Form S-1 Inpellis, Inc.
Table of Contents
As filed with the Securities and Exchange Commission on November 10, 2015
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
INPELLIS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 45-4772185 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
30 Washington Avenue, Suite F
Haddonfield, NJ 08033
(978) 750-0900
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Patrick T. Mooney, M.D.
President and Chief Executive Officer
30 Washington Avenue, Suite F
Haddonfield, NJ 08033
(978) 750-0900
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Thomas L. Barrette, Esq. Rodney H. Bell, Esq. Holland & Knight LLP 10 St. James Avenue Boston, MA 02116 (617) 523-2700 |
Juan M. Marcelino, Esq. Nelson Mullins Riley & Scarborough LLP One Post Office Square Boston, MA 02109 (617) 573-4700 |
Anthony J. Marsico, Esq. Greenberg Traurig, LLP 200 Park Avenue New York, NY 10166 (212) 801-9200 |
Approximate date of commencement of proposed sale to public: As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x | |||
The Registrant is an “emerging growth company,” as defines in Section 2(a) of the Securities Act. This registration statement complies with the requirement that apply to an issuer that is an emerging growth company.
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of each class of securities to be registered |
Proposed Maximum Offering Price(1) |
Amount of Registration Fee(2) | ||
| Common stock, par value $0.001 per share(3) |
$20,000,000 | $2,014 | ||
| Representative’s Warrants to Purchase Common Stock(3)(4) |
— | — | ||
| Common Stock Underlying Representative’s Warrants(3)(5) |
$1,680,000 | $169.18 | ||
| Total Registration Fee |
$21,680,000 | $2,183.18 | ||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
| (2) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price of the securities registered hereunder to be sold by the Registrant. |
| (3) | Pursuant to Rule 416 under the Securities Act, the shares of common stock registered hereby also include an indeterminate number of additional shares of common stock as may from time to time become issuable by reason of stock splits, stock dividends, recapitalizations or other similar transactions. |
| (4) | No registration fee pursuant to Rule 457(g) under the Securities Act. |
| (5) | Estimated solely for the purposes of calculating the registration fee pursuant to Rule 457(g) under the Securities Act of 1933, as amended. The proposed maximum aggregate offering price of the Representative’s warrants is $1,680,000, which is equal to 120% of $1,400,000 (7% of $20,000,000). |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION | DATED NOVEMBER 10, 2015 |
[ ] Shares
Common Stock

This is an initial public offering of [ ] shares of common stock of Inpellis, Inc. No public market currently exists for our shares. We anticipate that the initial public offering price of our shares of common stock will be between $[ ] and $[ ] per share.
We intend to apply to list our common stock on the NASDAQ Global Market under the symbol “INPL.” No assurances can be given that our application will be approved.
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, have elected to comply with certain reduced public company disclosure standards.
Investing in our common stock involves risks. See “Risk Factors” beginning on page 8 of this prospectus for a discussion of information that should be considered in connection with an investment in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
| Per share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting commissions(1) |
$ | $ | ||||||
| Proceeds to Inpellis, before expenses |
$ | $ | ||||||
| (1) | The underwriters will receive compensation in addition to the commissions. See “Underwriting” for a full description of compensation payable to the underwriters. |
The underwriters are selling the shares of common stock in this offering on a “best efforts” basis. The underwriters are not required to sell any specific number or dollar amount of common stock, but will use their best efforts to sell the securities offered. Because this is a best efforts offering, the underwriters do not have an obligation to purchase any shares of common stock, and, as a result, there is a possibility that we may not receive any proceeds from the offering.
The underwriters expect to deliver the securities to investors upon payment approximately three business days following acceptance of an order.
This offering shall terminate upon the earlier of [ ], 2015 or the receipt of a notice of termination from the underwriters.
Alexander Capital, L.P.
Prospectus dated , 2015
Table of Contents
| Page | ||||
| 1 | ||||
| 8 | ||||
| 40 | ||||
| 41 | ||||
| 42 | ||||
| 43 | ||||
| 45 | ||||
| 47 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results Of Operations |
48 | |||
| 57 | ||||
| 81 | ||||
| 86 | ||||
| 93 | ||||
| 95 | ||||
| 97 | ||||
| 102 | ||||
| Material U.S. Federal Income Tax Considerations For Non-U.S. Holders |
104 | |||
| 108 | ||||
| 117 | ||||
| 117 | ||||
| 117 | ||||
| F-1 | ||||
We are responsible for the information contained in this prospectus and in any free writing prospectus we prepare or authorize. We and the underwriters have not authorized anyone to provide you with different information, and we and the underwriters take no responsibility for any other information others may give you. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the cover of this prospectus.
We own or have rights to trademarks, service marks and trade names that we use in connection with the operation of our business, including our corporate name, logos and website names. Other trademarks, service marks and trade names appearing in this prospectus are the property of their respective owners. The trademarks that we own include Inpellis™. Solely for convenience some of the trademarks, service marks and trade names referred to in this prospectus are listed without the ® and ™ symbols, but we will assert, to the fullest extent under applicable law, our rights to our trademarks, service marks and trade names.
i
Table of Contents
This summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in shares of our common stock and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus carefully, especially “Risk factors” and “Management’s discussion and analysis of financial condition and results of operations,” before deciding to buy shares of our common stock. Unless the context requires otherwise, references in this prospectus to “Inpellis,” “the Company,” “we,” “us” and “our” refer to Inpellis, Inc.
Overview
Inpellis is a specialty pharmaceutical company developing transdermal product candidates for treating pain resulting from musculoskeletal disorders and peripheral neuropathy. Inpellis has acquired from BioChemics, Inc. (“BioChemics”) full or joint ownership of transdermal formulations which utilize the endel™ (vasoactive lipid encapsulated) transdermal drug delivery system for non-dermal pain, and to patents, patent applications, and know how related to the transdermal delivery system. Our goal is to apply this transdermal technology to provide rapid, safe and effective absorption of drugs for pain relief—through the skin—to the target tissues.
endel transdermal technology employs a transdermal delivery system that combines vasodilators, permeation enhancers, and active ingredients. Transdermal delivery has the potential to provide increased concentrations of the specified drug to target tissues, and avoid some of the acknowledged limitations of oral formulations including absorption, gastric issues, drug distribution, high serum concentrations and/or first-pass hepatic metabolism. endel technology also provides the potential to effectively transport molecules that were previously difficult to deliver transdermally. In addition, transdermal delivery, depending on the specific molecule, may result in increased safety, efficacy and/or convenience. The potential for mitigated development risk exists if the drugs that are selected for development are eligible for the streamlined 505(b)(2) regulatory-development pathway. 505(b)(2) provides an abbreviated regulatory path to US Food and Drug Administration or FDA approval for new or improved formulations, or new uses of previously approved products, by enabling an applicant to rely, in part, on the FDA’s findings of safety and efficacy for an existing product, or published literature, in support of the New Drug Application or NDA, translating into decreased regulatory costs and time to market. We believe the transdermal ibuprofen product we have in development, as described further below, may be eligible for the 505(b)(2) pathway, but until we formally seek to use 505(b)(2) we cannot be certain that it will be available to us.
We are dedicated to completing the development of two products in particular, as well as continuing work on a number of additional products in the pipeline. The two products are AX-IBU-01, a transdermal ibuprofen for moderate to severe osteoarthritis, and AX-DN-01, a transdermal benfotiamine for painful diabetic neuropathy. We are also conducting carefully focused continued research and development of other transdermal products for pain. We may develop products internally or may enter into co-development and co-commercialization of branded products, generic products, or new molecules in the therapeutic area of pain.
A Phase 2 study utilizing transdermal ibuprofen for the treatment of pain associated with moderate to severe osteoarthritis was conducted in Switzerland in 2011. A human proof of concept study with respect to vasodilation for diabetic neuropathy was conducted in the United States in 2002 and an animal proof of delivery study for transdermal benfotiamine was conducted in the United States in 2008. We have not yet filed an investigational new drug, or IND, application for either product in the United States or elsewhere. Based on the work that we have done with respect to our ibuprofen product candidate, we intend to perform additional studies regarding the formulation of that product in support of an IND application. Once we have done that work, we intend to submit an IND application for approval of a Phase 2B/3 study.
1
Table of Contents
The transdermal delivery system utilized for delivering transdermal ibuprofen for osteoarthritis pain and transdermal benfotiamine for painful diabetic neuropathy, as well as related formulations and methods, are covered by an International patent application. The application covers the system of transdermal drug delivery that combines vasodilators, chelators, penetration enhancers, and active ingredients.
We are open to forming strategic alliances for co-development and co-commercialization of drugs delivered transdermally for the treatment of pain. Inpellis will use third parties for manufacturing and marketing. Accordingly, the Inpellis strategy for the research, development, marketing and commercialization of its products entails entering into various arrangements with corporate partners, licensors, licensees and others; Inpellis will be dependent upon the subsequent success of these outside parties in performing their responsibilities. Inpellis may also rely on collaborative partners to conduct research efforts and clinical trials, to obtain regulatory approvals and to manufacture and market certain Inpellis products. In previous arrangements with manufacturing contractors, good manufacturing practices or GMP-quality human doses of AX-IBU-01 for Phase 1 and Phase 2 clinical trials have been produced, and we believe that this experience will allow us, through new arrangements with those, or similar, contractors, to produce GMP-quality AX-IBU-01 for our contemplated pivotal Phase 2B/3 clinical trial.
The Pain Market
The markets for pain medications are growing. By 2017, according to Nature Reviews Drug Discovery, the global pain management therapeutics market is forecast to generate sales of $35.1 billion, and is expected to grow substantially over the next decade due to population dynamics, an increase in the elderly, and co-morbidities associated with obesity and diabetes. The current market for prescription products for osteoarthritis is estimated by drugs.com at $8 billion, for oral non-steroidal anti-inflammatory drugs, or NSAIDs, $4 billion, and for transdermal NSAIDs $500 million. Neuropathic pain is estimated to be 16% of the pain market with sales of $3 billion, also according to Nature Reviews Drug Discovery.
Our Strategy
Our strategy is to build a specialty pharmaceutical company focused on developing and commercializing prescription therapeutics for pain using our proprietary transdermal technology. Our strategy includes:
| • | Rapidly advancing development of and seeking regulatory approval for endel based transdermal ibuprofen (AX-IBU-01) for the treatment of pain due to osteoarthritis. |
| • | Entering into agreements to commercialize AX-IBU-01 in the United States and build our organizational infrastructure to manage those agreements and advance the development and commercialization of AX-IBU-01 outside the United States. |
| • | Partnering with industry-leading contract manufacturing companies to ensure reliable product supply. |
| • | Collaborating with other key companies when desirable to build co-development and co-marketing relationships. |
| • | Leveraging our technology and approach to advance the additional products in our pipeline into clinical development—specifically endel based transdermal benfotiamine (AX-DN-01) for the treatment of painful diabetic neuropathy. |
| • | Develop a strategic pipeline of transdermally delivered drugs for pain utilizing existing branded and generic agents and drugs in-development. |
2
Table of Contents
Risk Factors
An investment in our common stock involves numerous risks. Any of the factors set forth under “Risk Factors” may limit our ability to successfully execute our business strategy. You should carefully consider all of the information set forth in this prospectus and, in particular, should evaluate the specific factors set forth under “Risk Factors” in deciding whether to invest in our common stock. Among these important risks are the following:
| • | We have incurred net losses of approximately $2,001,000 and $1,866,000 for the years ended December 31, 2014 and December 31, 2013, respectively, and a net loss of approximately $5,579,000 for the six months ended June 30, 2015. We anticipate that we will continue to incur substantial operating losses for the foreseeable future. We may never achieve or sustain profitability. As of June 30, 2015 and December 31, 2014 we had an accumulated deficit of approximately $10,982,000 and $5,403,000, respectively. |
| • | We have not submitted an application for, or obtained any FDA approval for, any product through the NDA process, which may impede our ability to obtain FDA approval in a timeframe that is consistent with our expectations and plans, or at all. |
| • | We will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed could force us to delay, limit, reduce or terminate our development or commercialization efforts of our product candidates. |
| • | If our drug candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities, we may incur additional costs or experience delays in completing, or we may ultimately be unable to obtain regulatory approval for our product candidates. |
| • | If our drug candidates are approved, our future commercial success will depend upon attaining significant market acceptance among physicians, patients and health care payors and, if we fail to do so, our business will be materially harmed. |
| • | We may not be able to manufacture our drug candidates in quantities sufficient for our clinical trials and/or any commercial launch of our product candidates. If we fail to meet deadlines or perform in an unsatisfactory manner our business could be harmed. |
| • | If we are unable to obtain or protect our intellectual property rights, including proprietary information and trade secrets, related to our drug candidates, we may not be able to prevent competitors with the same or similar drugs from entering our markets. |
| • | If we fail to attract and keep senior management and key scientific personnel, we may be unable to develop our drugs successfully, conduct our clinical trials and commercialize our drug candidates. |
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
| • | being permitted to provide only two years of audited financial statements in addition to any required unaudited interim financial statements with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| • | reduced disclosure obligations regarding our executive compensation arrangements; |
| • | not being required to hold a non-binding advisory vote on executive compensation or golden parachute arrangements; and |
3
Table of Contents
| • | exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
We may take advantage of these provisions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company on the date that is the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
Notwithstanding the above, we are also currently a “smaller reporting company” meaning that we are not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and have a public float of less than $75 million and annual revenues of less than $50 million during the most recently completed fiscal year. In the event that we are still considered a smaller reporting company at such time as we cease to be an emerging growth company, the disclosure we will be required to provide in our filings with the SEC will increase, but will still be less than it would be if we were not considered either an emerging growth company or a smaller reporting company. Specifically, similar to emerging growth companies, smaller reporting companies are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act of 2002, or the “Sarbanes-Oxley Act” requiring that independent registered public accounting firms provide an attestation report on the effectiveness of their internal control over financial reporting; and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in their annual reports.
Corporate Information
We were incorporated in the state of Delaware on March 13, 2012 as a wholly owned subsidiary of BioChemics. In recognition of the disgorgement and penalty obligations of BioChemics under the settlement terms of an enforcement action brought against BioChemics by the SEC, in January 2015, BioChemics transferred all of its shares in Inpellis to the Shareholder Resolution Trust, a Massachusetts trust (the “Trust”), which is currently the majority stockholder of Inpellis. The Trust is managed by three Trustees, all of whom must approve any decision. The three Trustees are Jack L. Altshuler, Esq., a Massachusetts attorney, Jan R. Schlichtmann, Esq., also a Massachusetts attorney, a director of BioChemics and the Managing Member of Sea Change Pharma LLC, which is the largest stockholder of BioChemics, and Daniel M. Glosband, a Massachusetts attorney. See “Risk Factors—Risks related to our former parent corporation,” “Business—Corporate History” and “Certain relationships and related party transactions” for additional information regarding our structure.
4
Table of Contents
THE OFFERING
| Common stock offered by us |
shares |
| Option to purchase additional shares |
The underwriter has an option for a period of 45 days to purchase up to additional shares of our common stock. |
| Common stock outstanding prior to this offering |
shares |
| Common stock to be outstanding immediately after this offering |
shares |
| Best efforts |
The underwriters are selling the shares of common stock offered in this prospectus on a “best efforts” basis and are not required to sell any specific number or dollar amount of the common stock offered by this prospectus, but will use their best efforts to sell the securities. |
| Use of proceeds |
Assuming we complete the maximum offering, we estimate that the net proceeds from this offering will be approximately $ million, or approximately $ million if the underwriters exercise their option to purchase additional shares in full, after deducting the underwriting commissions and estimated offering expenses payable by us. Since this is a “best efforts” offering, there is no assurance that any shares of common stock will be sold, and therefore no assurance that there will be any proceeds. We intend to use the net proceeds of this offering as follows: (1) approximately $ million to fund the continued development of AX-IBU-01, including our contemplated pivotal Phase 2B/3 clinical trial, approximately $ million to manufacture clinical supplies of AX-IBU-01, approximately $ million to fund other early stage pipeline development programs, and (4) the remainder for working capital and other general corporate purposes, including funding the costs of operating as a public company. See “Use of proceeds.” |
| Proposed NASDAQ Global Market Symbol |
We intend to apply to list our common stock on The NASDAQ Global Market under the symbol “INPL.” |
| Risk factors |
You should read the “Risk factors” section of this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. |
The number of shares of common stock to be outstanding after this offering is based on shares of common stock outstanding as of November , 2015 and excludes the following:
| • | shares of common stock issuable upon exercise of outstanding warrants as of November , 2015 at a weighted average exercise price of $ per share; and |
| • | shares of common stock reserved for future issuance under the Inpellis, Inc. 2015 Equity Incentive Plan, or the 2015 Equity Plan. |
Unless otherwise indicated, all information in this prospectus reflects or assumes the following:
| • | the filing and effectiveness of our amended and restated certificate of incorporation and the adoption of our amended and restated by-laws, which will occur upon the closing of this offering; |
| • | the automatic conversion of all outstanding convertible notes into shares of our common stock upon the closing of this offering; |
| • | no issuance or exercise of stock options or warrants on or after November , 2015; and |
| • | no exercise of the warrants to be issued to the Representative of the underwriters in connection with this offering as described in the “Underwriting- Representative’s Warrants” section of this prospectus. |
5
Table of Contents
SUMMARY FINANCIAL DATA
The following summary financial data for the six months ended June 30, 2015 and 2014, and for the years ended December 31, 2014 and 2013 are derived from our audited financial statements included elsewhere in this prospectus. You should read this data together with our audited financial statements and related notes included elsewhere in this prospectus and the information under the captions “Selected financial data” and “Management’s discussion and analysis of financial condition and results of operations.” The summary financial data in this section are not intended to replace our audited financial statements and related notes included elsewhere in this prospectus.
| For the six months ended June 30, |
For the years ended December 31, |
|||||||||||||||
| (in thousands, except share and per share data) | 2015 | 2014 | 2014 | 2013 | ||||||||||||
| Statements of operations data: |
||||||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
44 | 221 | 294 | 620 | ||||||||||||
| Research and development—Patents |
16 | 59 | 80 | 76 | ||||||||||||
| Business legal and consulting |
207 | 210 | 623 | — | ||||||||||||
| General and administrative |
5,590 | 105 | 164 | 350 | ||||||||||||
| Consulting services |
— | 410 | 830 | 820 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
$ | 5,857 | $ | 1,005 | $ | 1,991 | $ | 1,866 | ||||||||
| Other expense |
||||||||||||||||
| Change in fair value of derivative liabilities |
(278 | ) | (93 | ) | 10 | — | ||||||||||
| Net Loss |
(5,579 | ) | (912 | ) | $ | (2,001 | ) | $ | (1,866 | ) | ||||||
| Net Loss per Share; Basic and Diluted |
(0.11 | ) | (0.02 | ) | $ | (0.04 | ) | $ | (0.04 | ) | ||||||
| Weighted-Average Number of Shares used Per Common Share Calculations: |
||||||||||||||||
| Basic and Diluted |
50,000,000 | 50,000,000 | 50,000,000 | 50,000,000 | ||||||||||||
The table below presents our balance sheet data as of June 30, 2015 on an actual basis and:
on a pro forma basis to give effect to (1) the amendment of our certificate of incorporation to increase our authorized shares of common stock to 100,000,000, (2) the issuance of 1,384,616 shares of Common Stock to the Company’s Chief Executive Officer, (3) the issuance of $6,250,000 in aggregate principal amount of original issue discount convertible notes in August and September 2015 and the receipt of net proceeds therefrom, (4) the conversion of all of the original issue discount convertible notes issued in August and September 2015 into an aggregate of shares of our common stock, which will occur upon the closing of this offering; (5) the payment of a cash bonus to the Company’s Chief Executive Officer in the amount of $ within 180 days of the closing of this offering and (6) the filing of our restated certificate of incorporation upon the closing of this offering; and
on a pro forma as adjusted basis to give effect to the sale of shares of our common stock offered in this offering at an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting commissions and estimated offering expenses payable by us.
6
Table of Contents
| As of June 30, 2015 | ||||||||||||
| (in thousands) | Actual | Pro Forma | Pro Forma as adjusted(1) |
|||||||||
| Cash |
$ | 4 | $ | $ | ||||||||
| Total assets |
601 | |||||||||||
| Total liabilities |
11,583 | |||||||||||
| Common stock |
50 | |||||||||||
| Additional paid-in capital |
(50 | ) | ||||||||||
| Accumulated deficit |
(10,982 | ) | ( | ) | ||||||||
| Total stockholder’s deficiency |
(10,982 | ) | ||||||||||
| (1) | Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the range set forth on the cover page of this prospectus, would increase (decrease) each of pro forma as adjusted additional paid-in capital, stockholders’ equity and total capitalization by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) each of pro forma as adjusted additional paid-in capital, stockholders’ equity and total capitalization by approximately $ million, assuming the assumed initial public offering price per share, as set forth on the cover page of this prospectus, remains the same. |
7
Table of Contents
Investing in our common stock involves numerous risks. You should carefully consider the risks and uncertainties described below together with all of the other information contained in this prospectus, including our financial statements and related notes appearing at the end of this prospectus, before deciding to invest in our common stock. If any of the following risks actually occurs, our business, prospects, operating results and financial condition could suffer materially, the trading price of our common stock could decline and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business.
Risks related to our financial position and need for additional capital
We have incurred net losses since our inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future. We may never achieve or sustain profitability.
Our business plan is to develop, design and produce safe and effective products focusing on drug solutions for pain resulting from musculoskeletal disorders, cancer, and peripheral neuropathy. Our primary activities have been research and development, negotiating strategic alliances and other agreements, and raising capital. Expenses of the Company include those specifically identifiable to the Company, and allocations of expenses from the Company’s former parent. The allocated expenses were primarily based on the use of estimates. Expenses allocated from the Company’s parent were costs which benefitted the Company and were required for its operations. Certain general corporate expenses of the Company’s parent were not allocated because they did not provide a direct or material benefit to the business. In the opinion of management, the methods of allocating costs were reasonable; however, such costs did not necessarily equal costs that the Company would have incurred on a stand-alone basis. Therefore, the financial information included in this prospectus may not necessarily reflect assets and liabilities and cash flows of the Company if operated on a stand-alone basis. To date, the Company has not generated any revenues from its operations.
We have incurred net losses during each fiscal period since our inception. Our net loss was approximately $5,579,000 for the six months ended June 30, 2015, $2,001,000 for the year ended December 31, 2014 and approximately $1,866,000 for the year ended December 31, 2013. We do not know when or whether we will become profitable. To date, we have not commercialized any products or generated any revenues from the sale of products, and we do not expect to generate any product revenues in the foreseeable future. Our losses have resulted principally from costs incurred in our discovery and development activities. Our net losses may fluctuate significantly from quarter to quarter and year to year.
We have devoted most of our financial resources to research and development, including our clinical and preclinical development activities. To date, we have financed our operations only through advances from our former parent. The amount of our future net losses will depend, in part, on the rate of our future expenditures and our ability to obtain funding through equity or debt financings and strategic collaborations. We have not completed pivotal clinical trials for any product candidate and it may be several years, if ever, before we have a product candidate ready for commercialization. Even if we obtain regulatory approval to market a product candidate, our future revenues will depend upon the size of any markets in which our product candidates have received approval, and our ability to achieve sufficient market acceptance, reimbursement from third-party payors and adequate market share for our product candidates in those markets.
We expect to continue to incur significant expenses and increasing net losses for at least the next several years. We expect our expenses will increase substantially in connection with our ongoing activities, as we:
| • | submit an Investigational New Drug, or IND, application to the FDA for authorization to conduct our contemplated pivotal Phase 2B/3 clinical trial for AX-IBU-01; |
| • | seek regulatory approval for AX-IBU-01; |
8
Table of Contents
| • | submit an IND application to the FDA for authorization to begin Phase 1 clinical trials for AX-DN-01; |
| • | add personnel to support our product development and commercialization efforts; |
| • | continue our research and development efforts for new product opportunities; and |
| • | operate as a public company. |
If we are required by the FDA, or any equivalent foreign regulatory authority to perform clinical trials or studies in addition to those we currently expect to conduct, or if there are any delays in completing the clinical trials of AX-IBU-01 or AX-DN-01, our expenses could increase.
To become and remain profitable, we must succeed in developing our transdermal product candidates, obtaining regulatory approval for them, and manufacturing, marketing and selling those products for which we may obtain regulatory approval. We may not succeed in these activities, and we may never generate revenue from product sales that is significant enough to achieve profitability. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our failure to become or remain profitable would depress our market value and could impair our ability to raise capital, expand our business, discover or develop other transdermal product candidates or continue our operations. A decline in the value of our company could cause you to lose all or part of your investment.
The underwriters are offering the securities offered by this prospectus on a “best efforts” basis. The underwriters are not required to sell any specific number or dollar amount of common stock, but will use their best efforts to sell the securities. As a “best efforts” offering, there can be no assurance that the offering contemplated hereby will ultimately be consummated or will result in any proceeds being made to us. The success of this offering will impact our ability to finance operations over the next 12 months. If no shares of common stock are sold in this offering, or if we sell only a minimum number of shares of common stock yielding insufficient gross proceeds, we may be unable to successfully fund our operations, or execute on our business plan. This would result in a material adverse effect on our business, prospects, financial condition, and results of operations.
Our auditors have issued a going concern opinion.
Our independent auditors have indicated, in their report on our December 31, 2014 financial statements, that there is substantial doubt about our ability to continue as a going concern. A “going concern” opinion indicates that the financial statements have been prepared assuming we will continue as a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets, or the amounts and classification of liabilities that may result if we do not continue as a going concern. Therefore, you should not rely on our balance sheet as an indication of the amount of proceeds that would be available to satisfy claims of creditors, and potentially be available for distribution to shareholders, in the event of liquidation.
We will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed could force us to delay, limit, reduce or terminate our product development or commercialization efforts.
Our cash as of June 30, 2015 was $4,000 because we have funded all operations with advances from our former parent corporation, and we received an additional $5 million in cash proceeds from an original issue discount convertible note offering (the “Original Issue Discount Convertible Note Offering”) which we completed in September 2015. We believe that we will continue to expend substantial resources for the foreseeable future developing AX-IBU-01, AX-DN-01 and new transdermal product candidates. These expenditures will include costs associated with research and development, conducting preclinical studies and clinical trials, potentially obtaining regulatory approvals and manufacturing products, as well as marketing and selling products approved for sale, if any, and potentially acquiring new technologies. In addition, other unanticipated costs may arise. Because the outcome of our contemplated clinical trials is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our transdermal product candidates. Our costs will increase if we suffer any delays in our
9
Table of Contents
contemplated pivotal Phase 2B/3 clinical trial for AX-IBU-01, including delays in enrollment of patients. Upon the closing of this offering, we expect to incur additional costs associated with operating as a public company, hiring additional personnel and expanding our facilities.
Our future capital requirements depend on many factors, including:
| • | the scope, progress, results and costs of researching and developing AX-IBU-01, AX-DN-01 and our other potential transdermal product candidates and conducting preclinical studies and clinical trials; |
| • | the timing of, and the costs involved in, obtaining regulatory approvals for AX-IBU-01, AX-DN-01 and our other potential transdermal product candidates if clinical trials are successful; |
| • | the cost of commercialization activities for AX-IBU-01, AX-DN-01 and our other potential transdermal product candidates, if any of these transdermal product candidates are approved for sale, including marketing, sales and distribution costs; |
| • | the cost of manufacturing AX-IBU-01, AX-DN-01 and our other potential transdermal product candidates for clinical trials in preparation for regulatory approval and in preparation for commercialization; |
| • | our ability to establish and maintain strategic partnerships, licensing or other arrangements and the financial terms of such agreements; |
| • | our possible liability under a loan agreement to which we and our former parent are both parties and which is currently in default, as explained further below; |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and |
| • | the timing, receipt, and amount of sales of, or royalties on, our future products, if any. |
Based on our current operating plan, we believe that the net proceeds we receive from this offering, together with the $5 million in cash proceeds from the Original Issue Discount Convertible Note Offering which we completed in September 2015, will be sufficient to fund our projected operating requirements into the second half of 2016 and completion of our contemplated pivotal Phase 2B/3 clinical trial for AX-IBU-01. However, our operating plan may change as a result of many factors currently unknown to us. As a result of these factors, we may need additional funds sooner than planned. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate preclinical studies, clinical trials or other development activities for one or more of our transdermal product candidates or delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our transdermal product candidates.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or transdermal products on unfavorable terms to us.
We may seek additional capital through a variety of means, including through private and public equity offerings and debt financings. To the extent that we raise additional capital through the sale of equity instruments or convertible debt securities, your ownership interest will be diluted, and the terms of such equity or convertible debt securities may include liquidation or other preferences that are senior to or otherwise adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring additional debt, making capital expenditures, declaring dividends or encumbering our assets to secure future indebtedness. If we raise additional funds through strategic partnerships with third parties, we may have to relinquish valuable rights to our technologies or transdermal product candidates, or grant licenses on terms that are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts for AX-
10
Table of Contents
IBU-01, AX-DN-01 or any other transdermal product candidates, or grant rights to develop and market transdermal product candidates that we would otherwise prefer to develop and market ourselves.
We identified a material weakness in our internal control over financial reporting and if the remediation procedures we have undertaken are unable to successfully remediate the existing material weakness, then the accuracy and timing of our financial reports may be adversely affected.
In preparing our financial statements as of and for the year ended December 31, 2014, we identified control deficiencies in the design and operation of our internal control over financial reporting that together constituted a material weakness in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis. The material weaknesses identified were that we did not have adequate accounting systems and our accounting staff was inadequate both in terms of the number of personnel and their expertise in U.S. GAAP and SEC rules and regulations. As such, our controls over financial reporting were not designed or operating effectively.
Our independent certified public accounting firm is not required to perform an evaluation of our internal control over financial reporting in accordance with the provisions of the Sarbanes-Oxley Act by virtue of our status as an “emerging growth company” as defined in the JOBS Act. In light of the control deficiencies and the resulting material weakness that was identified as a result of the limited procedures we did perform, it is possible that additional material weaknesses and significant control deficiencies may have been identified if we and our independent registered public accounting firm performed an evaluation of our internal control over financial reporting in accordance with the provisions of the Sarbanes-Oxley Act. However, for as long as we remain an “emerging growth company” as defined in the JOBS Act, we intend to take advantage of the exemption permitting us not to comply with the requirement that our independent registered public accounting firm provide an attestation on the effectiveness of our internal control over financial reporting.
If we fail to remediate the material weakness or if in the future we fail to meet the demands that will be placed upon us as a public company, we may be unable to accurately report our financial results, or report them within the timeframes required by law or stock exchange regulations. Failure to comply with Section 404 of the Sarbanes-Oxley Act could also potentially subject us to sanctions or investigations by the SEC or other regulatory authorities.
Risks related to our former parent corporation
Our former parent corporation, BioChemics, recently settled claims against it by the SEC for violations of federal securities laws in connection with the private sale of securities of BioChemics.
Until January 2015, Inpellis was a wholly owned subsidiary of BioChemics. In December 2012, the SEC brought a civil enforcement action against BioChemics, its founder and then CEO, and other individuals in the United States District Court for the District of Massachusetts, entitled Securities and Exchange Commission v. BioChemics, Inc., et al., Civil Action No. 1:12-cv-12324 (District of Massachusetts), which we refer to as the BioChemics SEC Enforcement Action. In this action, the SEC alleged, among other things, that the defendants made false statements to investors about collaborations with major pharmaceutical companies and the status and results of drug trials of BioChemics’ products, and that they created fraudulent valuations of BioChemics’ stock in connection with the private sale of securities of BioChemics to certain investors. The founder and CEO of BioChemics was also sued in a 2004 SEC enforcement action alleging false and misleading statements by VASO Active Pharmaceuticals, a former BioChemics subsidiary, concerning, among other things, FDA approval of certain key products and the regulatory consequences of the future application of its primary product, which case resulted in a final judgment by consent against this individual that permanently enjoined him from violating the antifraud provisions of the federal securities laws.
In March 2015, the United States District Court for the District of Massachusetts entered judgment against BioChemics in the BioChemics SEC Enforcement Action pursuant to a consent settlement requiring BioChemics to comply with certain antifraud provisions of the federal securities laws and requiring it to pay disgorgement and penalties approximating $18 million for the benefit of certain investors in BioChemics.
11
Table of Contents
In recognition of the above-described BioChemics settlement with the SEC and its disgorgement and penalty obligations thereunder, and the prior SEC enforcement actions against BioChemics’ founder and former CEO, BioChemics has ceded all of the shares of Inpellis common stock previously issued to it to the Trust, which was established to resolve the claims arising from the settlement in the BioChemics SEC Enforcement Action. The Trust is now the majority stockholder of Inpellis and operates independently of BioChemics. The Trust is independent of BioChemics because it cannot act unless all three of its trustees approve the action and, of the three trustees, two have no connection to BioChemics. The third trustee, Jan Schlichtmann, is the Managing Member of a limited liability company that is the largest stockholder of BioChemics. See “Business—Corporate Structure.” As a result, BioChemics no longer has any ownership interest in Inpellis. Furthermore, all of the officers and directors of Inpellis are fully independent of BioChemics. In addition, the Trustees have agreed that the Trust shall not distribute any shares of Inpellis to any of the individuals who are defendants in the BioChemics SEC Enforcement Action or any entity controlled by any such individual.
The creditors of BioChemics could challenge certain transactions between us and BioChemics.
We recently acquired ownership or joint ownership of the BioChemics intellectual property as it relates to certain drugs for $750,000 in cash.
In addition, BioChemics recently agreed the Company would not be required to reimburse BioChemics for funds advanced to the Company. The creditors of BioChemics could attempt to assert that BioChemics did not receive adequate consideration for the sale of intellectual property and seek to require that the Company pay a greater amount to BioChemics. Similarly, the creditors of BioChemics could attempt to assert that BioChemics did not receive adequate consideration for its agreement not to be reimbursed and seek to require that the Company make payments to BioChemics. BioChemics has informed us that its decisions to sell intellectual property and to agree not to require reimbursement were made based on careful consideration of the alternatives, its conclusion that both arrangements would help Inpellis obtain capital it might not otherwise raise to pursue its business plan and its conclusion that the value of a well-funded Inpellis to the creditors and shareholders of BioChemics would likely exceed the value of a larger one-time cash payment and repayment of advances were Inpellis unable to obtain necessary capital. Nevertheless, if we were required, notwithstanding the conclusions of BioChemics, to pay BioChemics in connection with the sale of intellectual property, to repay the advances, or both, it could reduce the funds available to achieve our goals.
Risks related to regulatory review and approval of our products
Clinical failure may occur at any stage of clinical development, and we may never succeed in developing marketable products or generating product revenue.
Although the active ingredient in AX-IBU-01, ibuprofen, has been used safely as an oral therapeutic treatment for moderate to severe osteoarthritis for a number of years, it has not previously been approved or demonstrated to be safe over an extended period of time as a transdermal product. Early encouraging clinical results for AX-IBU-01 are not necessarily predictive of the results of our future clinical trials, including our contemplated pivotal Phase 2B/3 clinical trial. Promising results in preclinical studies of a drug candidate may not be predictive of similar results in humans during clinical trials. Any Phase 2B/3 or other clinical trials that we may conduct may not demonstrate the efficacy and safety necessary to obtain regulatory approval to market our product candidates. If the results of our clinical trials are inconclusive with respect to the efficacy of our transdermal product candidates or if we do not meet the clinical endpoints with statistical significance or if there are safety concerns associated with our transdermal product candidates, we may be prevented or delayed in obtaining marketing approval for our transdermal product candidates. In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the clinical trial protocols and the rate of dropout among clinical trial participants.
Alternatively, even if we obtain regulatory approval, that approval may be for indications or patient populations that are not as broad as intended or desired or may require labeling that includes significant use or
12
Table of Contents
distribution restrictions or safety warnings. We may also be required to perform additional or unanticipated clinical trials to obtain approval or be subject to additional post-marketing testing requirements to maintain regulatory approval. In addition, regulatory authorities may withdraw their approval of a product or impose restrictions on its distribution, such as in the form of a modified Risk Evaluation and Mitigation Strategy, or REMS. The failure to obtain timely regulatory approval of product candidates, any product marketing limitations or a product withdrawal would negatively impact our business, results of operations and financial condition.
Delays in the commencement, enrollment or completion of clinical trials of our transdermal product candidates could result in increased costs to us as well as a delay or failure in obtaining regulatory approval, or prevent us from commercializing our transdermal product candidates on a timely basis, or at all.
We cannot guarantee that clinical trials, including those associated with our contemplated pivotal Phase 2B/3 clinical trial for AX-IBU-01, will be conducted as contemplated or completed on schedule, if at all. A failure of one or more clinical trials can occur at any stage of testing. Events that may prevent successful or timely commencement, enrollment or completion of clinical development include:
| • | delays by us in reaching a consensus with regulatory agencies on trial design; |
| • | delays in reaching agreement on acceptable terms with prospective clinical research organizations, or CROs, and clinical trial sites; |
| • | delays in obtaining required Institutional Review Board, or IRB, approval at each clinical trial site; |
| • | delays in recruiting suitable patients to participate in clinical trials; |
| • | imposition of a clinical hold by regulatory agencies for any reason, including safety concerns or after an inspection of clinical operations or trial sites; |
| • | failure by CROs, other third parties or us to adhere to clinical trial requirements; |
| • | failure to perform in accordance with the FDA’s good clinical practices, or GCP, or applicable regulatory guidelines in other countries; |
| • | delays in the testing, validation, manufacturing and delivery of the transdermal product candidates to the clinical sites; |
| • | delays caused by patients not completing participation in a trial or not returning for post-treatment follow-up; |
| • | clinical trial sites or patients dropping out of a trial; |
| • | occurrence of serious adverse events, or AEs, in clinical trials that are associated with the transdermal product candidates that are viewed to outweigh its potential benefits; or |
| • | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
Delays, including delays caused by the above factors, can be costly and could negatively affect our ability to complete a clinical trial. If we are not able to successfully complete clinical trials, we will not be able to obtain regulatory approval and will not be able to commercialize our transdermal product candidates.
Clinical development, regulatory review and approval of the FDA and comparable foreign authorities are lengthy, time consuming, and inherently unpredictable. If we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
Our transdermal product candidates will be subject to extensive governmental regulations relating to, among other things, development, clinical trials, manufacturing and commercialization. In order to obtain regulatory approval for the commercial sale of any transdermal product candidate, we must demonstrate through extensive preclinical studies and clinical trials that the transdermal product candidate is safe and effective for use in each target indication.
13
Table of Contents
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable, typically takes many years following the commencement of clinical trials, and depends upon numerous factors. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions, which may cause delays in the approval or the decision not to approve an application. We have not obtained regulatory approval for any product candidate, and it is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval. In addition, we may gain regulatory approval for AX-IBU-01, AX-DN-01 or any other transdermal product candidate in some but not all of the territories available or some but not all of the target indications, resulting in limited commercial opportunity for the approved transdermal products.
Applications for our product candidates could be delayed or could fail to receive regulatory approval for many reasons, including but not limited to the following:
| • | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| • | the population studied in the clinical program may not be sufficiently broad or representative to assure safety in the full population for which we seek approval; |
| • | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from nonclinical studies or clinical trials; |
| • | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of a new drug application, or NDA, or to obtain regulatory approval in the United States or elsewhere; |
| • | the FDA may determine that we cannot rely on Section 505(b)(2) for any of our product candidates, for a number of reasons, including safety and efficacy issues which have already been raised; in which case we may be required to conduct additional clinical trials, provide additional data and information and meet additional standards for product approval, resulting in increased time and financial resources required to obtain FDA approval for our product candidates; |
| • | the FDA may determine that we have identified the wrong Reference Listed Drug, or RLD, or RLDs or that approval of a Section 505(b)(2) application for any of our product candidates is blocked by patent or non-patent exclusivity of the RLD or RLDs; |
| • | the FDA may require us to conduct additional clinical trials depending on the safety data from our contemplated future clinical trials, including our contemplated pivotal Phase 2B/3 clinical trial for AX-IBU-01; |
| • | we may be unable to demonstrate to the FDA or comparable foreign regulatory authorities that a product candidate’s risk benefit ratio for its proposed indication is acceptable; |
| • | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes, test procedures and specifications, or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; |
| • | we or any third-party service providers may be unable to demonstrate compliance with GMP to the satisfaction of the FDA or comparable foreign regulatory authorities which could result in delays in regulatory approval or require us to withdraw or recall products and interrupt commercial supply of our products; and |
| • | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
This lengthy approval process, as well as the unpredictability of the results of clinical trials, may result in our failing to obtain regulatory approval to market any of our product candidates, which would significantly harm our business, results of operations, and prospects.
14
Table of Contents
We are already aware that the FDA has safety and efficacy concerns regarding the results of our Phase 2 trial that we will need to address, and we know of at least three other areas where the FDA could require a more extensive review process with respect to the transdermal ibuprofen product. First, the product used in the Phase 2 trial used menthol as a vasodilator. Menthol, however, is also an analgesic. If the FDA concludes that further testing needs to be done to assure that the Phase 2 results were due to the ibuprofen and not the menthol, such additional testing could add significant time and expense to the commercialization of the transdermal ibuprofen product. Second, we believe that the FDA will agree with us that vasodilators are an active incipient, not an active ingredient. But if that agreement does not occur, we may have to perform much longer, multi-round clinical trials on our products. Finally, the Phase 2 trial of our transdermal ibuprofen product did not directly measure the ibuprofen levels in the blood because such measurement had been done in the Phase 1 trial. If the FDA determines that such testing needs to occur again, or if the FDA is not satisfied with further safety and efficacy testing, we would be delayed in obtaining required approvals and, therefore, commercialization.
We currently have only two transdermal product candidates, AX-IBU-01 and AX-DN-01, and are substantially dependent on these two product candidates. A failure of either product candidate in clinical development would adversely affect our business and may require us to discontinue development of other transdermal product candidates based on the same technology.
