / MBA; Extensive pharma drug development experience
Dr David Brown
Chief Scientific Officer
Twenty years of drug discovery and development experience
Dr Gordon Hirsch
Chief Medical Officer
Physician / MBA; Twenty years of pharmaceutical industry experience
Dr Peng Leong
Chief Business Officer
Eighteen years of business development and investment banking
experience
Dr Andrew Heaton
VP, Drug Discovery
Twenty years of medicinal chemistry experience
Cristyn Humphreys
Chief Financial Officer
Chartered accountant with twenty years of experience in corporate
roles
COMMERCIAL IN CONFIDENCE
6

Our newly-appointed Scientific Advisory Board brings global expertise and experience to Novogen
Professor Sir Murray Brennan
Dr Karen Ferrante
Professor Peter Gunning
Professor Alex Matter
Memorial Sloan Kettering Cancer Center
Takeda
MILLENNIUM
THE TAKEDA ONCOLOGY COMPANY
UNSW AUSTRALIA
NOVARTIS
PHARMACEUTICALS
NOVOGEN
COMMERCIAL IN CONFIDENCE
7
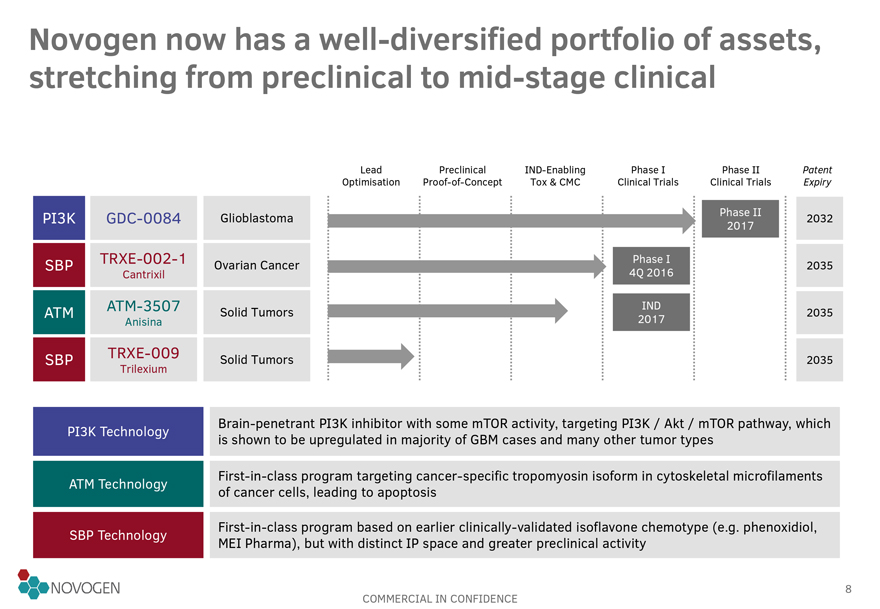
Novogen now has a well-diversified portfolio of assets, stretching from preclinical to mid-stage clinical
Lead Optimisation
Preclinical Proof-of-Concept
IND-Enabling Tox & CMC
Phase I Clinical Trials
Phase II Clinical Trials
Patent Expiry
PI3K
SBP
ATM
SBP
GDC-0084
TRXE-002-1
Cantrixil
ATM-3507
Anisina
TRXE-009
Trilexium
Glioblastoma
Ovarian Cancer
Solid Tumors
Solid Tumors
Phase I
4Q 2016
IND
2017
Phase 2017 II
2032
2035
2035
2035
PI3K Technology
Brain-penetrant PI3K inhibitor with some mTOR activity, targeting PI3K / Akt /
mTOR pathway, which is shown to be upregulated in majority of GBM cases and many other tumor types
ATM Technology
First-in-class program targeting cancer-specific tropomyosin isoform in cytoskeletal microfilaments