AX-IBU-01 is our lead development product candidate. AX-DN-01 has conducted a preclinical proof of concept study. While we have certain pre-clinical programs in development and intend to develop other product candidates, it will take several years and substantial additional investment for such programs to reach the same stage of development as AX-IBU-01 and AX-DN-01, respectively. If we were required to discontinue development of AX-IBU-01 or AX-DN-01 or if either product candidate does not receive regulatory approval or fails to achieve sufficient market acceptance, we would be delayed by many years in our ability to achieve profitability, if ever. In addition, since we anticipate that all of our transdermal product candidates will be based on the same endel technology, if AX-IBU-01 or AX-DN-01 fails in development as a result of any underlying problem with the endel technology, then we may be required to discontinue development of all transdermal product candidates that are based on the same technology. In such event, our business will be adversely affected.
If we fail to obtain regulatory approval in jurisdictions outside the United States, we will not be able to market our products in those jurisdictions.
We intend to market our transdermal product candidates, including AX-IBU-01 or AX-DN-01, if approved, in international markets either directly or through partnerships. Such marketing will require separate regulatory approvals in each market and compliance with numerous and varying regulatory requirements. The approval procedures vary from country to country and may require additional testing that we are not required to perform to obtain regulatory approval in the United States. Moreover, the time required to obtain approval may differ from that required to obtain FDA approval. In addition, in many countries outside the United States, a transdermal product must be approved for reimbursement before it can be approved for sale in that country. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market. If we or any future partner are unable to obtain regulatory approval for AX-IBU-01 or AX-DN-01 in one or more significant foreign jurisdictions, then the commercial opportunity for such product candidate, and our financial condition, will be adversely affected.
15
Table of Contents
Even if we receive regulatory approval for our transdermal product candidates, such products will be subject to ongoing regulatory review, which may result in significant additional expense. Additionally, our transdermal product candidates, if approved, could be subject to labeling and other restrictions, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our products.
Any regulatory approvals that we receive for our transdermal product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor safety and efficacy. In addition, if the FDA approves any of our transdermal product candidates, the manufacturing processes, labeling, packaging, distribution, AE reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with current good manufacturing practice, or GMP, and GCP, for any clinical trials that we conduct post-approval.
Later discovery of previously unknown problems with an approved transdermal product, including AEs of unanticipated severity or frequency, or with manufacturing operations or processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| • | fines, warning letters, or holds on clinical trials; |
| • | refusal by the FDA to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals; |
| • | product seizure or detention, condemnation and destruction, or refusal to permit the import or export of products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA’s policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our transdermal product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or not able to maintain regulatory compliance, we may lose any marketing approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
Our transdermal products may cause undesirable side effects or have other properties that delay or prevent their regulatory approval or limit their commercial potential.
Undesirable side effects caused by our transdermal products could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities and potential product liability claims. Serious AEs deemed to be caused by our transdermal products could have a material adverse effect on the development of our transdermal product candidates and our business as a whole. The most common AEs to date in the clinical trials evaluating the safety and efficacy of AX-IBU-01 have been a few incidents of skin redness. Our understanding of the relationship between AX-IBU-01 and these events may change as we gather more information, and additional unexpected AEs may occur. In addition, although ibuprofen has been in use for over 40 years, the long-term impact of using ibuprofen as a transdermal product is not well understood.
16
Table of Contents
If we or others identify undesirable side effects caused by our transdermal product candidates either before or after receipt of marketing approval, a number of potentially significant negative consequences could result, including:
| • | our clinical trials may be put on hold; |
| • | we may be unable to obtain regulatory approval for our transdermal product candidates; |
| • | regulatory authorities may withdraw approvals of our transdermal product candidates; |
| • | regulatory authorities may require additional warnings on the label; |
| • | a medication guide outlining the risks of such side effects for distribution to patients may be required; |
| • | we could be sued and held liable for harm caused to patients; and |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of our transdermal products and could substantially increase commercialization costs.
Risks related to manufacturing our transdermal product candidates and our reliance on third parties
We will rely on third-party manufacturers to manufacture our transdermal product candidates, including AX-IBU-01 and AX-DN-01, and any failure by a third-party manufacturer or supplier may delay or impair our ability to complete clinical trials or commercialize our transdermal product candidates, including AX-IBU-01 and AX-DN-01.
We have relied, and we expect to continue to rely, on third-party manufacturers to manufacture our transdermal product candidates for preclinical studies, including Phase 1 clinical trials and Phase 2 clinical trials of AX-IBU-01, and expect to continue to do so for our contemplated pivotal Phase 2B/3 clinical trial. We have relied, and we expect to continue to rely, on third-party suppliers of ibuprofen, the active pharmaceutical ingredient in AX-IBU-01. Similarly, we have relied, and we expect to continue to rely, on third-party manufacturers to manufacture our transdermal product candidates for preclinical studies of AX-DN-01, and expect to continue to do so for our clinical trials of AX-DN-01. We have relied, and we expect to continue to rely, on third-party suppliers of benfotiamine, the active pharmaceutical ingredient in AX-DN-01. We currently do not have contracts in place with any of these third parties. Our reliance on third parties for the manufacture of our product candidates, including AX-IBU-01 and AX-DN-01, increases the risk that we will not have sufficient quantities of our product candidates, or will not be able to obtain such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts. If our third-party manufacturer fails to produce GMP-quality AX-IBU-01 for our contemplated pivotal Phase 2B/3 clinical trial and we need to enter into alternative arrangements with a different manufacturer, it could delay our product development activities. If this failure of production were to occur after we received approval for and commenced commercialization of AX-IBU-01, we might be unable to meet the demand for this product and our business could be adversely affected. In addition, because we do not have any control over the manufacturing process or timing of the supply of the active pharmaceutical ingredient, there is greater risk that we will not have sufficient quantities of the active pharmaceutical ingredient at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts. The same risks exist for AX-DN-01 and other product candidates.
Our third-party manufacturers and suppliers may be subject to FDA inspection from time to time. Failure by our third-party manufacturers to pass such inspections and otherwise satisfactorily complete the FDA approval regimen with respect to our product candidates may result in regulatory actions such as the issuance of FDA Form 483 notices of observations, warning letters or injunctions or the loss of operating licenses. Based on the severity of the regulatory action, our clinical or commercial supply of plastic molds and other services could be interrupted or limited, which could have a material adverse effect on our business.
17
Table of Contents
Our transdermal product candidates have not been produced at a commercial scale. Our manufacturing partners may not be able to manufacture our transdermal product candidates in quantities sufficient for commercial launch of our product candidates, if our product candidates are approved, or for any future commercial demand for our product candidates.
Although contractors with whom we have dealt in the past have manufactured clinical quantities of AX-IBU-01, neither those contractors nor any other third company with whom we may contract in the future have manufactured commercial quantities of our product candidates. In addition, we have not scaled up manufacturing for AX-DN-01 to clinical quantities, although we do not anticipate difficulties in doing so. If AX-IBU-01 or AX-DN-01 is approved for commercialization and marketing, our manufacturing contractors may be required to manufacture the product in large quantities to meet demand. Producing product in commercial quantities requires developing and adhering to complex manufacturing processes that are different from the manufacture of a product in smaller quantities for clinical trials, including adherence to regulatory standards. Although we believe that we have developed processes and protocols that will enable our manufacturing contractors to manufacture commercial-scale quantities of product at acceptable costs, we cannot provide assurance that such processes and protocols will enable them to manufacture AX-IBU-01 or AX-DN-01 in quantities that may be required for commercialization of the product with yields and at costs that will be commercially attractive. If we are unable to establish or maintain commercial manufacture of the product or are unable to do so at costs that we currently anticipate, our business will be adversely affected.
If our manufacturing contractors are unable to use their manufacturing facilities for any reason, they would be unable to manufacture clinical supply and, if approved, commercial quantities of our transdermal product candidates for a substantial amount of time, which would harm our business.
We have relied, and we expect to continue to rely, on third-party manufacturers to manufacture our transdermal product candidates for preclinical studies, including Phase 1 clinical trials and Phase 2 clinical trials of AX-IBU-01, and expect to continue to do so for our contemplated pivotal Phase 2B/3 clinical trial and all commercial supplies of AX-IBU-01, if approved for commercial sale. Similarly, we have relied, and we expect to continue to rely, on third-party manufacturers to manufacture our transdermal product candidates for preclinical studies of AX-DN-01, and expect to continue to do so for our clinical trials of AX-DN-01. If our manufacturing contractors were to lose use of their facilities or equipment, their manufacturing facilities and manufacturing equipment would be difficult to replace and could require substantial replacement lead-time. Their facilities may be affected by natural disasters, such as floods or fire, or they may lose the use of their facilities due to manufacturing issues that arise at their facilities, such as contamination or regulatory concerns following a regulatory inspection. We do not currently have back-up capacity and there is only limited third-party manufacturing capacity that would be available to manufacture our products. In the event of a loss of the use of all or a portion of our manufacturing contractors’ facilities or equipment for the reasons stated above or any other reason, we would be unable to procure any of our transdermal product candidates until such time as the issues at the facility are remedied or we are able, if possible, to contract with an alternative third-party manufacturer.
We rely on third parties to conduct preclinical studies and clinical trials for AX-IBU-01, and if they do not properly and successfully perform their obligations to us, we may not be able to obtain regulatory approvals for AX-IBU-01 or any other transdermal product candidates that we may develop in the future.
We plan to design the clinical trials for AX-IBU-01 and for any future unpartnered transdermal product candidates that we may develop. However, we rely on CROs and other third parties to assist in managing, monitoring and otherwise carrying out many of these trials. We compete with many other companies for the resources of these third parties. The third parties on whom we rely generally may terminate their engagements at any time, and having to enter into alternative arrangements would delay development and commercialization of our transdermal product candidates.
The FDA and comparable foreign regulatory authorities require compliance with regulations and standards, including GCP, for designing, conducting, monitoring, recording, analyzing, and reporting the results of clinical
18
Table of Contents
trials to assure that the data and results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Although we rely on third parties to conduct many of our clinical trials, they are not our employees, and we are responsible for ensuring that each of these clinical trials is conducted in accordance with its general investigational plan, protocol and other requirements. Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibilities.
If these third parties do not successfully carry out their duties under their agreements, if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to clinical trial protocols or to regulatory requirements, or if they otherwise fail to comply with clinical trial protocols or meet expected deadlines, the clinical trials of our transdermal product candidates may not meet regulatory requirements. If clinical trials do not meet regulatory requirements or if these third parties need to be replaced, preclinical development activities or clinical trials may be extended, delayed, suspended or terminated. If any of these events occur, we may not be able to obtain regulatory approval of our transdermal product candidates on a timely basis or at all.
We will also rely on CROs and other third parties to assist in managing, monitoring and otherwise carrying out clinical trials for AX-DN-01, if the FDA authorizes us to proceed with our intended Phase 1 clinical trials, as well as any additional Phase 2 or Phase 3 trials that we may ultimately conduct. The same risks for this third party reliance described above will also apply to AX-DN-01.
We may not be successful in establishing and maintaining strategic partnerships, which could adversely affect our ability to develop and commercialize products, and have a negative effect on our operating results.
We continue to strategically evaluate and, as deemed appropriate, we expect to enter into, partnerships in the future when strategically attractive, including potentially with major biotechnology or pharmaceutical companies. In particular, we may enter into one or more partnerships for the development and commercialization of AX-IBU-01 in Europe or other countries outside of the United States. We face significant competition in seeking appropriate partners for our transdermal product candidates, and the negotiation process is time-consuming and complex. In order for us to successfully partner our transdermal product candidates, potential partners must view these transdermal product candidates as economically valuable in markets they determine to be attractive in light of the terms that we are seeking and other available products for licensing by other companies. Even if we are successful in our efforts to establish strategic partnerships, the terms that we agree upon may not be favorable to us, and we may not be able to maintain such strategic partnerships if, for example, development or approval of a transdermal product is delayed or sales of an approved product are disappointing. Any delay in entering into strategic partnership agreements related to our transdermal product candidates could delay the development and commercialization of such candidates and reduce their competitiveness even if they reach the market.
If we fail to establish and maintain strategic partnerships related to our transdermal product candidates, we will bear all of the risk and costs related to the development of any such transdermal product candidate, and we may need to seek additional financing, hire additional employees and otherwise develop expertise, such as regulatory expertise, for which we have not budgeted. This could negatively affect the development of any unpartnered transdermal product candidate.
19
Table of Contents
Risks related to commercialization of our transdermal product candidates
Our future commercial success depends upon attaining significant market acceptance of our transdermal product candidates, if approved, among physicians, patients and health care payors.
Even if we obtain regulatory approval for AX-IBU-01, AX-DN-01 or any other transdermal products that we may develop or acquire in the future, the product candidate may not gain market acceptance among physicians, health care payors, patients and the medical community. Market acceptance of any approved products depends on a number of factors, including:
| • | the efficacy and safety of the product, as demonstrated in clinical trials; |
| • | the indications for which the product is approved and the label approved by regulatory authorities for use with the product, including any warnings that may be required on the label; |
| • | acceptance by physicians and patients of the product as a safe and effective treatment; |
| • | the cost, safety and efficacy of treatment in relation to alternative treatments; |
| • | the availability of adequate reimbursement and pricing by third-party payors and government authorities; |
| • | relative convenience and ease of administration; |
| • | the prevalence and severity of adverse side effects; and |
| • | the effectiveness of our sales and marketing efforts. |
Market acceptance is critical to our ability to generate significant revenue and become profitable. Any therapeutic candidate, if approved and commercialized, may be accepted in only limited capacities or not at all. If any approved products are not accepted by the market to the extent that we expect, we may not be able to generate significant revenue and our business would suffer.
The market for our product candidate may not be as large as we expect.
Our estimates of the potential market opportunity for AX-IBU-01 and AX-DN-01 include several key assumptions based on our industry knowledge, industry publications, third-party research reports and other surveys. These assumptions include the prevalence and growth of the pain market, population dynamics, an increase in the elderly, and co-morbidities associated with obesity and diabetes. While we believe that our internal assumptions are reasonable, if any of these assumptions proves to be inaccurate, then the actual market for AX-IBU-01 or AX-DN-01 could be smaller than our estimates of our potential market opportunity. If the actual market for AX-IBU-01 or AX-DN-01 is smaller than we expect, our product revenue may be limited and it may be more difficult for us to achieve or maintain profitability.
If we are unable to establish sales, marketing and distribution capabilities, we may not be successful in commercializing our product candidates if and when they are approved.
We do not have a sales or marketing infrastructure and have no experience in the sale, marketing or distribution of products. To achieve commercial success for any product for which we have obtained marketing approval, we will need to establish partnerships with companies that have sales and marketing organizations.
Factors that may inhibit our partners’ efforts to commercialize our products include:
| • | the inability to recruit, train and retain adequate numbers of effective sales and marketing personnel; |
| • | the inability of sales personnel to obtain access to physicians; |
| • | the lack of adequate numbers of physicians to prescribe any future products; and |
20
Table of Contents
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines. |
We likely will have little control over the third parties which market and sell our products, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales, marketing and distribution capabilities successfully in collaboration with third parties, we will not be successful in commercializing our product candidates.
Reimbursement may be limited or unavailable in certain market segments for our transdermal product candidates, which could make it difficult for us to sell our products profitably.
In both domestic and foreign markets, sales of any of our product candidates, if approved, will depend, in part, on the extent to which the costs of our products will be covered by third-party payors, such as government health programs, commercial insurance and managed health care organizations. These third party payors decide which drugs will be covered and establish reimbursement levels for those drugs. The containment of health care costs has become a priority of foreign and domestic governments as well as private third party payors. The prices of drugs have been a focus in this effort. Governments and private third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability to sell our product candidates profitably. Cost-control initiatives could cause us to decrease the price we might establish for products, which could result in lower than anticipated product revenues.
Reimbursement by a third-party payor may depend upon a number of factors, including the third-party payor’s determination that use of a product is:
| • | a covered benefit under its health plan; |
| • | safe, effective and medically necessary; |
| • | appropriate for the specific patient; |
| • | cost-effective; and |
| • | neither experimental nor investigational. |
Adverse pricing limitations may hinder our ability to recoup our investment in AX-IBU-01, AX-DN-01 or any future product candidates, even if such product candidates obtain marketing approval.
Obtaining coverage and reimbursement approval for a product from a government or other third-party payor is a time consuming and costly process that could require us to provide supporting scientific, clinical and cost-effectiveness data for the use of our products to the payor. Further, there is significant uncertainty related to third-party payor coverage and reimbursement of newly approved drugs, including those with novel formulations such as AX-IBU-01 and AX-DN-01. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. We cannot be sure that coverage or adequate reimbursement will be available for any of our transdermal product candidates. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our products. If reimbursement is not available or is available only to limited levels, we may not be able to commercialize certain of our products. In addition, in the United States, third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new drugs. As a result, significant uncertainty exists as to whether and how much third-party payors will reimburse patients for their use of newly approved drugs, which in turn will put pressure on the pricing of drugs.
Price controls may be imposed in foreign markets, which may adversely affect our future profitability.
In some countries, particularly member states of the European Union, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take
21
Table of Contents
considerable time after receipt of marketing approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various European Union member states and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. In some countries, we may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our transdermal product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be adversely affected.
The impact of recent health care reform legislation and other changes in the health care industry and in health care spending on us is currently unknown, and may adversely affect our business model.
Our revenue prospects could be affected by changes in health care spending and policy in the United States and abroad. We operate in a highly regulated industry and new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, the method of delivery or payment for health care products and services could negatively impact our business, operations and financial condition.
The United States federal and state governments continue to propose and pass legislation designed to reduce the cost of health care. In March 2010, the U.S. Congress enacted the Patient Protection and Affordable Care Act, and the Health Care and Education Reconciliation Act which includes changes to the coverage and reimbursement of drug products under government health care programs such as:
| • | increasing drug rebates under state Medicaid programs for brand name prescription drugs and extending those rebates to Medicaid managed care; |
| • | extending discounted rates on drug products available under the Public Health Service pharmaceutical pricing program to additional hospitals and other providers; |
| • | assessing a fee on manufacturers and importers of brand name prescription drugs reimbursed under certain government programs, including Medicare and Medicaid; and |
| • | requiring drug manufacturers to provide a 50% discount on Medicare Part D brand name prescription drugs sold to Medicare beneficiaries whose prescription drug costs cause the beneficiaries to be subject to the Medicare Part D coverage gap (i.e., the so-called “donut hole”). |
It is likely that federal and state legislatures within the United States and foreign governments will continue to consider changes to existing health care legislation. We cannot predict the reform initiatives that may be adopted in the future or whether initiatives that have been adopted will be repealed or modified. The continuing efforts of the government, insurance companies, managed care organizations and other payors of health care services to contain or reduce costs of health care may adversely affect:
| • | the demand for any products for which we may obtain regulatory approval; |
| • | our ability to set a price that we believe is fair for our products; |
| • | our ability to obtain coverage and reimbursement approval for a product; |
| • | our ability to generate revenues and achieve or maintain profitability; and |
| • | the level of taxes that we are required to pay. |
In addition, other legislative changes have been proposed and adopted since the 2010 health care reform legislation. The Budget Control Act of 2011, as amended, or the Budget Control Act, includes provisions intended to reduce the federal deficit. The Budget Control Act resulted in the imposition of 2% reductions in
22
Table of Contents
Medicare payments to providers beginning in 2013. Recent legislation extends reductions through 2023. Any significant spending reductions affecting Medicare, Medicaid or other publicly funded or subsidized health programs that may be implemented, and/or any significant taxes or fees that may be imposed on us, as part of any broader deficit reduction effort or legislative replacement to the Budget Control Act, could have an adverse impact on our anticipated product revenues.
We face substantial competition, which may result in others discovering, developing or commercializing products before, or more successfully, than we do.
The development and commercialization of new drug products is highly competitive. Our future success depends on our ability to demonstrate and maintain a competitive advantage with respect to the design, development and commercialization of our transdermal product candidates. Transdermal products that we commercialize on or own or with our strategic partners may compete with existing, market-leading products.
We believe that the main competitors for AX-IBU-01 are therapies that can effectively treat the symptoms associated with osteoarthritis, or OA, with fewer AEs commonly associated with NSAIDs. These therapies include a number of topical formulations of NSAIDs (e.g., ibuprofen, diclofenac, and ketoprofen). Topical NSAIDs have been shown to provide analgesia through the same mechanism of action as oral NSAIDs, but because the activity of topical NSAIDs is effectively confined to the application site, systemic exposure—and consequently, the risk for gastrointestinal, cardiovascular, and renal toxicity—has been shown to be lower than that observed with oral NSAIDs. The current market for topical NSAIDS consists primarily of the four products: Voltaren ®, Pennsaid ®, Solaraze ® and Flector ®. If approved for the treatment of osteoarthritis, AX-IBU-01 would compete against these therapies.
One or more of our competitors may utilize their expertise in transdermal delivery of drugs to develop and obtain approval for transdermal delivery products that may compete with AX-IBU-01 and any other of our product candidates. These competitors may include smaller companies such as Endo Pharmaceuticals, Inc. and Horizon Pharma USA, Inc. and larger companies such as Pfizer Inc. and Novartis Consumer Health, Inc. If approved, our product candidates may face competition in the target commercial areas.
The availability of our competitors’ products could limit the demand, and the price we are able to charge, for any product candidates that we may develop and commercialize.
Risks related to our intellectual property
If we are unable to obtain or protect intellectual property rights related to our transdermal product candidates, we may not be able to compete effectively.
Our success depends in large part on our ability to obtain and maintain protection with respect to our intellectual property and proprietary technology. Our business is substantially dependent upon our intellectual property rights, which include patents, patent applications, trade secret protection and confidentiality agreements. We also use trade secret protection and confidentiality agreements to protect our intellectual property and our transdermal products candidates. The patent position of pharmaceutical companies is generally uncertain because it involves complex legal and factual considerations. The standards applied by the United States Patent and Trademark Office, or USPTO, and foreign patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in patents. The patent applications that we own may fail to result in issued patents, and if they do, such patents may not cover the endel technology and transdermal product candidates in the United States or in other countries. The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. There is no assurance that all potentially relevant prior art has been found relating
23
Table of Contents
to our patents and patent applications. We may be unaware of prior art that could be used to invalidate an issued patent or prevent our pending patent applications from issuing as patents. Even if patents do successfully issue and even if such patents cover the endel technology and transdermal product candidates, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our transdermal product candidates, prevent others from designing around our claims or otherwise provide us with a competitive advantage. Additionally, our confidentiality agreements and other contractual protections may not be adequate to protect our intellectual property from unauthorized disclosure, third-party infringement or misappropriation. We may not have adequate remedies in the case of a breach of any such agreements, and our trade secrets and other proprietary information could be disclosed to our competitors or others may independently develop substantially equivalent or superior proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technologies. Additionally, the laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology, which could make it difficult for us to stop the infringement of our patents and patents that we may own in the future. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
If patent applications that we own or jointly own with respect to endel or our transdermal product candidates fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity, it could dissuade companies from collaborating with us. We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patents or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Any successful challenge to these patents or any other patents owned by us could deprive us of rights necessary for the successful commercialization of any transdermal product candidate that we may develop. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we were the first to file any patent application related to a transdermal product candidate. Furthermore, if third parties have filed such patent applications, an interference proceeding in the United States can be initiated by the USPTO or a third-party to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. In addition, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. Various extensions may be available; however, the life of a patent and the protection it affords is limited. If we encounter delays in obtaining regulatory approvals, the period of time during which we could market a transdermal product under patent protection could be reduced. Even if patents covering our transdermal product candidates are obtained, once the patent life has expired for a product, we may be open to competition from similar or generic products. The launch of a generic version of one of our products in particular would be likely to result in an immediate and substantial reduction in the demand for our product, which could have a material adverse effect on our business.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted, redefine prior art, may affect patent litigation and switch the U.S. patent system from a “first-to-invent” system to a “first-to-file” system. The USPTO recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first-to-file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. The Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
24
Table of Contents
Any loss of patent protection could have a material adverse impact on our business. We may be unable to prevent competitors from entering the market with a product that is similar to or the same as our transdermal products.
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents or misappropriate or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, litigation may be necessary to enforce or defend our intellectual property rights, to protect our trade secrets and/or to determine the validity and scope of our own intellectual property rights or the proprietary rights of others. Such litigation can be expensive and time consuming, and any such claims could provoke defendants to assert counterclaims against us, including claims alleging that we infringe their patents or other intellectual property rights. Many of our current and potential competitors have the ability to dedicate substantially greater resources to litigate intellectual property rights than we can.
Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating our intellectual property. Litigation could result in substantial costs and diversion of management attention and resources, which could harm our business and financial results. In addition, in an infringement proceeding, a court may decide that a patent owned by or licensed to us is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Third-party claims of intellectual property infringement or misappropriation may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on us not infringing the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the pharmaceutical industries, including patent infringement lawsuits, interferences, inter partes reexamination proceedings, and oppositions before the USPTO and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the fields in which we are developing and may develop our transdermal product candidates. As the pharmaceutical industries expand and more patents are issued, the risk increases that our transdermal product candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we, our customers, licensees or parties indemnified by us are employing their proprietary technology without authorization or have infringed upon, misappropriated or otherwise violated their intellectual property or other rights. For example, we may be subject to claims that we are infringing the patent, trademark or copyright rights of third parties, or that our employees have misappropriated or divulged their former employers’ trade secrets or confidential information. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our transdermal product candidates, that we failed to identify. For example, applications filed before November 29, 2000 and certain applications filed after that date that will not be filed outside the United States remain confidential until issued as patents. Except for the preceding exceptions, patent applications in the United States and elsewhere are generally published only after a waiting period of approximately 18 months after the earliest filing, and sometimes not at all. Therefore, patent applications covering our endel technology or our transdermal product candidates could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our platform technologies, our transdermal product candidates or the use or manufacture of our transdermal product candidates.
25
Table of Contents
If any third-party patents were held by a court of competent jurisdiction to cover aspects of our product candidates, including the materials, formulations, methods of manufacture, methods of analysis, and/or methods for treatment, the holders of any such patents would be able to block our ability to develop and commercialize the applicable product candidate until such patent expired or unless we obtain a license. Such licenses may not be available on acceptable terms, if at all. Even if we were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our transdermal product candidates. Defending against claims of patent infringement or misappropriation of trade secrets could be costly and time consuming, regardless of the outcome. Thus, even if we were to ultimately prevail, or to settle at an early stage, such litigation could burden us with substantial unanticipated costs. In addition, litigation or threatened litigation could result in significant demands on the time and attention of our management team, distracting them from the pursuit of other company business. In the event of a successful claim of infringement against us, in addition to potential injunctive relief, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
We may face a claim of misappropriation if a third party believes that we inappropriately obtained and used trade secrets of such third party. If we are found to have misappropriated a third party’s trade secrets, we may be prevented from further using such trade secrets, limiting our ability to develop our transdermal product candidates, and we may be required to pay damages.
During the course of any patent or other intellectual property litigation, there could be public announcements of the results of hearings, rulings on motions, and other interim proceedings in the litigation. If securities analysts or investors regard these announcements as negative, the perceived value of our transdermal products, programs, or intellectual property could be diminished. Accordingly, the market price of our common stock may decline.
Confidentiality agreements with employees and third parties may not prevent unauthorized disclosure of trade secrets and other proprietary information.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our platform technology and discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, and outside scientific advisors, contractors and collaborators. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, or outside scientific advisors might intentionally or inadvertently disclose our trade secret information to competitors. In addition, competitors may otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques.
Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States sometimes are less willing than U.S. courts to protect trade secrets. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business.
26
Table of Contents
Risks related to our business and industry
If we fail to attract and keep senior management and key scientific personnel, we may be unable to successfully develop our transdermal products, conduct our clinical trials and commercialize our transdermal product candidates.
We are highly dependent on members of our senior management, including Patrick T. Mooney, M.D., our President and Chief Executive Officer and David Staskin, M.D., our Chief Strategy Officer and Secretary. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives. Also, each of these persons may terminate their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees.
Recruiting and retaining qualified scientific, clinical, manufacturing, sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors, including our scientific co-founders, may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
We may encounter difficulties in managing our growth and expanding our operations successfully.
As we seek to advance our transdermal product candidates through clinical trials and commercialization, we will need to expand our development, regulatory, manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for us. As our operations expand, we expect that we will need to manage additional relationships with various strategic partners, suppliers and other third parties. Future growth will impose significant added responsibilities on members of management. Our future financial performance and our ability to commercialize our transdermal product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train and integrate additional management, administrative and, if necessary, sales and marketing personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to accomplish these tasks, and our failure to accomplish any of them could prevent us from successfully growing our company or disrupt our operations.
27
Table of Contents
Our relationships with health care professionals, institutional providers, principal investigators, consultants, customers (actual and potential) and third-party payors are, and will continue to be, subject, directly and indirectly, to federal and state health care fraud and abuse, false claims, marketing expenditure tracking and disclosure, government price reporting, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face penalties, including, without limitation, civil, criminal, and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal health care programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations.
Our business operations and activities may be directly or indirectly subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act. If we obtain FDA approval for any of our transdermal product candidates and begin commercializing those products in the United States, our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. These laws may effect, among other things, our current activities with principal investigators and research subjects, as well as proposed and future sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by the federal government and state governments in which we conduct our business. The laws that may affect our ability to operate include, but are not limited to:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal health care program, such as the Medicare and Medicaid programs; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid, or other third-party payors that are false or fraudulent or knowingly making a false statement to improperly avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any health care benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any health care benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing, or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, health care benefits, items or services relating to health care matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 and their respective implementing regulations, which impose requirements on certain covered health care providers, health plans, and health care clearinghouses as well as their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization; |
| • | the federal physician self-referral law, commonly known as the Stark Law, which prohibits a physician from making a referral to an entity for certain designated health services reimbursed by Medicare or Medicaid if the physician or a member of the physician’s family has a financial relationship with the entity, and which also prohibits the submission of any claims for reimbursement for designated health services furnished pursuant to a prohibited referral; |
| • | the federal Physician Payments Sunshine Act, created under Section 6002 of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, |
28
Table of Contents
| collectively, or the ACA, and its implementing regulations requires manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the United States Department of Health and Human Services information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members, with data collection required beginning August 1, 2013 and reporting to the Centers for Medicare & Medicaid Services required by March 31, 2014 and by the 90th day of each subsequent calendar year; |
| • | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; |
| • | federal government price reporting laws, changed by the ACA to, among other things, increase the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program and offer such rebates to additional populations, that require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on our marketed drugs (participation in these programs and compliance with the applicable requirements may subject us to potentially significant discounts on our products, increased infrastructure costs, and potentially limit our ability to offer certain marketplace discounts); |
| • | the Foreign Corrupt Practices Act, a United States law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals); and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback, false claims, consumer protection and unfair competition laws which may apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements as well as submitting claims involving health care items or services reimbursed by any third-party payor, including commercial insurers; state laws that require biotech companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government that otherwise restricts payments that may be made to health care providers; state laws that require drug manufacturers to file reports with states regarding marketing information, such as the tracking and reporting of gifts, compensation and other remuneration and items of value provided to health care professionals and entities (compliance with such requirements may require investment in infrastructure to ensure that tracking is performed properly, and some of these laws result in the public disclosure of various types of payments and relationships, which could potentially have a negative effect on our business and/or increase enforcement scrutiny of our activities); and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways, with differing effects. |
In addition, the regulatory approval and commercialization of any of our product candidates outside the United States will also likely subject us to foreign equivalents of the health care laws mentioned above, among other foreign laws.
The ACA, among other things, amended the intent standard of the federal Anti-Kickback Statute and criminal health care fraud statutes to a stricter standard such that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act.
Efforts to ensure that our business arrangements will comply with applicable health care laws may involve substantial costs. It is possible that governmental and enforcement authorities will conclude that our business
29
Table of Contents
practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other health care laws and regulations. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, civil, criminal, and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal health care programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations, to provide accurate information to the FDA, to comply with manufacturing standards we have established, to comply with federal and state health care fraud and abuse laws and regulations, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the health care industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal health care programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our transdermal products.
We face an inherent risk of product liability as a result of the clinical testing of our transdermal product candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our transdermal product candidates. Even a successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| • | injury to our reputation; |
| • | decreased demand for our product candidates or products that we may develop; |
| • | withdrawal of clinical trial participants; |
| • | costs to defend the related litigations; |
| • | a diversion of management’s time and our resources; |
| • | substantial monetary awards to trial participants or patients; |
30
Table of Contents
| • | product recalls, withdrawals, or labeling, marketing or promotional restrictions; |
| • | loss of revenue; |
| • | the inability to commercialize our transdermal product candidates; and |
| • | a decline in our stock price. |
Failure to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. Although we plan to obtain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. If we are unable to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims, it could prevent or inhibit the development and commercial production and sale of our product candidates, which could adversely affect our business, financial condition, results of operations and prospects.
We and our third-party manufacturers must comply with environmental, health and safety laws and regulations, and failure to comply with these laws and regulations could expose us to significant costs or liabilities.
We and our third-party manufacturers are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the use, generation, manufacture, distribution, storage, handling, treatment, remediation and disposal of hazardous materials and wastes. Hazardous chemicals, including flammable and biological materials, are involved in certain aspects of our business, and we cannot eliminate the risk of injury or contamination from the use, generation, manufacture, distribution, storage, handling, treatment or disposal of hazardous materials and wastes. In the event of contamination or injury, or failure to comply with environmental, health and safety laws and regulations, we could be held liable for any resulting damages and any such liability could exceed our assets and resources. We could also incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations. We are uninsured for third-party injury from contamination.
Environmental, health and safety laws and regulations are becoming increasingly more stringent. We may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Further, with respect to the operations of our third-party manufacturers, it is possible that if they fail to operate in compliance with applicable environmental, health and safety laws and regulations or properly dispose of wastes associated with our products, we could be held liable for any resulting damages, suffer reputational harm or experience a disruption in the manufacture and supply of our product candidates or products.
31
Table of Contents
Risks related to our common stock and this offering
We are eligible to be treated as an “emerging growth company,” as defined in the JOBS Act, and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an emerging growth company, as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, (2) reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements and (3) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. In addition, as an emerging growth company, we are only required to provide two years of audited financial statements and two years of selected financial data in this prospectus.
We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700.0 million before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in non-convertible debt during any three-year period before that time, we would cease to be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” which would allow us to take advantage of many of the same exemptions from disclosure requirements, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We do not know whether a market will develop for our common stock or what the market price of our common stock will be and, as a result, it may be difficult for you to sell your shares of our common stock.
Before this offering, there was no public trading market for our common stock. If a market for our common stock does not develop or is not sustained, it may be difficult for you to sell your shares of common stock at an attractive price, or at all. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic partnerships or acquire companies or products by using our shares of common stock as consideration. We cannot predict the prices at which our common stock will trade. It is possible that in one or more future periods our results of operations may be below the expectations of public market analysts and investors and, as a result of these and other factors, the price of our common stock may fall.
32
Table of Contents
The market price of our common stock may be highly volatile, and you may not be able to resell your shares at or above the initial public offering price.
The initial public offering price for our shares will be determined by negotiations between us and the representatives of the underwriters and may not be indicative of prices that will prevail in the trading market. The market price of shares of our common stock could be subject to wide fluctuations in response to many risk factors listed in this “Risk factors” section, and others beyond our control, including:
| • | results and timing of clinical trials of our transdermal product candidates, including AX-IBU-01 and AX-DN-01; |
| • | results of clinical trials of our competitors’ products; |
| • | failure to adequately protect our trade secrets; |
| • | our inability to raise additional capital and the terms on which we raise it; |
| • | commencement or termination of any licensing arrangement, including but not limited to our licensing arrangements with BioChemics; |
| • | regulatory actions with respect to our products or our competitors’ products; |
| • | actual or anticipated fluctuations in our financial condition and operating results; |
| • | publication of research reports by securities analysts about us or our competitors or our industry; |
| • | our failure or the failure of our competitors to meet analysts’ projections or guidance that we or our competitors may give to the market; |
| • | additions and departures of key personnel; |
| • | strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy; |
| • | the passage of legislation or other regulatory developments affecting us or our industry; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • | sales of our common stock by us, our insiders or our other stockholders; |
| • | speculation in the press or investment community; |
| • | announcement or expectation of additional financing efforts; |
| • | changes in accounting principles; |
| • | terrorist acts, acts of war or periods of widespread civil unrest; |
| • | natural disasters and other calamities; |
| • | changes in market conditions for biopharmaceutical stocks; and |
| • | changes in general market and economic conditions. |
In addition, the stock market has frequently experienced significant volatility with respect to pharmaceutical, biotechnology and other life sciences company stocks. This volatility often does not relate to the operating performance of the companies represented by the stock. As a result of this volatility, you may not be able to sell your common stock at or above the initial public offering price. As we operate in a single industry, we are especially vulnerable to these factors to the extent that they affect our industry or our products, or to a lesser extent our markets. In the past, securities class action litigation has often been initiated against companies following periods of volatility in their stock price. This type of litigation could result in substantial costs and divert our management’s attention and resources, and could also require us to make substantial payments to satisfy judgments or to settle litigation.
33
Table of Contents
Our principal stockholder owns substantially all of our stock and, after this offering, will be able to exercise significant influence over matters subject to stockholder approval.
As of November , 2015, our principal stockholder, the Trust, owned 100% of our common stock, and we expect that upon completion of this offering, it will continue to beneficially hold at least a majority of our outstanding common stock. Accordingly, even after this offering, the Trust will be able to exert a significant degree of influence over our management and affairs and over matters requiring stockholder approval, including the election of our board of directors and approval of significant corporate transactions. This concentration of ownership could have the effect of entrenching our management and/or board of directors, delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material and adverse effect on the fair market value of our common stock.
A significant portion of our total outstanding shares may be sold into the public market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time after the expiration of the lock-up agreements described in the “Underwriting” section of this prospectus. These sales, or the market perception that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have shares of common stock outstanding. This includes the shares that we are selling in this offering, which may be resold in the public market immediately. The remaining shares will be able to be sold 180 days after the date of this prospectus, due to lock-up agreements between the holders of these shares and the underwriters. However, Alexander Capital, L.P., on behalf of the underwriters, can waive the provisions of these lock-up agreements by prior written consent and allow these stockholders to sell their shares at any time. In addition, the Trust, our largest stockholder, may decide to sell a significant number of its shares in Inpellis to make disgorgement and penalty payments for the benefit of certain stockholders of BioChemics pursuant to the BioChemics SEC Enforcement Action. If it sells such shares in the public market it is likely that such sale, or sales, would reduce the price of our common stock.
In addition, as of November , 2015, there were over 5,800,000 shares subject to outstanding warrants that will become eligible for sale in the public market, subject to the lock-up agreements and Rule 144 under the Securities Act. We also intend to register all shares of common stock that we may issue under our 2015 Equity Plan. Once we register these shares and they are issued in accordance with the terms of the plans, they can be freely sold in the public market upon issuance, subject to the lock-up agreements and the restrictions imposed on our affiliates under Rule 144. For more information, see “Shares eligible for future sale—Rule 144.”
You will incur immediate and substantial dilution as a result of this offering.
If you purchase common stock in this offering, assuming a public offering price of $ , the midpoint of the range set forth on the cover of this prospectus, you will incur immediate and substantial dilution of $ per share, representing the difference between the assumed initial public offering price of $ per share and our pro forma net tangible book value per share after giving effect to this offering. Moreover, we issued warrants in the past to acquire common stock at prices significantly below the assumed initial public offering price. As of November , 2015, there were over 5,800,000 shares subject to outstanding warrants. To the extent that these outstanding options are ultimately exercised, you will incur further dilution. As a result of the dilution to investors purchasing shares in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation. For more information on the dilution you may suffer as a result of investing in this offering, see “Dilution.”
34
Table of Contents
We have broad discretion in the use of net proceeds from this offering and may not use them effectively.
We currently intend to use the net proceeds from this offering to fund the continued development of AX-IBU-01 and AX-DN-01, including our contemplated pivotal Phase 2B/3 clinical trial of AX-IBU-01, to manufacture clinical supplies of AX-IBU-01 and AX-DN-01 and to fund other early stage pipeline development programs, as described in “Use of proceeds.” Any remaining amounts will be used for working capital and general corporate purposes, including funding the costs of operating as a public company, general and administrative expenses, capital expenditures and prosecution and maintenance of our intellectual property. Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of net proceeds will vary depending on numerous factors, including our ability to obtain additional financing, the relative success and cost of our research, preclinical and clinical development programs. As a result, management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds of this offering. In addition, we might decide to postpone or not pursue other clinical trials or preclinical activities if the net proceeds from this offering and the other sources of cash are less than expected.
We will incur increased costs as a result of being a public company, and our management will be required to devote substantial time to public company compliance programs.
As a public company, we will incur significant additional legal, insurance, accounting and other expenses that we did not incur as a private company. In addition, our administrative staff will be required to perform additional tasks. For example, in anticipation of becoming a public company, we will need to adopt additional internal controls and disclosure controls and procedures, retain a transfer agent, adopt an insider trading policy and bear all of the internal and external costs of preparing and distributing periodic public reports in compliance with our obligations under the securities laws. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment will result in increased general and administrative expenses and may divert management’s time and attention from product development activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed. In connection with this offering, we are increasing our directors’ and officers’ insurance coverage, which will increase our insurance cost. In the future, it will be more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
In addition, in order to comply with the requirements of being a public company, we may need to undertake various actions, including implementing new internal controls and procedures and hiring new accounting or internal audit staff. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file with the SEC is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that information required to be disclosed in reports under the Securities Exchange Act of 1934 as amended, or the Exchange Act, is accumulated and communicated to our principal executive and financial officers. Any failure to develop or maintain effective controls could adversely affect the results of periodic management evaluations. In the event that we are not able to demonstrate compliance with the Sarbanes-Oxley Act, that our internal control over financial reporting is perceived as inadequate, or that we are unable to produce timely or accurate financial statements, investors may lose confidence in our operating results and the price of our ordinary shares could decline. In addition, if we are unable to continue to meet these requirements, we may not be able to remain listed on the NASDAQ Global Market.
35
Table of Contents
We are not currently required to comply with the SEC’s rules that implement Section 404 of the Sarbanes-Oxley Act, and are therefore not yet required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose. Upon becoming a public company, we will be required to comply with certain of these rules, which will require management to certify financial and other information in our quarterly and annual reports and provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our second annual report. This assessment will need to include the disclosure of any material weaknesses in our internal control over financial reporting identified by our management or our independent registered public accounting firm. We are just beginning the costly and challenging process of implementing the system and processing documentation needed to comply with such requirements. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion.
Our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting until the later of our second annual report or the first annual report required to be filed with the SEC following the date we are no longer an “emerging growth company” as defined in the JOBS Act. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal controls in the future.
We do not expect to pay any cash dividends for the foreseeable future.
You should not rely on an investment in our common stock to provide dividend income. We do not anticipate that we will pay any cash dividends to holders of our common stock in the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our operations. In addition, any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any return on their investment. As a result, investors seeking cash dividends should not purchase our common stock.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend, in part, on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on our company. If no securities or industry analysts commence coverage of our company, the trading price for our stock would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. In addition, if our operating results fail to meet the forecast of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
The NASDAQ Global Market may not list our securities, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
We anticipate that our securities will be listed on The NASDAQ Global Market, a national securities exchange, upon consummation of this offering. Although, after giving effect to this offering, we expect to meet, on a pro forma, as adjusted basis, NASDAQ’s minimum initial listing standards, which generally mandate that we meet certain requirements relating to stockholders’ equity, market capitalization, aggregate market value of publicly held shares and distribution requirements, we cannot assure you that we will be able to meet those initial listing requirements. If The NASDAQ Global Market does not list our securities for trading on its exchange, we could face significant material adverse consequences, including:
| • | a limited availability of market quotations for our securities; |
| • | reduced liquidity with respect to our securities; |
36
Table of Contents
| • | a determination that our shares of common stock are “penny stock” which will require brokers trading in our shares of common stock to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for our shares of common stock; |
| • | a limited amount of news and analyst coverage for our company; and |
| • | a decreased ability to issue additional securities or obtain additional financing in the future. |
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Assuming our common stock will be listed on The NASDAQ Global Market, our common stock will be covered securities. Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. Further, if we were no longer listed on The NASDAQ Global Market, our common stock would not be covered securities and we would be subject to regulation in each state in which we offer our securities.
Our failure to meet the continued listing requirements of NASDAQ Global Market could result in a de-listing of our common stock.
If after listing we fail to satisfy the continued listing requirements of NASDAQ Global Market, such as the corporate governance requirements or the minimum closing bid price requirement, NASDAQ may take steps to de-list our common stock. Such a de-listing would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a de-listing, we would take actions to restore our compliance with NASDAQ’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the NASDAQ minimum bid price requirement or prevent future non-compliance with NASDAQ’s listing requirements.
If our shares become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not obtain or retain a listing on The NASDAQ Global Market and if the price of our common stock is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares.
Provisions in our corporate charter documents and under Delaware law could make an acquisition of our company, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and our amended and restated bylaws, which will become effective upon the closing of this offering, may discourage, delay or prevent a merger,
37
Table of Contents
acquisition or other change in control of our company that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions provide that:
| • | the authorized number of directors can be changed only by resolution of our board of directors; |
| • | our bylaws may be amended or repealed by our board of directors or our stockholders; |
| • | stockholders may not call special meetings of the stockholders or fill vacancies on the board of directors; |
| • | our board of directors is authorized to issue, without stockholder approval, preferred stock, the rights of which will be determined at the discretion of the board of directors and that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that our board of directors does not approve; |
| • | our stockholders do not have cumulative voting rights, and therefore our stockholders holding a majority of the shares of common stock outstanding will be able to elect all of our directors; and |
| • | our stockholders must comply with advance notice provisions to bring business before or nominate directors for election at a stockholder meeting. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful stockholder claims against us and may reduce the amount of money available to us.
As permitted by Section 102(b)(7) of the DGCL, our restated certificate of incorporation to be in effect upon the completion of this offering will limit the liability of our directors to the fullest extent permitted by law. In addition, as permitted by Section 145 of the DGCL, our restated certificate of incorporation and restated bylaws to be in effect upon the completion of this offering will provide that we shall indemnify, to the fullest extent authorized by the DGCL, each person who is involved in any litigation or other proceeding because such person is or was a director or officer of our company or is or was serving as an officer or director of another entity at our request, against all expense, loss or liability reasonably incurred or suffered in connection therewith. Our restated certificate of incorporation to be in effect upon the completion of this offering will provide that the right to indemnification includes the right to be paid expenses incurred in defending any proceeding in advance of its final disposition, provided, however, that such advance payment will only be made upon delivery to us of an undertaking, by or on behalf of the director or officer, to repay all amounts so advanced if it is ultimately determined that such director is not entitled to indemnification. If we do not pay a proper claim for indemnification in full within 60 days after we receive a written claim for such indemnification, except in the case of a claim for an advancement of expenses, in which case such period is 20 days, our restated certificate of incorporation and our restated bylaws authorize the claimant to bring an action against us and prescribe what constitutes a defense to such action.
Section 145 of the DGCL permits a corporation to indemnify any director or officer of the corporation against expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with any action, suit or proceeding brought by reason of the fact that such
38
Table of Contents
person is or was a director or officer of the corporation, if such person acted in good faith and in a manner that he reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, if he or she had no reason to believe his or her conduct was unlawful. In a derivative action, (i.e., one brought by or on behalf of the corporation), indemnification may be provided only for expenses actually and reasonably incurred by any director or officer in connection with the defense or settlement of such an action or suit if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, except that no indemnification shall be provided if such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the court in which the action or suit was brought shall determine that the defendant is fairly and reasonably entitled to indemnity for such expenses despite such adjudication of liability despite such adjudication of liability.
The rights conferred in the restated certificate of incorporation and the restated bylaws are not exclusive, and we are authorized to enter into indemnification agreements with our directors, officers, employees and agents and to obtain insurance to indemnify such persons. We have entered into or plan to enter into indemnification agreements with each of our officers and directors, the form of which is attached as an exhibit to the registration statement of which this prospectus is a part.
The above limitations on liability and our indemnification obligations limit the personal liability of our directors and officers for monetary damages for breach of their fiduciary duty as directors by shifting the burden of such losses and expenses to us. Although we plan to increase the coverage under our directors’ and officers’ liability insurance, certain liabilities or expenses covered by our indemnification obligations may not be covered by such insurance or the coverage limitation amounts may be exceeded. As a result, we may need to use a significant amount of our funds to satisfy our indemnification obligations, which could severely harm our business and financial condition and limit the funds available to stockholders who may choose to bring a claim against our company.
39
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our clinical results and other future conditions. The words “anticipate,” “believe,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
The forward-looking statements in this prospectus include, among other things, statements about:
| • | the timing of results of our contemplated clinical trials; |
| • | our plan is to continue development and gain regulatory approval for and commercialization of AX-IBU-01 and other products under development, including further development of AX-DN-01; |
| • | the potential benefits of strategic partnership agreements and our ability to enter into selective strategic partnership arrangements; |
| • | the rate and degree of market acceptance and clinical utility of any approved drug candidate; |
| • | our ability to quickly and efficiently develop drug candidates; |
| • | our commercialization, marketing and manufacturing capabilities and strategy; |
| • | our intellectual property position, including with respect to our trade secrets; and |
| • | our estimates regarding expenses, future revenues, capital requirements, the sufficiency of our current and expected cash resources and our need for additional financing. |
We may not achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this prospectus, particularly in the “Risk factors” section, that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
The forward-looking statements in this prospectus represent our views as of the date of this prospectus. We anticipate that subsequent events and developments will cause our views to change. However, although we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this prospectus.
40
Table of Contents
Assuming we complete the maximum offering, we estimate that our net proceeds from the sale of shares of common stock in this offering will be approximately $ million, after deducting underwriting commissions and estimated offering expenses payable by us and assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus. A $1.00 increase (decrease) in the assumed initial public offering price per share of $ , the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting commissions and estimated offering expenses payable by us.
The principal purposes of this offering are to obtain additional capital to support our operations, to create a public market for our common stock and to facilitate our future access to the public equity markets. We intend to use the net proceeds from this offering as follows:
| • | approximately $ million to fund the continued development of AX-IBU-01, including our contemplated pivotal Phase 2B/3 clinical trial; |
| • | approximately $ million to manufacture clinical supplies of AX-IBU-01; |
| • | approximately $ million to fund other pipeline development programs; and |
| • | the remainder for working capital and other general corporate purposes, including funding the costs of operating as a public company. |
The amount and timing of are actual expenditures will depend upon numerous factors, including the status and results of our AX-IBU-01 trials and research and development efforts. Furthermore, we anticipate that we will need to secure additional funding for the further development of AX-IBU-01 and for the development of our other product candidates.
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of net proceeds will vary depending on numerous factors, including our ability to obtain additional financing, the relative success and cost of our research, preclinical and clinical development programs. As a result, management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds of this offering. In addition, we might decide to postpone or not pursue other clinical trials or preclinical activities if the net proceeds from this offering and the other sources of cash are less than expected.
Pending their use, we plan to invest the net proceeds from this offering in a variety of capital preservation instruments, including short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
41
Table of Contents
We have never declared or paid cash dividends on our common stock. We currently intend to retain all available funds and any future earnings to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. In addition, future debt financing arrangements may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Any future determination to declare and pay dividends will be made at the discretion of our board of directors and will depend on then-existing conditions, including our results of operations, financial condition, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant. See “Risk Factors—Risks Relating to Our Common Stock and this Offering—We do not expect to pay any cash dividends for the foreseeable future.”
42
Table of Contents
The following table sets forth our cash and capitalization as of June 30, 2015:
| • | on an actual basis; |
| • | on a pro forma basis to give effect to (1) the amendment of our certificate of incorporation to increase our authorized shares of common stock to 100,000,000, (2) the issuance of 1,384,616 shares of Common Stock to the Company’s Chief Executive Officer, (3) the issuance of $6,250,000 in aggregate principal amount of original issue discount convertible notes in August and September 2015 and the receipt of net proceeds therefrom, (4) the conversion of all of the original issue discount convertible notes issued in August and September 2015 into an aggregate of shares of our common stock, which will occur upon the closing of this offering; (5) the payment of a cash bonus to the Company’s Chief Executive Officer in the amount of $ within 180 days of the closing of this offering and (6) the filing of our restated certificate of incorporation upon the closing of this offering; and |
| • | on a pro forma as adjusted basis to give effect to the sale of shares of our common stock offered in this offering at an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting commissions and estimated offering expenses payable by us. |
Our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of the offering determined at pricing. You should read this information together with our audited financial statements and related notes appearing elsewhere in this prospectus and the information set forth under the heading “Selected financial data” and “Management’s discussion and analysis of financial condition and results of operations.”
| As of June 30, 2015 | ||||||||||||
| (in thousands) | Actual | Pro Forma | Pro Forma as adjusted(1) |
|||||||||
| Cash |
$ | 4 | $ | $ | ||||||||
| Preferred stock, par value $[ ] per share; no shares authorized, issued or outstanding, actual; [ ] shares authorized and no shares issued or outstanding, pro forma and pro forma as adjusted |
||||||||||||
| Common stock, par value $0.001 per share, 50,000,000 shares authorized, issued and outstanding, actual; 100,000,000 shares authorized and 51,384,616 shares issued and outstanding, pro forma; and 100,000,000 shares authorized and [ ] shares issued and outstanding pro forma as adjusted |
50 | |||||||||||
| Additional paid-in capital |
(50 | ) | ||||||||||
| Accumulated deficit |
(10,982 | ) | ( | ) | ( | ) | ||||||
| Total stockholder’s deficiency |
(10,982 | ) | ||||||||||
| (1) | Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the range set forth on the cover page of this prospectus, would increase (decrease) each of pro forma as adjusted cash, additional paid-in capital and stockholders’ equity by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) each of pro forma as adjusted cash, additional paid-in capital and stockholders’ equity by approximately $ million, assuming the assumed initial public offering price per share, as set forth on the cover page of this prospectus, remains the same. |
43
Table of Contents
The number of shares in the table above does not include:
| • | shares of our common stock reserved for future issuance under our 2015 Equity Plan, which will become effective on the day prior to the public trading date of our common stock; |
| • | 2,000,000 shares of common stock issuable upon exercise of warrants outstanding as of June 30, 2015, at an exercise price of $5.00 per share; and |
| • | any shares of common stock that will underlie the Representative’s warrants. |
44
Table of Contents
If you invest in our common stock, your ownership interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock in this offering and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
As of June 30, 2015, we had a historical net tangible book value of $( ), or $( ) per share of common stock. Historical net tangible book value per share is equal to our total tangible assets, less total liabilities divided by the number of outstanding shares of our common stock.
As of June 30, 2015, the pro forma net tangible book value of our common stock was $ , or $ per share of common stock, taking into account the issuance of $6,250,000 in aggregate principal amount of original issue discount convertible notes in August and September 2015 and the receipt of net proceeds therefrom and the expected conversion of all of the original issue discount convertible notes issued in August and September 2015 into an aggregate of shares of our common stock, prior to the completion of this offering. After giving further effect to the sale of shares of common stock in this offering, after deducting estimated underwriting commissions and estimated offering expenses payable by us, at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, our pro forma as adjusted net tangible book value as of June 30, 2015 would have been approximately $ , or approximately $ per share of common stock. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to our existing stockholders and an immediate dilution of $ per share to investors participating in this offering.
The following table illustrates this per share dilution:
| Assumed initial public offering price per share |
$ | |||
| Historical net tangible book value per share as of June 30, 2015 |
||||
| Increase attributable to the issuance and sale reclassification of warrants to purchase common stock |
Pro forma net tangible book value per share after this offering:
Dilution per share to new investors-
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) our pro forma as adjusted net tangible book value by approximately $ million, the pro forma as adjusted net tangible book value per share by approximately $ per share and the dilution to investors purchasing shares in this offering by approximately $ per share, assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting the underwriting commissions and estimated offering expenses payable by us.
45
Table of Contents
The following table summarizes, on a pro forma as adjusted basis as of June 30, 2015, the differences between the number of shares of common stock purchased from us, the total consideration and the average price per share paid by existing stockholders (giving effect to the issuance of $6,250,000 in aggregate principal amount of original issue discount convertible notes in August and September 2015 and the conversion of all of the original issue discount convertible notes issued in August and September 2015 into an aggregate of shares of our common stock prior to the completion of this offering) and by investors participating in this offering, after deducting underwriting commissions and estimated offering expenses, at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus.
| Shared Purchased | Total Consideration | Average Price Per Share |
||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||
| Existing Stockholders |
% | $ | % | $ | ||||||||||||||
| New investors |
||||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
| Total |
100 | % | 100 | % | ||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
The number of shares of common stock to be outstanding after this offering is based on the number of shares outstanding as of June 30, 2015 and excludes the following:
| • | shares of our common stock reserved for future issuance under our 2015 Equity Plan, which will become effective on the day prior to the public trading date of our common stock; |
| • | 2,000,000 shares of common stock issuable upon exercise of outstanding warrants as of June 30, 2015 at an exercise price of $5.00 per share; and |
| • | any shares of common stock that will underlie warrants issued after June 30, 2015, including the Representative’s warrants. |
To the extent any of the outstanding warrants is exercised, there will be further dilution to new investors. If all of such outstanding warrants had been exercised as of June 30, 2015, the pro forma as adjusted net tangible book value per share after this offering would be $ , and total dilution per share to new investors would be $ .
Furthermore, due to market conditions or strategic considerations, we may choose to raise additional capital through the sale of equity or convertible debt securities, even if we believe we have sufficient funds for our current or future operating plans. New investors will experience further dilution if any of our outstanding options or warrants is exercised, new options are issued and exercised under our equity incentive plans, or we issue additional shares of common stock, other equity securities or convertible debt securities in the future. See “Risk Factors—You will incur immediate and substantial dilution as a result of this offering.”
46
Table of Contents
The information set forth below should be read in conjunction with the “Management’s discussion and analysis of financial condition and results of operations” section of this prospectus and with our financial statements and notes thereto included elsewhere in this prospectus.
The selected statements of operations data for the six months ended June 30, 2015 and 2014 and for the years ended December 31, 2014 and 2013 and the balance sheets as of December 31, 2014 and 2013 have been derived from our audited financial statements included elsewhere in this prospectus. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period.
| For the six months ended June 30, |
For the years ended December 31, |
|||||||||||||||
| (in thousands, except share and per share data) | 2015 | 2014 | 2014 | 2013 | ||||||||||||
| Statements of operations data: |
||||||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
44 | 221 | 294 | 620 | ||||||||||||
| Research and development—Patents |
16 | 59 | 80 | 76 | ||||||||||||
| Business legal and consulting |
207 | 210 | 623 | — | ||||||||||||
| General and administrative |
5,590 | 105 | 164 | 350 | ||||||||||||
| Consulting services |
— | 410 | 830 | 820 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
$ | 5,857 | $ | 1,005 | $ | 1,991 | $ | 1,866 | ||||||||
| Other expense |
||||||||||||||||
| Change in fair value of derivative liabilities |
(278 | ) | (93 | ) | 10 | — | ||||||||||
| Net Loss |
(5,579 | ) | (912 | ) | $ | (2,001 | ) | $ | (1,866 | ) | ||||||
| Net Loss per Share; Basic and Diluted |
(0.11 | ) | (0.02 | ) | $ | (0.04 | ) | $ | (0.04 | ) | ||||||
| Weighted-Average Number of Shares used Per Common Share Calculations: |
||||||||||||||||
| Basic and Diluted |
50,000,000 | 50,000,000 | 50,000,000 | 50,000,000 | ||||||||||||
| June 30, 2015 |
December 31, 2014 |
|||||||
| Cash |
$ | 4 | $ | — | ||||
| Total assets |
601 | 115 | ||||||
| Total liabilities |
11,583 | 5,518 | ||||||
| Common stock |
50 | 50 | ||||||
| Additional paid-in capital |
(50 | ) | (50 | ) | ||||
| Accumulated deficit |
(10,982 | ) | (5,403 | ) | ||||
| Total stockholder’s deficiency |
(10,982 | ) | (5,403 | ) | ||||
47
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with “Selected Financial Data” and our financial statements and the related notes appearing elsewhere in this prospectus. In additional to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included elsewhere in this prospectus. All amounts in this report are in U.S. dollars, unless otherwise noted.
Overview
Inpellis is a specialty pharmaceutical company developing transdermal product candidates for treating pain resulting from musculoskeletal disorders and peripheral neuropathy. Inpellis has acquired from BioChemics full or joint ownership of transdermal formulations which utilize the endel (vasoactive lipid encapsulated) transdermal drug delivery system for non-dermal pain, and to patents, patent applications, and know how related to the transdermal delivery system. Our goal is to apply this transdermal technology to provide rapid, safe and effective absorption of drugs for pain relief—through the skin—to the target tissues.
endel transdermal technology employs a transdermal delivery system that combines vasodilators, permeation enhancers, and active ingredients. Transdermal delivery has the potential to provide increased concentrations of the specified drug to target tissues, and avoid some of the acknowledged limitations of oral formulations including absorption, gastric issues, drug distribution, high serum concentrations and/or first-pass hepatic metabolism. endel technology also provides the potential to effectively transport molecules that were previously difficult to deliver transdermally. In addition, transdermal delivery, depending on the specific molecule, may result in increased safety, efficacy and/or convenience. The potential for mitigated development risk exists if the drugs that are selected for development are eligible for the 505(b)(2) regulatory-development pathway, translating into decreased regulatory costs and time to market.
The Company is dedicated to completing the development of two products in particular, as well as continuing work on a number of additional products in the pipeline. The two products are transdermal ibuprofen for moderate to severe osteoarthritis and transdermal benfotiamine for painful diabetic neuropathy. We are also conducting carefully focused continued research and development of other transdermal products for pain. We may develop products internally or may enter into co-development and co-commercialization of branded products, generic products, or new molecules in the therapeutic area of pain. A Phase 2 study utilizing transdermal ibuprofen for the treatment of pain associated with moderate to severe osteoarthritis was conducted in Switzerland in 2011. A human proof of concept study with respect to vasodilation for diabetic neuropathy was conducted in the United States in 2002 and an animal proof of delivery study for transdermal benfotiamine was conducted in the United States in 2008.
We have not yet filed an investigational new drug, or IND, application for either product in the United States or elsewhere. Based on the work that we have done with respect to our ibuprofen product candidate, we intend to perform additional studies regarding the formulation of that product in support of an IND application. Once we have done that work, we intend to submit an IND application for approval of either a Phase 2B or Phase 2B/3 study.
The transdermal delivery system that is utilized for delivering transdermal ibuprofen for osteoarthritis pain and transdermal benfotiamine for painful diabetic neuropathy, as well as related formulations and methods, are covered by an International patent application. The application covers the system of transdermal drug delivery that combines vasodilators, chelators, penetration enhancers, and active ingredients.
48
Table of Contents
Financial operations overview and analysis for the six months ended June 30, 2015 and June 30, 2014
Revenue. We did not recognize product sales for the six months ended June 30, 2015 and June 30, 2014. Our ability to generate product revenues in the future will depend almost entirely on our ability to successfully develop, obtain regulatory approval for and then successfully commercialize AX-IBU-01 in the United States. In the event we choose to pursue a partnering arrangement to commercialize AX-IBU-01 or other products outside the United States, we would expect to initiate additional research and development and clinical trial activities in the future.
Cost of revenue. We did not recognize cost of product sales for the six months ended June 30, 2015 or June 30, 2014.
Research and development expense. Research and Development expense was approximately $61,000 for the six months ended June 30, 2015 and approximately $280,000 for the six months ended June 30, 2014. Research and development expense consists of costs related to the research and development of AX-IBU-01 including the cost of filing and maintaining the patents we are utilizing currently, or anticipate utilizing in the future relating to our pipeline products; the development and testing of new drug formulations; preclinical studies; consulting fees; personnel related costs, including salaries, and benefits; and in the preparations for clinical trials which are designed to obtain FDA drug approvals for AX-IBU-01. Research and development expense is charged as incurred. Until June 30, 2015, research and development expense was incurred by our then parent corporation and allocated to the Company.
We expect that research and development expense will continue to increase substantially in future years as we seek pivotal trial enrollment and pursue regulatory approvals for AX-IBU-01. Based on these anticipated timelines and the resources we have allocated, we expect the total operating expense to bring AX-IBU-01 through our goal of FDA approval will be approximately $10 million. In addition, we expect to expand the scope of our new product development, which may also result in substantial increases in research and development expense.
Business, legal and consulting expense. Business, legal and consulting expense was approximately $207,000 for the six months ended June 30, 2015 and approximately $210,000 for the six months ended June 30, 2014. Business, legal and consulting expense in 2014 was all attributable to consulting services provided by consultants in the areas of management, finance and business development. In 2015 these expenses continued and the Company began incurring significant legal expenses in connection with the preparation for its initial public offering.
We expect that business, legal and consulting expense will decrease in the future as we increase our personnel and therefore become less reliant on consultants.
General and administrative expense. General and administrative expense was $5,590,000 for the six months ended June 30, 2015 and $105,000 for the six months ended June 30, 2014. General and administrative expense consists of personnel related costs, including salaries, bonuses and the agreement to grant to our Chief Executive Officer 1,384,616 shares of Common Stock; and development expenses associated with AX-IBU-01 marketing preparations; costs related to administrative personnel and senior management; costs related to the completion of the license agreement with BioChemics; and costs associated with our plans and preparations for a future potential capital raise. These expenses also include the costs of conducting market research, attending and/or participating in industry conferences and seminars, business development activities, and other general business and outside consulting activities. General and administrative expense also includes travel costs, for employees and third-party consultants, legal and accounting fees and other professional and administrative costs.
We expect that general and administrative expense will increase in the future as we increase our personnel and expand our infrastructure to support the requirements of being a public company.
49
Table of Contents
Consulting services expense. Consulting services expense was $0 for the six months ended June 30, 2015 and $410,000 for the six months ended June 30, 2014. This expense is due exclusively to the value of warrants issued to a single consultant which vested in 2014 and 2015. See “Critical Accounting Policies—Stock Based Compensation” below.
Other expense. Other expense was $(278,000) for the six months ended June 30, 2015 and $(93,000) for the six months ended June 30, 2014. This negative expense reflects the change in value of warrants issued to a single consultant which vested in 2014 and 2013. See “Critical Accounting Policies—Stock Based Compensation” below.
Financial operations overview and analysis for the years ended December 31, 2014 and December 31, 2013
Revenue. We did not recognize product sales for the years ended December 31, 2014 and December 31, 2013. Our ability to generate product revenues in the future will depend almost entirely on our ability to successfully develop, obtain regulatory approval for and then successfully commercialize AX-IBU-01 in the United States. In the event we choose to pursue a partnering arrangement to commercialize AX-IBU-01 or other products outside the United States, we would expect to initiate additional research and development and clinical trial activities in the future.
Cost of revenue. We did not recognize cost of product sales for the years ended December 31, 2014 or December 31, 2013.
Research and development expense. Research and Development expense was approximately $375,000 for the year ended December 31, 2014 and approximately $696,000 for the year ended December 31, 2013. Research and development expense consists of costs related to the research and development of AX-IBU-01 including the cost of filing and maintaining the patents we are utilizing currently, or anticipate utilizing in the future relating to our pipeline products; the development and testing of new drug formulations; preclinical studies; consulting fees; personnel related costs, including salaries, and benefits; and in the preparations for clinical trials which are designed to obtain FDA drug approvals for AX-IBU-01. Research and development expense is charged as incurred. Until December 31, 2014, research and development expense has been incurred by our then parent corporation and allocated to the Company. In the future, that expense will be incurred directly by the Company.
We expect that research and development expense will continue to increase substantially in future years as we seek pivotal trial enrollment and pursue regulatory approvals for AX-IBU-01. Based on these anticipated timelines and the resources we have allocated, we expect the total operating expense to bring AX-IBU-01 through our goal of FDA approval will be approximately $10 million. In addition, we expect to expand the scope of our new product development, which may also result in substantial increases in research and development expense.
Business, legal and consulting expense. Business, legal and consulting expense was approximately $623,000 for the year ended December 31, 2014. Business, legal and consulting expense in 2014 was all attributable to consulting services provided by consultants in the areas of management, finance and business development. There was no comparable expense for the year ended December 31, 2013.
We expect that business, legal and consulting expense will decrease in the future as we increase our personnel and therefore become less reliant on consultants.
General and administrative expense. General and administrative expense was approximately $164,000 for the year ended December 31, 2014 and approximately $350,000 for the year ended December 31, 2013. General and administrative expense consists of personnel related costs, including salaries and bonuses; and development expenses associated with AX-IBU-01 marketing preparations; costs related to administrative personnel and senior management; costs related to the completion of the license agreement with BioChemics; and costs
50
Table of Contents
associated with our plans and preparations for a future potential capital raise. These expenses also include the costs of conducting market research, attending and/or participating in industry conferences and seminars, business development activities, and other general business and outside consulting activities. General and administrative expense also includes travel costs, for employees and third-party consultants, legal and accounting fees and other professional and administrative costs.
We expect that general and administrative expense will increase in the future as we increase our personnel and expand our infrastructure to support the requirements of being a public company.
Consulting services expense. Consulting services expense was $830,000 for the year ended December 31, 2014 and $820,000 for the year ended December 31, 2013. This expense is due exclusively to the value of warrants issued to a single consultant which vested in 2014 and 2013. See “Stock Based Compensation” below.
Other expense. Other expense was $10,000 for the year ended December 31, 2014, due exclusively to the change in value of warrants issued to a single consultant which vested in 2014 and 2013. See “Critical Accounting Policies—Stock Based Compensation” below. There was no comparable expense for the year ended December 31, 2013.
Liquidity and Going Concern
We reported a loss of approximately $5,579,000 for the six months ended June 30, 2015 and a loss of approximately $912,000 for the six months ended June 30, 2014. We reported a loss of approximately $2,001,000 for the year ended December 31, 2014 and a loss of approximately $1,866,000 for the year ended December 31, 2013. At June 30, 2015 and June 30, 2014, our accumulated deficit amounted to approximately $10,982,000 and $4,314,000 respectively. We had a working capital deficit of $11,546,000 as of June 30, 2015. We have not yet achieved profitability. These conditions raise substantial doubt about our ability to continue as a going concern. We expect that our research and development and general and administrative expenses will continue to increase and, as a result, we will need to generate significant product revenues to achieve profitability. We may never achieve profitability.
Sources of liquidity
Since our inception, most of our operations have been financed through approximately $3,518,000 in advances from our related party, BioChemics, as well as an additional $5,000,000 in cash proceeds from the Original Issue Discount Convertible Note Offering which we completed in September 2015. For the six months ended June 30, 2015, all of our operations were financed through increases in our accounts payable and accrued expenses. For the year ended December 31, 2014, all of our operations were financed through $797,000 from BioChemics. In August 2015 BioChemics agreed that the Company is not obligated to repay any of the advances made to the Company by BioChemics. BioChemics has no obligation to provide advances in the future.
Cash flows
Net cash used in operating activities was approximately $52,000 for the six months ended June 30, 2015, and approximately $(697,000) and $(895,000) for the years ended December 31, 2014 and 2013, respectively. The net cash used in these periods primarily reflects net losses for these years. For each year, the net loss was offset in part by changes in operating assets and liabilities.
Net cash used in financing activities was approximately $56,000 for the six months ended June 30, 2015, and $697,000 and $895,000 for the years ended December 31, 2014 and 2013, respectively. The net cash provided by financing activities for the six months ended June 30, 2015 and for the years ended December 31, 2014 and 2013 was attributable to advances made by BioChemics.
51
Table of Contents
Contractual obligations
On September 17, 2015, the Company entered into a lease agreement, relating to the lease of approximately 3,513 square feet of space at a facility located in Beverly, Massachusetts. The lease commenced on October 15, 2015, with a term of five years. The monthly rental rate starts at approximately $5,400 in the first year with customary increases. In addition, the Company is required to pay its proportionate share of all increases in real estate taxes and real property surcharges and special assessments from the base year.
On September 18, 2015, the Company entered into a lease agreement, relating to the lease of approximately 1,509 square feet of space at a facility located in Haddonfield, New Jersey. The lease commenced on November 1, 2015, with an initial term of two years. The Company has two options to renew the lease for successive terms of two years under the same rent terms, conditions and provisions. The monthly rental rate starts at approximately $2,700 in the first year and escalates by 3.0% per year each year thereafter. In addition, the Company is required to pay its proportionate share of all increases in real estate taxes and fire and casualty and liability insurance from base year.
Operating capital and capital expenditure requirements
We expect to continue to incur substantial operating losses in the future and to make capital expenditures to support the expansion of our research and development programs, establishment of a research and development facility and to initiate commercial operations. We anticipate using a portion of the net proceeds from this offering to finance these activities. It may take several years to obtain the necessary regulatory approvals to commercialize AX-IBU-01 as a drug in the United States. There is no assurance that such approvals will be obtained.
Our future funding requirements will depend on many factors, including:
| • | the scope, rate of progress and cost of our clinical trials and other research and development activities; |
| • | future clinical trial results; |
| • | the terms and timing of any collaborative, licensing and other arrangements that we may establish; |
| • | the cost and timing of regulatory approvals; |
| • | the cost and delays in product development as a result of any changes in regulatory oversight applicable to our products; |
| • | the cost and timing of establishing sales, marketing and distribution capabilities; |
| • | the effect of competing technological and market developments; |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| • | the extent to which we acquire or invest in businesses, products and technologies. |
We may seek to raise additional funds through public or private equity offerings, debt financings, capital lease transactions, corporate collaborations or other means. We may seek to raise additional capital due to favorable market conditions or strategic considerations even if we have sufficient funds for planned operations. The sale of additional equity or convertible debt securities could result in dilution to our stockholders. To the extent that we raise additional funds through collaborative arrangements, it may be necessary to relinquish some rights to our technologies or grant licenses on terms that are not favorable to us. We do not know whether additional funding will be available on acceptable terms, or at all. A failure to secure additional funding when needed may require us to curtail certain operational activities, including regulatory trials, sales and marketing, and international operations and would have a material adverse effect on our future business and financial condition.
52
Table of Contents
Off-balance sheet arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements as defined under Securities and Exchange Commission rules.
Critical accounting policies
The preparation of our financial statements in conformity with accounting principles generally accepted in the United States requires management to exercise its judgment. We exercise considerable judgment with respect to establishing sound accounting policies and in making estimates and assumptions that affect the reported amounts of our assets and liabilities, our recognition of expenses, and disclosure of commitments and contingencies at the date of the financial statements.
On an ongoing basis, we evaluate our estimates and judgments. We base our estimates and judgments on a variety of factors including our historical experience, knowledge of our business and industry, current and expected economic conditions, the attributes of our products, the regulatory environment, and in certain cases, the results of outside appraisals. We periodically re-evaluate our estimates and assumptions with respect to these judgments and modify our approach when circumstances indicate that modifications are necessary.
While we believe that the factors we evaluate provide us with a meaningful basis for establishing and applying sound accounting policies, we cannot guarantee that the results will always be accurate. Since the determination of these estimates requires the exercise of judgment, actual results could differ from such estimates.
A description of significant accounting policies that require us to make estimates and assumptions in the preparation of our financial statements is as follows:
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent liabilities at the dates of the financial statements and the reported amounts of expenses during the reporting periods. Actual results could differ from these estimates. Significant estimates and assumptions include the recoverability of long-lived assets, expense allocations from the Company’s parent, valuation allowance related to the Company’s deferred tax assets, stock based compensation for warrants and derivative liabilities.
Stock based compensation
We account for share-based awards exchanged for employee services based on the estimated fair value of the award on the grant date. We estimate the fair value of employee awards using the Black-Scholes valuation model. We amortize the fair value of employee stock awards on a straight-line basis over the requisite service period of the awards. Compensation expense includes the impact of an estimate for forfeitures for all stock awards.
We account for equity instruments issued to non-employees based on the estimated fair value of the instrument on the measurement date. We estimate the fair value of these equity instruments using the Black-Scholes valuation model. The measurement of stock-based compensation is subject to periodic adjustment as the underlying equity instrument vests or becomes non-forfeitable. Non-employee stock-based compensation charges are amortized over the vesting period or as earned.
During the year ended December 31, 2013, the Company granted 4,000,000 warrants to purchase common stock to a consultant for services rendered. The warrants have a life of five years and expire on December 1, 2018. 1,000,000 shares vested immediately on December 1, 2013, the effective date of the services agreement,
53
Table of Contents
and 1,000,000 shares vest on each of the next three anniversaries of the services agreement; provided, however that in the event of a public offering of the Company’s securities, the warrants shall immediately vest in their entirety. In June 2015, the Company terminated the consulting agreement, at which point vesting of the warrants ceased. As of November , 2015, 2,000,000 warrants have vested. The warrants are exercisable at $5.00 per share. For the years ended December 31, 2014 and 2013 the Company recorded compensation expense for $830,000 and $820,000 respectively. For the three month periods ended June 30, 2015 and 2014 compensation expense was $0 and $410,000 respectively.
In addition, prior to an amendment entered into in June 2015, under the terms of the warrant agreement, in the event the Company entered into a qualified financing in which shares of stock are sold at a price less than the warrant exercise price, the warrant price will be adjusted accordingly. In accordance with the provisions of ASC 815-40, these warrants are subject to derivative accounting treatment and are recorded as a liability which is marked to market at each reporting date. Any change in fair value is recorded as a change in fair value of derivative liabilities in the accompanying statements of operations. The Company reassesses the classification at each balance sheet date, if the classification changes as a result of events during the period, the contract is reclassified as of the date of the event that caused the reclassification.
During the six months ended June 30, 2015, the Company committed to issuing 1,384,616 shares of common stock to the Chief Executive Officer under an employment agreement. The Company has recorded a stock based compensation charge of approximately $5.4 million which represents the fair value of those shares.
The Company’s common stock is not listed on any exchange and, accordingly, the Company used a valuation model to arrive at an estimated fair value of the Company’s equity instruments for the years ended December 31, 2014 and 2013. To assist us with the valuation of the equity instruments a third party valuation specialist was utilized to obtain the fair value of the Company’s equity instruments.
The Company and valuation specialist considered the following significant qualitative factors in preparing the fair value analyses:
| • | The nature and history of the entity’s business; |
| • | The general economic conditions and specific industry outlook; |
| • | The book value of the entity and its financial condition; |
| • | The future earnings capacity of the entity; |
| • | The entity’s distribution potential; |
| • | The existence of intangible value (patents) within the business; and |
| • | Capital resource requirements |
The Company is considered an operating entity expected to generate future cash flows. Accordingly, the Company and the valuation specialist primarily relied on the Income Approach in arriving at a value for the Company’s equity. Given the significant risk associated with the development process and clinical trials, there is a very low rate of success for companies in the biotechnology industry to successfully develop and market their treatments. To capture this low success rate, the probability-weighted forecasted cash flows based on assumptions developed by the Company as well as observed historical success rates at each stage of clinical trials were developed. The discount rates were determined from the weighted average cost of capital from discount rates appropriate for companies in the early development stage not yet conducting clinical trials. These rates were derived from analyzing similar guideline companies in the industry. The concluded value for the Company’s total equity was approximately $196.8 million and $150.9 million as of December 31, 2014 and 2013, respectively. In addition the Black-Scholes valuation model was used to allocate the Company’s total equity value among the Company’s outstanding equity instruments to determine the fair value of the common stock and warrants.
54
Table of Contents
The significant assumptions used in the valuation model were as follows:
| December 31, | ||||
| 2014 |
2013 | |||
| Fair market value of total equity |
$196.8 million | $150.9 million | ||
| Fair market value of common stock per share |
$3.90 | $2.98 | ||
| Fair market value of price per warrant |
$0.83 | $0.82 | ||
| Volatility |
65% | 75% | ||
| Discount rates |
18.5% | 19.5% | ||
| Risk—free rate |
0.44% | 0.76% | ||
| Term to liquidity |
1.92 years | 2.92 years | ||
A summary of changes in outstanding warrants during the years ended December 31, 2014 and 2013 is as follows:
| Number of Shares |
Weighted Average Exercise Price Per Share |
Weighted Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding at January 1, 2013 |
— | |||||||||||||||
| Granted |
4,000,000 | $ | 5.00 | 5.00 | — | |||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited |
— | — | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Outstanding at December 31, 2013 |
4,000,000 | $ | 5.00 | 4.92 | — | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Granted |
— | — | ||||||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited |
— | — | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Outstanding at December 31, 2014 |
4,000,000 | $ | 5.00 | 3.92 | — | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Granted |
— | — | ||||||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited |
2,000,000 | — | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Outstanding at June 30, 2015 (Unaudited) |
2,000,000 | $ | 5.00 | 3.67 | — | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Exercisable at June 30, 2015 (Unaudited) |
2,000,000 | $ | 5.00 | — | ||||||||||||
| Exercisable at December 31, 2014 |
2,000,000 | $ | 5.00 | — | ||||||||||||
| Exercisable at December 31, 2013 |
1,000,000 | $ | 5.00 | — | ||||||||||||
Income taxes
We are required to determine the aggregate amount of income tax expense or loss based upon tax statutes in jurisdictions in which we conduct business. In making these estimates, we adjust our results determined in accordance with generally accepted accounting principles for items that are treated differently by the applicable taxing authorities. Deferred tax assets and liabilities resulting from these differences are reflected on our balance sheet for temporary differences in loss and credit carry forwards that will reverse in subsequent years. We also establish a valuation allowance against deferred tax assets when it is more likely than not that some or all of the deferred tax assets will not be realized. Valuation allowances are based, in part, on predictions that management must make as to our results in future periods. The outcome of events could differ over time which would require that we make changes in our valuation allowance.
Research and development
Research and development expense is charged to operations as incurred and consists primarily of personnel expenses, clinical and regulatory services and supplies.
55
Table of Contents
Jobs Act
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act for complying with new or revised accounting standards. In other words, an emerging growth company” can delay complying with new or revised accounting standards until those standards would otherwise apply to private companies.
We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404 (b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission.
56
Table of Contents
Overview
Inpellis is a specialty pharmaceutical company developing transdermal product candidates for treating pain resulting from musculoskeletal disorders and peripheral neuropathy. Inpellis has acquired from BioChemics, Inc. (“BioChemics”) full or joint ownership of transdermal formulations which utilize the endel (vasoactive lipid encapsulated) transdermal drug delivery system for non-dermal pain, and to patents, patent applications, and know how related to the transdermal delivery system. Our goal is to apply this transdermal technology to provide rapid, safe and effective absorption of drugs for pain relief—through the skin—to the target tissues.
endel transdermal technology employs a transdermal delivery system that combines vasodilators, permeation enhancers, and active ingredients. Transdermal delivery has the potential to provide increased concentrations of the specified drug to target tissues, and avoid some of the acknowledged limitations of oral formulations including absorption, gastric issues, drug distribution, high serum concentrations and/or first-pass hepatic metabolism. endel technology also provides the potential to effectively transport molecules that were previously difficult to deliver transdermally. In addition, transdermal delivery, depending on the specific molecule, may result in increased safety, efficacy and/or convenience. The potential for mitigated development risk exists if the drugs that are selected for development are eligible for the 505(b)(2) regulatory-development pathway, translating into decreased regulatory costs and time to market. We believe the transdermal ibuprofen product we have in development, as described further below, may be eligible for the 505(b)(2) pathway, but until we formally seek to use 505(b)(2) we cannot be certain that it will be available to us.