of cancer cells, leading to apoptosis
SBP Technology
First-in-class program based on earlier clinically-validated isoflavone chemotype (e.g. phenoxidiol, MEI Pharma), but with distinct IP space and greater preclinical activity
NOVOGEN
COMMERCIAL IN CONFIDENCE
8
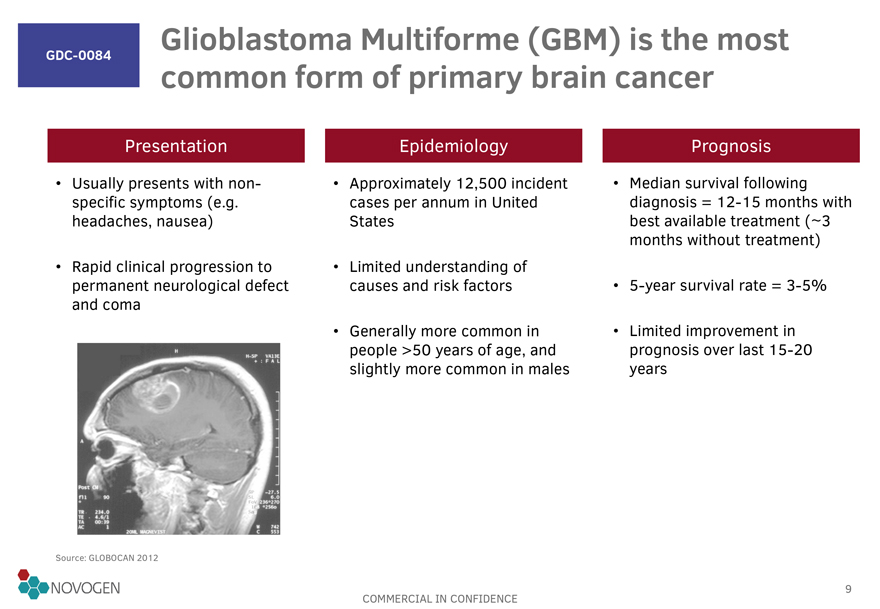
GDC-0084
Glioblastoma Multiforme (GBM) is the most common form of primary brain cancer
Presentation
Usually presents with non-specific symptoms (e.g. headaches, nausea)
Rapid clinical progression to permanent neurological defect and coma
Epidemiology
Approximately 12,500 incident cases per annum in United States
Limited understanding of causes
and risk factors
Generally more common in people >50 years of age, and slightly more common in males
Prognosis
Median survival following diagnosis = 12-15
months with best available treatment ( ~3 months without treatment)
5-year survival rate =
3-5%
Limited improvement in prognosis over last 15-20 years
Source: GLOBOCAN 2012
NOVOGEN
COMMERCIAL IN CONFIDENCE 9
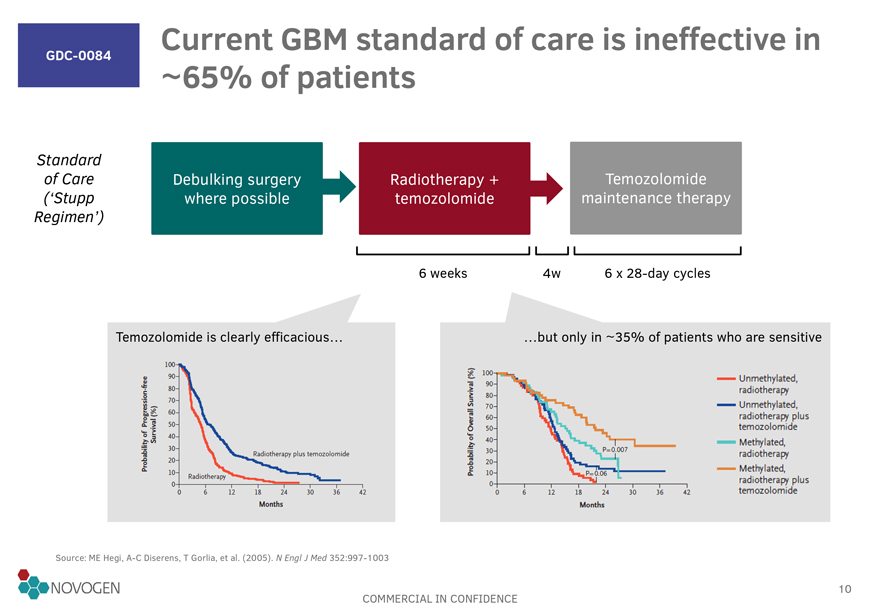
GDC-0084
Current GBM standard of care is ineffective in ~65% of patients
Standard of Care (‘Stupp
Regimen’)
Debulking surgery where possible
Radiotherapy + temozolomide
Temozolomide maintenance therapy
6 weeks
4w
6 x 28-day cycles
Probability of Progression-free Survival (%)
Temozolomide is clearly efficacious
Probability of Overall Survival (%)
but only in
35% of patients who are sensitive
Unmethylated, radiotherapy
Unmethylated radiotherapy plus temozolomide
Methylated, radiotherapy
Methylated, radiotherapy plus temozolomide
Radiotherapy plus temozolomide
Radiotherapy
Months
Months
Source: ME Hegi, A-C Diserens, T Gorlia, et al.