The Company is dedicated to completing the development of two products in particular, as well as continuing work on a number of additional products in the pipeline. The two products are transdermal ibuprofen for moderate to severe osteoarthritis and transdermal benfotiamine for painful diabetic neuropathy. We are also conducting carefully focused continued research and development of other transdermal products for pain. We may develop products internally or may enter into co-development and co-commercialization of branded products, generic products, or new molecules in the therapeutic area of pain. A Phase 2 study utilizing transdermal ibuprofen for the treatment of pain associated with moderate to severe osteoarthritis was conducted in Switzerland in 2011. A human proof of concept study with respect to vasodilation for diabetic neuropathy was conducted in the United States in 2002 and an animal proof of delivery study for transdermal benfotiamine was conducted in the United States in 2008.
The transdermal delivery system that is utilized for delivering transdermal ibuprofen for osteoarthritis pain and transdermal benfotiamine for painful diabetic neuropathy, as well as related formulations and methods, are covered by an International patent application. The application covers the system of transdermal drug delivery that combines vasodilators, chelators, penetration enhancers, and active ingredients.
The Company is open to forming strategic alliances for co-development and co-commercialization of drugs which are delivered transdermally for the treatment of pain. Inpellis will use third parties for manufacturing and marketing. Accordingly, the Inpellis strategy for the research, development, marketing and commercialization of its products entails entering into various arrangements with corporate partners, licensors, licensees and others, Inpellis will be dependent upon the subsequent success of these outside parties in performing their responsibilities. Inpellis may also rely on collaborative partners to conduct research efforts and clinical trials, to obtain regulatory approvals and to manufacture and market certain Inpellis products. Through previous arrangements with manufacturing contractors, we have produced GMP-quality human doses of AX-IBU-01 for Phase 1 and Phase 2 clinical trials, and we believe that this experience will allow us, through manufacturing contractors with whom we have worked in the past, or with new such contractors, to produce GMP-quality AX-IBU-01 for our contemplated pivotal Phase 2B/3 clinical trial.
57
Table of Contents
Corporate History
Inpellis was formed as a wholly owned subsidiary of BioChemics in March 2012. BioChemics formed Inpellis as part of its overall strategy to commercialize its transdermal technology. Under that strategy, BioChemics planned to create subsidiaries to which it would license its technology for use with specified products which BioChemics believed would be good candidates for commercialization. In the case of Inpellis, the focus is on products for pain resulting from musculoskeletal disorders and peripheral neuropathy. In addition, the plan was to recruit an independent, well qualified management team for Inpellis to pursue commercialization. Until June 2014, the sole officer and director of Inpellis was John Masiz, the founder and Chief Executive Officer of BioChemics. Mr. Masiz was not compensated by Inpellis for his service in those capacities. Mr. Masiz resigned all of his positions with Inpellis in June 2014. The current management team now runs all aspects of the Inpellis business. Because of his deep expertise in transdermal technology, current management does consult with Mr. Masiz and expects to continue to do so. There is no formal consulting contract in place with Mr. Masiz and he is not being compensated by Inpellis for his consulting services.
BioChemics first implemented its strategy to license products to a subsidiary for commercialization in 2003 when it formed a corporation known as Vaso Active Pharmaceuticals, Inc., or Vaso Active. BioChemics licensed three over-the-counter products candidates to Vaso Active in 2001 for a combination of equity and future royalty payments. None of these products are part of the Inpellis portfolio. Furthermore, there is no relation between Vaso Active and Inpellis other than each company having, or having had, BioChemics as its parent company and a licensor. In December 2003, Vaso Active completed an initial public offering of shares of its stock. In April 2004, the SEC commenced an investigation into Vaso Active. The SEC brought a formal action against Vaso Active in August 2004 alleging that Vaso Active’s public statements regarding products having FDA approval were false, which was immediately settled with Vaso Active agreeing to neither admit nor deny wrongdoing and to an injunction against future securities violations. Vaso Active did not pay a fine in connection with that settlement. After the settlement, Vaso Active filed a claim against the law firm which represented it in its initial public offering and drafted the relevant statements. That claim was settled in 2009 for $2.5 million. In March 2010, Vaso Active filed a voluntary petition for relief under Chapter 11 of the United States Bankruptcy Code.
In February 2014, a settlement was reached between BioChemics and the SEC in the BioChemics SEC Enforcement Action. In January 2015, in light of the on-going proceedings regarding the settlement and BioChemics disgorgement and penalty obligations thereunder, BioChemics transferred all of its shares in Inpellis to the Shareholder Resolution Trust, a Massachusetts trust, which was established to resolve the claims arising from the settlement in the BioChemics SEC Enforcement Action. The Trust is now the majority stockholder of Inpellis. The Trust is managed by three Trustees, all of whom must approve any decision. The three Trustees are Jack L. Altshuler, Esq., a Massachusetts attorney, Jan R. Schlichtmann, Esq., also a Massachusetts attorney, a director of BioChemics and the Managing Member of a limited liability company which is the largest stockholder of BioChemics, and Daniel M. Glosband, a Massachusetts attorney. The Trust is independent of BioChemics because it cannot act unless all three of its trustees approve the action and, of the three trustees, two have no connection to BioChemics. In addition, the Trustees have agreed that the Trust shall not distribute any shares of Inpellis to any of the individuals who are defendants in the BioChemics SEC Enforcement Action or any entity controlled by any such individual.
The Trust was formed in a proceeding in the Essex County, Massachusetts, Probate Court. In September 2014, Mr. Schlichtmann, in his role as a director of BioChemics, petitioned the court for an order approving the establishment of the Trust and the court issued that order on September 8, 2014. The Trust is an irrevocable trust for the benefit of the persons and entities with an ownership interest in BioChemics in the form of shares, warrants, or debt convertible into an ownership interest. The Trust was formed as a Qualified Settlement Fund under Section 468B of the Internal Revenue Code for the purpose of resolving the actual or potential controversies or claims of the beneficiaries arising out of or relating to the transactions referred to in the SEC Enforcement Action. In its order approving the Trust, the court also appointed Mr. Schlichtmann as the court appointed trustee. Under the terms of the Trust, the court appointed trustee can appoint up to two additional
58
Table of Contents
trustees and Mr. Schlichtmann has done so, resulting in the Trust having the three trustees described above. With three trustees in place, all decisions to be made by the trustees must be unanimous. The court appointed trustee may remove either of the other trustees only with court assent for sufficient cause. We believe that this requirement of court assent to removal makes the Trust independent of BioChemics. The court may remove any of the trustees for sufficient cause. The Trust does not provide for the beneficiaries to vote on any matters except that it requires the Trustees to endeavor to ensure that any distribution to the beneficiaries be fair and equitable and in accordance with a plan in which the beneficiaries have had full and fair opportunity to consider and provide their assent.
In March 2015, the United States District Court for the District of Massachusetts entered judgment against BioChemics in the BioChemics SEC Enforcement Action pursuant to a consent settlement requiring BioChemics to comply with certain antifraud provisions of the federal securities law and requiring it to pay disgorgement and penalties approximating $18 million. Inpellis is not a party to this settlement. See “Risk Factors” for a discussion of risks that we may face as a result of the foregoing.
The Pain Market
The market for pain medications is growing. By 2017, the global pain management therapeutics market is forecast to generate sales of $35.1 billion, and is expected to grow substantially over the next decade due to population dynamics, an increase in the elderly, and co-morbidities associated with obesity and diabetes. The current market for prescription products for osteoarthritis is estimated at $8 billion, for oral NSAIDs, $3.6 billion, and for transdermal NSAIDs, $500 million. Neuropathic pain is estimated to be 16% of the pain market with sales of $3 billion.
Transdermal Ibuprofen for Moderate to Severe Osteoarthritis
The lead drug in development is AX-IBU-01—transdermally delivered ibuprofen for pain associated with moderate to severe osteoarthritis. In November 2013, Phase 2 results for AX-IBU-01 were published in the journal Pain Physician (Randomized Clinical Trial Evaluating Transdermal Ibuprofen for Moderate to Severe Knee Osteoarthritis, 2013; 16:E749-E762). The published results were from a 14-day Phase 2 independent contract research organization, or CRO, monitored multicenter, randomized, double-blind, placebo-controlled study in a European country with FDA reciprocity. The study included patients with primary osteoarthritis in a single knee joint with a progression level of moderate to severe based in part on a grade II or III designation according to the Kellgren and Lawrence classification system. Patients received the corresponding, randomly assigned study formulation (AX-IBU-01 or placebo) for application to the target knee at a dose of 2.0 grams of drug product (200 mg ibuprofen) twice daily for 14 days. The evaluation of the efficacy of the treatments utilized the widely accepted methods of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index and the Visual Analog Scale (VAS) scores for the patients. The results indicated that the AX-IBU-01 formulation was very well tolerated from a safety perspective during the two week trial. In particular, the WOMAC Total and WOMAC Physical Functioning for AX-IBU-01 were superior and statistically significant compared to the placebo, with the WOMAC Total P Value equal to 0.0283 and the WOMAC Physical Functioning P Value equal to 0.0201. Other clinical endpoints, including the WOMACPain, WOMACStiffness, and VASResting scores, were greater than those obtained from the placebo group (trending towards statistical significance). Based on the Patient and Physician Global Impression of Change survey, patient satisfaction slightly improved across both groups (no statistical significance was detectable as compared to the baseline).
In light of the results of the Phase 2 trial in Europe, we have concluded that we should proceed with a pivotal trial. We have not yet filed an investigational new drug, or IND, application for either product in the United States or elsewhere. Based on the work that we have done with respect to our ibuprofen product candidate, we intend to perform additional studies regarding the formulation of that product in support of an IND application. We will also have to demonstrate to the FDA that the safety and efficacy work which we have done support a pivotal Phase 2B/3 trial. We know from prior experience and from discussions with the FDA that they will raise significant questions about our work. We believe we can address all such concerns effectively, but
59
Table of Contents
there can be no assurance that we will be successful in doing so, or that we will be able to do so in the time period we have planned. Once we have done that work, we intend to submit an IND application for approval of either a Phase 2B or Phase 2B/3 study.
The pivotal trial is being structured. We currently anticipate (although it could change significantly) this pivotal trial would consist of 300 total patients and, assuming recruitment proceeds as anticipated, could be completed within 12 months of commencement. We already have a detailed proposal and budget from a CRO to conduct that trial with 300 subjects in 15 locations across the United States.
Painful Diabetic Neuropathy
In diabetic conditions, hyperglycemia induces an altered cellular metabolism and, through a series of events, results in the creation of damaging cellular metabolites by influencing a number of glucose metabolizing pathways. This results in diabetic peripheral neuropathy. To date, FDA approved and marketed products treat the pain component of diabetic neuropathy without directly addressing the underlying pathophysiology of altered glucose metabolism on the nervous system.
The Rationale for the Development of Transdermal Benfotiamine for Diabetic Neuropathy
Benfotiamine has been utilized for the treatment of painful diabetic neuropathy. Benfotiamine is currently offered as a prescriptive drug for the treatment of diabetic neuropathy in Europe in an oral form. The typical oral dose recommended is 40-450 mg/day. Benfotiamine is a pro-drug for thiamine, an active metabolite of which is accepted as the active drug in the target tissue. The topical application of benfotiamine is proposed in order to allow more prodrug to be introduced into the target tissues and, as a result, potentially generate a greater therapeutic effect. The limiting factor of utilizing oral thiamine is the rapid metabolism to inactive agents by the liver, the broad tissue distribution which dilutes the active agent, and decreased blood flow to the target tissues secondary to associated diabetic peripheral vascular disease. Once benfotiamine has been transported to the target tissue, it is converted through a series of metabolic and enzymatic steps to S-benzoylthiamine, which, after internalization into the cell, forms thiamine. This latter then serves as a substrate for enzymatic conversion to form thiamine mono-, di-, and triphosphates.
The application of benfotiamine and the resulting activation of the enzyme transketolase in the diabetic, hyperglycemic system can also produce beneficial effects on general nerve health. Benfotiamine has been shown in many preclinical as well as clinical studies to prevent the formation of advanced glycation end-products, or AGEs, and eventually with continued treatment to reverse the symptoms associated with the neuropathy.
Transdermal Benfotiamine Delivery—A Proof of Concept Study for Increasing Target Tissue Concentrations
Our transdermal system was utilized to serve as a delivery platform for benfotiamine utilizing the attributes of passive penetration enhancing molecules to penetrate through the outer layers of skin combined with the advance of incorporating various peripherally-acting vasodilators to enhance drug uptake. Benfotiamine, incorporated into this transdermal formulation, was applied to skin in an animal model in order to determine the ability to deliver this thiamine pro-drug effectively to the sub-epithelial layers. In a proof of concept study in guinea pigs in 2008, it was found that a single topical application of either a solubilized form of benfotiamine (15 mg) or a microcrystalline suspension form (25 mg) resulted in considerable increases of the dephosphorylated benfotiamine (S-benzoylthiamine) in the skin tissue as well as in significant increases in the thiamine and thiamine phosphate pools compared to control animals. The presence of a ~8000x increase in thiamine and increases in its phosphorylated derivatives in the epidermis and dermis tissue of the test animals gives a strong indication that the topical treatment with benfotiamine works very well for the desired outcome of producing an intracellular increase of the activating cofactor pool for transketolase enzyme, which is implicated in the pathophysiology of diabetic neuropathy.
60
Table of Contents
In light of the results of the proof of concept study, we plan to submit an IND application shortly after the closing of this offering, meet with the FDA, then initiate a combined Phase 1 and 2 clinical trial evaluating the safety and efficacy of AX-DN-01 in treating painful diabetic neuropathy. We anticipate that the study will require 12 months to complete.
Our Strategy
Our strategy is to build a specialty pharmaceutical company focused on developing and commercializing transformative prescription therapeutics for pain using our proprietary transdermal technology in combination with ibuprofen, benfotiamine, gabapentin, celecoxib and rofecoxib with an initial focus on treating osteoarthritis with ibuprofen. Key elements of our strategy include:
| • | rapidly advance development of and seek regulatory approval for AX-IBU-01 for the treatment of pain due to osteoarthritis. |
| • | enter in to agreements to commercialize AX-IBU-01 in the United States and build our organizational infrastructure to manage those agreements and advance the development and commercialization of AX-IBU-01 outside the United States. |
| • | partner with industry-leading contract manufacturing companies to ensure reliable product supply. |
| • | collaborate with other key companies when desirable to build co-development and co-marketing relationships. |
| • | leverage our technology and approach to advance the additional products in our pipeline into clinical development—specifically AX-DN-01 for the treatment of painful diabetic neuropathy. |
| • | develop a strategic pipeline of transdermally delivered drugs for pain utilizing existing branded and generic agents and drugs in-development. |
Background
Osteoarthritis overview and issues with current forms of treatment
Osteoarthritis (OA) is a chronic, painful, degenerative joint condition that most commonly affects the hips, knees, and hands and often requires long-term treatment to manage acute symptoms and prevent long-term complications. These complications include the destruction of articular cartilage and subchondral bone, bone re-modelling, atrophy of periarticular muscles, capsular stretching, and synovitis in weight-bearing joints.
Additional health-related problems associated with OA include emotional stress, fatigue, and impaired sleep all of which may significantly diminish the quality of a patient’s life. Many patients with OA also commonly experience multiple comorbidities, such as cardiovascular, gastrointestinal, and endocrine disorders.
OA is estimated to affect nearly 27 million people in the United States, with annual US healthcare costs stemming from the treatment of OA estimated at $185 billion. Inflammatory processes have been shown to promote disease progression in OA. Consequently, NSAIDs have become integral to the management of OA. However, the use of these agents has been linked to an increase in the risk of gastrointestinal adverse events (AEs). The safety of oral NSAIDs is further compromised with increasing patient age and by the use of higher doses, extended periods of use, the presence of comorbid medical conditions, and the coadministration of certain medications.
Selective cyclo-oxygenase-2 (COX-2) inhibitors were developed because they were thought to be safe alternatives for patients at a greater risk for peptic ulcer disease or gastrointestinal bleeding; however, population-based analyses have called their safety into question. Because COX-2 has substantial expression in cardiovascular and renal tissue, concerns have been raised regarding potential adverse cardiovascular and renal effects associated with the use of these agents.
61
Table of Contents
Attempts to address these issues have led to the development profiles in relation to earlier generations of NSAIDs. As a result, a number of NSAIDs are available worldwide as topical formulations (e.g., ibuprofen, diclofenac, and ketoprofen). Topical NSAIDs have been shown to provide analgesia through the same mechanism of action as oral NSAIDs, but because the activity of topical NSAIDs is effectively confined to the application site, systemic exposure—and consequently, the risk for gastrointestinal, cardiovascular, and renal toxicity—has been shown to be much lower than that observed with oral NSAIDs.
A Cochrane Database review of 34 studies has demonstrated that topical NSAIDs, in particular those containing diclofenac, produced fewer systemic AEs while maintaining similar effectiveness for treating chronic musculoskeletal conditions as compared to oral formulations.
The current market for topical NSAIDS consists primarily of the four products described in the following table.
| Product |
Formulation |
Date of Approval |
Label Indication | |||
| Voltaren® |
Gel (1% diclofenac) | 2007 | Pain from osteoarthritis in knees and hands1 | |||
| Pennsaid® |
Topical solution (1.5% diclofenac) |
2009 | Osteoarthritis of the knee2 | |||
| Solaraze® |
Gel (3% diclofenac) | 2000 | Actinic keratosis3 | |||
| Flector® |
Transdermal patch (1.3% diclofenac) |
2007 | Acute pain from minor sprains, strains, and contusions4 | |||
| 1 | Included two efficacy trials in hands and knees that did not demonstrate a significant treatment effect, in addition to the successful trials upon which the approval was based. |
| 2 | Used multi-arm Phase III trial: Phase III noninferiority study vs. oral; Phase III active vs. placebo; six Phase III trials since 2004. |
| 3 | Not approved for pain. No attempt has been made to achieve approval for use in pain. |
| 4 | The only approved transdermal patch system. Initial NDA did not show sufficient separation from placebo, additional efficacy trials, redefinition of endpoints, and additional imputation methods were required for approval. |
Source: www.biopharminsight.com
Worldwide sales for Voltaren (2GMs x4/day—relief of the pain of osteoarthritis of joints amenable to topical treatment) , Pennsaid (40drops x 4/day—indicated for the treatment of the pain of osteoarthritis of the knee(s), and Flector (1 patch 2x/day—acute pain due to minor strains, sprains, and contusions) were approximately $450 million in 2011.
Prescription Osteoarthritis Transdermal Product (AX-IBU-01)
AX-IBU-01 Overview
In a recent review, Balmaceda stated, “Nonsteroidal anti-inflammatory drugs (NSAIDs) are a standard treatment for osteoarthritis (OA), but the use of oral NSAIDs has been linked to an elevated risk for cardiovascular and gastrointestinal adverse events and renal toxicity. Topical NSAIDs are thought to afford efficacy that is comparable to oral formulations while reducing widespread systemic drug exposure, which may provide a benefit in terms of safety and tolerability. As a result, European treatment guidelines have, for many years, recommended the use of topical NSAIDs as a safe and effective treatment option for OA. Following the recent approval of several topical NSAID formulations by the US Food and Drug Administration, US treatment guidelines are increasingly recommending the use of topical NSAIDs as an alternative therapy and, in some cases, as a first-line option for OA.
62
Table of Contents
Clinical guidelines on the use of topical NSAIDs as safe and effective alternatives to oral NSAIDs should be regularly evaluated as new OA research emerges. Many current treatment guidelines for OA already suggest minimizing NSAID exposure and the risk of complications by prescribing the lowest effective oral dose for the shortest duration of time, but initiating treatment with topical NSAIDs, as recommended in the NICE guidelines, may help to even more effectively mitigate such risks. If long-term tolerability data demonstrate that there is a significant reduction in the frequency of cardiovascular and gastrointestinal AEs with topical NSAIDs compared to oral NSAIDs, revising or removing the black box warning requirement for topical NSAIDs also may be warranted.
Currently, only the National Institute for Health and Clinical Excellence (NICE) and the American Academy of Orthopaedic Surgeons (AAOS) recommend topical NSAIDs as first-line treatment for OA of the knee. Additionally, the European League Against Rheumatism (EULAR) guidelines recommend these agents as a first-line option for OA of the hand. Other guidelines, such as the American College of Rheumatology (ACR), recommend topical NSAIDs as first-line treatment only for select, high-risk patient populations. However, several US guidelines have not been updated since the recent FDA approval of topical diclofenac formulations. Reevaluation of those guidelines on the basis of emerging research should help to streamline OA management recommendations and further improve patient outcomes in the safest possible manner. Recommendations for the management of OA will likely continue to evolve to include the increased and earlier use of topical NSAIDs.” (see Table 1 below)
Thus we believe that the multifaceted benefits of AX-IBU-01 should translate into rapid and substantial market penetration if we are successful in obtaining regulatory approval.
TABLE 1. ORGANIZATION GUIDELINES AND RECOMMENDATIONS ON TOPICAL NSAIDs
| ORGANIZATION |
RECOMMENDATION | |
| American Association of Orthopaedic Surgeons (AAOS) 2013(1) |
Knee OA: Strongly recommend oral or topical NSAIDs or tramadol for the pharmacologic management of patients with symptomatic OA of the knee. | |
| American College of Rheumatology (ACR) 2012(2) |
Knee OA: Initial management of knee OA should include one of the following: acetaminophen, oral NSAIDs, topical NSAIDs, tramadol, intra-articular corticosteroid injections. Topical rather than oral NSAIDs should be used in patients with hand or knee OA aged > 75 years. | |
| American Geriatric Society (AGS) 2009(3) |
Localized, non-neuropathic persistent pain: Patients with localized, non-neuropathic persistent pain may be candidates for topical NSAIDs. | |
| European League Against Rheumatism (EULAR) 2003(4) |
Knee OA: Topical NSAIDs and capsaicin have clinical efficacy and are safe in the treatment of hand or knee OA. | |
| National Institute for Health and Clinical Excellence(5) (NICE, United Kingdom) 2008 |
Hand or Knee OA: Topical NSAIDs should be considered for pain relief in addition to non-pharmacologic treatment. Topical NSAIDs and/or acetaminophen should be considered ahead of oral NSAIDs, COX-2 inhibitors, or opioids. | |
63
Table of Contents
| ORGANIZATION |
RECOMMENDATION | |
| Osteoarthritis Research Society International (OARSI) 2008(6) |
Knee OA: Topical NSAIDs and capsaicin may be effective as adjunctives and alternatives to oral analgesics/anti-inflammatory agents in patients with knee OA | |
| 1) | Treatment of Osteoarthritis of the Knee Evidence-Based Guideline 2nd Edition. American Academy of Orthopaedic Surgeons; 2013. |
| 2) | Hochberg MC, et. al.: American college of rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies for osteoarthritis of the hand, hip and knee. Arthritis Care Res (Hoboken) 2012, 64: 465–474. |
| 3) | American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons: Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009, 57:1331–1346. |
| 4) | Jordan KM, et.al. on behalf of the Standing Committee for International Clinical Studies Including Therapeutic Trials ESCISIT: EULAR recommendations 2003. |
| 5) | National Collaborating Centre for Chronic Conditions: Osteoarthritis: National Clinical Guideline for Care and Management in Adults. London: Royal College of Physicians; 2008 |
| 6) | OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008, 16:137–162. |
AX-IBU-01 Clinical Trials
AX-IBU-01 has undergone a Phase 2 clinical trial, completed in 2011. The clinical data generated to date for AX-IBU-01 is presented below.
For the Phase 1 human clinical trial, which took place in 2007, endel was formulated to promote localized tissue drug delivery. The trial, in addition to the evaluation of safety considerations following the application of a single 200 mg dose of endel-ibuprofen, also determined the amount of ibuprofen in the bloodstream. The results indicate that endel-ibuprofen delivers ibuprofen into the body, with minimal systemic penetration as designed. In addition, the data indicated that the ibuprofen is slowly released from the skin and joint tissue with a peak plasma concentration noted at 24 hours after the application.
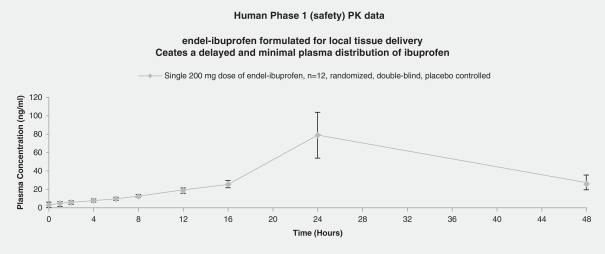
64
Table of Contents
The goals of the Phase 1 clinical trial for AX-IBU-01 were to assess safety, AE, skin reactions, and the plasma profile of ibuprofen over time. The target of AX-IBU-01 was the local tissue with limited systemic circulation. The trial included 12 subjects and a single dose of 200 mg topical application in the randomized, double blind, placebo controlled study. The results showed no adverse effects. The PK data indicated that a low-level delayed distribution of ibuprofen into the plasma, suggesting that ibuprofen accumulates in the local tissue (i.e. in the arthritic joint). The results indicate that AX-IBU-01 should meet the product profile required for both on demand and once daily application. We cannot be certain that the product will meet required safety criteria, however, until we complete FDA-mandated safety studies.
Using an identical formulation of endel-ibuprofen from the Phase 1 human clinical trial, a study was performed on guinea pigs to determine the tissue distribution of the ibuprofen. For the human clinical trial, ibuprofen was formulated with endel to promote localized tissue drug delivery. The Phase 1 clinical trial data indicated a slow release of a small amount of ibuprofen into the plasma from the administration site. Results, shown below, indicate that endel-ibuprofen delivers ibuprofen into the tissues of the synovial tissue as well as the skin. The repeated dose study (twice daily for four days) also resulted in significant plasma levels. The assumption from the study results is that the bulk of the ibuprofen is concentrated in the local tissue surrounding the application site.
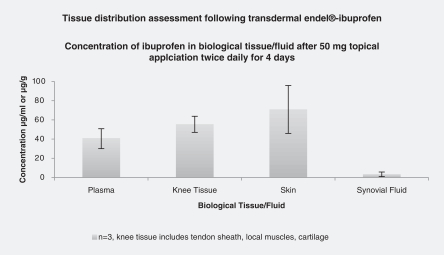
The concentration of ibuprofen in biological tissue/fluid was measured after 50 mg of topical application was given twice daily for four days. The 50 mg of ibuprofen was formulated with the endel delivery system. The formulation was applied onto the knee of healthy guinea pigs. Samples of blood and skin corresponding to the application site, synovial fluid, tendon, cartilage, and muscles surrounding the joint were removed. The tissues were homogenized, extracted and analyzed for the presence of ibuprofen. The results from the guinea pig study indicate that the endel-ibuprofen delivers ibuprofen into inflamed arthritic tissue and cartilage as well as into the synovial fluid of the knee with no adverse events other than fleeting dermal redness.
The Phase 2 study of AX-IBU-01 was a randomized, placebo-controlled, double blind, multi-center clinical trial. It was conducted at an academic medical center, and private rheumatology and interventional pain management practices in Massachusetts and in Switzerland. The study included patients with primary osteoarthritis in a single knee joint with a progression level of moderate to severe based in part on a grade II or III designation according to the Kellgren and Lawrence classification system. Patients received the corresponding, randomly assigned study formulation (endel-ibuprofen or placebo) for application to the target knee at a dose of 2.0 grams of drug product (200 mg ibuprofen) twice daily for 14 days. The evaluation of the efficacy of the treatments utilized the widely accepted methods of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index and the Visual Analog Scale (VAS) scores for the patients.
65
Table of Contents
The results indicated that the transdermal endel-ibuprofen formulation was very well tolerated from a safety perspective during the two-week trial and also produced significant, positive clinical improvements superior to the placebo in all clinical endpoints tested. In particular, the WOMACTotal and WOMACPhysical Functioning, for the endel-ibuprofen, were superior compared to the placebo (P = 0.0283 and P = 0.0201, respectively). Other clinical endpoints including the WOMACPain, WOMACStiffness, and VASResting scores were superior to those obtained from the placebo group, trending towards statistical significance compared to placebo (P = 0.0811, 0.1103, and 0.0785, respectively). Based on the Patient and Physician Global Impression of Change survey, patient satisfaction slightly improved across both groups; however, no statistical significance was detectable as compared to the baseline. We acknowledge that the sample size of 64 subjects in the final data analysis and the lack of including an orally administered drug group were limitations of the study.
Our final conclusions from the study were that the use of transdermal endel-ibuprofen has beneficial clinical effects on the pain levels experienced in some patients with moderate to severe osteoarthritis of the knee as measured by the WOMAC Osteoarthritis Indices for stiffness, pain, physical function, and total. Visual Analog Scales (VAS) tests, VASMotion and VASWeight-bearing, while appearing superior to placebo, were not statistically different from placebo. Notwithstanding our conclusions, we will have to demonstrate to the FDA that the safety and efficacy work which we have done support a pivotal Phase 2B/3 trial. We know from prior experience and from discussions with the FDA that they will raise significant questions about our work. We believe we can address all such concerns effectively, but there can be no assurance that we will be successful in doing so, or that we will be able to do so in the time period we have planned.
FDA Meeting Regarding Ibuprofen Product
BioChemics met with the FDA’s Division of Anesthesia, Analgesia and Addition Products in August 2012. The purpose of the meeting was to determine if the clinical trial data for acute pain which had been generated through the Phase 2 study could be used to support a chronic Phase 2B/3 clinical trial for chronic pain. The FDA indicted that further steps would be needed in order to pursue a clinical trial for chronic pain. Inpellis management has determined, however, that an acute severe osteoarthritis claim is unique for an osteoarthritis treatment. Accordingly, Inpellis will first pursue a Phase 2B/3 clinical trial for an acute claim and thereafter determine if a chronic claim should be added to the development program.
Prescription Diabetic Neuropathy Transdermal Product (AX-DN-01)
Diabetic neuropathy is a peripheral nerve disorder that may affect any patient with diabetes, including the approximately 25.8 million people in the US with diabetes. The symptoms of diabetic neuropathy include numbness, pain or tingling in the feet or legs and may lead to weakness in the muscles of the feet after several years. The disease is quite serious. For example, 25% of diabetics, or more than six million Americans, will develop foot ulcers and about 60% of those patients, or over three million Americans, get infections. Currently, 80% of all U.S. amputations are related to diabetic neuropathy. At present, there is no cure for the nerve damage that causes diabetic neuropathy. Current treatments for this condition are largely directed at relieving discomfort from the symptoms.
AX-DN-01 is a transdermal formulation in clinical development for diabetic neuropathy. It is comprised of a transketolase activator in the endel drug delivery system. The transdermal formulation for diabetic neuropathy has been developed to protect endothelial and neuronal cells from glucose damage and may also promote vascular health of affected tissues. Because AX-DN-01 is delivered transdermally, it is expected to be safer due to its targeted tissue delivery (meaning limited side effects and a decreased total body dose), and more effective, than current products. Those products are unable to get to the neuropathy due to poor blood flow and can have first pass metabolism effects. The trials conducted to date have shown positive results in terms of increasing blood flow and accumulation of the active in the targeted tissue indicating transdermal AX-DN-01 will be a significantly improved therapy for patients with diabetic neuropathy. We estimate that the potential U.S. market
66
Table of Contents
for this product is approximately $10 billion based on a projected annual cost per patient of $1,800. According to the American Diabetes Association, over 29 million Americans have diabetes and 60% to 70% of those individuals have mild to severe forms of neuropathy. Peripheral neuropathy and neuropathic pain affect an estimated 170 to 270 million individuals globally.
AX-DN-01 is designed to be topically administered to the foot or neuropathic tissue of the diabetic one to two times daily with the goal of reversing symptoms associated with the neuropathy. The formulation is also designed to treat the skin in a way to improve the general health of the tissue and to prevent the repeated dermal damage as a result of xerosis and severe dryness. AX-DN-01 contains a transketolase activator that inhibits the formation of AGEs, the main components responsible for nerve damage and cell death. AGEs inhibit the function of metabolic processes in different cells, including peripheral neurons. The active ingredient Inpellis has chosen has been shown to be efficacious in treating diabetic neuropathy but requires substantial doses when given orally, drastically reducing patient compliance and efficacy. The compound has not been successfully delivered topically without the endel transdermal drug delivery system due to its hydrolytic instability. Inpellis’s topical formulation is designed for accumulation in the dermis and epidermis for absorption into the peripheral nerves and to preserve the presence and function of the small fiber nerve cells. The composition has been developed to protect endothelial and neuronal cells from glucose damage by normalizing cell replication rates, decreasing apoptosis and correcting imbalances in the polyol pathway (i.e., reducing aldose reductase activity, sorbitol concentrations and intracellular glucose).
The addition of a topically applied vasodilator in AX-DN-01 may assist in the administration of other critical components to the skin. Stimulation of blood flow into the region with the vasodilator would allow for several beneficial effects to the foot including the delivery of oxygen and nutrients to allow the tissue to remain healthy as well as promoting hydration of the skin tissue, critical to the health of the foot. Increased blood flow would assist in the removal of cellular waste products again improving the general health of the foot. The inclusion of micronutrient active ingredients, antioxidants, and vitamins would be delivered to further enhance the maintenance of healthy tissue and to eliminate any free radicals that may have developed.
67
Table of Contents
AX-DN-01 Proof of Concept Results
A proof of concept study in 2003 showed that endel vasodilators could increase blood flow up to 300-400% in neuropathic patients, which supports the goals of AX-DN-01. The study was conducted to determine the ability of the vasodilator in the endel drug delivery system, methyl nicotinate, to elicit a vasodilatory response. The human study included ten healthy subjects and ten neuropathic diabetic patients. Subjects applied a single dose of the endel-based formulation to their forearms and feet and blood flow was measured in the area using a Laser Doppler system. Each patient was his or her own control and blood flow measurements were taken prior to application and then periodically after application. The endel vasodilator increased blood flow by 450% (max flow) as compared to iontophoretically-delivered sodium nitroprusside or acetylcholine (vasodilators) controls. The study showed that the endel formulation can increase blood flow up to 300-400% above starting blood flow for a period of up to two hours. The results of the study were published in the peer-reviewed Journal of Diabetes and its Complications. Applied twice daily, morning and evening, there may be a sufficient increase in blood flow for a sufficient period of time to allow for the benefits of promoting endothelial vascular health of affected tissues to occur by delivering oxygen and nutrients.
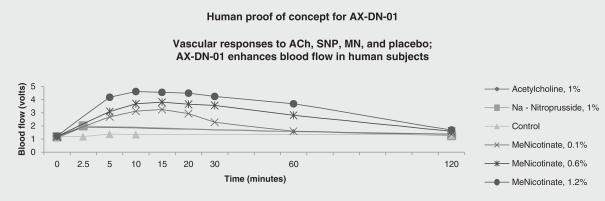
A skin irritation trial for AX-DN-01 showed the inherent safety of the product. The studies have shown that the drug, when delivered in the endel system, has been deposited deep into the epidermal and dermal tissue with minimal exposure to blood/plasma. This results in a long residence time in the skin, which serves as a reservoir for the neuronal target tissue. Compared to oral, AX-DN-01 has a 400-fold increase in the target tissue and compared to other topical technologies, as mentioned previously, it has shown a 3,200-fold increase.
Prescription Diabetic Neuropathy Products and Competitors
The two FDA-approved and widely prescribed treatments for diabetic nerve pain are Eli Lilly and Co.’s Cymbalta® and Pfizer Inc.’s Lyrica® and both products are designed to treat the burning, stabbing, and shooting pain caused by diabetic neuropathy. While Cymbalta® only needs to be taken orally once daily, Lyrica® is usually orally ingested two or three times daily and besides more common side effects such as nausea, sleepiness, dizziness, constipation, increased sweating, dry mouth, decreased appetite, blurry vision, weight gain, and trouble concentrating, more severe side effects have been reported, such as suicidal thinking.
Other Products in Development
In addition to AX-IB-01 and AX-DN-01, work has been done on our transdermal delivery system with nine other potential products. For example, we have conducted animal proof of concept studies for products using celecoxib in 2011 and gabapentin in 2010. Our strategy is to focus our efforts on the two lead products in order to bring them to market as rapidly as possible. We will, however, use some resources to continue development of the other products and seek opportunities to bring them to market profitably as well. It will likely be several years before we are ready to pursue clinical trials for regulatory approval and commercial development of these products.
68
Table of Contents
Operating Plan and Key Milestones
Our most important goal over the 18 months following the closing of this offering is to complete a successful Phase 2B/3 pivotal clinical trial of our ibuprofen product, AX-IBU-01. We believe that the proceeds of this offering will be sufficient to fund that effort. As noted above, our first step in this process will be to perform additional studies in support of an IND application. We expect to begin that work shortly after this offering is completed and complete it approximately six months later. At that point, if the studies are favorable, we will submit the IND and the proposed Phase 2B/3 clinical trial protocol to the FDA with the expectation that we will be able to begin patient enrollment thereafter.
At the same time we are doing the studies and other work necessary to submit the IND for AX-IBU-01, we will be identifying a manufacturing partner and working with that partner to help it put into place the production resources necessary to manufacture the amount of product necessary for the planned clinical trial.
We expect to complete the Phase 2B/3 trial and report top line data from it six to nine months after the trial begins. Assuming successful completion of that clinical trial, we would submit our New Drug Application, or NDA, to the FDA thereafter. Based on these anticipated timelines and the resources we have allocated, we expect the total operating expense to bring AX-IBU-01 through our goal of FDA approval will be approximately $10 million. The single greatest area of cost to reach this goal will be the payments we make to contract research organizations for their help in designing the trial, conducting it and helping to analyze its results. We estimate that amount to be approximately $6 million. Other significant expenses will be compensation of employees, both management and our scientific staff, and general overhead, such as rent, insurance, legal and accounting.
Sales and Marketing
Our strategy for the marketing and commercialization of our products entails entering into various arrangements with corporate partners, licensors, licensees and others. We intend to establish license agreements with established marketing companies (e.g., pharmaceutical and biotechnology companies) for sales, marketing and distribution of our products. There can be no assurance that these agreements will be maintained by Inpellis and, if maintained, will be profitable for Inpellis. Inpellis may also rely on collaborative partners to conduct research efforts and clinical trials, to obtain regulatory approvals and to manufacture and market certain of Inpellis’ products. There can be no assurance that Inpellis will be successful in establishing collaborative arrangements or that, if established, the arrangements will be successful.
Manufacturing
We will use third parties for manufacturing. Through previous arrangements with manufacturing contractors, GMP-quality human doses of AX-IBU-01 for Phase 1 and Phase 2 clinical trials have been produced, and we believe that this experience will allow us, through contractors with whom we have worked in the past, or with new such contractors, to produce GMP-quality AX-IBU-01 for our contemplated pivotal Phase 2B/3 clinical trial.
Government regulation
Government authorities in the United States, at the federal, state and local level, and in other countries and jurisdictions, including the European Union, extensively regulate, among other things, the research, development, testing, manufacture, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, and import and export of pharmaceutical products. The processes for obtaining regulatory approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
69
Table of Contents
Review and approval of drugs in the United States
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant and/or sponsor to a variety of administrative or judicial sanctions, including refusal by the FDA to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters and other types of letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, or civil or criminal investigations and penalties brought by the FDA and the Department of Justice or other governmental entities.
An applicant seeking approval to market and distribute a new drug product in the United States must typically undertake the following:
| • | completion of preclinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s GLP regulations; |
| • | submission to the FDA of an investigational new drug application, or IND, which must take effect before human clinical trials may begin or, alternatively, following an analogous process outside the United States that is subject to the same standards as clinical trials conducted inside the United States, such as how the Company conducted its Phase 2 trial for its transdermal ibuprofen product in Switzerland; |
| • | approval by an independent IRB representing each clinical site before each clinical trial may be initiated; |
| • | performance of adequate and well-controlled human clinical trials in accordance with GCP to establish the safety and efficacy of the proposed drug product for each indication; |
| • | preparation and submission to the FDA of an NDA; |
| • | review of the product by an FDA advisory committee, where appropriate or if applicable; |
| • | satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with GMP requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; |
| • | payment of user fees and securing FDA approval of the NDA; and |
| • | compliance with any post-approval requirements, including a REMS, and post-approval studies required by the FDA, and regulations applicable to post-approval marketing, e.g., compliance with good manufacturing practices and adverse event reporting. |
Preclinical studies
Preclinical studies include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess safety of the drug for initial testing in humans and to establish a rationale for therapeutic use. The conduct of preclinical studies is subject to federal regulations and requirements, including GLP regulations. The results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical trials, among other things, are submitted to the FDA as part of an IND. Some long-term preclinical testing, such as 9 tests of reproductive AEs and carcinogenicity, may continue after the IND is submitted.
70
Table of Contents
Human clinical trials in support of an NDA
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include, among other things, the requirement that all research subjects provide their informed consent in writing before their participation in any clinical trial. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to a proposed clinical trial and places the trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects. An IRB must operate in compliance with FDA regulations. Information about certain clinical trials must be submitted within specific timeframes to the NIH for public dissemination.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
Phase 1: The drug is initially introduced into healthy human subjects or patients with the target disease (e.g., PD) or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness and to determine optimal dosage.
Phase 2: The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
Phase 3: The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and, more frequently, if serious AEs occur. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
Submission of an NDA to the FDA
Assuming successful completion of required clinical testing and other requirements, the results of the preclinical and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA requesting approval to market the drug product for one or more indications. Under federal law, the submission of most NDAs is additionally subject to an application user fee, currently exceeding $2.1 million.
NDAs for most new drug products are based on two full clinical trials that must contain substantial evidence of the safety and efficacy of the proposed new product. These applications are submitted under Section 505(b)(1) of the FDCA. The FDA is, however, authorized to approve an alternative type of NDA under Section 505(b)(2) of the FDCA. Section 505(b)(2) of the FDCA provides an alternate regulatory pathway to FDA approval for new or improved formulations or new uses of previously approved drug products. Specifically, Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies
71
Table of Contents
not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely upon the FDA’s findings of safety and efficacy for an approved product that acts as the RLD. The FDA may also require 505(b)(2) applicants to perform additional studies or measurements to support the change from the RLD. The FDA may then approve the new product candidate for all or some of the labeled indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
Thus, Section 505(b)(2) authorizes the FDA to approve an NDA based on safety and effectiveness data that were not developed by the applicant. If the 505(b)(2) applicant can establish that reliance on the FDA’s previous approval is scientifically appropriate, the applicant may eliminate the need to conduct certain preclinical or clinical trials of the new product. We cannot predict whether the FDA will allow us to use 505(b)(2) for any of our proposed products and we are already aware that they will require significant additional work from us in connection with using it for our transdermal ibuprofen product.
The FDA conducts a preliminary review of an NDA within 60 days of its receipt and informs the sponsor by the 74th day after the FDA’s receipt of the submission whether the application is sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission has been accepted for filing, the FDA begins an in-depth substantive review. The FDA has agreed to specified performance goals in the review process of NDAs. For standard and priority NDAs concerning new molecular entities, the FDA’s performance goal is to review and act on 90% of such NDAs within 10 months or six months of the 60-day filing date, respectively. For standard and priority NDAs that do not concern a new molecular entity, the FDA’s performance goal is to review and act on 90% of such NDAs within 10 months or six months of receipt, respectively.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with GMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to ensure compliance with GCP.
The FDA may also require submission of a REMS plan to mitigate any identified or suspected serious risks. The REMS plan could include medication guides, physician communication plans, assessment plans, and elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools.
If the FDA approves a product, it may limit the approved indications for use of the product, require that contraindications, warnings, or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess the drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs.
Post-approval requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
72
Table of Contents
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with GMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from GMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain GMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including AEs of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| • | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| • | fines, warning letters or holds on post-approval clinical trials; |
| • | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals; |
| • | product seizure or detention, condemnation and destruction, or refusal to permit the import or export of products; or |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Abbreviated new drug applications for generic drugs
In 1984, with the passage of the Hatch-Waxman Act, Congress authorized the FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the NDA provisions of the statute. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application, or ANDA, to the agency.
The FDCA provides a period of five years of non-patent data exclusivity for a new drug containing a new chemical entity. In cases where such exclusivity has been granted, an ANDA or 505(b)(2) application referencing that drug may not be filed with the FDA until the expiration of five years, unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the
73
Table of Contents
original product approval. CVT-301 will not be eligible for this type of exclusivity. The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted by or for the applicant and are essential to the approval of the application.
Hatch-Waxman patent certification and the 30-month stay
Upon approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant’s product or an approved method of using the product. Each of the patents listed by the NDA sponsor is published in the Orange Book. When an ANDA applicant files its application with the FDA, the applicant is required to certify to the FDA concerning any patents listed for the reference product in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval. To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would.
Specifically, the applicant must certify with respect to each patent that:
| • | the required patent information has not been filed; |
| • | the listed patent has expired; |
| • | the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or |
| • | the listed patent is invalid, unenforceable or will not be infringed by the new product. |
A certification that the new product will not infringe the already approved product’s listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification.
If the ANDA or 505(b)(2) applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA or 505(b)(2) application has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA or 505(b)(2) application until the earlier of 30 months after the receipt of the Paragraph IV notice, expiration of the patent, or a decision in the infringement case that is favorable to the ANDA applicant.
Combination products
AX-IBU-01 and other products in our pipeline are combination products. Combination products include products comprised of two or more FDA-regulated components (i.e., drug/device, biologic/device, drug/biologic or drug/device/biologic) that are physically, chemically, or otherwise combined or mixed and produced as a single entity.
FDA regulations require that the FDA determine the combination product’s primary mode of action, or PMOA, which is the single mode of a combination product that provides the most important therapeutic action of the combination product. The branch of the FDA, or Center, that regulates that portion of the product that generates the PMOA or that has expertise in the relevant therapeutic area becomes the lead evaluator. When evaluating an application, a lead Center may consult other Centers but still retain complete reviewing authority, or it may collaborate with another Center, by which the lead Center assigns review of a specific section of the
74
Table of Contents
application to another Center, delegating its review authority for that section. Typically, the FDA requires a single marketing application submitted to the Center selected to be the lead evaluator, although the agency has the discretion to require separate applications to more than one Center. The marketing application for AX-IBU-01 and other products will be reviewed by the Pain and Analgesic Center.
Review and approval of drug products in the European Union
Whether or not we obtain FDA approval for a product, we would need to obtain the necessary approvals by the comparable foreign regulatory authorities before we can commence clinical trials or marketing of the product in those countries or jurisdictions. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
To obtain marketing approval of a drug under European Union regulatory systems, an applicant must submit a marketing authorization application either under a centralized or decentralized procedure.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all European Union member states. The centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases.
The decentralized procedure is available to applicants who wish to market a product in various European Union member states where such product has not received marketing approval in any European Union member states before. The decentralized procedure provides for approval by one or more other, or concerned, member states of an assessment of an application performed by one member state designated by the applicant, known as the reference member state.
In the European Union, new chemical entities qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. The overall 10-year period will be extended to a maximum of 11 years if, during the first eight of those 10 years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. AX-IBU-01 will not be eligible for this type of exclusivity.
In the European Union, a marketing application for a product that, although similar to an approved medicinal product does not qualify as a generic, may also seek to rely to some degree on the data in the dossier for the approved product. As with a generic product, the application may not be submitted until expiration of the data exclusivity period, and the product, if approved, may not be placed on the market until expiration of the market exclusivity period. Such an application must also contain data specific to the proposed product, however. The extent to which such a “hybrid” application requires new data is determined on a case-by-case basis by the competent authorities, based on the differences between the innovative medicinal product and the medicinal product subject to the hybrid application for marketing authorization. The purpose of the pre-clinical tests and clinical trials is to generate additional data that complement the data relating to the innovative medicinal product and to demonstrate the quality, safety and efficacy of the medicinal product for which authorization is sought.
Pharmaceutical coverage, pricing and reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of products approved by the FDA and other government authorities. Sales of any of our product candidates, if approved, will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government health
75
Table of Contents
programs such as Medicare and Medicaid, commercial health insurers and managed care organizations. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Third-party reimbursement may not be sufficient to enable us to maintain price levels high enough to realize an appropriate return on our investment in product development.
The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. Adoption of such controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular drug candidate to currently available therapies.
New legislation and regulations
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations and policies are often revised or interpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether further legislative changes will be enacted, or FDA regulations, guidance, policies or interpretations changed or what the impact of such changes, if any, may be.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, an increasing emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on drug pricing. Coverage policies, third-party reimbursement rates and drug pricing regulation may change at any time. In particular, the ACA was enacted in the United States in March 2010 and contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal health care programs.
Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
76
Table of Contents
Health care law and regulation
Health care providers, physicians and third-party payors play a primary role in the recommendation and prescription of drug products that are granted marketing approval. Arrangements with third-party payors and customers are subject to broadly applicable fraud and abuse and other health care laws and regulations. Such restrictions under applicable federal and state healthcare laws and regulations, include the following:
| • | the federal health care Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal health care program such as Medicare and Medicaid; |
| • | the federal False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | HIPAA imposes criminal and civil liability for executing a scheme to defraud any health care benefit program or making false statements relating to health care matters; |
| • | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for health care benefits, items or services; |
| • | the federal transparency requirements under the Health Care Reform Law will require manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals and physician ownership and investment interests; and |
| • | analogous state and foreign laws and regulations. |
Efforts to ensure that our business arrangements with third parties will comply with applicable health care laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other health care laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded health care programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded health care programs.
Environmental, health and safety laws and regulations
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the use, generation, manufacture, distribution, storage, handling, treatment, remediation and disposal of hazardous materials and wastes. Our operations involve the use of hazardous materials, and the risk of injury, contamination or noncompliance with environmental, health and safety requirements cannot be eliminated. Although compliance with such laws and regulations has not had a material effect on our capital expenditures, earnings or competitive position, environmental, health and safety laws and regulations have tended to become increasingly stringent and, to the extent legal or regulatory changes occur in the future, they could result in, among other things, increased costs to us.
77
Table of Contents
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our transdermal technologies and scientific knowledge provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any drug candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. For example, we believe that the main competitors for AX-IBU-01 are therapies that can effectively treat the symptoms associated with osteoarthritis, or OA, with fewer AEs commonly associated with NSAIDs. These therapies include a number of topical formulations of NSAIDs (e.g., ibuprofen, diclofenac, and ketoprofen). Topical NSAIDs have been shown to provide analgesia through the same mechanism of action as oral NSAIDs, but because the activity of topical NSAIDs is effectively confined to the application site, systemic exposure—and consequently, the risk for gastrointestinal, cardiovascular, and renal toxicity—has been shown to be lower than that observed with oral NSAIDs. The current market for topical NSAIDS consists primarily of the four products: Voltaren ®, Pennsaid ®, Solaraze ® and Flector ®. If approved for the treatment of osteoarthritis, AX-IBU-01 would compete against these therapies. The key competitive factors affecting the success of AX-IBU-01 and any other drug candidates that we develop, if approved, are likely to be their efficacy, safety, convenience, price, the level of generic competition and the availability of reimbursement from government and other third-party payors.
Many of our competitors may have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
Specific competitors include, but are not limited to Johnson & Johnson, Novartis, Abbott, Boehringer Ingelheim, Bayer, GlaxoSmithKline, Pfizer, Bristol-Myers Squibb, Merck, and specialty/drug delivery pharmaceutical companies including Altea Therapeutics, Avanir Pharmaceuticals, Anacor, Endo Pharmaceuticals, IDEA AG, Immunosyn Corporation, MacroChem, MediQuest Therapeutics, Noven Pharmaceuticals, Quigley Corporation, Transdel Pharmaceuticals and Vyteris. The market for transdermal drug delivery systems is large and growing rapidly and is likely to attract new entrants.
Intellectual property
Pursuant to an October 2015 Intellectual Property Purchase Agreement, we paid $750,000 to purchase from BioChemics, our former parent, joint ownership or, in some cases, full ownership, of patents, patent applications and know how related to transdermal drug delivery as it relates to six pharmaceutical products for the prescriptive prophylactic or therapeutic treatment of non-dermal pain in humans. The products are transdermal ibuprofen, benfotiamine, gabapentin, celecoxib, rofecoxib and lidocaine (including products similar to lidocaine). The purchase covers 24 issued patents and pending patent applications in the United States or foreign countries. Of those patents and patent applications, we have purchased full ownership of six, all relating to ibuprofen. The Intellectual Property Purchase Agreement also provides that BioChemics has a right of first offer if we determine to sell our ownership of the intellectual property we purchased from them. BioChemics must notify us within 10 days of receiving notice whether it wishes to negotiate to purchase such property from us. Upon receipt of such notice from BioChemics, we must, prior to selling such property to another party, negotiate in good faith with BioChemics for a period not to exceed 30 days. In addition, BioChemics has the option to purchase back from us the intellectual property related to lidocaine (and similar products) conveyed pursuant to the Intellectual Property
78
Table of Contents
Purchase Agreement for $750,000, provided that we would retain joint ownership of that intellectual property as it relates to the treatment of shingles and similar conditions. We will continue to pursue patent protection in connection with development efforts and advances.
The remaining patents, patent applications and other intellectual property are owned jointly with BioChemics under a Joint Ownership Agreement. Under the terms of that agreement, we will use the jointly owned intellectual property in connection with six pharmaceutical products described above. Currently, one pending patent application, which we own jointly, covers the ibuprofen and benfotiamine transdermal products for which regulatory approval is being sought. Inpellis will manage the prosecution and maintenance of all patents relating to the jointly owned intellectual property. However, we may only use, and BioChemics is excluded from using, the jointly owned intellectual property in connection with the following products:
| • | Transdermal Ibuprofen |
| • | Transdermal Benfothiamine |
| • | Transdermal Gabapentin |
| • | Transdermal Celecoxib |
| • | Transdermal Rofecoxib |
| • | Transdermal Lidocaine and any similar products derived from the same chemical including, without limitation, Benzocaine, |
| • | Dibucaine, Oxybuprocaine, Proparacaine, Proxymetacaine, Tetracaine, Amethocaine, Xylocaine and Lignocaine |
All of the patents and patent applications which we own or own jointly relate to technology to deliver drugs transdermally. In the portfolio of 24, there are two issued United States patents and seven issued foreign patents. The issued patents generally are expected to expire between 2020 and 2030. The remainder consist of one United States patent application and 14 foreign patent applications.
In addition, it should be noted that the creditors of BioChemics could attempt to assert that BioChemics did not receive adequate consideration for the sale of intellectual property and seek to require that the Company pay a greater amount to BioChemics. BioChemics has informed us that its decision to sell intellectual property was made based on careful consideration of the alternatives, its conclusion that the arrangement would help Inpellis obtain capital it might not otherwise raise to pursue its business plan and its conclusion that the value of a well-funded Inpellis to the creditors and shareholders of BioChemics would likely exceed the value of a larger one-time cash payment were Inpellis unable to obtain necessary capital.
The term of individual patents depends upon the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the date of filing the non-provisional application or PCT application. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier-filed patent. The patent term of a patent that covers an approved drug may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the development and regulatory review process. To obtain a patent extension in the United States, the term of the relevant patent must not have expired before the extension application, the patent cannot have been extended previously under this law, an application for extension must be submitted, the product must be subject to regulatory review prior to its commercialization, and the permission for the commercial marketing or use of the product after such regulatory review period must be the first permitted commercial marketing or use of the product.
79
Table of Contents
Trade secrets
We endeavor to maintain trade secret protection for certain aspects of our technology. In some situations, maintaining information as a trade secret is more appropriate than filing a patent application. For example, we have a number of trade secrets relating to the manufacturing process.
Third-party rights
Many pharmaceutical companies, biotechnology companies and academic institutions are competing with us in the field of transdermal drug delivery and filing patent applications potentially relevant to our business. In order to contend with the strong possibility of third-party intellectual property conflicts, we periodically conduct freedom-to-operate studies, but such studies may not uncover all patents relevant to our business.
From time to time, we find it necessary or prudent to obtain licenses from third-party intellectual property holders. Where licenses are readily available at reasonable cost, such licenses are considered a normal cost of doing business. In other instances, however, we may use the results of freedom-to-operate studies to guide our early-stage research away from areas where we are likely to encounter obstacles in the form of third-party intellectual property. For example, where a third party holds relevant intellectual property and is a direct competitor, a license might not be available on commercially reasonable terms or available at all. We strive to identify potential third-party intellectual property issues in the early stages of research of our research programs, in order to minimize the cost and disruption of resolving such issues.
In spite of these efforts to avoid obstacles and disruptions arising from third-party intellectual property, it is impossible to establish with certainty that our technology or our product programs will be free of claims that we infringe, misappropriate or otherwise violate the rights of third-party intellectual property holders. Even with modern databases and on-line search engines, freedom-to-operate searches are imperfect and may fail to identify relevant patents and published applications. Even when a third-party patent is identified, we may conclude that we do not infringe the patent or that the patent is invalid. If the third- party patent owner disagrees with our conclusion and we continue with the business activity in question, patent litigation may result. We might decide to initiate litigation in an attempt to have a court declare the third-party patent invalid or non-infringed by our activity. In either scenario, patent litigation typically is costly and time consuming, and the outcome is uncertain. Ultimately, in the case of an adverse outcome in litigation, we could be prevented from commercializing a product or using certain aspects of our technology as a result of patent infringement claims asserted against us. This could have a material adverse effect on our business. For further discussion of the risks relating to intellectual property see “Risk factors—Risks related to our intellectual property.”
Employees
As of November , 2015, we had six full time employees. Of these employees, three are engaged in research and development and three are engaged in finance, human resources, facilities and business and general management. We have no collective bargaining agreements with our employees and we have not experienced any work stoppages. We consider our relations with our employees to be good.
Legal proceedings
From time to time, we are subject to various legal proceedings and claims that arise in the ordinary course of our business activities. Although the results of litigation and claims cannot be predicted with certainty, as of the date of this prospectus, we do not believe we are party to any claim or litigation, the probable outcome of which, if determined adversely to us, would individually or in the aggregate be reasonably expected to have a material adverse effect on our business. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
80
Table of Contents
Executive officers and directors
Below is a list of the names, ages as of November , 2015 and positions, and a brief account of the business experience of the individuals who serve as our executive officers and directors as of the date of this prospectus.
| INDIVIDUAL |
POSITION |
AGE | ||||
| Patrick T. Mooney, M.D. |
President, Chief Executive Officer and Director | 47 | ||||
| David R Staskin, M.D. |
Chief Strategy Officer, Secretary and Director | 61 | ||||
| Frank A. Manguso |
Chief Financial Officer and Treasurer | 74 | ||||
| John J. Clarke, Jr. |
Director, Chairman | 72 | ||||
| Frederic J. de Bure |
Director | 42 | ||||
| Harry G McCoy, Pharm.D. |
Director | 63 | ||||
Patrick T. Mooney, M.D. became our Chief Executive Officer in June 2015 and was elected to our board of directors in the same month. He became our President in September 2015. Prior to joining Inpellis, Dr. Mooney was an independent consultant to Alexander Capital, L.P., the representative of the underwriters in this offering, from April 2014 to June 2015. Prior to that time, Dr. Mooney served as President, Chief Executive Officer and Chairman of the Board of Echo Therapeutics, Inc. (NASDAQ: ECTE) from 2007 to 2013. Dr. Mooney previously served as President, Chief Executive Officer and Director of Echo Therapeutics, Inc. (a privately-held pharmaceutical company prior to its merger with Sontra Medical Corporation) from 2006 to 2007. Prior to joining Echo Therapeutics, Inc., Dr. Mooney was President, Chief Executive Officer and Chairman of Aphton Corporation (NASDAQ: APHT), a biopharmaceutical company, from 2004 to 2006. Dr. Mooney was a Senior Biotechnology Analyst at Thomas Weisel Partners, LLC, a full service merchant banking firm, and a Senior Biotechnology Analyst at Janney Montgomery Scott, LLC, a full services investment banking firm. He graduated from the Jefferson Medical College of Thomas Jefferson University and trained as a surgical resident at Thomas Jefferson University Hospital. Dr. Mooney served on the Board of Directors of Metastat, a cancer therapy company, from March 2012 to October 2014. Dr. Mooney’s knowledge of the capital markets, his experience as the Chief Executive Officer and Chief Medical Officer of Aphton, and his medical training provide invaluable expertise to our Board and executive management team in matters regarding our operations, product development, capital requirements and strategic direction. During Dr. Mooney’s tenure at Aphton Corporation, on May 23, 2006, Aphton Corporation declared bankruptcy under Chapter 11 of the United Sates Bankruptcy Code.
David Staskin, M.D. served as our President from January to September 2015, when he became Chief Strategy Officer. He has served as Secretary, and as a director, since January 2015. Prior to joining the Company, Dr. Staskin practiced as an academic urologist beginning in 1985. (1985-89 Faculty, Boston University Medical Center, University Hospital; 1989-2002 Faculty, Harvard Medical School, Beth Israel Medical Center; 2002-08 Faculty, Weill-Cornell Medical College, New York Presbyterian Hospital; 2008-present Faculty, Tufts University, St. Elizabeth’s Medical Center. In addition, Dr. Staskin has been an active consultant in the medical and device industries since 1987. He has multiple device patents which have resulted in four successful commercial products and served as a consultant for development and commercialization of these products with American Medical Systems since 1999. Recently, Dr. Staskin has served as a consultant on the Allergan, National Medical Advisory Board—Executive Council, and the Astellas Pharma, National and International Medical Advisory Boards. He has published extensively, including more than 125 peer-reviewed publications and four textbooks. Dr. Staskin has also served on the board of directors of a number of medical societies, including the Society for Urodynamics and Female Urology, the American Urogynecological Society and the American Association Clinical Urologists. Dr. Staskin holds a B.A. from Cornell University and an M.D. from Hahnemann Medical College of Drexel University. Dr. Staskin performed his residency at the University of Pennsylvania, where he was a National Kidney Foundation Scholar in pharmacology, and completed his post-graduate fellowship at the University of California Los Angeles. We believe that Dr. Staskin’s extensive experience as a consultant to the pharmaceutical and medical device industry advising on all stages of drug and devise development as well as his academic and scientific background qualifies him to serve as a member of our board of directors.
81
Table of Contents
Frank A. Manguso, CPA, M.B.A. has served as our Treasurer and Chief Financial Officer since January 2015. Prior to joining the Company, Mr. Manguso was a self-employed business consultant from 2001 to 2014, and a SCORE mentor from 2004 to 2014. Prior to that, Mr. Manguso served as Chief Financial Officer of Star Solutions, Inc. from 1999-2001. Prior to Star Solutions, Mr. Manguso served as Chief Executive Officer and Chief Financial Officer of Z-LOK Industries, Inc. from 1990-1999. Prior to Z-LOK Industries, Mr. Manguso served as Director of Management Information Consulting at Bank Boston Corporation from 1970-1990. Before that, Mr. Manguso also held management positions at two Gillette companies, Sterilon and Gillette of Puerto Rico. Mr. Manguso holds an A.B. from Harvard College and an M.B.A. from Babson College.
John J. Clarke, Jr. has served on the Board of Directors of the Company since May 2015. Mr. Clarke currently serves as a Co-Founder of The Baldwin & Clarke Companies, a diversified financial services organization made up of four independent companies established in 1972. Mr. Clarke was a founder and director of two New Hampshire state chartered commercial banks, Centerpoint Bank, from 1990 to 1997, and Centrix Bank, from 1999 /to 2014. In 2003 Mr. Clarke started The Triumph Investment Funds which invested in commercial banks around the United States. Mr. Clarke has been a director for a number of for-profit and nonprofit organizations for the past 30 years. Mr. Clarke holds a B.A. degree from Northeastern University and is a registered principal with the Financial Industry Regulatory Authority. We believe that Mr. Clarke extensive experience in finance and management with a special emphasis on startups, healthcare and manufacturing qualifies him to serve as a member of our board of directors.
Frederic J. de Bure has served on the Board of Directors of the Company since January 2015. Since 2006, Mr. de Bure has served as a Partner with IDG Ventures SEA. Prior to joining IDG Ventures, Mr. de Bure served in a number of roles for eBay, Inc. culminating in South East Asia Managing Director from 2001 to 2005. Mr. de Bure also served as a Founder of Commonstream.com from 1999 to 2001. Prior to CommonStream, Mr. de Bure served as an Associate for the Cowen Group, Inc. from 1998 to 1999, and served as an analyst in the corporate finance group of the Bear Stearns Companies, Inc. in New York and Hong Kong from 1993 to 1996. Mr. de Bure currently sits or has sat on the boards of MJ Group (since 2012), The Rubicon Network (2012 to 2014), IDG Ventures SEA (since 2007), In2Nite (since 2012) and YWS International (2010 to 2014). Mr. de Bure holds a B.A. from Vassar College and an M.B.A. from the University of Chicago School of Business. We believe that Mr. de Bure’s extensive experience in investment management and healthcare technology as a former healthcare and technology banker as well as a technology venture capitalist qualifies him to serve as a member of our board of directors.
Harry G. McCoy, Pharm.D. has served on the Board of Directors of the Company since January 2015 and served as our interim Chief Executive Officer from January 2015 to June 2015. Prior to joining our Company, Dr. McCoy served as President and Chief Executive Officer of Thorne Diagnostics, Inc. from March 2008 to March 2015 and President of Hamilton Thorne Biosciences, Inc. from February 2001 to March 2008. Dr. McCoy was also a Founder, President and Executive Chairman of MEDTOX Scientific, Inc., formerly known as Editek, Inc., from January 1984 to October 2000. In addition to his responsibilities with the Company, Dr. McCoy is currently Co-Founder and Chairman of the Board of Directors of North Shore InnoVentures, Inc., a non-profit incubator of early-stage biotechnology and clean technology companies, as well as a Director at Attogen, Inc., an early-stage drug discovery company. Dr. McCoy previously served on the board of AppTec, a biopharmaceutical manufacturing company. Dr. McCoy also previously served as an Associate Professor at the University of Minnesota College of Pharmacy and a Clinical Assistant Professor at the University of North Dakota School of Medicine. Dr. McCoy holds a B.A. in Biology from the University of California, San Diego and a Pharm.D. from the University of California, San Francisco and completed his post-doctoral fellowship in pharmacokinetics at the University of Minnesota. We believe that Dr. McCoy’s extensive experience as a pharmaceutical industry leader as well as his academic and scientific background qualifies him to serve as a member of our board of directors.
82
Table of Contents
Board composition
Our board of directors is currently comprised of five members. Our directors hold office until their successors have been elected and qualified or until their earlier death, resignation or removal. There are no family relationships among any of our directors or executive officers.
Our amended and restated certificate of incorporation will provide that our board of directors will be divided into three classes of directors, with the classes as nearly equal in number as possible. Upon completion of this offering, each of our directors identified above will serve in the class indicated. Subject to any earlier resignation or removal in accordance with the terms of our amended and restated certificate of incorporation and amended and restated by-laws that we expect to be in effect upon the closing of this offering, our Class I directors will serve until the first annual meeting of stockholders following the completion of this offering; our Class II directors will serve until the second annual meeting of stockholders following the completion of this offering; and our Class III directors will serve until the third annual meeting of stockholders following the completion of this offering.
Board committees
Our board of directors has three standing committees: the audit committee, the compensation committee and the nominating and corporate governance committee.
Audit committee
Our audit committee is composed of Mr. Clarke, Dr. McCoy and Mr. de Bure, with Mr. Clarke serving as chairman of the committee. Our board of directors has determined that each member of the audit committee meets the independence requirements of Rule 10A-3 under the Exchange Act and the applicable listing standards of the NASDAQ Global Market. Our board of directors has determined that Mr. Clarke is an “audit committee financial expert” within the meaning of the SEC regulations and applicable listing standards of the NASDAQ Global Market. The audit committee’s responsibilities include:
| • | appointing, approving the compensation of, and assessing the qualifications, performance and independence of our independent registered public accounting firm; |
| • | pre-approving audit and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm; |
| • | reviewing the internal audit plan with the independent registered public accounting firm and members of management responsible for preparing our financial statements; |
| • | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us; |
| • | setting policies for our hiring of employees or former employees of our independent registered public accounting firm; |
| • | reviewing our significant risks or exposures and assessing the steps that management has taken or should take to monitor and minimize such risks or exposures; |
| • | reviewing the adequacy of our internal control over financial reporting, including information system controls and security; |
| • | coordinating our board of director’s oversight of our code of business conduct and our disclosure of controls and procedures; |
| • | monitoring developments in income tax laws and regulations; |
83
Table of Contents
| • | establishing policies and procedures for the receipt and retention of accounting-related complaints and concerns; |
| • | recommending, based upon the audit committee’s review and discussions with management and the independent registered public accounting firm, whether our audited financial statements shall be included in our Annual Report on Form 10-K; |
| • | monitoring our compliance with legal and regulatory requirements as they relate to our financial statements and accounting matters; |
| • | preparing the audit committee report required by the rules of the SEC to be included in our annual proxy statement; |
| • | reviewing all related party transactions for potential conflict of interest situations and approving all such transactions; and |
| • | reviewing and discussing with management and our independent registered public accounting firm our earnings releases and scripts. |
Compensation committee
Our compensation committee is composed of Dr. McCoy, Mr. de Bure and Mr. Clarke, with Mr. Clarke serving as chairman of the committee.
Our board of directors has determined that each member of the compensation committee is “independent” as defined under the applicable listing standards of the NASDAQ Global Market. The compensation committee’s responsibilities include:
| • | annually reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer; |
| • | evaluating the performance of our chief executive officer in light of such corporate goals and objectives and determining and approving the compensation of our chief executive officer; |
| • | reviewing and approving the compensation of our other executive officers; |
| • | appointing, compensating and overseeing the work of any compensation consultant, legal counsel or other advisor retained by the compensation committee; |
| • | conducting the independence assessment outlined in the listing standards of the NASDAQ Global Market with respect to any compensation consultant, legal counsel or other advisor retained by the compensation committee; annually reviewing and reassessing the adequacy of the committee charter; |
| • | reviewing and establishing our overall management compensation, and our compensation philosophy and policy; |
| • | overseeing and administering our compensation and other compensatory plans; |
| • | reviewing and approving our equity and incentive policies and procedures for the grant of equity-based awards and approving the grant of such equity-based awards; |
| • | reviewing and making recommendations to our board of directors with respect to non-employee director compensation; and |
| • | producing a report on executive compensation to be included in our annual proxy statement or Annual Report on Form 10-K. |
84
Table of Contents
Nominating and corporate governance committee
Our nominating and corporate governance committee is composed of Mr. Clarke, Mr. de Bure and Dr. McCoy, with Mr. de Bure serving as chairman of the committee. Our board of directors has determined that each member of the nominating and corporate governance committee is “independent” as defined under the applicable listing standards of the NASDAQ Global Market. The nominating and corporate governance committee’s responsibilities include:
| • | developing and recommending to our board of directors criteria for board and committee membership; |
| • | establishing procedures for identifying and evaluating board of director candidates, including nominees recommended by stockholders; |
| • | identifying individuals qualified to become members of our board of directors; |
| • | recommending to our board of directors the persons to be nominated for election as directors and to each of our board’s committees; |
| • | developing and recommending to our board of directors a set of corporate governance principles; |
| • | articulating to each director what is expected, including reference to the corporate governance principles and directors’ duties and responsibilities; |
| • | reviewing and recommending to our board of directors practices and policies with respect to directors; |
| • | reviewing and recommending to our board of directors the functions, duties and compositions of the committees of our board of directors; |
| • | reviewing and assessing the adequacy of the committee charter and submitting any changes to our board of directors for approval; |
| • | consider and report to our board of directors any questions of possible conflicts of interest of board of directors members; |
| • | provide for new director orientation and continuing education for existing directors on a periodic basis; |
| • | performing an evaluation of the performance of the committee; and |
| • | overseeing the evaluation of our board of Directors and management. Our board of directors may establish other committees from time to time. |
Compensation committee interlocks and insider participation
None of the members of our compensation committee has at any time during the prior three years been one of our officers or employees. None of our executive officers currently serves, or in the past fiscal year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee. For a description of transactions between us and members of our compensation committee and affiliates of such members, please see “Certain Relationships and Related Party Transactions.”
Code of business conduct and ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Upon the closing of this offering, our code of business conduct and ethics will be available on our website, http://www.Inpellisrx.com/. We intend to disclose any amendments to the code, or any waivers of its requirements, on our website, http://www.Inpellisrx.com/.
85
Table of Contents
EXECUTIVE AND DIRECTOR COMPENSATION
Summary Compensation Table
The following table sets forth information regarding the compensation awarded to, earned by, or paid to certain of our executive officers during the year ended December 31, 2014. The executive officers listed below are our principal executive officer, our former principal executive officer, and the two most highly compensated executive officers other than our principal executive officer. These officers are referred to as our “named executive officers.”
| Name & Principal Position |
Year | Salary ($) |
Bonus ($) |
Stock Awards ($) |
Option Awards ($) |
Non-equity incentive plan compensation (4) |
Non- qualified deferred compensation earnings ($) |
All other compensation ($) |
Total ($) |
|||||||||||||||||||||||||||
| Patrick T. Mooney, M.D. |
2014 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Harry G McCoy, Pharm.D., |
2014 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| David R Staskin, M.D., |
2014 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Frank A. Manguso, |
2014 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| * | Dr. McCoy served as our interim Chief Executive Officer from January 2015 to June 2015. |
Narrative Disclosure to Summary Compensation Table
Elements of executive compensation
Other than Dr. Mooney, none of our named executive officers received any compensation from the Company during the period ended June 30, 2015. During the six months ended June 30, 2015, the Company committed to issuing 1,384,616 shares of common stock to Dr. Mooney under an employment agreement. The Company has recorded a stock based compensation charge of approximately $5.4 million which represents the fair value of those shares. The compensation of our named executive officers in fiscal year 2015 will consist of base salary and equity awards as well as employee benefits that are made available to substantially all salaried employees.
Base salaries. Base salaries for our named executive officers are determined annually by our board of directors after reviewing the recommendation of our compensation committee, unless otherwise provided for in the agreements described below. When making its base salary recommendations to our board of directors, our compensation committee takes factors into account such as each executive’s experience and individual performance, the company’s performance as a whole, including our Chief Executive Officer’s assessment of the company’s performance, survey data for comparable life science companies, cost of living adjustments and other industry conditions, but does not assign any specific weighting to any factor. Dr. Mooney’s base salary is established by contract at $480,000 per year. Dr. Staskin’s base salary is established by contract at $240,000 per year. Our board of directors has approved a base salary for Mr. Manguso of $180,000 per year.
Equity awards. Our named executive officers will participate in our 2015 Equity Plan. See “Equity and Incentive plans—2015 Equity Plan” below for a description of this plan. These awards will generally be subject to time-based vesting conditions.
Cash bonuses. The Company does not now have a cash bonus plan, but may implement one during 2015.
86
Table of Contents
Benefits. We provide modest benefits to our named executive officers, which are limited to participation in our tax-qualified retirement plan, or our 401(k) plan, and basic health and welfare benefit coverage. These benefits are available to substantially all of our salaried employees.
Employment agreements. We have entered into employment agreements with our Chief Executive Officer and Chief Strategic Officer. See “Employment Agreements” below for a description of these agreements.
Grants of plan-based awards at fiscal year-end
Other than Dr. Mooney, none of our named executive officers received any grants of stock, option awards or other plan-based awards during the year ended December 31, 2014 or the six months ended June 30, 2015. During the six months ended June 30, 2015, the Company committed to issuing 1,384,616 shares of common stock to Dr. Mooney under an employment agreement. The Company has recorded a stock based compensation charge of approximately $5.4 million which represents the fair value of those shares.
Options exercised and stock vested at fiscal year-end
None of our named executive officers exercised any stock options, and no restricted stock units, if any, held by our named executive officers vested during the year ended December 31, 2014 or the six months ended June 30, 2015. The Company has no activity with respect to these awards.
Outstanding equity awards at fiscal year-end
There were no equity awards held by our named executive officers as of December 31, 2014 or the six months ended June 30, 2015.
Employment agreements
There were no formal employment agreements with any of our named executive officers as of December 31, 2014.
In June 2015, we entered into an employment agreement with Dr. Mooney. The agreement provides for participation in the benefit plans the Company provides to its employees generally, reimbursement of reasonable business travel, business development and other business expenses, and participation in the 2015 Equity Plan. It also contains restrictive covenants, including those pertaining to proprietary information, assignment of developments, non-competition and non-solicitation.
With respect to salary, Dr. Mooney, our Chief Executive Officer, will be paid an annual salary of $480,000, provided that the salary does not begin to be paid until the month in which the Company receives proceeds from the sale of shares of stock, or promissory notes convertible into shares of stock, which total $3,000,000 or more in the aggregate. Prior to that month, the Company is to pay him a monthly stipend of $10,000 until the month in which the Company receives proceeds from the sale of shares of stock, or promissory notes convertible into shares of stock, which total $500,000 or more in the aggregate. Commencing with the month in which the Company receives the $500,000 in proceeds described in the preceding sentence, the Company will pay Dr. Mooney a monthly stipend of $20,000. If, prior to the Company meeting the $3,000,000 threshold described above, it has received proceeds from the sale of shares of stock, or promissory notes convertible into shares of stock, which total $1,500,000 or more in the aggregate, the Company will pay Dr. Mooney a monthly stipend of $30,000 commencing with the month in which the aggregate proceeds received equal or exceed $1,500,000. Dr. Mooney is eligible to receive an annual bonus in an amount up to 100% of his base salary based on the Company’s financial performance in each calendar year. Pursuant to the 2015 Equity Plan, Dr. Mooney will receive a stock option for the purchase of 3,461,540 shares of Common Stock, which number will be subject to
87
Table of Contents
adjustment in the event of a stock split, reverse stock split, stock dividend or similar event, at 100% of the then fair market value of the common stock. The stock option grant notice will include a vesting schedule as follows: (i) 692,308 shares vest on closing of this offering, (ii) 692,308 shares vest on the date the first patient is enrolled in the contemplated pivotal Phase 2B/3 clinical trial of AX-IBU-01, (iii) 692,308 shares vest on the date the first patient is enrolled in the contemplated Phase 1/2 clinical trial of AX-DN-01, and (iv) 1,384,616 shares vest on the submission of a new drug application for AX-IBU-01. In addition, the Company also committed to issuing 1,384,616 shares of common stock to Dr. Mooney pursuant to a restricted stock agreement.
The term of Dr. Mooney’s employment agreement is for three years unless earlier terminated upon the first to occur of the following: (i) the employee’s death or permanent disability, (ii) the agreement is terminated for Cause (as defined in the agreement) by the Company; (iii) the agreement is terminated for Good Reason (as defined in the agreement) by the employee, (iv) the agreement is terminated without Cause by the Company, or (v) the agreement is terminated by the employee without Good Reason. Upon the termination of Dr. Mooney’s employment with Inpellis, Dr. Mooney is entitled to receive all earned or accrued but unpaid salary, accrued vacation and reimbursement of approved business expenses as well as all amounts or benefits to which Dr. Mooney is entitled under any applicable employee benefit plan in which he was a participant during his employment with Inpellis. If Dr. Mooney’s employment agreement is terminated by the Company without Cause or by Dr. Mooney with Good Reason, Dr. Mooney is entitled to receive a lump sum equal to his base salary and benefits for a period of 24 months and a lump sum equal to the annual bonus he received for the preceding year.
In September 2015, we entered into an employment agreement with Dr. Staskin. The agreement provides for participation in the benefit plans the Company provides to its employees generally, reimbursement of reasonable business travel, business development and other business expenses, and participation in the 2015 Equity Plan. It also contains restrictive covenants, including those pertaining to proprietary information, assignment of developments, non-competition and non-solicitation.
With respect to salary, Dr. Staskin, will be paid an annual salary of $240,000, provided that the salary does not begin to be paid until the month in which the Company closes its initial public offering. Dr. Staskin is eligible to receive an annual bonus in an amount determined by the board of directors in good faith. Pursuant to the 2015 Equity Plan, Dr. Staskin will receive a fully vested Incentive Stock Option for the purchase of 1,384,616 shares of Common Stock, which number will be subject to adjustment in the event of a stock split, reverse stock split, stock dividend or similar event, at 100% of the then fair market value of the common stock.
Dr. Staskin’s employment agreement terminates upon the first to occur of the following: (i) the employee’s death or permanent disability, (ii) the agreement is terminated for Cause (as defined in the agreement) by the Company; (iii) the agreement is terminated for Good Reason (as defined in the agreement) by the employee, (iv) the agreement is terminated without Cause by the Company, or (v) the agreement is terminated by the employee without Good Reason. Upon the termination of Dr. Staskin’s employment, he is entitled to receive all earned or accrued but unpaid salary, accrued vacation and reimbursement of approved business expenses as well as all amounts or benefits to which he is entitled under any applicable employee benefit plan in which he was a participant during his employment with the Company. If Dr. Staskin’s employment agreement is terminated by the Company without Cause or by Dr. Staskin with Good Reason, he is entitled to continue to receive his then current salary for 60 days after the date of termination.
Retirement plans
We do not maintain any qualified or non-qualified defined benefit plans or supplemental executive retirement plans that cover our named executive officers.
88
Table of Contents
Non-Employee Director Compensation Policy
Our board of directors has adopted a non-employee director compensation policy, effective in connection with this offering, which is designed to enable us to attract and retain, on a long-term basis, highly qualified non-employee directors. Under the policy, all non-employee directors will be paid cash compensation from and after the completion of this offering, as set forth in the following table:
| Annual Retainer |
||||
| Board of Directors: |
||||
| All non-employee members |
$ | 25,000 | ||
| Additional retainer for chair |
$ | 25,000 | ||
Under our non-employee director compensation policy, each non-employee director who is initially appointed or elected to our board of directors will be eligible to receive a grant of stock options to purchase shares of our common stock under the 2015 Equity Plan at the time of his or her initial appointment or election to our board of directors, which will vest annually in equal installments over a three-year period, subject to the director remaining in service on the applicable vesting date. In addition, each continuing non-employee director will be eligible to receive, at each annual meeting of our shareholders, an annual stock option grant to purchase shares of our common stock, which will vest in full on the first anniversary of the grant date, subject to the director remaining in service on the vesting date. The stock options will be granted with an exercise price equal to the fair market value of a share of our common stock on the date of grant and have a 10-year term. In connection with this offering, each non-employee director will receive the initial grant of stock options described above, which will have an exercise price equal to the offering price.
Equity and incentive plans
2015 Equity Plan
In connection with this offering, our board of directors will adopt the 2015 Equity Plan, and, following this offering, all equity-based awards will be granted under the 2015 Equity Plan. The following summary describes the material terms of the 2015 Equity Plan. This summary of the 2015 Equity Plan is not a complete description of all provisions of the 2015 Equity Plan and is qualified in its entirety by reference to the 2015 Equity Plan, which is filed as an exhibit to the registration statement of which this prospectus is a part.
Administration. The 2015 Equity Plan will be administered by our compensation committee. Our compensation committee has the authority to, among other things, interpret the 2015 Equity Plan, determine eligibility for, grant and determine the terms of awards under the 2015 Equity Plan, determine the form of settlement of awards (whether in cash, shares of our common stock or other property), and do all things necessary or appropriate to carry out the purposes of the 2015 Equity Plan. Our compensation committee’s determinations under the 2015 Equity Plan will be conclusive and binding.
Eligibility. Our key employees, directors, consultants and advisors will be eligible to participate in the 2015 Equity Plan.
Authorized Shares. Subject to adjustment, as described below, the maximum number of shares of our common stock that may be delivered in satisfaction of awards under the 2015 Equity Plan will be shares. The number of shares of our common stock available for issuance under the 2015 Equity Plan will be automatically increased annually on each January 1st, from January 1, through January 1, 2025, in an amount equal to the lesser of 4% of outstanding shares of our common stock as of the close of business on the immediately preceding December 31st or the number of shares determined by our board of directors. Subject to adjustment, as described below, no more than shares of our common stock may be delivered in satisfaction of incentive stock options, or ISOs, awarded under the 2015 Equity Plan.