(2005). N Engl J Med 352:997-1003
10 COMMERCIAL IN CONFIDENCE
NOVOGEN
100 90 80 70 60 50 40 30 20 10 0 0 6 12 18 24 30 36 42
100 90 80 70 60 50 40 30 20 10 0 0 6 12 18 24 30 36 42
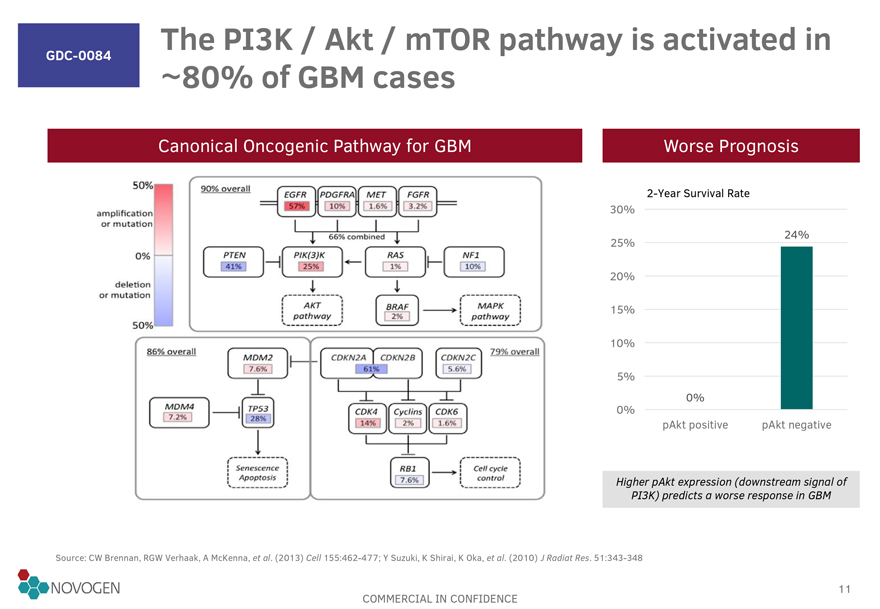
GDC-0084 The PI3K / Akt / mTOR pathway is activated in 80% of GBM cases
Canonical Oncogenic Pathway for GBM Worse Prognosis
50% 90% overall EGFR 57%
PDGFRA 10% MET 1.6% FGFR 3.2%
amplication or mutation 66% combined 2-Year Survival Rate
0% PTIN 41% PIK(3)K 25% RAS 1% NF1 10% 30%
deletion or mutation AKT pathway BRAF 2% MAPK
pathway 25% 24%
50% 86% overall MDM2 7.6% CDKN2A CDKN2B 61% CDKN2C 5.6% 20%
MDM4 7.2% TP53 28% CDK4 14% Cyclins 2% CDK6 1.6% 15%
Senescene Apotosis RB1
7.6% Cell cycle control 10%
5%
0%
0%
pAkt positive pAkt negative
Higher pAkt expression (downstream signal of
PI3K) predicts a worse response in GBM
Source: CW Brennan, RGW Verhaak, A McKenna, et al. (2013) Cell 155:462-477; Y Suzuki, K Shirai, K Oka, et al.
(2010) J Radiat Res. 51:343-348
11
NOVOGEN
COMMERCIAL IN CONFIDENCE

PI3K inhibitors are well-validated, with one
GDC-0084 marketed product and extensive clinical data
Zydelig (idelalisib) on market Other PI3K inhibitors in clinical trials
Review Nature Reviews Clinical Oncology 10, 143-153 (March 2013) | doi:10.1038/ Development of PI3K inhibitors: lessons
learned from early clinical trials Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers Elisavet Paplomala Wmship Cancer Institute of Emory University, Atlanta, GA
USA Ruth O’Regan [email protected] Wmship Cancer Institute of Emory University. Atlanta GA. USA Abstract The phosphoinostlide 3 kinase (P13KyMtr mammalian (or mectonnUc) targel of rapamydn (mTOR> pathway is a complicated intracellular
pathway, which leads to cell growlh and tumor proliferation and ploys a significant role in endocrine resistance In breast cancer Multiple compounds targeting this pathway are being evaluated in clinical trials These agents are generally well
tolerated and can be used In combi nation with targeted therapies, endocrine therapy or cytotoxic agents. The identification of subtypes of tumors more likely to respond to these therapeutics cannot be overemphasized, since breast canoer is a very
heterogeneous malignancy. Activation of
GDC-0084 differentiated from other PI3K inhibitors by:-
Ability to cross blood-brain barrier
Optimised balance of PI3K and mTOR activity
NOVOGEN
12 COMMERCIAL IN CONFIDENCE
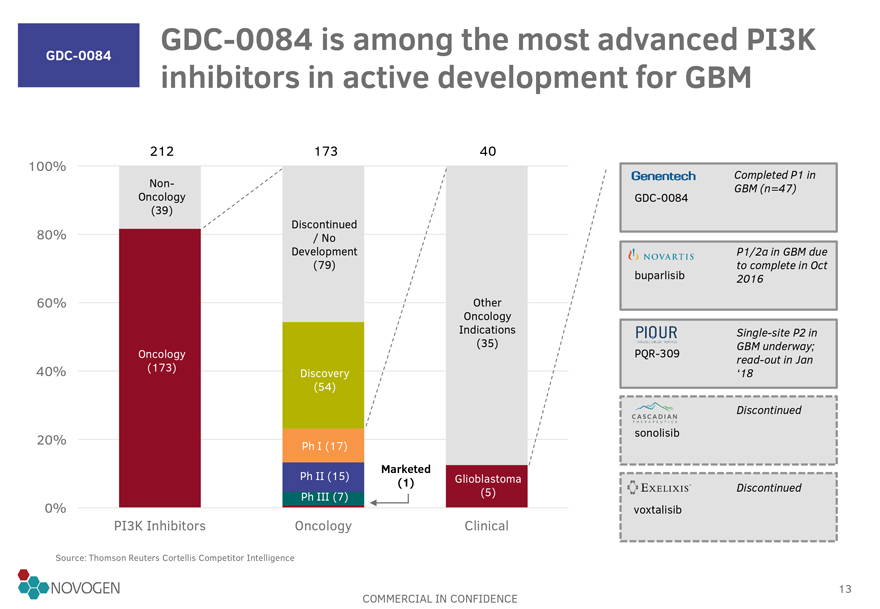
GDC-0084 is among the most advanced PI3K
GDC-0084 inhibitors in active development for GBM
212
173 40
100% Completed P1 in
Non-GBM (n=47)
Oncology GDC-0084
(39)
Discontinued
80% / No
Development P1/2a in GBM due
(79) to complete in Oct
buparlisib 2016
60% Other
Oncology
Indications Single-site P2 in
(35) GBM underway;
Oncology PQR-309 read-out in Jan
40% (173) Discovery ‘18
(54)
Discontinued
20% sonolisib
Ph I (17)
Marketed
Ph II (15) (1) Glioblastoma
Ph III (7) (5) Discontinued
0% voxtalisib
PI3K Inhibitors Oncology Clinical
Source: Thomson Reuters Cortellis Competitor Intelligence
NOVOGEN
13 COMMERCIAL IN CONFIDENCE
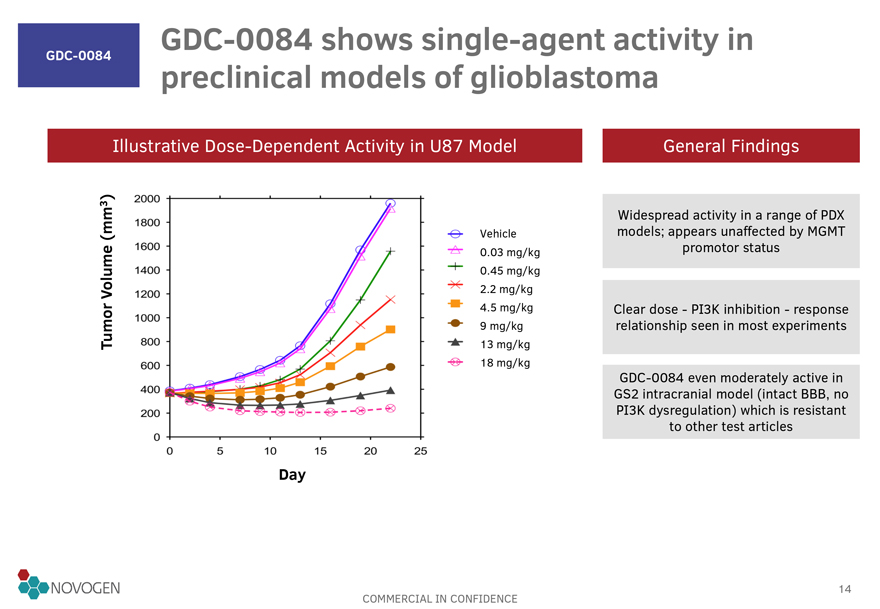
GDC-0084 GDC-0084 shows
single-agent activity in preclinical models of glioblastoma
Illustrative Dose-Dependent Activity in U87 Model General Findings
Tumor Volume (mm3)
Widespread activity in a range of PDX
Vehicle models; appears unaffected by MGMT
0.03 mg/kg promotor status
0.45 mg/kg
2.2 mg/kg
4.5 mg/kg Clear dose - PI3K inhibition - response
9 mg/kg relationship seen in most
experiments
13 mg/kg
18 mg/kg
GDC-0084 even moderately active in
GS2 intracranial
model (intact BBB, no
PI3K dysregulation) which is resistant
to other test
articles
Day
2000 1800 1600 1400 1200 1000 600 400 200 0 0 5 10 15 20 25
NOVOGEN
14 COMMERCIAL IN CONFIDENCE
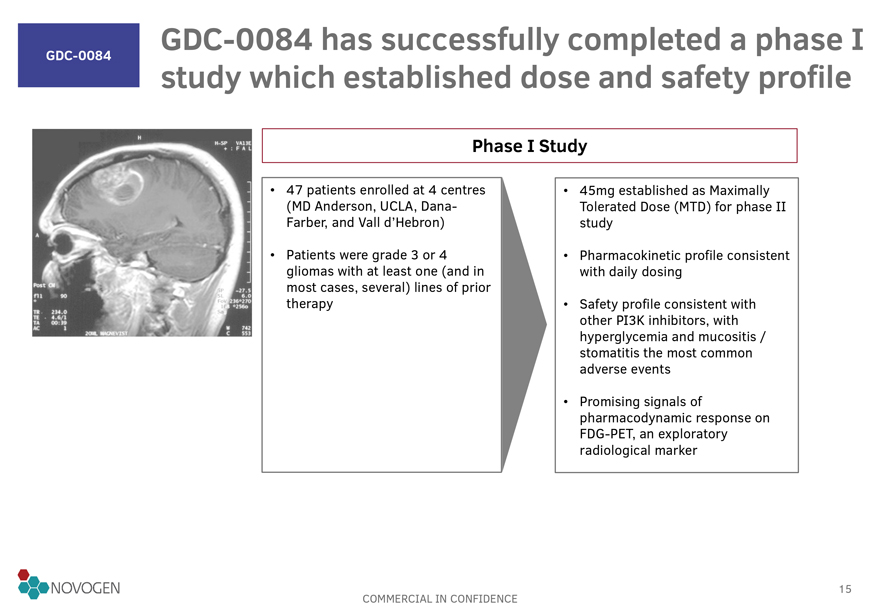
GDC-0084
GDC-0084 has successfully completed a phase I study which established dose and safety profile
Phase I Study
47 patients enrolled at 4 centres (MD Anderson, UCLA, Dana-Farber, and Vall
d’Hebron)
Patients were grade 3 or 4 gliomas with at least one (and in most cases, several) lines of prior therapy