89
Table of Contents
The shares of our common stock to be issued under the 2015 Equity Plan may be authorized but unissued shares of our common stock or previously issued shares of our common stock acquired by us. Any shares of our common stock underlying awards that are settled in cash, expire or become unexercisable without having been exercised or that are forfeited or repurchased by us will again be available for issuance under the 2015 Equity Plan. In addition, the number of shares of our common stock delivered in satisfaction of awards will be determined net of shares withheld by us in payment of the exercise price of an award or in satisfaction of tax withholding requirements with respect to an award.
Individual limits. The maximum number of shares of our common stock subject to stock options and the maximum number of shares of our common stock subject to stock appreciation rights, or SARs, that may be granted to any participant in the 2015 Equity Plan in any calendar year will be shares each. The maximum number of shares of our common stock subject to other awards that may be granted to any participant in the 2015 Equity Plan in any calendar year will be shares. A participant who is a non- employee director may not receive awards with respect to the greater of an aggregate of shares of our common stock or $ in aggregate grant date fair value in any calendar year.
Types of awards. The 2015 Equity Plan will provide for awards of stock options, SARs, restricted stock, unrestricted stock, stock units, performance awards and other awards convertible into or otherwise based on shares of our common stock. Eligibility for stock options intended to be ISOs will be limited to our employees. Dividend equivalents may also be provided in connection with an award under the 2015 Equity Plan.
Stock options and SARs. The exercise price of a stock option, and the base price against which a SAR is to be measured, may not be less than the fair market value (or, in the case of an ISO granted to a ten percent shareholder, 110% of the fair market value) of a share of our common stock on the date of grant. Our compensation committee will determine the time or times at which stock options or SARs become exercisable and the terms on which such awards remain exercisable.
| • | Restricted and unrestricted stock. A restricted stock award will be an award of shares of our common stock subject to forfeiture restrictions, while an unrestricted stock award will not be subject to such restrictions. |
| • | Stock units. A stock unit award will be an award denominated in shares of our common stock that entitles the participant to receive shares of our common stock or cash measured by the value of shares of our common stock in the future. The delivery of shares or cash under a stock unit may be subject to the satisfaction of performance conditions or other vesting conditions. |
| • | Performance awards. A performance award will be an award the vesting, settlement or exercisability of which is subject to specified performance criteria. |
| • | Other awards. Other awards will be awards that are convertible into or otherwise based on shares of our common stock. |
Performance awards. The 2015 Equity Plan will provide for the grant of performance awards that are made based upon, and subject to achieving, performance objectives. Performance objectives with respect to those awards that are intended to qualify as “performance-based compensation” for purposes of Section 162(m) of the Code, or Section 162(m), to the extent applicable, are limited to an objectively determinable measure or measures of performance relating to any or any combination of the following (measured either absolutely or by reference to an index or indices and determined either on a consolidated basis or, as the context permits, on a divisional, subsidiary, line of business, project or geographical basis or in combinations thereof): sales; revenues; assets; expenses; earnings before or after deduction for all or any portion of interest, taxes, depreciation, amortization or equity expense, whether or not on a continuing operations or an aggregate or per share basis; return on equity, investment, capital, capital employed or assets; one or more operating ratios; operating income or profit, including on an after-tax basis; net income; borrowing levels, leverage ratios or credit rating; market share; capital expenditures; cash flow; stock price; stockholder return; sales of particular products or services;
90
Table of Contents
customer acquisition or retention; acquisitions and divestitures (in whole or in part); joint ventures, strategic alliances, licenses or collaborations; spin-offs, split-ups and the like; reorganizations; recapitalizations, restructurings, financings (issuance of debt or equity) or refinancings; manufacturing or process development; or achievement of clinical trial or research objectives, regulatory or other filings or approvals or other product development milestones.
To the extent consistent with the requirements for satisfying the performance-based compensation exception under Section 162(m), to the extent applicable, our compensation committee may provide in the case of any award intended to qualify for such exception that one or more of the performance objectives applicable to an award will be adjusted in an objectively determinable manner to reflect events (for example, the impact of charges for restructurings, discontinued operations, mergers, acquisitions, extraordinary items, and other unusual or non-recurring items, and the cumulative effects of tax or accounting changes, each as defined by U.S. generally accepted accounting principles) occurring during the performance period that affect the applicable performance objectives.
Vesting; Termination of employment or service. Our compensation committee will have the authority to determine the vesting schedule applicable to each award, and to accelerate the vesting or exercisability of an award. Our compensation committee will determine the effect of a termination of employment or service on an award. Unless otherwise provided by our compensation committee, upon a termination of a participant’s employment or service, all unvested stock options and SARs then held by the participant will terminate and all other unvested awards will be forfeited and all vested stock options and SARs then held by the participant will remain outstanding for three months following such termination, or one year in the case of death, or, in each case, until the applicable expiration date, if earlier. All stock options and SARs held by a participant immediately prior to the participant’s termination of employment or service will immediately terminate if such termination is for cause, as defined in the 2015 Equity Plan, or occurs in circumstances that would have constituted grounds for the participant’s employment or service to be terminated for cause.
Non-transferability of awards. Awards under the 2015 Equity Plan may not be transferred other than by the laws of descent and distribution, unless, for awards other than ISOs, otherwise provided by our compensation committee.
Recovery of compensation. Our compensation committee may cancel, rescind, withhold or otherwise limit or restrict any award at any time under the 2015 Equity Plan if the participant is not in compliance with the provisions of the 2015 Equity Plan or any award thereunder or if the participant breaches any agreement with our company with respect to non-competition, non- solicitation or confidentiality. Our compensation committee also may recover any award or payments or gain in respect of any award under the 2015 Equity Plan in accordance with any applicable company recoupment policy or as otherwise required by applicable law or applicable stock exchange listing standards.
Certain transactions; Certain adjustments. In the event of a consolidation, merger or similar transaction or series of related transactions, including a sale or other disposition of shares of our common stock, in which our company is not the surviving corporation or that results in the acquisition of all or substantially all of our then outstanding shares of common stock by a single person or entity or by a group of persons and/or entities acting in concert, a sale of all or substantially all of our assets or our dissolution or liquidation, our compensation committee may, among other things, provide for the continuation or assumption of outstanding awards, for new grants in substitution of outstanding awards, for the accelerated vesting or delivery of shares under awards or for a cash-out of outstanding awards, in each case on such terms and with such restrictions as it deems appropriate. Except as our compensation committee may otherwise determine, awards not assumed in connection with such a transaction will terminate automatically and, in the case of outstanding restricted stock, will be forfeited automatically upon the consummation of such covered transaction.
91
Table of Contents
In the event of a stock dividend, stock split or combination of shares, including a reverse stock split, recapitalization or other change in our capital structure that constitutes an equity restructuring within the meaning of FASB ASC 718, our compensation committee will make appropriate adjustments to the maximum number of shares of our common stock that may be delivered under, and the ISO and individual share limits included in, the 2015 Equity Plan, and will also make appropriate adjustments to the number and kind of shares or securities subject to awards, the exercise prices of such awards or any other terms of awards affected by such change. Our compensation committee will also make the types of adjustments described above to take into account distributions and other events other than those listed above if it determines that such adjustments are appropriate to avoid distortion in the operation of the 2014 Equity Plan.
Amendment; Termination. Our compensation committee will be able to amend the 2015 Equity Plan or outstanding awards, or terminate the 2015 Equity Plan as to future grants of awards, except that our compensation committee will not be able to alter the terms of an award if it would affect materially and adversely a participant’s rights under the award without the participant’s consent (unless expressly provided in the 2015 Equity Plan or expressly reserved by our compensation committee). Shareholder approval will be required for any amendment to the 2015 Equity Plan to the extent such approval is required by law, including applicable stock exchange requirements.
92
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a description of transactions, since January 1, 2012, to which we have been a party, in which the amount involved exceeded or will exceed the lesser of $120,000 or 1% of the average of our total assets at year end for our last two completed fiscal years, and one or more of our executive officers, directors, director nominees or 5% stockholders, or their immediate family members, each of whom we refer to as a “related person,” had a direct or indirect material interest.
Indemnification agreements
We have entered into indemnification agreements with each of our directors. Prior to the completion of this offering, we expect to enter into amended and restated employment agreements with each of our executive officers. These indemnification and employment agreements will require us to indemnify these individuals and, in certain cases, affiliates of such individuals, to the fullest extent permissible under Delaware law against liabilities that may arise by reason of their service to us or at our direction, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified.
Purchase of Patents, Patent Applications and Intellectual Property
In October 2015 we paid $750,000 to purchase from BioChemics, our former parent, joint ownership or, in some cases, full ownership, of patents, patent applications and know how related to transdermal drug delivery as it relates to six pharmaceutical products for the prescriptive prophylactic or therapeutic treatment of non-dermal pain in humans. The products are transdermal ibuprofen, benfotiamine, gabapentin, celecoxib, rofecoxib and lidocaine (including products similar to lidocaine). The purchase covers 24 issued patents and pending patent applications in the United States or foreign countries. Of those patents and patent applications, we have purchased full ownership of six, all relating to ibuprofen. The remaining patents, patent applications and other intellectual property is owned jointly with BioChemics under a Joint Ownership Agreement. Under the terms of that agreement, we will use the jointly owned intellectual property in connection with six pharmaceutical products described above. Currently, one pending patent application, which we own jointly, covers the ibuprofen and benfotiamine transdermal products for which regulatory approval is being sought. We will continue to pursue patent protection in connection with development efforts and advances. We have also agreed with BioChemics that it will have a right of first offer if we determine to sell our ownership of the intellectual property we purchased from them. In addition, BioChemics has the option to purchase back from us the intellectual property related lidocaine (and similar products) for $750,000, provided that we would retain joint ownership of that intellectual property as it relates to the treatment of shingles and similar conditions. The Company’s Board of Directors made the determination as to the amount to pay for the assets purchased from BioChemics. The Board made the determination that the amount paid was fair and reasonable based on its knowledge of the effort that had gone into developing the intellectual property purchased and the value to the Company of owning that intellectual property rather than licensing it.See “Risk Factors—Risks related to our former parent corporation” and “Business—Intellectual Property.”
BioChemics
Inpellis was formed as a wholly owned subsidiary of BioChemics in March 2012. BioChemics formed Inpellis as part of its overall strategy to commercialize its transdermal technology. Under that strategy, BioChemics planned to create subsidiaries to which it would license its technology for use with specified products which BioChemics believed would be good candidates for commercialization. In the case of Inpellis, the focus was (and is) on products for pain resulting from musculoskeletal disorders and peripheral neuropathy. In addition, the plan was to recruit an independent, well qualified management team for each subsidiary (including Inpellis) to pursue commercialization, both in terms of research and financing. Until June 2014, the sole officer and director of Inpellis was John Masiz, then the Chief Executive Officer of BioChemics. Mr. Masiz resigned all of his positions with Inpellis in June 2014. The current management team now runs all aspects of the
93
Table of Contents
Inpellis business. They do consult with Mr. Masiz and expect to continue to do so. There is no formal consulting contract in place with Mr. Masiz and he is not being compensated by Inpellis or BioChemics for his consulting services. In recognition of the disgorgement and penalty obligations of BioChemics under the BioChemics SEC Enforcement Action, BioChemics transferred all of its shares in Inpellis to the Trust, which is currently the majority stockholder of Inpellis.
From our inception until our Original Issue Discount Convertible Note Offering in August 2015, all of our operations were financed through advances from BioChemics and direct payment of expenses by BioChemics on our behalf. Marshall Sterman, who serves as the Chief Executive Officer of BioChemics, was responsible for determining whether to make the above-referenced advances and direct payments of expenses. Prior to June 2014, Mr. Masiz made these determinations. BioChemics made the advances based on what amounts Inpellis needed to fund operations. BioChemics was interested in providing such funding because it wanted to benefit its shareholders who are beneficiaries of the Trust and it wanted to demonstrate that its commercialization strategy described above can be successful. In August 2015 BioChemics agreed that the Company is not obligated to repay these advances.
Based on the foregoing, BioChemics and John Masiz were promoters of Inpellis as such term is defined in Rule 405 of Regulation C promulgated under the Securities Act.
Related person transactions policy
We have adopted a related person transaction approval policy that will govern the review of related person transactions following the closing of this offering. Pursuant to this policy, if we want to enter into a transaction with a related person or an affiliate of a related person, our will review the proposed transaction to determine, based on applicable rules of the NASDAQ Global Market and the SEC, if such transaction requires pre-approval by our audit committee and/or our board of directors. If pre-approval is required, such matters will be reviewed at the next regular or special meeting of our audit committee and/or our board of directors. We may not enter into a related person transaction unless our audit committee or our board of directors has either specifically confirmed in writing that no further reviews are necessary or that all requisite corporate reviews have been obtained.
94
Table of Contents
The following table sets forth information relating to the beneficial ownership of our common stock as of November , 2015, by:
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our outstanding shares of common stock; |
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | all directors and executive officers as a group. |
Inpellis was formed as a wholly owned subsidiary of BioChemics in March 2012. In February 2014, a settlement was reached between BioChemics and the SEC in the BioChemics SEC Enforcement Action. BioChemics is still in discussions with the SEC regarding whether BioChemics stockholders who receive payments pursuant to the settlement will be required to relinquish their shares of BioChemics stock. In January 2015, in light of the on-going proceedings regarding the settlement and BioChemics disgorgement and penalty obligations thereunder, BioChemics transferred all of its shares in Inpellis to the Shareholder Resolution Trust, a Massachusetts trust, which was established to resolve the claims arising from the settlement in the BioChemics SEC Enforcement Action. The Trust is now the majority stockholder of Inpellis. The Trust is managed by three Trustees, all of whom must approve any decision. The three Trustees are Jack L. Altshuler, Esq., a Massachusetts attorney, Jan R. Schlichtmann, Esq., also a Massachusetts attorney, a director of BioChemics and the Managing Member of a limited liability company which is the largest stockholder of BioChemics, and Daniel M. Glosband, a Massachusetts attorney. The Trust is independent of BioChemics because it cannot act unless all three of its trustees approve the action and, of the three trustees, two have no connection to BioChemics. In addition, the Trustees have agreed that the Trust shall not distribute any shares of Inpellis to any of the individuals who are defendants in the BioChemics SEC Enforcement Action or any entity controlled by any such individual.
The number of shares beneficially owned by each entity, person, director or executive officer is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has the right to acquire within 60 days of November , 2015 through the exercise of any stock option, warrant or other right. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock held by that person.
95
Table of Contents
The percentage of shares beneficially owned is computed on the basis of 50,000,000 shares of our common stock outstanding as of November , 2015. Shares of our common stock that a person has the right to acquire within 60 days of November , 2015 are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless otherwise indicated below, the address for each beneficial owner listed is c/o Frank Manguso, Inpellis, Inc., 100 Cummings Center, Suite 463E, Beverly, MA 01915.
| Number of Shares Beneficially Owned |
Percentage of Common Stock Beneficially Owned |
|||||||||||
| Beneficial Owner |
Before Offering | After Offering | ||||||||||
| Directors and Executive Officers |
||||||||||||
| Patrick T. Mooney, M.D. |
2,076,924 | [ | ]% | [ | ]% | |||||||
| David R. Staskin, M.D. |
1,384,616 | [ | ]% | [ | ]% | |||||||
| Frank A. Manguso |
— | * | * | |||||||||
| John J Clarke Jr. |
— | * | * | |||||||||
| Frederic J. de Bure |
— | * | * | |||||||||
| Harry G McCoy, Pharm.D. |
— | * | * | |||||||||
| All current executive officers and directors as a group (8 persons) |
— | * | * | |||||||||
| Five Percent Stockholders |
||||||||||||
| Shareholder Resolution Trust, a Massachusetts trust(1) P.O. Box 233 Prides Crossing, MA 01965 |
50,000,000 | [ | ]% | [ | ]%(2) | |||||||
| Adec Private Equity Investments, LLC c/o SCS Financial, 919 3rd Avenue, 30th Floor New York, NY 10022 |
2,840,909 | (3) | [ | ]% | [ | ]%(2) | ||||||
| * | Represents beneficial ownership of 0% |
| (1) | The trustees of the Trust, by unanimous action, have voting control of the shares owned by the Trust. The trustees are: Jan R. Schlichtmann, Daniel M. Glosband and Jack L. Altshuler. |
| (2) | Assuming the underwriters do not exercise their option to acquire additional shares, as described in the section “Underwriting” below. If they do exercise in full their option to acquire additional shares, the Trust will own approximately [ ]% of our outstanding shares of common stock immediately after this offering. |
| (3) | Issuable upon conversion of Original Issue Discount Convertible Note at the option of the holder at any time. |
On the completion of this offering, we will plan to grant our named executive officers and directors stock options, SARs, restricted stock, unrestricted stock, stock units, performance awards or other awards convertible into or otherwise based on shares of our common stock under our 2015 Equity Plan.
96
Table of Contents
General
The following description of our capital stock is intended as a summary only and is qualified in its entirety by reference to our amended and restated certificate of incorporation and amended and restated by-laws that will be in effect at the closing of this offering, which will be filed as exhibits to the registration statement of which this prospectus is a part, and to the applicable provisions of the DGCL. We refer in this section to our amended and restated certificate of incorporation as our certificate of incorporation, and we refer to our amended and restated by-laws as our by-laws. The description of our capital stock reflects changes to our capital structure that will occur upon the closing of this offering.
Upon the closing of this offering and the filing of our amended and restated certificate of incorporation, our authorized capital stock will consist of shares of our common stock, par value $0.001 per share.
As of November , 2015, we had issued and outstanding:
| • | 51,384,616 shares of our common stock; |
| • | 2,000,000 warrants to purchase our common stock at an exercise price of $5.00 per share; |
| • | warrants to purchase our common stock at an exercise price per share to be determined based on the terms of this offering; and |
| • | warrants to purchase our common stock at an exercise price of $1.58 per share. |
As of November , 2015, we had two stockholders of record.
Common Stock Dividend Rights
Subject to preferences that may apply to shares of stock outstanding at the time, holders of outstanding shares of common stock will be entitled to receive dividends out of assets legally available at the times and in the amounts as our board of directors may from time to time determine.
Voting rights
Each outstanding share of common stock will be entitled to one vote on all matters submitted to a vote of stockholders. Holders of shares of our common stock shall have no cumulative voting rights.
Preemptive rights
Our common stock will not be entitled to preemptive or other similar subscription rights to purchase any of our securities.
Conversion or redemption rights
Our common stock will be neither convertible nor redeemable.
Liquidation rights
Upon our liquidation, the holders of our common stock will be entitled to receive pro rata our assets which are legally available for distribution, after payment of all debts and other liabilities
Preferred Stock
Our board of directors may direct the issuance of shares of preferred stock in series and may, at the time of issuance, determine the designations, powers, preferences, privileges, and relative participating, optional or special rights as well as the qualifications, limitations or restrictions thereof, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be
97
Table of Contents
greater than the rights of the common stock. Satisfaction of any dividend preferences of outstanding shares of preferred stock would reduce the amount of funds available for the payment of dividends on shares of our common stock. Holders of shares of preferred stock may be entitled to receive a preference payment in the event of our liquidation before any payment is made to the holders of shares of our common stock. Under certain circumstances, the issuance of shares of preferred stock may render more difficult or tend to discourage a merger, tender offer or proxy contest, the assumption of control by a holder of a large block of our securities or the removal of incumbent management. Upon the affirmative vote of a majority of the total number of directors then in office, our board of directors, without stockholder approval, may issue shares of preferred stock with voting and conversion rights which could adversely affect the holders of shares of our common stock and the market value of our common stock. Upon consummation of this offering, there will be no shares of preferred stock outstanding, and we have no present intention to issue any shares of preferred stock.
Warrants and Convertible Notes
On December 1, 2013, in connection with a consulting agreement for management and advisory service, we granted 4,000,000 warrants to purchase common stock to a consultant. The warrants have a life of five years and therefore expire on December 1, 2018. 1,000,000 warrants vested immediately on December 1, 2013 and 1,000,000 warrants were to vest on each of the next three anniversaries of the services agreement; provided, however that in the event of a public offering of the Company’s securities, the warrants shall immediately vest in their entirety. The warrants are exercisable at $5.00 per share. On June 10, 2015, the Company terminated the consulting agreement, at which point vesting of these warrants ceased with 2,000,000 warrants having vested.
On September 2, 2015, the Company completed its Original Issue Discount Convertible Note offering for gross proceeds of approximately $5 million. The principal amount of the notes in the aggregate was approximately $6.25 million. The maturity dates of the notes range from August 17, 2016 to September 2, 2016. The notes are convertible into shares of common stock at the option of the holder at any time at a conversion price of $1.32.
In the event the Company enters into a public offering of not less than $10 million the notes will automatically convert to shares of common stock at the lesser of $1.32 per share or the per share price of the common stock representing the pre-money valuation immediately prior to any shares being sold in the Company’s initial public offering, multiplied by 80%.
In addition the Company initially granted the note holders 3,551,135 warrants to purchase common stock at the lesser of $1.52 per share or at a 15% premium per share to the pre money initial public offering valuation if below $75,000,000. In the event the Company does not consummate its initial public offering or become public in some other manner on or before February 18, 2016, the Company will grant the note holders additional warrants to purchase common stock by increasing the warrant coverage from 75% to 100%.
The Company has also agreed that all shares issuable upon conversion of the notes be registered for public resale in the initial public offering registration statement at the discretion of the managing underwriter. If at least 25% of the shares of common stock issuable on conversion of the notes are not permitted to be included in the registration statement, the warrant coverage will increase from 75% to 100%. The managing underwriter has not made a determination as to whether to include such shares in the registration statement. If the determination is made to include some or all of such shares, the registration statement will be amended accordingly.
As compensation for acting as placement agent in connection with the Original Issue Discount Convertible Note Offering, we agreed to issue to Alexander Capital and/or its designees warrants to purchase up to 8% of the maximum number of shares of the Company’s common stock underlying the convertible notes sold to investors in this offering, up to a cap of 1,500,000 shares of common stock (the “Placement Agent Warrants”). The Placement Agent Warrants will be exercisable at any time, and from time to time, in whole or in part, during the four-year period commencing one year from the effective date of the offering, which period shall not extend further than five years from the effective date of the offering in compliance with FINRA Rule 5110(f)(2)(G)(i).
98
Table of Contents
The Placement Agent Warrants are exercisable at a fixed per share price equal to $1.58. The Placement Agent Warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. Alexander Capital (or permitted assignees under Rule 5110(g)(1)) will not sell, transfer, assign, pledge, or hypothecate these Placement Agent Warrants or the securities underlying these Placement Agent Warrants, nor will it engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Placement Agent Warrants or the underlying securities for a period of 180 days from the date of effectiveness of this offering. The exercise price and number of shares issuable upon exercise of the Placement Agent Warrants may be adjusted in certain circumstances including in the event of a stock dividend, stock split, or our recapitalization, reorganization, merger or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
The following table sets forth additional information regarding the above-referenced warrants and convertible notes.
| Total possible number of shares of common stock underlying the convertible notes(1) |
Minimum: 4,734,848 Maximum: 7,352,941 | |
| Total possible number of shares of common stock underlying the warrants(2) |
Minimum: 3,586,648 Maximum: 7,352,941 | |
| Shares of common stock outstanding prior to the transaction |
51,384,616 | |
| Shares of common stock outstanding prior to the transaction and held by persons other than the investors and affiliates of the Company |
0 | |
| Shares of common stock issuable in connection with the transaction(3) |
14,705,802 | |
| Percentage of total issued and outstanding securities issuable in the transaction(4) |
— |
| (1) | Minimum is $6,250,000 divided by the stated conversion price of $1.32. Maximum is $6,250,000 divided by 80% of $1.06 ($0.85). |
| (2) | Minimum is the current number of shares of common stock underlying the warrants. Maximum assumes the number of shares underlying the convertible notes have increased to their maximum and that the owners of the convertible notes have not been permitted to include any of the shares underlying those notes in this registration statement. |
| (3) | Assumes that the maximum numbers of shares (as set forth above) for each of the convertible notes and the warrants are issued. |
| (4) | The number of shares issued and outstanding prior to the transaction and held by persons other than the investors and affiliates of the Company was, as noted in the table, 0. |
Anti-takeover effects of our certificate of incorporation and our by-laws
Our certificate of incorporation and by-laws will contain certain provisions that are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and which may have the effect of delaying, deferring or preventing a future takeover or change in control of us unless such takeover or change in control is approved by our board of directors.
These provisions include:
Classified board. Our certificate of incorporation will provide that our board of directors will be divided into three classes of directors, with the classes as nearly equal in number as possible. As a result, approximately one-third of our board of directors will be elected each year. The classification of directors will have the effect of making it more difficult for stockholders to change the composition of our board. Our certificate of incorporation will also provide that, subject to any rights of holders of preferred stock to elect additional directors under specified circumstances, the number of directors will be fixed exclusively pursuant to a resolution adopted by our board of directors.
99
Table of Contents
Action by written consent; Special meetings of stockholders. Our certificate of incorporation will provide that stockholder action can be taken only at an annual or special meeting of stockholders and cannot be taken by written consent in lieu of a meeting. Our certificate of incorporation and the by-laws will also provide that, except as otherwise required by law, special meetings of the stockholders can only be called pursuant to a resolution adopted by a majority of our board of directors. Except as described above, stockholders will not be permitted to call a special meeting or to require our board of directors to call a special meeting.
Removal of directors. Our certificate of incorporation will provide that our directors may be removed only for cause by the affirmative vote of at least two-thirds of the voting power of our outstanding shares of capital stock, voting together as a single class. This requirement of a supermajority vote to remove directors could enable a minority of our stockholders to prevent a change in the composition of our board.
Advance notice procedures. Our by-laws will establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to the board of directors. Stockholders at an annual meeting will only be able to consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of our board of directors or by a stockholder who was a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given our Secretary timely written notice, in proper form, of the stockholder’s intention to bring that business before the meeting. Although the by-laws will not give our board of directors the power to approve or disapprove stockholder nominations of candidates or proposals regarding other business to be conducted at a special or annual meeting, the by-laws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempting to obtain control of us.
Authorized but unissued shares. Our authorized but unissued shares of common stock and preferred stock will be available for future issuance without stockholder approval. These additional shares may be utilized for a variety of corporate purposes, including future public offerings to raise additional capital, corporate acquisitions and employee benefit plans. The existence of authorized but unissued shares of common stock and preferred stock could render more difficult or discourage an attempt to obtain control of a majority of our common stock by means of a proxy contest, tender offer, merger or otherwise.
Exclusive forum. Our certificate of incorporation will provide that, subject to limited exceptions, the state or federal court located within the State of Delaware will be the sole and exclusive forum for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders, (3) any action asserting a claim against us arising pursuant to any provision of the DGCL, our certificate of incorporation or our by-laws, or (4) any other action asserting a claim against us that is governed by the internal affairs doctrine. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and to have consented to the provisions of our certificate of incorporation described above. Although we believe these provisions benefit us by providing increased consistency in the application of Delaware law for the specified types of actions and proceedings, the provisions may have the effect of discouraging lawsuits against our directors and officers. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with one or more actions or proceedings described above, a court could find the choice of forum provisions contained in our certificate of incorporation to be inapplicable or unenforceable.
Super majority approval requirements. The DGCL generally provides that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s certificate of incorporation, unless a corporation’s certificate of incorporation or by-laws requires a greater percentage. Our certificate of incorporation and by-laws will provide that the affirmative vote of holders of at least two-thirds of the total votes eligible to be cast in the election of directors will be required to amend, alter, change or repeal the provisions
100
Table of Contents
described above. This requirement of a supermajority vote to approve amendments to our certificate of incorporation could enable a minority of our stockholders to exercise veto power over any such amendments.
Section 203 of the DGCL
Upon completion of this offering, we will be subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested stockholder status, 15% or more of the corporation’s voting stock.
Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions: before the stockholder became interested, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances; or at or after the time the stockholder became interested, the business combination was approved by our board of directors of the corporation and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder.
A Delaware corporation may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or by-laws resulting from a stockholders’ amendment approved by at least a majority of the outstanding voting shares. We have not opted out of these provisions. As a result, mergers or other takeover or change in control attempts of us may be discouraged or prevented.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. The transfer agent and registrar’s address is 250 Royall Street, Canton, Massachusetts 02021.
Stock Market Listing
We intend to apply to list our common stock on the NASDAQ Global Market under the symbol “INPL”.
101
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock. Future sales of our common stock, including shares issued upon the exercise of outstanding options or warrants, in the public market after this offering, or the perception that those sales may occur, could cause the prevailing market price for our common stock to fall or impair our ability to raise equity capital in the future. As described below, only a limited number of shares of our common stock will be available for sale in the public market for a period of several months after completion of this offering due to contractual and legal restrictions on resale described below. Future sales of our common stock in the public market either before (to the extent permitted) or after restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price of our common stock at such time and our ability to raise equity capital at a time and price we deem appropriate.
Sale of restricted shares
As of November , 2015, based on the number of shares of our common stock then outstanding, upon the closing of this offering and assuming (1) no exercise of the underwriters’ option to purchase additional shares of common stock, and (2) no exercise of outstanding options or warrants, we would have had outstanding an aggregate of approximately [ ] shares of common stock. Of these shares, all of the shares of common stock to be sold in this offering, and any shares sold upon exercise of the underwriters’ option to purchase additional shares, will be freely tradable in the public market without restriction or further registration under the Securities Act, unless the shares are held by any of our “affiliates” as such term is defined in Rule 144 under the Securities Act. All remaining shares of common stock held by existing stockholders immediately prior to the completion of this offering will be “restricted securities” as such term is defined in Rule 144. These restricted securities were issued and sold by us, or will be issued and sold by us, in private transactions and are eligible for public sale only if registered under the Securities Act or if they qualify for an exemption from registration under the Securities Act, including the exemptions provided by Rule 144 or Rule 701, which rules are summarized below.
As a result of the lock-up agreements referred to below and the provisions of Rule 144 and Rule 701 under the Securities Act, [ ] shares of our common stock (excluding the shares sold in this offering) will be available for sale in the public market 180 days after the date of this prospectus upon expiration of the lock-up agreements referred to below, subject in some cases to applicable volume limitations under Rule 144.
Rule 144
In general, under Rule 144, as currently in effect, once we have been subject to the public company reporting requirements of the Exchange Act for at least 90 days, a person (or persons whose shares are required to be aggregated) who is not deemed to have been one of our “affiliates” for purposes of Rule 144 at any time during the three months preceding a sale, and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six months, including the holding period of any prior owner other than one of our “affiliates,” is entitled to sell those shares in the public market (subject to the lock-up agreement referred to above, if applicable) without complying with the manner of sale, volume limitations or notice provisions of Rule 144, but subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than “affiliates,” then such person is entitled to sell such shares in the public market without complying with any of the requirements of Rule 144 (subject to the lock-up agreements referred to above, if applicable). In general, under Rule 144, as currently in effect, once we have been subject to the public company reporting requirements of the Exchange Act for at least 90 days, our “affiliates,” as defined in Rule 144, who have beneficially owned the shares proposed to be sold for at least six months are entitled to sell in the public market, upon expiration of any applicable lock-up agreements and within any three-month period, a number of those shares of our common stock that does not exceed the greater of: 1% of the number of common shares then outstanding, which will equal approximately [ ] shares of common stock immediately after this offering (calculated on the basis of the number of shares of our common stock outstanding as of December 31, 2014 and
102
Table of Contents
the assumptions described above); or the average weekly trading volume of our common stock on the NASDAQ Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale.
Such sales under Rule 144 by our “affiliates” or persons selling shares on behalf of our “affiliates” are also subject to certain manner of sale provisions, notice requirements and to the availability of current public information about us. Notwithstanding the availability of Rule 144, the holders of substantially all of our restricted securities have entered into lock-up agreements as referenced above and their restricted securities will become eligible for sale (subject to the above limitations under Rule 144) upon the expiration of the restrictions set forth in those agreements.
Rule 701
In general, under Rule 701, as currently in effect, any of our employees, directors, officers, consultants or advisors who acquired common stock from us in connection with a written compensatory stock or option plan or other written agreement in compliance with Rule 701 under the Securities Act before the effective date of the registration statement of which this prospectus is a part (to the extent such common stock is not subject to a lock-up agreement) is entitled to rely on Rule 701 to resell such shares beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act in reliance on Rule 144, but without compliance with the holding period requirements contained in Rule 144. Accordingly, subject to any applicable lock-up agreements, beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act, under Rule 701 persons who are not our “affiliates,” as defined in Rule 144, may resell those shares without complying with the minimum holding period or public information requirements of Rule 144, and persons who are our “affiliates” may resell those shares without compliance with Rule 144’s minimum holding period requirements (subject to the terms of the lock-up agreements referred to above).
Equity incentive plans
We intend to file with the SEC a registration statement under the Securities Act covering the shares of common stock that we may issue upon exercise of outstanding options reserved for issuance under our 2015 Equity Plan, which will be established upon the closing of this offering. Such registration statement is expected to be filed as soon as practicable after the completion of this offering. Accordingly, shares registered under such registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations and the lock-up agreements described above.
Lock-Up Agreements
Our officers, directors and all holders of our outstanding shares, who hold an aggregate of approximately [●] shares of our common stock and/or shares underlying outstanding options to purchase common stock, have agreed, subject to limited exceptions, not to offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock held prior to the offering for a period of 180 days after the date of this prospectus, without the prior written consent of Alexander Capital, L.P., the representative of the underwriters. Alexander Capital, L.P., as the representative of the underwriters, may in its sole discretion choose to release any or all of these shares from these restrictions prior to the expiration of the 180-day period.
Following the lock-up periods set forth in the agreements described above, and assuming that the representatives of the underwriters do not release any parties from these agreements, all of the shares of our common stock that are restricted securities or are held by our affiliates as of the date of this prospectus will be eligible for sale in the public market in compliance with Rule 144 under the Securities Act.
103
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
The following is a summary of the material U.S. federal income and estate tax considerations relating to the purchase, ownership and disposition of our common stock by Non-U.S. Holders (defined below). This summary does not purport to be a complete analysis of all the potential tax considerations relevant to Non-U.S. Holders of our common stock. This summary is based upon the Internal Revenue Code of 1986, as amended, or the Code, the Treasury regulations promulgated thereunder and administrative and judicial interpretations thereof, all as of the date hereof and all of which are subject to differing interpretations and to change at any time, possibly on a retroactive basis.
This summary assumes that shares of our common stock are held as “capital assets” within the meaning of Section 1221 of the Code (generally, property held for investment). This summary does not purport to deal with all aspects of U.S. federal income and estate taxation that might be relevant to particular Non-U.S. Holders in light of their particular investment circumstances or status, nor does it address specific tax considerations that may be relevant to particular persons (including, for example, financial institutions, broker-dealers, insurance companies, partnerships or other pass-through entities, certain U.S. expatriates, tax-exempt organizations, pension plans, “controlled foreign corporations,” “passive foreign investment companies,” corporations that accumulate earnings to avoid U.S. federal income tax, persons in special situations, such as those who have elected to mark securities to market or those who hold common stock as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment, or holders subject to the alternative minimum tax or the 3.8% Medicare tax on “net investment income”). In addition, except as explicitly addressed herein with respect to estate tax, this summary does not address estate and gift tax considerations or considerations under the tax laws of any state, local or non-U.S. jurisdiction.
For purposes of this summary, a “Non-U.S. Holder” means a beneficial owner of common stock that for U.S. federal income tax purposes is not classified as a partnership and is not:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation or any other organization taxable as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate, the income of which is included in gross income for U.S. federal income tax purposes regardless of its source; or |
| • | a trust if (1) a U.S. court is able to exercise primary supervision over the trust’s administration and one or more U.S. persons have the authority to control all of the trust’s substantial decisions or (2) the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
If an entity that is classified as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of persons treated as its partners for U.S. federal income tax purposes will generally depend upon the status of the partner and the activities of the partnership. Partnerships and other entities that are classified as partnerships for U.S. federal income tax purposes and persons holding our common stock through a partnership or other entity classified as a partnership for U.S. federal income tax purposes are urged to consult their own tax advisors.
There can be no assurance that the IRS will not challenge one or more of the tax consequences described herein, and we have not obtained, nor do we intend to obtain a ruling from the IRS with respect to the U.S. federal income or estate tax consequences to a Non-U.S. Holder of the purchase, ownership or disposition of our common stock.
THIS SUMMARY IS FOR GENERAL INFORMATION ONLY AND IS NOT INTENDED TO BE TAX ADVICE. NON-U.S. HOLDERS ARE URGED TO CONSULT THEIR TAX ADVISORS
104
Table of Contents
CONCERNING THE U.S. FEDERAL INCOME AND ESTATE TAXATION, STATE, LOCAL AND NON-U.S. TAXATION AND OTHER TAX CONSEQUENCES TO THEM OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK.
Distributions on our common stock
As discussed under “Dividend policy” above, we do not currently expect to pay dividends. In the event that we do make a distribution of cash or property with respect to our common stock, any such distributions generally will constitute dividends for U.S. federal income tax purposes to the extent of our current and accumulated earnings and profits, if any, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will constitute a return of capital and will first reduce the holder’s adjusted tax basis in our common stock, but not below zero. Any remaining excess will be treated as capital gain, subject to the tax treatment described below in “—Gain on sale, exchange or other taxable disposition of our common stock.” Any such distribution would also be subject to the discussions below under the sections titled “—Additional withholding and reporting requirements” and “—Backup withholding and information reporting.”
Dividends paid to a Non-U.S. Holder generally will be subject to a 30% U.S. federal withholding tax unless such Non-U.S. Holder provides us or our agent, as the case may be, with the appropriate IRS Form W-8, such as:
| • | IRS Form W-8BEN or IRS Form W-8BEN-E (or successor forms) certifying, under penalties of perjury, an exemption from or a reduction in withholding under an applicable income tax treaty, or |
| • | IRS Form W-8ECI (or successor form) certifying that a dividend paid on common stock is not subject to withholding tax because it is effectively connected with a trade or business in the United States of the Non-U.S. Holder (in which case such dividend generally will be subject to regular graduated U.S. tax rates as described below). |
The certification requirement described above must be provided to us or our agent prior to the payment of dividends and must be updated periodically. The certification also may require a Non-U.S. Holder that provides an IRS form or that claims treaty benefits to provide its U.S. taxpayer identification number. Special certification and other requirements apply in the case of certain Non-U.S. Holders that hold shares of our common stock through intermediaries or are pass-through entities for U.S. federal income tax purposes.
Each Non-U.S. Holder is urged to consult its own tax advisor about the specific methods for satisfying these requirements. A claim for exemption will not be valid if the person receiving the applicable form has actual knowledge or reason to know that the statements on the form are false.
If dividends are effectively connected with a trade or business in the United States of a Non-U.S. Holder (and, if required by an applicable income tax treaty, attributable to a U.S. permanent establishment or fixed base), the Non-U.S. Holder, although exempt from the withholding tax described above (provided that the certifications described above are satisfied), generally will be subject to U.S. federal income tax on such dividends on a net income basis in the same manner as if it were a resident of the United States. In addition, if a Non- U.S. Holder is treated as a corporation for U.S. federal income tax purposes, the Non-U.S. Holder may be subject to an additional “branch profits tax” equal to 30% (unless reduced by an applicable income treaty) of its earnings and profits in respect of such effectively connected dividend income.
Non-U.S. Holders that do not timely provide us or our agent with the required certification, but which are eligible for a reduced rate of U.S. federal withholding tax pursuant to an income tax treaty, may obtain a refund or credit of any excess amount withheld by timely filing an appropriate claim for refund with the IRS.
105
Table of Contents
Gain on sale, exchange or other taxable disposition of our common stock
Subject to the discussions below under the sections titled “—Additional withholding and reporting requirements” and “—Backup withholding and information reporting,” in general, a Non-U.S. Holder will not be subject to U.S. federal income tax or withholding tax on gain realized upon such holder’s sale, exchange or other taxable disposition of shares of our common stock unless (1) such Non-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of disposition, and certain other conditions are met, (2) we are or have been a “United States real property holding corporation,” as defined in the Code, or a USRPHC, at any time within the shorter of the five-year period preceding the disposition and the Non-U.S. Holder’s holding period in the shares of our common stock, and certain other requirements are met, or (3) such gain is effectively connected with the conduct by such Non-U.S. Holder of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base maintained by such Non-U.S. Holder in the United States).
If the first exception applies, the Non-U.S. Holder generally will be subject to U.S. federal income tax at a rate of 30% (or at a reduced rate under an applicable income tax treaty) on the amount by which such Non-U.S. Holder’s capital gains allocable to U.S. sources exceed capital losses allocable to U.S. sources during the taxable year of the disposition. If the third exception applies, the Non-U.S. Holder generally will be subject to U.S. federal income tax with respect to such gain in the same manner as described above under “—Distributions on our common stock.”
Generally, a corporation is a USRPHC only if the fair market value of its U.S. real property interests (as defined in the Code) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance in this regard, we believe that we are not, and do not anticipate becoming, a USRPHC. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property interests relative to the fair market value of other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we became a USRPHC, a Non-U.S. Holder would not be subject to U.S. federal income tax on a sale, exchange or other taxable disposition of our common stock by reason of our status as USRPHC so long as our common stock is regularly traded on an established securities market at any time during the calendar year in which the disposition occurs and such Non-U.S. Holder does not own and is not deemed to own (directly, indirectly or constructively) more than 5% of our common stock at any time during the shorter of the five year period ending on the date of disposition and the holder’s holding period. However, no assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described above. Prospective investors are encouraged to consult their own tax advisors regarding the possible consequences to them if we are, or were to become, a USRPHC.