45mg established as Maximally Tolerated Dose (MTD) for phase II study
Pharmacokinetic profile
consistent with daily dosing
Safety profile consistent with other PI3K inhibitors, with hyperglycemia and mucositis / stomatitis the most common adverse events
Promising signals of pharmacodynamic response on FDG-PET, an exploratory radiological marker
15 COMMERCIAL IN CONFIDENCE
NOVOGEN
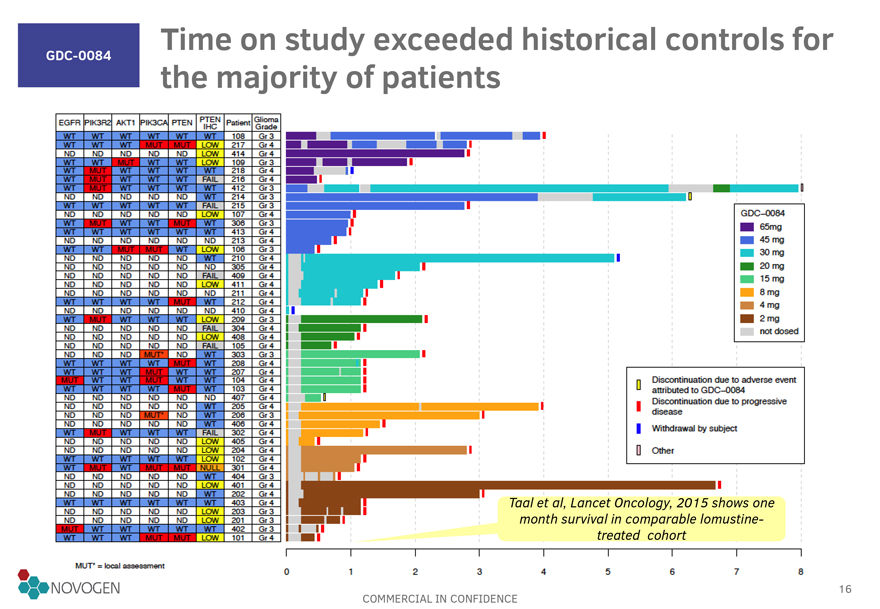
GDC-0084 Time on study exceeded historical controls for the majority of
patients
EGFR PIK3R2 AKT1 PIK3CA PTEN PTEN IHC Patient Glioma Grade
WT WT WT
WT WT WT 108 Gr 3
WT WT WT MUT MUT LOW 217 Gr 4
ND ND ND ND ND LOW 414 Gr 4
WT WT MUT WT AT LOW 109 Gr 3
WT MUT WT WT WT WT 218 Gr 4
WT MUT WT WT WT FAIL 216 Gr 4
WT MUT WT WT WT WT 412 Gr 3
ND ND ND ND ND WT 214 Gr 3
WT WT WT WT WT FAIL 215 Gr 3
ND ND ND ND ND LOW 107 Gr 4
WT MUT WT WT MUT WT 306 Gr 3
WT WT WT WT WT WT 413 Gr 4
ND ND ND ND ND ND 213 Gr 4
WT WT MUT MUT WT LOW 106 Gr 3
ND ND ND ND ND WT 210 Gr 4
ND ND ND ND ND ND 305 Gr 4
ND ND ND ND ND FAIL 409 Gr 4
ND ND ND ND ND LOW 411 Gr 4
ND ND ND ND ND ND 211 Gr 4
WT WT WT WT MUT WT 212 Gr 4
ND ND ND ND ND ND 410 Gr 4
WT MUT WT WT WT LOW 209 Gr 3
ND ND ND ND ND FAIL 304 Gr 4
ND ND ND ND ND LOW 408 Gr 4
ND ND ND ND ND FAIL 105 Gr 4
ND ND ND MUT* ND WT 303 Gr 3
WT WT WT WT MUT WT 208 Gr 4
WT WT WT MUT WT WT 207 Gr 4
MUT WT WT MUT WT WT 104 Gr 4
WT WT WT WT M JT WT 103 Gr 4
ND ND ND ND ND ND 407 Gr 4
ND ND ND ND ND WT 205 Gr 4
ND ND ND MUT* ND WT 206 Gr 3
ND ND ND ND ND WT 406 Gr 4
WT MUT WT WT WT FAIL 302 Gr 4
ND ND ND ND ND LOW 405 Gr 4
ND ND ND ND ND LOW 204 Gr 4
WT WT WT WT WT LOW 102 Gr 4
WT MUT WT MUT MUT NULL 301 Gr 4
ND ND ND ND ND WT 404 Gr 3
ND ND ND ND ND LOW 401 Gr 4
ND ND ND ND ND WT 202 Gr 4
WT WT WT WT WT WT 403 Gr 4
ND ND ND ND ND LOW 203 Gr 3
ND ND ND ND ND LOW 201 Gr 3
MUT WT WT WT WT WT 402 Gr 3
WT WT WT MUT MUT LOW 101 Gr 4
GDC-0084
65mg
45 mg
30 mg
20 mg
15 mg
&mg
4 mg
2 mg
Discontinuation due to adverse event attributed to
GDC-OOB4
Discontinuation due to progressive disease
Withdrawal by subject 0
Other
Taal et al, Lancet Oncology, 2015 shows one month survival in comparable lomustine-treated cohort
16
COMMERCIAL IN CONFIDENCE
NOVOGEN
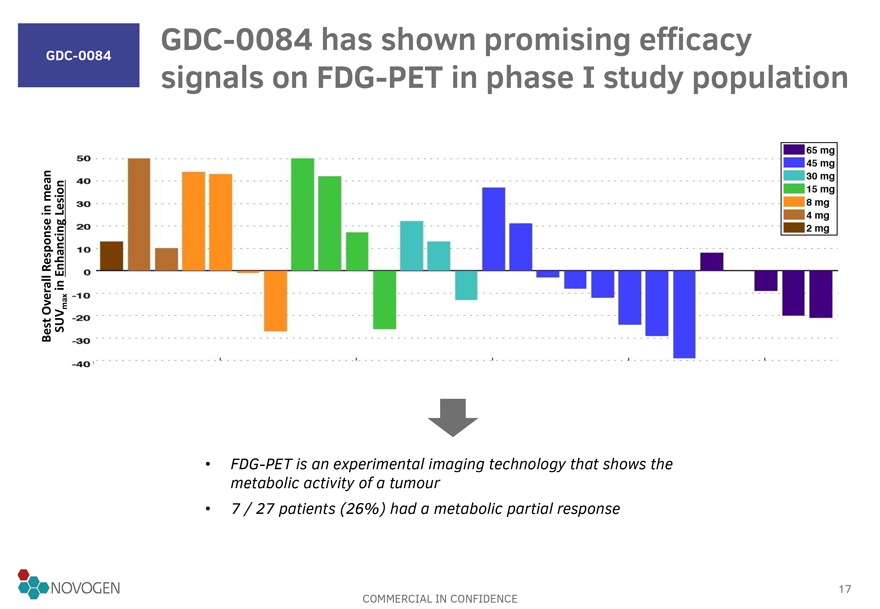
GDC-0084
GDC-0084 has shown promising efficacy signals on FDG-PET in phase I study population
Best Overall Response in mean
SUVmax in Enhancing Lesion
50
40
30
20
10
0
-10
-20
-30
-40
65 mg
45 mg
30 mg
15 mg
8 mg
4 mg
2 mg
FDG-PET is an experimental imaging technology that shows the metabolic activity of a tumour
7 / 27 patients (26%) had a metabolic partial response
17 COMMERCIAL IN CONFIDENCE
NOVOGEN
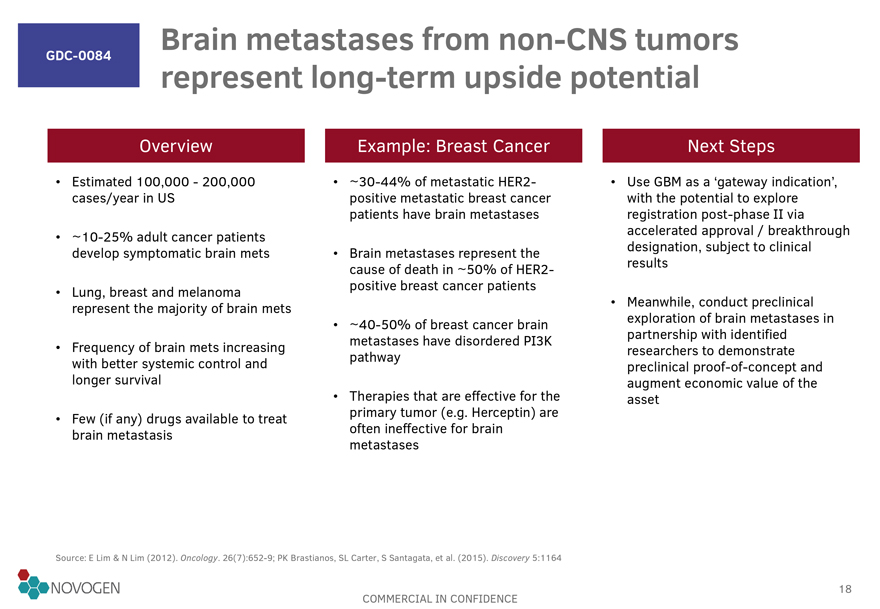
GDC-0084 Brain metastases from
non-CNS tumors represent long-term upside potential
Overview
Estimated 100,000 - 200,000 cases/year in US
~10-25%
adult cancer patients develop symptomatic brain mets
Lung, breast and melanoma represent the majority of brain mets
Frequency of brain mets increasing with better systemic control and longer survival
Few (if
any) drugs available to treat brain metastasis
Example: Breast Cancer
~30-44% of metastatic HER2-positive metastatic breast cancer patients have brain metastases
Brain metastases represent the cause
of death in ~50% of HER2-positive breast cancer patients
~40-50% of breast cancer brain metastases have disordered PI3K
pathway
Therapies that are effective for the primary tumor (e.g. Herceptin) are often ineffective for brain metastases
Next Steps
Use GBM as a ‘gateway indication’, with the potential to explore
registration post-phase II via accelerated approval / breakthrough designation, subject to clinical results
Meanwhile, conduct preclinical exploration of brain
metastases in partnership with identified researchers to demonstrate preclinical proof-of-concept and augment economic value of the asset
Source: E Lim & N Lim (2012). Oncology. 26(7):652-9; PK Brastianos, SL Carter, S Santagata, et al. (2015). Discovery 5:1164
18 COMMERCIAL IN CONFIDENCE
NOVOGEN
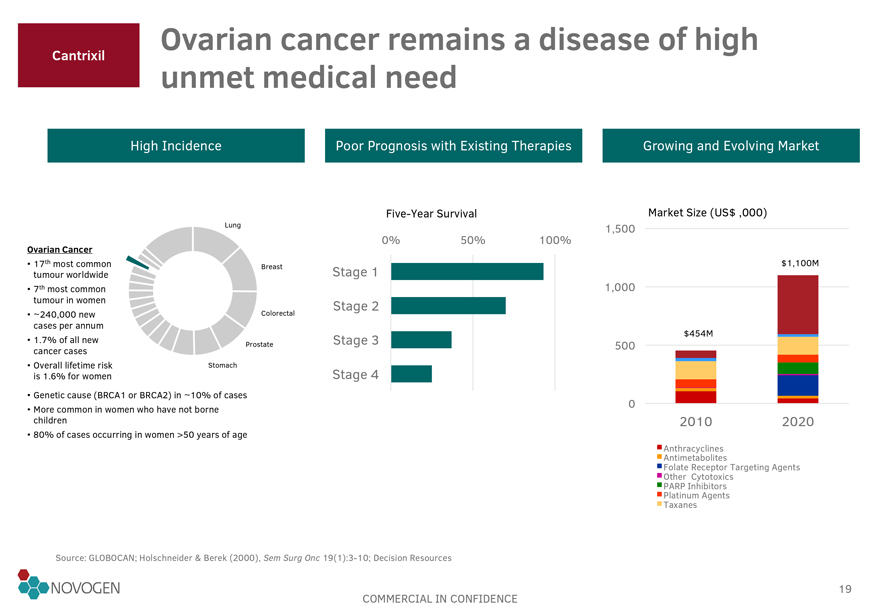
Cantrixil Ovarian cancer remains a disease of high unmet medical need
High Incidence Poor Prognosis with Existing Therapies Growing and Evolving Market