Additional withholding and reporting requirements
Sections 1471 through 1474 of the Code and Treasury regulations promulgated thereunder (commonly referred to as FATCA) impose, in certain circumstances, U.S. federal withholding at a rate of 30% on payments of (1) dividends on our common stock, and (2) gross proceeds from the sale or other disposition of our common stock on or after January 1, 2017. In the case of payments made to a “foreign financial institution” as defined under FATCA (including, among other entities, an investment fund), as a beneficial owner or as an intermediary, the tax generally will be imposed, subject to certain exceptions, unless such institution enters into (or is otherwise subject to) and complies with an agreement with the U.S. government, or a FATCA Agreement, or complies with an applicable intergovernmental agreement between the United States and a foreign jurisdiction (including any foreign law enacted in connection therewith, an “IGA”), in either case to, among other things, collect and provide to the U.S. or other relevant tax authorities certain information regarding U.S. account holders of such institution. In the case of payments made to a foreign entity that is not a foreign financial institution (as a beneficial owner), the tax generally will be imposed, subject to certain exceptions, unless such foreign entity provides the withholding agent with a certification that it does not have any “substantial U.S. owners” (generally, any
106
Table of Contents
specified U.S. person that directly or indirectly owns more than a specified percentage of such entity) or that identifies its substantial U.S. owners. If our common stock is held through a foreign financial institution that enters into (or is otherwise subject to) a FATCA Agreement, such foreign financial institution (or, in certain cases, a person paying amounts to such foreign financial institution) generally will be required, subject to certain exceptions, to withhold such tax on payments of dividends and proceeds described above made to (1) a person (including an individual) that fails to comply with certain information requests or (2) a foreign financial institution that has not entered into (and is not otherwise considered compliant with) a FATCA Agreement or complied with FATCA pursuant to an applicable IGA.
Prospective investors should consult their own tax advisors regarding the possible impact of these rules on their investment in our common stock, and the entities through which they hold our common stock, including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of this 30% withholding tax under FATCA.
Backup withholding and information reporting
We must report annually to the IRS and to each Non-U.S. Holder the gross amount of the distributions on our common stock paid to the holder and the tax withheld, if any, with respect to the distributions. Non-U.S. Holders may have to comply with specific certification procedures to establish that the holder is not a United States person (as defined in the Code) in order to avoid backup withholding at the applicable rate, currently 28%, with respect to dividends on our common stock. Dividends paid to Non-U.S. Holders subject to the U.S. withholding tax, as described above under the section titled “—Distributions on our common stock,” generally will be exempt from U.S. backup withholding.
Information reporting and backup withholding will generally apply to the proceeds of a disposition of our common stock by a Non-U.S. Holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a Non-U.S. Holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Prospective investors should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the Non-U.S. Holder resides or, in which the Non- U.S. Holder is incorporated, under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder can be refunded or credited against the Non-U.S. Holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
Federal estate tax
Common stock owned (or treated as owned) by an individual who is not a citizen or a resident of the United States (as defined for U.S. federal estate tax purposes) at the time of death will be included in the individual’s gross estate for U.S. federal estate tax purposes unless an applicable estate or other tax treaty provides otherwise, and therefore, may be subject to U.S. federal estate tax.
107
Table of Contents
Alexander Capital, L.P. is acting as the sole book-running manager of the offering and as representative of the underwriters, or the Representative. We have entered into an underwriting agreement, dated [ ], 2015, with the Representative. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to each underwriter named below, and each underwriter named below has severally and not jointly agreed to offer and sell on a best effort basis, the number of shares of common stock listed next to its name in the following table.
| Underwriters |
Number of Shares | |
| Alexander Capital, L.P. |
||
|
| ||
| Total |
||
|
|
This offering is being completed on a “best efforts” basis and the underwriters shares have no obligation to buy any shares of common stock from us or to arrange for the purchase or sale of any specific number or dollar amount of common stock. The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriters’ of officers’ certificates and legal opinions.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act of 1933, and to contribute to payments the underwriters may be required to make in respect thereof.
Commissions. The following table shows the public offering price, underwriting commissions, non-accountable expense allowance, and proceeds, before expenses, to us.
| Per Share | Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discount and commissions (7%) |
$ | $ | ||||||
| Non-accountable expense allowance (1%)(1) |
$ | $ | ||||||
| Proceeds, before expenses, to us |
$ | $ | ||||||
108
Table of Contents
The underwriters propose to offer the shares to the public at the public offering price per share set forth on the cover of this prospectus. In addition, the underwriters may offer some of the shares to other securities dealers at such price less a concession of $[ ] per share. After the initial public offering, the public offering price and concession to dealers may be changed.
Expenses. We have paid an aggregate expense deposit of $55,000 to the Representative for out-of-pocket accountable expenses, which will be applied against the accountable expense allowance (in compliance with FINRA Rule 5110(f)(2)(c)) that will be paid by us to the Representative in connection with this offering. The underwriting agreement, however, provides that in the event the offering is terminated, the $55,000 expense deposit paid to the Representative will be returned to the extent such out-of-pocket accountable expenses are not actually incurred in accordance with FINRA Rule 5110(f)(2)(C).
We have also agreed to pay the Representative’s expenses relating to the offering, including (a) all fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed $5,000 in the aggregate; (b) all filing fees associated with the review of this offering by FINRA; (c) all fees, expenses and disbursements relating to the registration or qualification of securities offered under the “blue sky” securities laws of such states and other jurisdictions designated by the Representative, including up to a maximum payment of $15,000 in legal fees to the Representative’s counsel in connection with fees, expenses and disbursements related only to “blue sky” filings for an offering commenced on the Over the Counter Bulletin Board; (d) all fees, expenses and disbursements relating to the registration, qualification or exemption of securities offered under the securities laws of foreign jurisdictions designated by the Representative; (e) the costs associated with commemorative mementos and Lucite tombstones in an amount not to exceed $5,000; (f) the reasonable fees and expenses of the Representative’s legal counsel; (g) the $21,775 cost associated with the use of Ipreo’s book-building, prospectus tracking and compliance software for this offering; and (h) up to $20,000 of the Representative’s actual accountable road show expenses for this offering. Notwithstanding the foregoing, the total aggregate amount of accountable expenses to be reimbursed by us will not exceed $175,000.
We estimate that the total expenses of the offering payable by us, excluding the total underwriting commission, will be approximately $[ ].
Discretionary Accounts. The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements. Pursuant to certain “lock-up” agreements, we, our executive officers and directors, and all holders of our outstanding shares of common stock on a fully diluted basis (including shares underlying options, warrants and convertible securities) have agreed, subject to certain exceptions, not to offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic risk of ownership of, directly or indirectly, engage in any short selling of any common stock or securities convertible into or exchangeable or exercisable for any common stock, whether currently owned or subsequently acquired, without the prior written consent of the representative, for a period of 180 days from the date of effectiveness of the offering.
Representative’s Warrants. We have agreed to issue to the Representative and/or its designees warrants to purchase up to a total of [ ] shares of common stock (7% of the shares of common stock sold in this offering). The warrants will be exercisable at any time, and from time to time, in whole or in part, during the four-year period commencing one year from the effective date of the offering , which period shall not extend further than five years from the effective date of the offering in compliance with FINRA Rule 5110(f)(2)(G)(i). The warrants are exercisable at a per share price equal to $[ ] per share, or 120% of the public offering price per share in the offering. The warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The Representative (or permitted assignees under Rule 5110(g)(1)) will not sell, transfer, assign, pledge, or hypothecate these warrants or the securities
109
Table of Contents
underlying these warrants, nor will it engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the warrants or the underlying securities for a period of 180 days from the date of effectiveness. In addition, the warrants provide for registration rights upon request, in certain cases. The demand registration right provided will not be greater than five years from the effective date of the offering in compliance with FINRA Rule 5110(f)(2)(G)(iv). The piggyback registration right provided will not be greater than seven years from the effective date of the offering in compliance with FINRA Rule 5110(f)(2)(G)(v). We will bear all fees and expenses attendant to registering the securities issuable on exercise of the warrants other than underwriting commissions incurred and payable by the holders. The exercise price and number of shares issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend, stock split, or our recapitalization, reorganization, merger or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
Right of First Refusal. For a period of 18 months from the effective date of this offering, we will grant to the Representative an irrevocable right of first refusal to provide any financing arrangements to the Company during such 18-month period, with the role of the Representative to be determined at the time of such financings, on terms and conditions customary to the Representative for such transactions.
Electronic Offer, Sale and Distribution of Shares. A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The Representative may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Determination of the Initial Public Offering Price
Prior to this offering, there has been no public market for our common stock. The initial public offering price was determined through negotiations between us and the Representative. In addition to prevailing market conditions, the factors considered in determining the initial public offering price included the following:
| • | the information included in this prospectus and otherwise available to the Representative; |
| • | the valuation multiples of publicly traded companies that the Representative believes to be comparable to us; |
| • | our financial information; |
| • | our prospects and the history and the prospectus of the industry in which we compete; |
| • | an assessment of our management, its past and present operations, and the prospects for, and timing of, our future revenues; |
| • | the present state of our development; and |
| • | the above factors in relation to market values and various valuation measures of other companies engaged in activities similar to ours. |
An active trading market for our common stock may not develop. It is also possible that, after the offering, the shares will not trade in the public market at or above the initial public offering price.
110
Table of Contents
Stabilization. In connection with this offering, the underwriters may engage in stabilizing transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
| • | Stabilizing transactions permit bids to purchase shares of common stock so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the shares of common stock while the offering is in progress. |
| • | Syndicate covering transactions involve purchases of shares of common stock in the open market after the distribution has been completed in order to cover syndicate short positions. |
| • | Penalty bids permits the Representative to reclaim a selling concession from a syndicate member when the shares of common stock originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our shares of common stock or preventing or retarding a decline in the market price of our shares of common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on the NASDAQ Global Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Other Relationships. The Representative has provided, and certain underwriters and their affiliates, including the Representative, may in the future provide, various investment banking, commercial banking and other financial services for us and our affiliates for which they have received, and may in the future receive, customary fees. However, except as disclosed in this prospectus, we have no present arrangements with any of the underwriters for any further services.
On September 2, 2015, the Company completed the Original Issue Discount Convertible Note Offering and issued $6,250,000 in aggregate principal amount of original issue discount convertible notes (the “Convertible Bridge Notes”), which shall convert into shares of the Company’s common stock upon the closing of the Offering, together with certain warrants to purchase the Company’s common stock, for gross proceeds of approximately $5 million. The Representative acted as placement agent in connection with the Original Issue Discount Convertible Note Offering. Neither the Representative, nor any of its associated persons or affiliates, purchased any securities in the Original Issue Discount Convertible Note Offering.
As compensation for acting as placement agent in connection with the Original Issue Discount Convertible Note Offering, the Company paid to the Representative a cash placement fee in the aggregate amount of $500,000 and issued to the Representative Placement Agent Warrants to purchase up to 8% of the maximum number of shares of the Company’s common stock underlying the Convertible Bridge Notes sold to investors in the Original Issue Discount Convertible Note Offering, up to a cap of 1,500,000 shares of common stock. The Placement Agent Warrants will be exercisable at any time, and from time to time, in whole or in part, during the four-year period commencing one year from the effective date of the offering, which period shall not extend further than five years from the effective date of the offering in compliance with FINRA Rule 5110(f)(2)(G)(i). The Placement Agent Warrants are exercisable at a fixed per share price equal to $1.58. The Placement Agent Warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The Representative (or permitted assignees under Rule 5110(g)(1)) will not sell, transfer, assign, pledge, or hypothecate these Placement Agent Warrants or the securities underlying these Placement Agent Warrants, nor will it engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Placement Agent Warrants or the underlying securities for a period of 180 days from the date of effectiveness of this offering. The exercise price and number of shares issuable upon exercise of the Placement Agent Warrants may be adjusted in certain circumstances including in the event of a stock dividend, stock split, or our recapitalization, reorganization, merger or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
111
Table of Contents
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction.
Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer for the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area—Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of common stock will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of common stock has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
| • | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| • | to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statement); |
112
Table of Contents
| • | to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)I of the Prospectus Directive) subject to obtaining the prior consent of the company or any underwriter for any such offer; or |
| • | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of common stock shall result in a requirement for the publication by the company of a prospectus pursuant to Article 3 of the Prospectus Directive. |
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The common stock has not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the common stock has not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs non-qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the common stock cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The common stock has not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The common stock offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the ISA), or ISA, nor have such common stock been registered for sale in Israel. The shares and warrants may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the common stock being offered. Any resale in Israel, directly or indirectly, to the public of the common stock offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
113
Table of Contents
Italy
The offering of the common stock in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, “CONSOB”) pursuant to the Italian securities legislation and, accordingly, no offering material relating to the common stock may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| • | to Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
| • | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the common stock or distribution of any offer document relating to the common stock in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
| • | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
| • | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the common stock in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such common stock being declared null and void and in the liability of the entity transferring the common stock for any damages suffered by the investors.
Japan
The common stock has not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the common stock may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires common stock may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of common stock is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The common stock has not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the common stock has not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissão do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of common
114
Table of Contents
stock in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the common stock be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of common stock in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The common stock may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the common stock may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the common stock has been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of common stock will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the common stock have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor have we received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the common stock within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the common stock, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by us.
No offer or invitation to subscribe for common stock is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the common stock. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the common stock may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances that do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
115
Table of Contents
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the common stock has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to us.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
116
Table of Contents
The validity of the issuance of our common stock offered in this prospectus will be passed upon for us by Holland & Knight LLP, Boston, Massachusetts. Certain legal matters in connection with this offering will be passed upon for the underwriters by Greenberg Traurig, LLP, New York, New York.
The audited financial statements of Inpellis Inc. (then known as Alterix Inc.) as of December 31, 2014 and 2013, and for the years then ended, included in this prospectus have been so included in reliance on the report of Marcum LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting. The report on the financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information with respect to us and the common stock offered hereby, reference is made to the registration statement and the exhibits and schedules filed therewith. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. A copy of the registration statement and the exhibits and schedules filed therewith may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street N.E., Washington, D.C. 20549, and copies of all or any part of the registration statement may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address is www.sec.gov.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act and, in accordance therewith, will file periodic reports, proxy statements and other information with the SEC. Such periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and website of the SEC referred to above.
117
Table of Contents
INPELLIS INC.
| F-2 | ||||
| Balance Sheets as of June 30, 2015 (unaudited) and December 31, 2014 and 2013 |
F-3 | |||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 |
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Alterix Inc.
We have audited the accompanying balance sheets of Alterix Inc. (the “Company”) as of December 31, 2014 and 2013, and the related statements of operations, stockholders deficiency and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Alterix Inc. as of December 31, 2014 and 2013, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As more fully discussed in Note 2, the Company has incurred net losses since inception and needs to raise additional funds to meet its obligations and sustain its operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Marcum LLP
Marcum LLP
New York, NY
April 8, 2015
F-2
Table of Contents
INPELLIS, INC.
(formerly known as Alterix Inc.)
| As of June 30, 2015 (Unaudited) |
As of December 31, 2014 |
As of December 31, 2013 |
||||||||||
| ASSETS | ||||||||||||
| Current Assets: |
||||||||||||
| Cash |
$ | 4,395 | $ | 50 | $ | — | ||||||
| Prepaid expenses |
32,788 | 8,635 | 16,212 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Current Assets |
37,183 | 8,685 | 16,212 | |||||||||
|
|
|
|
|
|
|
|||||||
| Equipment, net |
4,045 | 7,054 | 17,019 | |||||||||
| Deferred offering costs |
559,470 | 99,256 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Assets |
$ | 600,698 | $ | 114,995 | $ | 33,231 | ||||||
|
|
|
|
|
|
|
|||||||
| LIABILITIES AND STOCKHOLDER’S DEFICIENCY | ||||||||||||
| Current Liabilities: |
||||||||||||
| Accounts payable |
$ | 588,760 | $ | 165,722 | $ | 137,644 | ||||||
| Accrued compensation and expenses |
6,094,136 | 594,316 | 176,506 | |||||||||
| Derivative liabilities |
1,382,000 | 1,660,000 | 820,000 | |||||||||
| Payable to parent |
3,517,948 | 3,097,891 | 2,301,308 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Current Liabilities |
11,582,844 | 5,517,929 | 3,435,458 | |||||||||
|
|
|
|
|
|
|
|||||||
| Commitments and Contingencies |
— | — | — | |||||||||
| Stockholder’s Deficiency: |
||||||||||||
| Common Stock, $0.001 par value; 50,000,000 shares authorized; 50,000,000 shares issued and outstanding at June 30, 2015 (unaudited), December 31, 2014 and December 31, 2013 |
50,000 | 50,000 | 50,000 | |||||||||
| Additional paid in capital |
(50,000 | ) | (50,000 | ) | (50,000 | ) | ||||||
| Accumulated deficit |
(10,982,146 | ) | (5,402,934 | ) | (3,402,227 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total Stockholder’s Deficiency |
(10,982,146 | ) | (5,402,934 | ) | (3,402,227 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total Liabilities and Stockholder’s Deficiency |
$ | 600,698 | $ | 114,995 | $ | 33,231 | ||||||
|
|
|
|
|
|
|
|||||||
The Accompanying Footnotes are an Integral Part of these Financial Statements
F-3
Table of Contents
INPELLIS, INC.
(formerly known as Alterix Inc.)
| For the six months ended | For the years ended | |||||||||||||||
| June 30, 2015 | June 30, 2014 | |||||||||||||||
| (Unaudited) | (Unaudited) | December 31, 2014 | December 31, 2013 | |||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Operating Expenses |
||||||||||||||||
| Research and development |
44,165 | 220,734 | 294,234 | 620,013 | ||||||||||||
| Research and development—patents |
16,570 | 59,013 | 80,287 | 76,385 | ||||||||||||
| Business legal and consulting |
206,725 | 210,000 | 622,500 | — | ||||||||||||
| General and administrative |
5,589,752 | 104,826 | 163,686 | 349,878 | ||||||||||||
| Consulting services |
— | 410,000 | 830,000 | 820,000 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Operating Expenses |
5,857,212 | 1,004,573 | 1,990,707 | 1,866,276 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other (income) expense |
||||||||||||||||
| Change in fair value of derivative liabilities |
(278,000 | ) | (93,000 | ) | 10,000 | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net Loss |
$ | (5,579,212 | ) | $ | (911,573 | ) | $ | (2,000,707 | ) | $ | (1,866,276 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net Loss per Share; Basic and Diluted |
$ | (0.11 | ) | $ | (0.02 | ) | $ | (0.04 | ) | $ | (0.04 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-Average Number of Shares Used per Common Share Calculations: |
||||||||||||||||
| Basic and Diluted |
50,000,000 | 50,000,000 | 50,000,000 | 50,000,000 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The Accompanying Footnotes are an Integral Part of these Financial Statements
F-4
Table of Contents
INPELLIS, INC.
(formerly known as Alterix Inc.)
STATEMENT OF CHANGES IN STOCKHOLDER’S DEFICIENCY
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
AND FOR THE SIX MONTHS ENDED JUNE 30, 2015 (UNAUDITED)
| Common Stock | Additional Paid in Capital |
Accumulated Deficit |
Total Stockholder’s Deficiency |
|||||||||||||||||
| Shares | Amount | |||||||||||||||||||
| Balance—January 1, 2013 |
50,000,000 | $ | 50,000 | $ | (50,000 | ) | $ | (1,535,951 | ) | $ | (1,535,951 | ) | ||||||||
| Stock based compensation |
— | — | 820,000 | — | 820,000 | |||||||||||||||
| Reclassification of warrants to derivative liability |
— | — | (820,000 | ) | — | (820,000 | ) | |||||||||||||
| Net loss |
— | — | — | (1,866,276 | ) | (1,866,276 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance December 31, 2013 |
50,000,000 | 50,000 | (50,000 | ) | (3,402,227 | ) | (3,402,227 | ) | ||||||||||||
| Stock based compensation |
— | — | 830,000 | — | 830,000 | |||||||||||||||
| Reclassification of warrants to derivative liability |
— | — | (830,000 | ) | — | (830,000 | ) | |||||||||||||
| Net Loss |
— | — | — | (2,000,707 | ) | (2,000,707 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance December 31, 2014 |
50,000,000 | 50,000 | (50,000 | ) | (5,402,934 | ) | (5,402,934 | ) | ||||||||||||
| Net Loss |
— | — | — | (5,579,212 | ) | (5,579,212 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance June 30, 2015 (Unaudited) |
50,000,000 | $ | 50,000 | $ | (50,000 | ) | $ | (10,982,146 | ) | $ | (10,982,146 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The Accompanying Footnotes are an Integral Part of these Financial Statements
F-5
Table of Contents
INPELLIS, INC.
(formerly known as Alterix Inc.)
| For the six months ended | ||||||||||||||||
| June 30, 2015 | June 30, 2014 | For the years ended | ||||||||||||||
| (Unaudited) | (Unaudited) | December 31, 2014 | December 31, 2013 | |||||||||||||
| Cash Flows from Operating Activities |
||||||||||||||||
| Net Loss |
$ | (5,579,212 | ) | $ | (911,573 | ) | $ | (2,000,707 | ) | $ | (1,866,276 | ) | ||||
| Adjustments to Reconcile Net Loss to Net Cash Used in Operating Activities: |
||||||||||||||||
| Depreciation and amortization |
3,009 | 4,983 | 9,965 | 11,605 | ||||||||||||
| Stock based compensation |
5,400,002 | 410,000 | 830,000 | 820,000 | ||||||||||||
| Change in fair value of derivative liabilities |
(278,000 | ) | (93,000 | ) | 10,000 | — | ||||||||||
| Changes in Operating Assets and Liabilities: |
||||||||||||||||
| Prepaid Expenses |
(24,153 | ) | 14,535 | 7,577 | (10,916 | ) | ||||||||||
| Accounts Payable |
423,038 | (53,401 | ) | 28,078 | 51,426 | |||||||||||
| Accrued compensation and expenses |
3,762 | 196,104 | 417,810 | 99,623 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net Cash Used in Operating Activities |
(51,554 | ) | (432,352 | ) | (697,277 | ) | (894,538 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash Flows from Financing Activities |
||||||||||||||||
| Deferred offering costs |
(364,158 | ) | — | (99,256 | ) | — | ||||||||||
| Advances from parent |
420,057 | 432,352 | 796,583 | 894,538 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net Cash Provided by Financing Activities |
55,859 | 432,352 | 697,327 | 894,538 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net Increase in Cash |
4,345 | — | 50 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash, Beginning of Period |
50 | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash, End of Period |
$ | 4,395 | $ | — | $ | 50 | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Non-cash investing and financing activities Reclassification of warrants to derivative liability |
$ | — | $ | — | $ | 830,000 | $ | 820,000 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Deferred offering costs included in accrued compensation and expenses |
$ | 96,056 | $ | — | — | $ | — | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The Accompanying Footnotes are an Integral Part of these Financial Statements
F-6
Table of Contents
INPELLIS, INC.
(formerly known as Alterix Inc.)
Note 1. Business Organization
Organization
Alterix, Inc. (the “Company” or Inpellis) is a wholly-owned subsidiary of BioChemics, Inc. (“BioChemics”) and was incorporated under the laws of the State of Delaware on March 13, 2012. The Company’s principal office is located in Danvers, Massachusetts. The Company is a clinically late stage healthcare company that intends to develop, design and produce safe and effective products focusing on drug solutions for chronic and acute pain resulting from musculoskeletal disorders, cancer, and peripheral neuropathy. In January 2015, Biochemics transferred all of its common shares of Inpellis to the Shareholder Resolution Trust, a Massachusetts Trust.
The Company’s primary activities have been the research and development of its business plan, negotiating strategic alliances and other agreements, and raising capital. Expenses of the Company include those specifically identifiable to the Company, and allocations of expenses from the Company’s parent. The allocated expenses were primarily based on the use of estimates. Expenses allocated from the Company’s parent were costs which benefitted the Company and were required for its operations. Certain general corporate expenses of the Company’s parent were not allocated because they did not provide a direct or material benefit to the business. In the opinion of management, the methods of allocating costs were reasonable; however, such costs did not necessarily equal costs that the Company would have incurred on a stand-alone basis. Therefore, the financial information included herein may not necessarily reflect assets and liabilities and cash flows of the Company if operated on a stand-alone basis. To date, the Company has not generated any revenues from its operations.
Unaudited Interim Results
The accompanying balance sheet as of June 30, 2015, statements of operations and cash flows for the six months ended June 30, 2015 and 2014 and statement of changes in stockholder’s deficiency for the six months ended June 30, 2015 are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to present fairly the Company’s financial position as of June 30, 2015 and the results of operations and cash flows for the six months ended June 30, 2015 and 2014. The financial data and other information disclosed in the notes to the financial statements related to the six months ended June 30, 2015 and 2014 are unaudited. The results for the six months ended June 30, 2015 are not indicative of the results to be expected for the year ended December 31, 2015 or for any other interim period or for any future year.
Note 2. Liquidity and Going Concern
The Company has not generated any revenue, has recurring net losses, a working capital deficiency as of June 30, 2015, December 31, 2014 and 2013 of approximately $11,546,000, $5,509,000 and $3,419,000, respectively, and had cash used in operations of approximately $52,000, $697,000 and $895,000 for the six months ended June 30, 2015, and for the years ended December 31, 2014 and 2013, respectively. In addition, as of June 30, 2015, and December 31, 2014 and 2013, the Company had an accumulated deficit of approximately $10,982,000, $5,403,000 and $3,402,000, respectively. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The Company’s former parent, BioChemics, Inc., has historically funded the operations of Inpellis. Subsequent to June 30, 2015, the Company has continued to be funded by its former parent through cash advances and direct payment of expenses. In addition, on September 2, 2015, the Company completed an Original Issue Discount Convertible Note Offering and issued approximately $6.25 million in aggregate principal amount of original issue discount convertible notes for gross proceeds of approximately $5 million.
F-7
Table of Contents
In addition, the Company needs to raise additional capital from either its parent BioChemics, Inc. or from external sources in order to sustain its operations while continuing the longer term efforts contemplated under its business plan. The Company expects to continue incurring losses for the foreseeable future and must raise additional capital to pursue its product development initiatives, penetrate markets for the sale of its products and continue as a going concern. The Company cannot provide any assurance that it will raise additional capital. Management believes that the Company has access to capital resources through possible public or private equity offerings, debt financings, corporate collaborations or other means; however, the Company has not secured any commitment for new financing at this time nor can it provide any assurance that new financing will be available on commercially acceptable terms, if at all. If the Company is unable to secure additional capital, it may be required to curtail its research and development initiatives and take additional measures to reduce costs in order to conserve its cash in amounts sufficient to sustain operations and meet its obligations. These measures could cause significant delays in the Company’s clinical and regulatory efforts, which is critical to the realization of its business plan and the future operations of the Company. The accompanying financial statements do not include any adjustments that may be necessary should the Company be unable to continue as a going concern.
The Company has undertaken efforts to commence an IPO of its equity securities (Note 5). In its efforts to complete the IPO, the Company incurred and capitalized approximately $99,000 of deferred IPO costs through December 31, 2014 and $559,000 through June 30, 2015.
Note 3. Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from these estimates. Significant estimates and assumptions include the expense allocations from the Company’s parent, valuation allowance related to the Company’s deferred tax assets, stock based compensation for warrants, the fair value of common stock to be issued and derivative liabilities.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less to be cash equivalents. As of June 30, 2015, December 31, 2014 and 2013, the Company had no cash equivalents.
Equipment, Net
Equipment is stated at cost less accumulated depreciation and amortization. Depreciation of equipment is computed using the straight-line method over the estimated useful lives of the respective assets. The Company’s primary asset is manufacturing, lab and other equipment and is depreciated over 5 years. Expenditures for maintenance and repairs, which do not extend the economic useful life of the related assets, are charged to operations as incurred, and expenditures, which extend the economic life, are capitalized. When assets are retired, or otherwise disposed of, the costs and related accumulated depreciation or amortization are removed from the accounts and any gain or loss on disposal is recognized.
Fair Value of Financial Instruments
Financial instruments, including cash, accounts payable and accrued liabilities are carried at cost, which management believes approximates fair value due to the short-term nature of these instruments.
Stock-Based Compensation
The Company accounts for share-based awards exchanged for employee services at the estimated grant date fair value of the award. The Company estimates the fair value of employee stock awards using the Black-Scholes
F-8
Table of Contents
valuation model. The Company amortizes the fair value of employee stock awards on a straight-line basis over the requisite service period of the awards. Compensation expense includes the impact of an estimate for forfeitures for all stock awards. During the six months ended June 30, 2015, and the years ended December 31, 2014 and 2013 the Company did not grant any options to employees.
The Company accounts for equity instruments issued to non-employees at their fair value on the measurement date. The measurement of stock-based compensation is subject to periodic adjustment as the underlying equity instrument vests or becomes non-forfeitable. Non-employee stock-based compensation charges are amortized over the vesting period or as earned.
The Company recorded stock-based compensation charges (for warrants issued), net of estimated forfeitures of approximately $0, $830,000 and $820,000 for the six months ended June 30, 2015, and for the years ended December 31, 2014 and 2013, respectively, which have been included as consulting services.
The Company recorded stock-based compensation charges (for common stock to be issued) of approximately $5,400,000 for the six months ended June 30, 2015 which have been included as general and administrative expenses in the accompanying statement of operations.
Fair Value Accounting of Equity-Based Compensation
The Company used the Income Approach to arrive at an estimated fair value of the Company’s common stock for the years ended December 31, 2014 and 2013.
The Income Approach (for determining the fair value of the Company’s common stock) is based on the economic principle of competition (i.e., in a free market, forces of demand and supply will direct the values of businesses to a particular balance). Valuation under the Income Approach entails both the application of appropriate market-based multiples selected from guideline public companies (GPCs) to parameters such as level of earnings, cash flow, revenues, invested capital or other financial factors (financial metrics) that represent the subject company’s future financial performance and or from cash transactions related to the sale of securities of the Company. This method is based on the idea of determination of the price at which the company will be exchanged in the public market, and is particularly useful for valuing companies that are either currently profitable or expected to be making profits in the foreseeable future.
Derivative Liabilities
In connection with the issuance of certain warrants that include price protection reset or anti-dilution provisions, the Company determined that these provision features are derivative instruments pursuant to FASB ASC 815 “Derivatives and Hedging.”
The accounting treatment of derivative financial instruments requires that the Company record the warrants as a liability at fair value and mark-to-market the instruments at fair values as of each subsequent balance sheet date. Any change in fair value is recorded as a change in the fair value of derivative liabilities for each reporting period at each balance sheet date. The Company reassesses the classification at each balance sheet date. If the classification changes as a result of events during the period, the contract is reclassified as of the date of the event that caused the reclassification.
Research and Development
Research and Development expense is charged to operations as incurred and consists primarily of personnel expenses, patent related legal expenses and filing fees, clinical and regulatory services and supplies. Research and development expense was approximately $60,000, $375,000 and $696,000 for the six months ended June 30, 2015, and for the years ended December 31, 2014 and 2013, respectively.
F-9
Table of Contents
Net Loss per Share
The Company computes basic and diluted earnings per share by dividing net loss by the weighted average number of common shares outstanding during the period. Basic and diluted net loss per common share were the same since the inclusion of common shares issuable pursuant to the exercise of warrants in the calculation of diluted net loss per common shares would have been anti-dilutive.
The following table summarizes the number of common share equivalents excluded from the calculation of diluted net loss per common share for the six months ended June 30, 2015 and the years ended December 31, 2014 and 2013:
| As of June 30, 2015 (unaudited) |
As of December 31, | |||||||||||
| 2014 | 2013 | |||||||||||
| Warrants |
2,000,000 | 4,000,000 | 4,000,000 | |||||||||
| Common stock to be issued |
1,384,616 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total common stock equivalents |
3,384,616 | 4,000,000 | 4,000,000 | |||||||||
|
|
|
|
|
|
|
|||||||
Income Taxes
The Company accounts for income taxes under Accounting Standards Codification (“ASC”) 740 Income Taxes (“ASC 740”). Under ASC 740, deferred tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities and net operating loss and credit carryforwards using enacted tax rates in effect for the year in which the differences are expected to impact taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
ASC 740 also clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return.
Tax benefits claimed or expected to be claimed on a tax return are recorded in the Company’s financial statements. A tax benefit from an uncertain tax position is only recognized if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution. Uncertain tax positions have had no impact on the Company’s financial condition, results of operations or cash flows.
Recent Accounting Pronouncements
The FASB has issued ASU No. 2014-12, Compensation—Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. This ASU requires that a performance target that affects vesting, and that could be achieved after the requisite service period, be treated as a performance condition. As such, the performance target should not be reflected in estimating the grant date fair value of the award. This update further clarifies that compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. The amendments in this ASU are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. Earlier adoption is permitted. The Company is currently evaluating the effect of the ASU on its financial position, results of operations and cash flows.
In August, 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties About an Entities Ability to Continue as a Going Concern. The standard is intended to define management’s responsibility to decide whether there is substantial doubt about an
F-10
Table of Contents
organization’s ability to continue as a going concern and to provide related footnote disclosures. The standard requires management to decide whether there are conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. The standard provides guidance to an organization’s management, with principles and definitions that are intended to reduce diversity in the timing and content of disclosures that are commonly provided by organizations in the footnotes. The standard becomes effective in the annual period ending after December 15, 2016, with early application permitted. The adoption of this pronouncement is not expected to have a material impact on the financial statements.
The FASB and the SEC have issued certain other accounting standards updates and regulations that will become effective in subsequent periods; however, management of the Company does not believe that any of those updates would have significantly affected the Company’s financial statements or disclosures had they been in effect during 2015 or 2014, and does not believe that any of those standards will have a significant impact on the Company’s financial statements at the time they become effective.
Subsequent Events
Management has evaluated subsequent events or transactions occurring through the date these financial statements were issued (See Note 11 and 12).
Note 4. Equipment, Net
As of June 30, 2015, December 31, 2014 and 2013, equipment consists of the following:
| As of June 30, 2015 (unaudited) |
As of December 31, | |||||||||||
| 2014 | 2013 | |||||||||||
| Lab Equipment |
$ | 143,386 | $ | 143,386 | $ | 143,386 | ||||||
| Less: Accum. Depreciation |
$ | (139,341 | ) | (136,332 | ) | (126,367 | ) | |||||
|
|
|
|
|
|
|
|||||||
| Total Equipment, Net |
$ | 4,045 | $ | 7,054 | $ | 17,019 | ||||||
|
|
|
|
|
|
|
|||||||
The Company recorded a charge for depreciation expense of $3,000 and $5,000 for the six months ended June 30, 2015 and 2014, respectively, and a charge of approximately $10,000 and $11,600 for the years ended December 31, 2014 and 2013, respectively.
Note 5. Accrued Expenses
As of June 30, 2015, December 31, 2014 and 2013, accrued expenses consist of the following:
| As of June 30, 2015 (unaudited) |
As of December 31, | |||||||||||
| 2014 | 2013 | |||||||||||
| Legal Expenses |
$ | 96,056 | $ | 69,257 | — | |||||||
| Salaries and Related Costs |
584,903 | 164,400 | $ | 175,216 | ||||||||
| Research & Development-Patents |
2,096 | 200 | 1,290 | |||||||||
| Consulting Fees |
10,000 | 360,000 | — | |||||||||
| Fair Value of Common Stock to be Issued |
5,400,002 | — | — | |||||||||
| Other |
1,079 | 459 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 6,094,136 | $ | 594,316 | $ | 176,506 | ||||||
|
|
|
|
|
|
|
|||||||
Deferred offering costs consist of legal fees in connection with the filing of the Company’s Form S-1 Registration Statement with the Securities and Exchange Commission.
Salaries and related costs consist of accrued unpaid salaries and benefits for employees.
F-11
Table of Contents
Consulting fees all relate to fees accrued under a contract with Logic International. Logic International was contracted by the Company to provide management and consulting services for assistance with functional operation, corporate governance, and to assist with financing and merger and acquisition activities.
Note 6 Fair Value
ASC 820 “Fair Value Measurements and Disclosures” defines fair value, establishes a framework for measuring fair value and requires enhanced disclosures about fair value measurements. As defined in ASC 820, fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Standard clarifies that the exchange price is the price in an orderly transaction between market participants to sell an asset or transfer a liability at the measurement date and emphasizes that fair value is a market-based measurement and not an entity-specific measurement.
ASC 820 establishes the following hierarchy used in fair value measurements and expands the required disclosures of assets and liabilities measured at fair value:
Level 1—Inputs use quoted prices in active markets for identical assets or liabilities that the Company has the ability to access.
Level 2—Inputs that are observable, either directly or indirectly. These inputs include quoted prices for similar assets and liabilities in active markets as well as other inputs such as interest rates and yield curves that are observable at commonly quoted intervals.
Level 3—Inputs are unobservable, including inputs that are available in situations where there is little, if any, market activity for the related asset or liability.
In instances where inputs used to measure fair value fall into different levels in the above fair value hierarchy, fair value measurements in their entirety are categorized based on the lowest level input that is significant to the valuation. The Company’s assessment of the significance of particular inputs to these fair measurements requires judgment and considers factors specific to each asset or liability.
Liabilities measured at fair value on a recurring basis at June 30, 2015, December 31, 2014 and 2013 are as follows:
| Quoted Prices in Active Markets for Identical Liabilities (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Balance | |||||||||||||
| Fair value of common stock to be issued |
$ | — | $ | — | $ | 5,400,002 | $ | 5,400,002 | ||||||||
| Warrant liability |
$ | — | $ | — | $ | 1,382,000 | $ | 1,382,000 | ||||||||
| Total at June 30, 2015 (unaudited) |
$ | — | $ | — | $ | 6,782,002 | $ | 6,782,002 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Warrant liability—December 31, 2014 |
$ | — | $ | — | $ | 1,660,000 | $ | 1,660,000 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Warrant liability—December 31, 2013 |
$ | — | $ | — | $ | 820,000 | $ | 820,000 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable. The Company’s Level 3 liabilities consist of derivative liabilities associated with warrants that include price protection reset or Anti Dilution Provisions.
The Company’s management is responsible for determining the inputs for its level 3 instruments. An increase or decrease in these inputs could significantly increase or decrease the fair values as reported.
F-12
Table of Contents
The following table provides a summary of the changes in fair value, including net transfers in and/or out, of all financial assets measured at fair value on a recurring basis using significant unobservable inputs during the six months ended June 30, 2015 and for the years ended December 31, 2014 and 2013.
| Warrant Liability |
||||
| Balance at—January 1, 2013 |
$ | — | ||
| Change in fair value of derivative liabilities |
— | |||
| Reclassification of derivative liabilities from equity |
820,000 | |||
|
|
|
|||
| Balance at December 31, 2013 |
820,000 | |||
| Change in fair value of derivative liabilities |
10,000 | |||
| Reclassification of derivative liabilities from equity |
830,000 | |||
|
|
|
|||
| Balance at December 31, 2014 |
1,660,000 | |||
| Stock based compensation |
5,400,002 | |||
| Change in fair value of derivative liabilities |
(278,000 | ) | ||
|
|
|
|||
| Balance at June 30, 2015 (unaudited) |
$ | 6,782,002 | ||
|
|
|
|||
Note 7. Commitments and Contingencies
Litigation
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company, but which will only be resolved when one or more future events occur or fail to occur. The Company assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment.
In assessing loss contingencies related to legal proceedings that are pending against the Company, or unasserted claims that may result in such proceedings, the Company evaluates the perceived merits of any legal proceedings or unasserted claims, as well as the perceived merits of the amount of relief sought or expected to be sought therein.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potentially material loss contingency is not probable, but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability and an estimate of the range of possible losses, if determinable and material, would be disclosed.
Loss contingencies considered remote are generally not disclosed, unless they involve guarantees, in which case the guarantees would be disclosed. There can be no assurance that such matters will not materially and adversely affect the Company’s business, financial position, and results of operations or cash flows. As of June 30, 2015, December 31, 2014 and 2013, the Company is not involved in any litigation and accordingly has not accrued any amounts for contingencies.
The Company is a co-signer under a credit arrangement entered into by its parent. The arrangement functions as a guarantee of up to $2.1 million with its parent. As of June 30, 2015, the Company has asserted it has no obligation with respect to this arrangement and therefore no liability under this arrangement has been reflected in the June 30, 2015, December 31, 2014 and 2013 balance sheets.
In February 2014, a settlement was reached between BioChemics and the SEC in the BioChemics SEC Enforcement Action. On March 24, 2015, a final judgment was entered against BioChemics where by BioChemics is required to disgorge approximately $17 million of funds. Inpellis is not a party to this judgment. In January 2015 BioChemics transferred all of its common stock to the shareholder resolution trust, a Massachusetts trust.
F-13
Table of Contents
Credit Risk
The Company maintains the cash in bank deposit amounts which, at times, may exceed federally insured limits. The Company has not experienced any losses on such accounts and does not believe it is exposed to any significant credit risk on cash.
Consulting Agreement
On December 1, 2013, the Company entered into a consulting agreement for management and advisory service. The agreement requires the Company to pay the consultant a minimum of $30,000 per month. The term of the services agreement is for one year, with automatic renewal thereafter for additional 180 day periods unless either party provides written notice of termination 90 days prior to the expiration date. In addition, the Company granted 4,000,000 warrants to purchase common stock to the consultant. The warrants have a life of five years and therefore expire on December 1, 2018. 1,000,000 warrants vested immediately on December 1, 2013 and 1,000,000 warrants vest on each of the next three anniversaries of the services agreement; provided, however that in the event of a public offering of the Company’s securities, the warrants shall immediately vest in their entirety. As of June 30, 2015, 2,000,000 warrants have vested. The warrants are exercisable at $5.00 per share, however, in the event that the Company issues any new securities in a material transaction at a price per share less than $5.00, the warrants then become exercisable at such lower price per share, subject to certain exceptions.