Five-Year
Survival Market Size (US$ ,000)
Lung 1,500
0% 50% 100%
Ovarian Cancer
17th most common tumour worldwide Breast Stage 1 $1,100M
7th most common tumour in women 1,000
Stage 2
~240,000 new cases per annum Colorectal
1.7% of all new cancer cases Stage 3 $454M
Prostate 500
Overall lifetime risk is 1.6% for women Stomach
Stage 4
Genetic cause (BRCA1 or BRCA2) in ~10% of cases
More common in women who have not borne children 0
2010 2020
80% of cases occurring in women >50 years of age
Anthracyclines
Antimetabolites
Folate Receptor Targeting Agents
Other Cytotoxics
PARP Inhibitors
Platinum Agents
Taxanes
Source: GLOBOCAN; Holschneider & Berek (2000), Sem Surg Onc 19(1):3-10; Decision Resources
19 COMMERCIAL IN CONFIDENCE
NOVOGEN
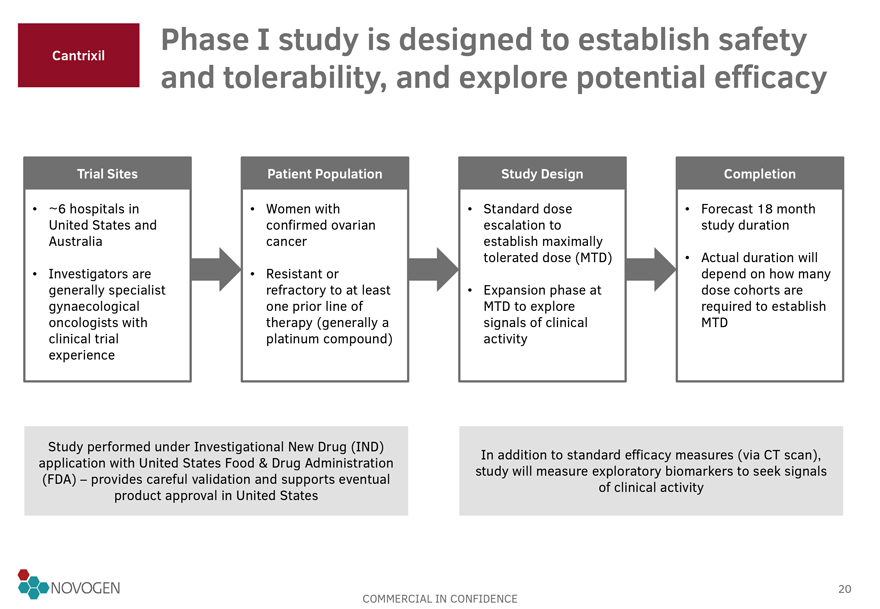
Cantrixil Phase I study is designed to establish safety and tolerability, and explore potential efficacy
Trial Sites
~6 hospitals in United States and Australia
Investigators are generally specialist gynaecological oncologists with clinical trial experience
Patient Population
Women with confirmed ovarian cancer
Resistant or refractory to at least one prior line of therapy (generally a platinum compound)
Study Design
Standard dose escalation to establish maximally tolerated dose
(MTD)
Expansion phase at MTD to explore signals of clinical activity
Completion
Forecast 18 month study duration
Actual duration will depend on how many dose cohorts are required to establish MTD
Study
performed under Investigational New Drug (IND) application with United States Food & Drug Administration (FDA) – provides careful validation and supports eventual product approval in United States
In addition to standard efficacy measures (via CT scan), study will measure exploratory biomarkers to seek signals of clinical activity
20 COMMERCIAL IN CONFIDENCE
NOVOGEN
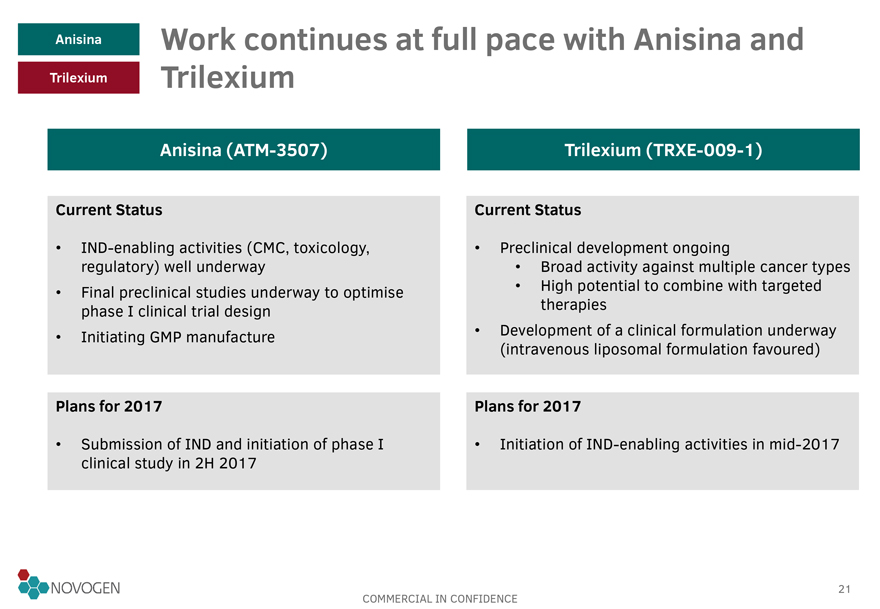
Anisina Work continues at full pace with Anisina and Trilexium
Trilexium
Anisina (ATM-3507)
Current Status
IND-enabling activities (CMC,
toxicology, regulatory) well underway
Final preclinical studies underway to optimise phase I clinical trial design
Initiating GMP manufacture
Plans for 2017
Submission of IND and initiation of phase I clinical study in 2H 2017
Trilexium (TRXE-009-1)
Current Status
Preclinical development ongoing
Broad activity against multiple cancer types
High potential to combine with targeted therapies
Development of a clinical formulation
underway
(intravenous liposomal formulation favoured)
Plans for 2017
Initiation of IND-enabling activities in mid-2017
21 COMMERCIAL IN CONFIDENCE
NOVOGEN

2017 will be an important year for Novogen, with a rich series of value-driving events
Key Milestones for 2017
IND submission and approval for Anisina Initiation of Anisina phase I
study Initiation of GDC-0084 phase II study Full recruitment of Cantrixil phase I study Initiation of IND-enabling activities for Trilexium
22 COMMERCIAL IN CONFIDENCE
NOVOGEN

Novogen now has a diversified portfolio and is positioned for growth
Focus on unmet need: pipeline of novel therapies, targeting oncology patients, poorly served by existing treatment options
Building a sustainable model: leveraging oncology expertise, developing commercially attractive, in-house and external assets
Diversified portfolio:
Multiple assets in various stages of development – from pre-clinical through to phase II-ready
Across technologies / development platforms
Strong management and board: lean team of internationally-experienced pharma executives
Financially sound: listed on ASX and NASDAQ, with cash runway
News flow: rich series of
value-driving milestones over 12-18 months
23 COMMERCIAL IN CONFIDENCE
NOVOGEN

NOVOGEN
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Actions Technology's Smartwatch Chip Powers HONOR Band 9 to Achieve 14-Day Long Battery Life
- Wego and Tourism Authority of Thailand Partner for The Fourth Consecutive Year, Showcasing the Beauty of Thailand
- Walgreens Launches Gene and Cell Services as Part of Newly Integrated Walgreens Specialty Pharmacy Business
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share