On June 10, 2015, the Company amended its consulting agreement whereby the consultant agreed to release the Company from all obligations to it (BioChemics assumed those obligations) and terminated its service contract with the Company, at which point vesting of these warrants ceased. In addition the warrant agreement was amended to eliminate the section of that warrant that adjusted the exercise price in the event of a subsequent sale of shares at a purchase price less than that of the warrant. As of June 30, 2015 the fair value of 2,000,000 warrants has been included in derivative liabilities as the Company does not have sufficient authorized shares to settle the contract. Upon completion of increasing the authorized shares, the fair value of the warrants will be reclassified to equity.
Fair Value of Common Stock to be Issued
In June 2015, the Company entered into an employment agreement with the Chief Executive Officer of the Company for an annual salary of $480,000 the salary does not begin to be paid or accrued until the month in which the Company receives proceeds from the sale of shares of stock, or promissory notes convertible into shares of stock, which total $3,000,000 or more in the aggregate. Prior to that month, the Company is to pay or accrue him a monthly stipend of $10,000. In addition under the terms of the agreement the Chief Executive Officer is entitled to receive an annual bonus in an amount up to 100% of his base salary based on the Company’s financial performance in each calendar year. The Company will also pay the Chief Executive Officer a one-time bonus within 180 days of the closing of the Company’s initial public offering calculated under the terms of the agreement. Pursuant to the completion of the Company’s 2015 Equity Plan, the Chief Executive Officer will receive an Incentive Stock Option for the purchase of 3,461,540 shares of Common Stock, exercisable at 100% of the then fair market value of the common stock. The Incentive Stock Option Grant will vest based on performance criterial as defined in the terms of the agreement. The Company also committed to issuing the Chief Executive Officer 1,384,616 shares of common stock. The shares of common stock were fully vested on the date of the agreement and are non-forfeitable. As of June 30, 2015 the Company has recorded a stock based compensation charge of approximately $5.4 million in the statement of operations for the six months ended June 30, 2015. The fair value of the common stock has been included in accrued compensation and expenses as the Company did not have sufficient authorized shares to issue the common stock and will therefore be marked to market at each reporting period. Upon the completion of the increase in the authorized shares the fair value of the common stock will be reclassified to equity. (See Note 12)
F-14
Table of Contents
Proposed Initial Public Offering
On April 6, 2015 the Board of Directors authorized the Company to file a registration statement with the U.S. Securities and Exchange Commission in connection with its IPO. The Company incurred approximately $559,000 of cumulative IPO costs through June 30, 2015 and $99,000 of such costs through December 31, 2014 consisting of professional fees in preparation of filing the registration statement on Form S-1. These amounts are presented as deferred offering costs in the accompanying balance sheets at June 30, 2015 and December 31, 2014.
The Company cannot provide any assurance that it will complete its proposed IPO. The Company expects to incur substantial additional costs in connection with its efforts to complete this offering. Upon the Company’s closing of its IPO, these costs will be recorded as a reduction of the proceeds received. If the Company does not successfully complete its IPO, the costs will be recorded as a charge to operations.
License Agreement
On November 30, 2012, the Company entered into a worldwide, royalty-free, exclusive license agreement with BioChemics pursuant to which BioChemics has agreed to license certain pharmaceutical products to the Company for utilization in the prescriptive prophylactic or therapeutic treatment of pain in humans. In consideration of this license, Inpellis issued to BioChemics 50,000,000 shares of Common Stock of Inpellis in connection with the formation of the Company.
The agreement contains representations and warranties of the parties regarding its enforceability, no conflict with agreement to which the parties are bound, and no violations of law, and representations of BioChemics that it has not granted any other license with respect to the Products for use in the Field or Territory. Inpellis has agreed to indemnify BioChemics with respect to third party claims arising from Inpellis breach of representations and warranties or negligence or willful misconduct. Either party may terminate the agreement for an uncured material breach, but only after undergoing a dispute resolution process. In addition, either party may terminate the agreement if the other party ceases to do business, makes an assignment for the benefit of creditors or voluntarily files, fails to contest an involuntary filing or is adjudicated bankrupt or insolvent under bankruptcy, insolvency, receivership or similar law.
Reimbursement Agreement
On June 1, 2015 the Company entered into a reimbursement agreement with BioChemics to repay operating expenses that were allocated from BioChemics as follows: on or before April 15 of each year beginning on April 15, 2016, the Company shall pay BioChemics an amount equal to five percent of net revenue for the preceding calendar year until such time as the amounts due to BioChemics is fully repaid. (See Note 12)
Note 8. Common Stock
The Company is authorized to issue up to 50,000,000 shares of common stock with a par value of $0.001 per share. Each share of common stock has the right to one vote. The holders of common stock are entitled to dividends when funds are legally available and when declared by the board of directors. Upon the formation of the Company 50,000,000 shares were originally issued to BioChemics. In January 2015, BioChemics transferred all of its common shares of Inpellis to the Shareholder Resolution Trust, a Massachusetts Trust.
Note 9. Compensation Expense—Warrants
During the year ended December 31, 2013, the Company granted 4,000,000 warrants to purchase common stock to a consultant for services rendered. The warrants have a life of five years and expire on December 1, 2018. 1,000,000 shares vested immediately on December 1, 2013, the effective date of the services agreement, and 1,000,000 shares vest on each of the next three anniversaries of the services agreement; provided, however that in the event of a public offering of the Company’s securities, the warrants shall immediately vest in their
F-15
Table of Contents
entirety. The warrants are exercisable at $5.00 per share, however, in the event that the Company issues any new securities in a material transaction at a price per share less than $5.00, the warrants then become exercisable at such lower price per share, subject to certain exceptions. For the six months ended June 30, 2015 and the years ended December 31, 2014 and 2013 the Company recorded compensation expense of $0, $830,000 and $820,000 respectively, in accordance with ASC 505-50 “Equity Based Payments to Non-Employees.” (See Note 12.)
In addition, under the terms of the warrant agreement, in the event the Company enters into a qualified financing in which shares of stock are sold at a price less than the warrant exercise price, the warrant price will be adjusted accordingly. In accordance with the provisions of ASC 815-40, these warrants are subject to derivative accounting treatment once performance is completed, the warrants are vested and no longer subject to ASC 505-50 and are recorded as a liability which is marked to market at each reporting date. Any change in fair value is recorded as a change in fair value of derivative liabilities in the accompanying statements of operations. The Company reassesses the classification at each balance sheet date to determine if liability treatment is appropriate, if the classification changes as a result of events during the period, the contract is reclassified to equity as of the date of the event that caused the reclassification.
For the years ended December 31, 2014 and 2013 the Company recorded compensation expense of $830,000 and $820,000 respectively, in accordance with ASC 505-50 “Equity Based Payments to Non-Employees.”
On June 10, 2015, the Company amended its consulting agreement whereby the consultant agreed to release the Company from all obligations to it (BioChemics assumed those obligations) and terminated its service contract with the Company, at which point vesting of these warrants ceased. In addition the warrant agreement was amended to eliminate the section of that warrant that adjusted the exercise price in the event of a subsequent sale of shares at a purchase price less than that of the warrant. In accordance with this amendment the Company assessed the classification of the warrant and deemed the warrant to be a liability instrument since it did not have sufficient authorized shares to settle the contract and has continued to report the warrant as a derivative liability.
As of June 30, 2015, 2,000,000 warrants have vested. For the six months ended June 30, 2015 and 2014 the Company recorded compensation expense of $0, and $410,000, respectively, in accordance with ASC 505-50 “Equity Based Payments to Non-Employees.”
The Company’s common stock is not listed on any exchange and, accordingly, the Company used a valuation model to arrive at an estimated fair value of the Company’s equity instruments for the years ended December 31, 2014 and 2013. To assist us with the valuation of the equity instruments a third party valuation specialist was utilized to obtain the fair value of the Company’s equity instruments.
The Company and valuation specialist considered the following significant qualitative factors in preparing the fair value analyses:
| • | The nature and history of the entity’s business; |
| • | The general economic conditions and specific industry outlook; |
| • | The book value of the entity and its financial condition; |
| • | The future earnings capacity of the entity; |
| • | The entity’s distribution potential; |
| • | The existence of intangible value (patents) within the business; and |
| • | Capital resource requirements |
The Company is considered an operating entity expected to generate future cash flows. Accordingly, the Company and the valuation specialist primarily relied on the Income Approach in arriving at a value for the Company’s equity. Given the significant risk associated with the development process and clinical trials, there is
F-16
Table of Contents
a very low rate of success for companies in the biotechnology industry to successfully develop and market their treatments. To capture this low success rate, the probability-weighted forecasted cash flows based on assumptions developed by the Company as well as observed historical success rates at each stage of clinical trials were developed. The discount rates were determined from the weighted average cost of capital from discount rates appropriate for companies in the early development stage not yet conducting clinical trials. These rates were derived from analyzing similar guideline companies in the industry. The concluded value for the Company’s total equity was approximately $196.8 million and $150.9 million as of December 31, 2014 and 2013, respectively. In addition the Black-Scholes valuation model was used to allocate the Company’s total equity value among the Company’s outstanding equity instruments to determine the fair value of the common stock and warrants. The Company determined the binomial lattice model and the Black-Scholes valuation model to be materially the same.
The significant assumptions used in the valuation model were as follows:
| December 31, | ||||
| 2014 | 2013 | |||
| Fair value of total equity |
$196.8 million | $150.9 million | ||
| Fair value of common stock per share |
$3.90 | $2.98 | ||
| Fair value of price per warrant |
$0.83 | $0.82 | ||
| Volatility |
65% | 75% | ||
| Discount rates |
18.5% | 19.5% | ||
| Risk—free rate |
0.44% | 0.76% | ||
| Term to liquidity |
1.92 years | 2.92 years | ||
A summary of changes in outstanding warrants during the years ended December 31, 2014 and 2013 and for the six months ended June 30, 2015 is as follows:
| Number of Shares |
Weighted Average Exercise Price Per Share |
Weighted Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding at January 1, 2013 |
— | |||||||||||||||
| Granted |
4,000,000 | $ | 5.00 | 5.00 | — | |||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited |
— | — | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Outstanding at December 31, 2013 |
4,000,000 | $ | 5.00 | 4.92 | — | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Granted |
— | — | ||||||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited |
— | — | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Outstanding at December 31, 2014 |
4,000,000 | $ | 5.00 | 3.92 | — | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Granted |
— | — | ||||||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited |
(2,000,000 | ) | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Outstanding at June 30, 2015 (Unaudited) |
2,000,000 | $ | 5.00 | 3.67 | — | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Exercisable at June 30, 2015 (Unaudited) |
2,000,000 | $ | 5.00 | — | ||||||||||||
| Exercisable at December 31, 2014 |
2,000,000 | $ | 5.00 | — | ||||||||||||
| Exercisable at December 31, 2013 |
1,000,000 | $ | 5.00 | — | ||||||||||||
F-17
Table of Contents
Note 10. Income Taxes
The following summarizes the income tax provision (benefit):
| 2014 | 2013 | |||||||
| Current |
||||||||
| Federal |
— | |||||||
| State |
— | |||||||
| Deferred |
||||||||
| Federal |
$ | (680,136 | ) | $ | (634,534 | ) | ||
| State |
$ | (105,621 | ) | $ | (98,539 | ) | ||
| Change in Valuation Allowance |
$ | 785,757 | $ | 733,073 | ||||
|
|
|
|
|
|||||
| Income Tax Provision (Benefit) |
— | — | ||||||
|
|
|
|
|
|||||
The reconciliation between the U.S. statutory federal income tax rate and the Company’s effective tax rate for the year’s ended December 31, 2014 and 2013 is as follows:
| 2014 | 2013 | |||||||
| U.S. Federal at Statutory Rate |
(34.0 | )% | (34.0 | )% | ||||
| State Income Taxes, Net of Federal Benefit |
(5.3 | )% | (5.3 | )% | ||||
| Permanent Differences |
0.7 | % | ||||||
| Change in Valuation Allowance |
38.6 | % | 39.3 | % | ||||
| Income Tax Provision |
0.0 | % | 0.0 | % | ||||
As of December 31, 2014 and 2013, the Company’s deferred tax assets consisted of the effects of temporary differences attributable to the following:
| 2014 | 2013 | |||||||
| Net Operating Loss Carryovers |
$ | 2,122,151 | $ | 1,336,394 | ||||
| Less: Valuation Allowance |
$ | (2,122,151 | ) | $ | (1,336,394 | ) | ||
|
|
|
|
|
|||||
| Deferred Tax Asset, Net of Valuation Allowance |
— | — | ||||||
|
|
|
|
|
|||||
The Company is included in U.S. federal and state tax returns with its parent. These tax returns are subject to examination by tax authorities for periods beginning with the year ended December 31, 2012, however, this footnote has been presented as if the Company is filing its tax returns on a single company basis.
As of December 31, 2014, the Company determined that there has been no change of control since the Company’s formation for purposes of Internal Revenue Code Section 382. The Company, after considering all available evidence, fully reserved its deferred tax assets since it is more likely than not that such benefits will not be realized in future periods. The valuation allowance increased by $785,757 at December 31, 2014. The Company has incurred losses for both financial reporting and income tax purposes for the period from March 13, 2012 (inception) to December 31, 2014. For the years ended December 31, 2014 and 2013, the Company had approximately $5,403,000 and $3,402,000 of federal and state net operating loss carryforwards, respectively, which begin to expire in 2032. The Company will continue to evaluate its deferred tax assets to determine whether any changes in circumstances could affect the realization of their future benefit. If it is determined in future periods that portions of the Company’s deferred income tax assets satisfy the realization standards, the valuation allowance will be reduced accordingly.
The Company has identified its federal tax return and its state tax return in Massachusetts as major tax jurisdictions. The Company’s evaluation of uncertain tax matters was performed for the tax period from March 13, 2012 (inception) to December 31, 2014. The Company has elected to reflect interest and penalties attributable to income taxes, to the extent they arise, as a component of its income tax provision or benefit, as well as its outstanding income tax assets and liabilities.
F-18
Table of Contents
The Company only recognizes tax benefits from an uncertain tax position if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution. To date, the Company has not recognized such tax benefits in its financial statements.
The Company does not have any uncertain tax positions for which it is reasonably possible that the total amount of gross unrecognized tax benefits will increase or decrease within 12 months of December 31, 2014. The unrecognized tax benefits may increase or change during the next year for items that arise in the ordinary course of business.
Note 11. Subsequent Events
Subsequent to December 31, 2014, the Company has continued to be funded by its former parent through cash advances and direct payment of expenses.
On January 25, 2015, the Company entered into an Amended and Restated License Agreement with BioChemics, Inc. The primary purpose for entering into an amended and restated agreement was to revise the agreement to cover five pharmaceutical products for the prescriptive prophylactic or therapeutic treatment of non-dermal pain in humans. The products are ibuprofen, benfotiamine, gabapentin, celecoxib and rofecoxib.
Note 12. Subsequent Events (Unaudited)
On August 13, 2015, the Company amended its Certificate of Incorporation to increase the authorized shares of its Common Stock from 50,000,000 to 100,000,000.
On August 13, 2015 the Company issued 1,384,616 shares of Common Stock to its Chief Executive Officer.
On August 13, 2015, the Company entered into an agreement with BioChemics to terminate the Reimbursement Agreement between BioChemics and the Company and to release the Company from any obligations under that agreement (SEE NOTE 7).
On August 16, 2015, the Company entered into an agreement with BioChemics to assume the obligations of the Company to pay a former Consultant and the Chief Financial Officer of the Company for services rendered to the Company during the six months ended June 30, 2015.
On September 2, 2015, the Company completed its Original Issue Discount Convertible Note Offering for gross proceeds of approximately $5 million. The principal amount of the notes in the aggregate was approximately $6.25 million. The maturity dates of the notes range from August 17, 2016 to September 2, 2016. The notes are convertible into shares of common stock at the option of the holder at any time at a conversion price of $1.32.
In the event the Company enters into a public offering of not less than $10 million the notes will automatically convert to shares of common stock at the lesser of $1.32 per share or the per share price of the common stock representing the pre-money valuation immediately prior to any shares being sold in the Company’s initial public offering, multiplied by 80%.
In addition the Company initially granted the note holders 3,557,135 warrants to purchase common stock at the lesser of $1.52 per share or at a 15% premium per share to the pre money initial public offering valuation if below $75,000,000. In the event the Company does not consummate its initial public offering or become public in some other manner on or before February 18, 2016, the Company will grant the note holders additional warrants to purchase common stock by increasing the warrant coverage from 75% to 100%.
F-19
Table of Contents
The Company has also agreed that all shares issuable upon conversion of the notes be registered for public resale in the initial public offering registration statement at the discretion of the managing underwriter. If at least 25% of the shares of common stock issuable on conversion of the notes are not permitted to be included in the registration statement, the warrant coverage will increase from 75% to 100%. The managing underwriter has not made a determination as to whether to include such shares in the registration statement. If the determination is made to include some or all of such shares, the registration statement will be amended accordingly.
As compensation for acting as placement agent in connection with the notes, the Company has agreed to issue to Alexander Capital and/or its designees warrants to purchase up to 8% of the maximum number of shares of the Company’s common stock underlying the convertible notes sold to investors in this offering, up to a cap of 1,500,000 shares of common stock. The warrants will be exercisable at any time, and from time to time, in whole or in part, during the four-year period commencing one year from the effective date of the offering. The warrants are exercisable at a fixed per share price equal to $1.58.
On September 22, 2015, the Company amended its Certificate of Incorporation to change its name from Alterix Inc. to Inpellis, Inc.
On September 17, 2015, the Company entered into a lease agreement, relating to the lease of approximately 3,513 square feet of space at a facility located in Beverly, Massachusetts. The lease commences on October 15, 2015, with a term of five years. The monthly rental rate starts at approximately $5,400 in the first year with customary increases. In addition, the Company is required to pay its proportionate share of all increases in real estate taxes and real property surcharges and special assessments from the base year.
On September 18, 2015, the Company entered into a lease agreement, relating to the lease of approximately 1,509 square feet of space at a facility located in Haddonfield, New Jersey. The lease commenced on November 1, 2015, with an initial term of two years. The Company has two options to renew the lease for successive terms of two years under the same rent terms, conditions and provisions. The monthly rental rate starts at approximately $2,700 in the first year and escalates by 3.0% per year each year thereafter. In addition, the Company is required to pay its proportionate share of all increases in real estate taxes and fire and casualty and liability insurance from base year.
On September 1, 2015, the Company entered into an Employment Agreement with the Chief Strategy Officer of the Company for an annual salary of $240,000. In addition, pursuant to the completion of the Company’s 2015 Equity Plan, the Chief Strategy Officer will receive a stock option for the purchase of 1,384,616 shares of common stock, exercisable at 100% of the then fair market value of the common stock. The stock option grant will vest as defined in the terms of the 2015 Equity Plan.
On October 26, 2015, the Company entered into (a) an Intellectual Property Purchase Agreement and (b) a Joint Ownership Agreement with BioChemics. Under the Intellectual Property Purchase Agreement, the Company paid BioChemics $750,000 to acquire joint ownership or, in some cases, full ownership, of patents, patent applications and know how related to transdermal drug delivery as it relates to six pharmaceutical products for the prescriptive prophylactic or therapeutic treatment of non-dermal pain in humans. The products are transdermal ibuprofen, benfotiamine, gabapentin, celecoxib, rofecoxib and lidocaine (including products similar to lidocaine). The purchase covers 24 issued patents and pending patent applications in the United States or foreign countries. Of those patents and patent applications, the Company purchased full ownership of six, all relating to ibuprofen. The remaining patents, patent applications and other intellectual property are owned jointly with BioChemics under the Joint Ownership Agreement. Under the terms of that agreement, the Company will use the jointly owned intellectual property in connection with six pharmaceutical products described above. The Company has also agreed with BioChemics that it will have a right of first offer if the Company determines to sell its ownership of the intellectual property purchased from BioChemics. In addition, BioChemics has the option to purchase back from the Company the intellectual property related to lidocaine (and similar products) for $750,000, provided that the Company would retain joint ownership of that intellectual property as it relates to the treatment of shingles and similar conditions.
F-20
Table of Contents
[ ] Shares
Common Stock

Alexander Capital, L.P.
PROSPECTUS
, 2015
Through and including , 2015 (the 25th day after the date of this offering), all dealers effecting transactions in these securities or not participating in this offering may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment subscription.
Table of Contents
PART II—INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution |
The following table sets forth all expenses, other than the underwriting commissions, payable by the registrant in connection with the sale of the common stock being registered. All the amounts shown are estimates except the SEC registration fee and the FINRA filing fee.
| Amount to be paid | ||||
| SEC registration fee |
$ | |||
| FINRA filing fee |
$ | |||
| The NASDAQ Global Market initial listing fee |
$ | |||
| Blue sky qualification fees and expenses |
$ | |||
| Transfer agent and registrar fees |
$ | |||
| Accounting fees and expenses |
$ | |||
| Legal fees and expenses |
$ | |||
| Printing and engraving expenses |
$ | |||
| Miscellaneous |
$ | |||
|
|
|
|||
| Total |
$ | |||
|
|
|
|||
| Item 14. | Indemnification of Directors and Officers |
Section 102 of the General Corporation Law of the State of Delaware permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificate of incorporation provides that, to the fullest extent permitted by the General Corporation Law of the State of Delaware, a director of the Registrant shall not be liable to the Registrant or its stockholders for monetary damages for breach of fiduciary duty as a director.
Section 145 of the General Corporation Law of the State of Delaware provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party to any threatened, ending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
Our bylaws provide that we shall indemnify any director or officer of the Registrant, and may indemnify any other person, who was or is a party or is threatened to be made a party to any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the Registrant) by reason of the fact that he is or was a director, officer, employee, or agent of the Registrant, or is or was serving at the request of the Registrant as a director, officer, employee, or agent of another Registrant, partnership, joint venture, trust, or other enterprise, against expenses (including
II-1
Table of Contents
attorneys’ fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with such action, suit, or proceeding if he acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the Registrant, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. The termination of any action, suit, or proceeding by judgment, order, settlement, or conviction, or upon a plea of nolo contendere or its equivalent, shall not, of itself, create a presumption that the person did not act in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the Registrant, and, with respect to any criminal action or proceeding, had reasonable cause to believe that his conduct was unlawful.
We shall also indemnify any director or officer, and may indemnify any other person, who was or is a party or is threatened to be made a party to any threatened, pending, or completed action or suit by or in the right of the Registrant to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee, or agent of the Registrant, or is or was serving at the request of the Registrant as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise against expenses (including attorneys’ fees) actually and reasonably incurred by him in connection with the defense or settlement of such action or suit if he acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the Registrant and except that no indemnification shall be made in respect of any claim, issue, or matter as to which such person shall have been adjudged to be liable to the Registrant unless and only to the extent that the Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses as the Court of Chancery or such other court shall deem proper.
To the extent that a present or former director or officer of the Registrant has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to in this Item 14, or in defense of any claim, issue or matter therein, such person shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection therewith. Expenses (including attorneys’ fees) must also be advanced under certain circumstances.
We maintain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
| Item 15. | Recent Sales of Unregistered Securities |
On September 2, 2015, the Company completed the Original Issue Discount Convertible Note Offering and issued $6,250,000 in aggregate principal amount of Convertible Bridge Notes to accredited investors, which shall convert into shares of the Company’s common stock upon the closing of the Offering, together with certain warrants to purchase the Company’s common stock, for gross proceeds of approximately $5 million. The Representative acted as placement agent in connection with the Original Issue Discount Convertible Note Offering. Neither the Representative, nor any of its associated persons or affiliates, purchased any securities in the Original Issue Discount Convertible Note Offering.
As compensation for acting as placement agent in connection with the Original Issue Discount Convertible Note Offering, the Company paid to the Representative a cash placement fee in the aggregate amount of $500,000 and issued to the Representative Placement Agent Warrants to purchase up to 8% of the maximum number of shares of the Company’s common stock underlying the Convertible Bridge Notes sold to investors in the Original Issue Discount Convertible Note Offering, up to a cap of 1,500,000 shares of common stock. The Placement Agent Warrants will be exercisable at any time, and from time to time, in whole or in part, during the four-year period commencing one year from the effective date of the offering, which period shall not extend further than five years from the effective date of the offering in compliance with FINRA Rule 5110(f)(2)(G)(i). The Placement Agent Warrants are exercisable at a fixed per share price equal to $1.58. The Placement Agent Warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The Representative (or permitted assignees under Rule 5110(g)(1)) will not sell,
II-2
Table of Contents
transfer, assign, pledge, or hypothecate these Placement Agent Warrants or the securities underlying these Placement Agent Warrants, nor will it engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Placement Agent Warrants or the underlying securities for a period of 180 days from the date of effectiveness of this offering. The exercise price and number of shares issuable upon exercise of the Placement Agent Warrants may be adjusted in certain circumstances including in the event of a stock dividend, stock split, or our recapitalization, reorganization, merger or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
The sales of the Convertible Bridge Notes and the Placement Agent Warrants were exempt from registration pursuant to Rule 506 and Section 4(a)(2) of the Securities Act.
| Item 16. | Exhibits and Financial Statement Schedules |
(a) Exhibits
See the Exhibit Index on the page immediately preceding the exhibits for a list of exhibits filed as part of this registration statement on Form S-1, which Exhibit Index is incorporated herein by reference.
(b) Financial Statement Schedules
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
| Item 17. | Undertakings |
| (a) | The undersigned registrant hereby undertakes: |
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than
II-3
Table of Contents
registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter);
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(f) The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
(h) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described under Item 14 above, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(i) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized in the Town of Beverly, Commonwealth of Massachusetts, on the 10th of November, 2015.
| INPELLIS INC. | ||||||
| By: |
/s/ Patrick T. Mooney, M.D. | |||||
| Name: |
Patrick T. Mooney, M.D. | |||||
| Title: |
President, Chief Executive Officer | |||||
POWER OF ATTORNEY
We, the undersigned officers and directors of Inpellis Inc., hereby severally constitute and appoint Patrick T. Mooney and Frank A. Manguso, and each of them singly, our true and lawful attorneys with full power to any of them, and to each of them singly, to sign for us and in our names, in the capacities indicated below, the Registration Statement on Form S-1 filed herewith and any and all pre-effective and post-effective amendments to said registration statement and any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same with all exhibits thereto, and the other documents in connection therewith, with the Securities and Exchange Commission, and generally to do all such things in our name and behalf in our capacities as officers and directors to enable Inpellis Inc. to comply with the provisions of the Securities Act of 1933, as amended, and all requirements of the Securities and Exchange Commission, hereby ratifying and confirming our signatures as they may be signed by our said attorneys, or any of them, to said registration statement and any and all amendments thereto.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement on Form S-1 has been signed by the following persons in the capacities and on the dates indicated below.
| Signature Date |
Title | |||
| /s/ Patrick T. Mooney, M.D. |
President, Chief Executive Officer and Director | |||
| Patrick T. Mooney, M.D. November 10, 2015 |
(Principal Executive Officer) | |||
| /s/ Frank A. Manguso |
Chief Financial Officer | |||
| Frank A. Manguso November 10, 2015 |
(Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ David R. Staskin, M.D. |
Chief Strategy Officer, Secretary and Director | |||
| David R. Staskin, M.D. November 10, 2015 |
||||
| /s/ John J Clarke Jr. |
Director | |||
| John J Clarke Jr. November 10, 2015 |
||||
II-5
Table of Contents
| Signature Date |
Title | |||
| /s/ Frederic J. de Bure |
Director | |||
| Frederic J. de Bure November 10, 2015 |
||||
| /s/ Harry G. McCoy, Pharm.D. |
Director | |||
| Harry G. McCoy, Pharm.D. November 10, 2015 |
||||
II-6
Table of Contents
EXHIBIT INDEX
| Exhibit No. |
Description | |
| 1.1* | Underwriting Agreement. | |
| 3.1 | Second Amended and Restated Certificate of Incorporation. | |
| 3.2 | Bylaws. | |
| 4.1 | Reference is made to exhibits 3.1 and 3.2. | |
| 4.2* | Form of Common Stock Certificate. | |
| 4.3(a) | Common Stock Purchase Warrant, dated December 1, 2013, issued by Alterix Inc. to Logic International Consulting Group, L.L.C. | |
| 4.3(b) | Amendment to Common Stock Purchase Warrant, dated June 10, 2015, by and between Logic International Consulting Group, L.L.C. and Alterix Inc. | |
| 4.4 | Securities Purchase Agreement, dated August 17, 2015. | |
| 5.1* | Legal Opinion of Holland & Knight LLP. | |
| 10.1 | Reference is made to exhibit 4.3. | |
| 10.2#* | Inpellis Inc. 2015 Equity Incentive Plan. | |
| 10.3#* | Form of Stock Option Agreement. | |
| 10.4#* | Form of Restricted Stock Agreement. | |
| 10.5# | Employment Agreement, effective June 11, 2015, by and between Patrick T. Mooney, M.D. and Alterix Inc. | |
| 10.6# | Employment Agreement, effective September 1, 2015, by and between David Staskin, M.D. and Alterix Inc. | |
| 10.7# | Proprietary Information, Assignment of Developments and Non-Competition Agreement, effective June 11, 2015, by and between Patrick T. Mooney, M.D. and Alterix Inc. | |
| 10.8#* | Proprietary Information, Assignment of Developments and Non-Competition Agreement, by and between David Staskin, M.D. and Alterix Inc. | |
| 10.9 | Lease, dated September 18, 2015, by and between Streamwood Associates/Haddonfield, LLC T/A 30 Washington Avenue and Alterix Inc. | |
| 10.10 | Intellectual Property Purchase Agreement, dated October 24, 2015, by and between BioChemics, Inc. and Inpellis, Inc. | |
| 10.11 | Joint Ownership Agreement, dated October 24, 2015, by and between BioChemics, Inc. and Inpellis, Inc. | |
| 23.1 | Consent of Marcum LLP, independent registered public accounting firm. | |
| 23.2* | Consent of Holland & Knight LLP (included in Exhibit 5.1). | |
| 24.1 | Power of Attorney (included on signature page). | |
| * | To be filed by amendment. |
| # | Denotes management compensation plan or contract. |
Exhibit 3.1
SECOND AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
ALTERIX INC.
Alterix Inc. (the “Corporation”), a corporation organized and existing under and by virtue of the General Corporation Law of the State of Delaware, does hereby certify as follows:
| 1. | The current name of the Corporation is Alterix Inc. The original Certificate of Incorporation was filed with the Secretary of State of Delaware on March 13, 2012 and the Amended and Restated Certificate of Incorporation was filed with the Secretary of State of Delaware. |
| 2. | This Second Amended and Restated Certificate of Incorporation, which restates and integrates and also further amends the provisions of the Corporation’s Amended and Restated Certificate of Incorporation, was duly adopted in accordance with the provisions of Sections 242 and 245 of the General Corporation Law of the State of Delaware (the “DGCL”) and by the written consent of its sole stockholder in accordance with Section 228 of the DGCL. |
| 3. | The Corporation’s Amended and Restated Certificate of Incorporation is hereby amended, integrated and restated to read in its entirety as follows: |
FIRST: The name of the Corporation is Inpellis, Inc.
SECOND: The address of the registered office of the Corporation in the State of Delaware is 16192 Coastal Highway, Lewes, Delaware 19958, County of Sussex, and the name of the registered agent of the Corporation in the State of Delaware at such address is Harvard Business Services, Inc.
THIRD: The purpose of the Corporation is to engage in any lawful activity for which corporations may be organized under the DGCL.
FOURTH: The total number of shares of stock that the Corporation is authorized to issue is one hundred million (100,000,000) shares having a par value of $0.001 per share.
FIFTH: The business and affairs of the Corporation shall be managed by or under the direction of the board of directors, and the directors need not be elected by ballot unless required by the bylaws of the Corporation.
SIXTH: This Corporation shall be perpetual unless otherwise decided by a majority of the board of directors.
SEVENTH: In furtherance and not in limitation of the powers conferred by the laws of Delaware, the board of directors is authorized to amend or repeal the bylaws.
EIGHTH: The Corporation reserves the right to amend or repeal any provision in this Certificate in the manner prescribed by the laws of Delaware.
NINTH: To the fullest extent permitted by the DGCL a director of this Corporation shall not be liable to the Corporation or its stockholders for monetary damages for breach of fiduciary duty as a director.
IN WITNESS WHEREOF, the Corporation has caused this Second Amended and Restated Certificate of Incorporation to be signed by its Chief Executive Officer this 21st day of September, 2015.
| /s/ Patrick T. Mooney |
| Patrick T. Mooney, Chief Executive Officer |
2
Exhibit 3.2
BY-LAWS
OF
ALTERIX INC.
ARTICLE I
OFFICES
SECTION 1.01. Registered Office. The registered office of the corporation in the State of Delaware shall be in the City of Wilmington, County of New Castle, and the name of its registered agent shall be Corporation Service Company.
SECTION 1.02. Other Offices. The corporation may also have offices at such other places both within and without the State of Delaware as the Board of Directors may from time to time determine or the business of the corporation may require.
ARTICLE II
MEETINGS OF STOCKHOLDERS
SECTION 2.01. Annual Meeting. The annual meeting of stockholders for the election of directors, and for the transaction of any other proper business, shall be held at such date and time as shall be designated from time to time by the Board of Directors and stated in the notice of the meeting.
SECTION 2.02. Special Meeting. Special meetings of the stockholders, for any purpose or purposes, unless otherwise prescribed by statute or by the certificate of incorporation, may be called at any time by the Chairman of the Board, the Chief Executive Officer or the President of the corporation or by the Board of Directors or by written order of a majority of the directors and shall be called by the President, the Chief Executive Officer or the Secretary at the request in writing of stockholders owning not less than ten percent of the capital stock of the corporation issued and outstanding and entitled to vote. Such request shall state the purposes of the proposed meeting.
SECTION 2.03. Place of Meeting. All meetings of stockholders shall be held at such place, if any, either within or without the State of Delaware, as shall be designated from time to time by the Board of Directors and stated in the notice of such meeting. The Board of Directors may, in its sole discretion and subject to such guidelines and procedures as the Board of Directors may from time to time adopt, determine that the meeting shall not be held at any specific place, but may instead be held solely by means of remote communication.
SECTION 2.04. Notice of Meeting. Written or other proper notice of any meeting of stockholders, stating the place, if any, date and hour of the meeting, the means of remote communications, if any, by which stockholders and proxyholders may be deemed to be present in person and vote at such adjourned meetings, and, in the case of a special meeting, the purpose or purposes thereof, shall be given to each stockholder entitled to vote thereat, not less than 10 nor more than 60 days before the meeting. Business transacted at any special meeting of stockholders shall be limited to the purposes stated in the notice.
SECTION 2.05. Voting List. The officer who has charge of the stock ledger of the corporation shall prepare and make, at least 10 days before every meeting of stockholders, a complete list of the stockholders entitled to vote at the meeting, arranged in alphabetical order, and showing the address of each stockholder and the number of shares registered in the name of each stockholder. Such list shall be open to the examination of any stockholder, for any purpose germane to the meeting, during ordinary business hours, for a period of at least 10 days prior to the meeting (i) on a reasonably accessible electronic network, provided that the information required to gain access to such list is provided with the notice of the meeting, or (ii) during ordinary business hours, at the principal place of business of the corporation. If the meeting is to be held at a specific place, then the list shall be produced and kept at the time and place of the meeting during the whole time thereof, and may be inspected by any stockholder who is present. If the meeting is to be held solely by means of remote communication, then the list shall also be open to the examination of any stockholder during the whole time of the meeting on a reasonably accessible electronic network, and the information required to access such list shall be provided with the notice of the meeting.
SECTION 2.06. Quorum. At any meeting of the stockholders, the holders of a majority of the shares issued and outstanding and entitled to vote thereat, present in person or represented by proxy, shall constitute a quorum for the transaction of business, except as otherwise provided by statute, by the certificate of incorporation or by these by-laws. If, however, such quorum shall not be present or represented at any meeting of the stockholders, the stockholders entitled to vote thereat, present in person or represented by proxy, shall have power to adjourn the meeting from time to time, without notice other than announcement at the meeting, until a quorum shall be present or represented. At such adjourned meeting at which a quorum shall be present or represented any business may be transacted which might have been transacted at the meeting as originally notified. If the adjournment is for more than 30 days, or if after the adjournment a new record date is fixed for the adjourned meeting, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting.
SECTION 2.07. Voting. When a quorum is present at any meeting of the stockholders, the vote of the holders of a majority of the shares entitled to vote on the subject matter and present in person or represented by proxy shall decide any question brought before such meeting, unless the question is one upon which, by express provision of applicable statutes, of the certificate of incorporation or of these by-laws, a different vote is required, in which case such express provision shall govern and control the decision of such question. Except as otherwise provided in the certificate of incorporation, each stockholder entitled to vote at any meeting of the stockholders shall be entitled to one vote for each share of capital stock held by the stockholder.
SECTION 2.08. Proxies. Each stockholder entitled to vote at a meeting of the stockholders may authorize, by an instrument in writing subscribed by such stockholder, bearing a date not more than three years prior to voting, unless such instrument provides for a longer period, and filed with the Secretary of the corporation before, or at the time of the meeting, another person or persons to act for him by proxy.
-2-
SECTION 2.09. Consent of Stockholders. Unless otherwise provided in the certificate of incorporation, any action required to be taken at any annual or special meeting of stockholders of the corporation, or any action which may be taken at any annual or special meeting of such stockholders, may be taken without a meeting, without prior notice and without a vote, if a consent in writing, setting forth the action so taken, shall be signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted. Prompt notice of the taking of the corporate action without a meeting by less than unanimous written consent shall be given to those stockholders who have not consented in writing. A telegram, cablegram or other electronic transmission consenting to an action to be taken and transmitted by a stockholder or proxyholder, or by a person or persons authorized to act for a stockholder or proxyholder, shall be deemed to be written, signed and dated, for the purposes of this Section to the extent permitted by law.
SECTION 2.10. Voting of Stock of Certain Holders. Shares of the corporation’s capital stock standing in the name of another corporation, domestic or foreign, may be voted by such officer, agent, or proxy as the by-laws of such corporation may prescribe, or in the absence of such provision, as the Board of Directors of such corporation may determine. Shares standing in the name of a deceased person may be voted by the executor or administrator of such deceased person, either in person or by proxy. Shares standing in the name of a guardian, conservator, or trustee may be voted by such fiduciary, either in person or by proxy, but no such fiduciary shall be entitled to vote shares held in such fiduciary capacity without a transfer of such shares into the name of such fiduciary. Shares standing in the name of a receiver may be voted by such receiver. A stockholder whose shares are pledged shall be entitled to vote such shares, unless in the transfer by the pledgor on the books of the corporation, he has expressly empowered the pledgee to vote thereon, in which case only the pledgee, or his proxy, may represent the stock and vote thereon.
SECTION 2.11. Treasury Stock. The corporation shall not vote, directly or indirectly, shares of its own capital stock owned by it; and such shares shall not be counted in determining the total number of outstanding shares of the corporation’s capital stock.
SECTION 2.12. Fixing Record Date. The Board of Directors may fix in advance a date, which shall not be more than 60 days nor less than 10 days preceding the date of any meeting of stockholders, nor more than 60 days preceding the date for payment of any dividend or distribution, or the date for the allotment of rights, or the date when any change, or conversion or exchange of capital stock shall go into effect, or a date in connection with obtaining a consent, as a record date for the determination of the stockholders entitled to notice of, and to vote at, any such meeting and any adjournment thereof, or entitled to receive payment of any such dividend or distribution, or to receive any such allotment of rights, or to exercise the rights in respect of any such change, conversion or exchange of capital stock, or to give such consent, and in such case such stockholders and only such stockholders as shall be stockholders of record on the date so fixed, shall be entitled to such notice of, and to vote at, any such meeting and any adjournment thereof, or to receive payment of such dividend or distribution, or to receive such allotment of rights, or to exercise such rights, or to give such consent, as the case may be, notwithstanding any transfer of any stock on the books of the corporation after any such record date fixed as aforesaid.
-3-
ARTICLE III
BOARD OF DIRECTORS
SECTION 3.01. Powers. The business and affairs of the corporation shall be managed by or under the direction of its Board of Directors, which may exercise all such powers of the corporation and do all such lawful acts and things as are not by statute or by the certificate of incorporation or by these by-laws directed or required to be exercised or done by the stockholders.
SECTION 3.02. Number, Election and Term. The number of directors that shall constitute the whole Board of Directors shall not be less than one. Subject to the Company’s Certificate of Incorporation, such number of directors shall from time to time be fixed and determined by the directors and shall be set forth in the notice of any meeting of stockholders held for the purpose of electing directors. The directors shall be elected at the annual meeting of stockholders, except as provided in Section 3.03, and each director elected shall hold office until his successor shall be elected and shall qualify or, if earlier, his death, resignation, retirement, disqualification or removal. The vote of any stockholder on an election of directors may be taken in any manner and no such vote shall be required to be taken by written ballot or by electronic transmission unless otherwise required by law. Directors need not be residents of Delaware, citizens of the United States or stockholders of the corporation.
SECTION 3.03. Vacancies, Additional Directors, and Removal from Office. If any vacancy occurs in the Board of Directors caused by death, resignation, retirement, disqualification, or removal from office of any director, or otherwise, or if any new directorship is created by an increase in the authorized number of directors, a majority of the directors then in office, though less than a quorum, or a sole remaining director, may choose a successor or fill the newly created directorship; and a director so chosen shall hold office until the next election and until his successor shall be duly elected and shall qualify, unless sooner displaced. Any director may be removed either for or without cause at any special meeting of stockholders duly called and held for such purpose.
SECTION 3.04. Resignation. Any director may resign at any time upon notice given in writing or by electronic transmission to the corporation. A resignation from the Board of Directors shall be deemed to take effect immediately upon receipt of such notice or at such other time as the director may specify in the notice.
SECTION 3.05. Regular Meetings. A regular meeting of the Board of Directors shall be held each year, without other notice than this bylaw, at the place of, and immediately following, the annual meeting of stockholders; and other regular meetings of the Board of Directors may be held at such places (within or without the State of Delaware), if any, and at such times as the Board of Directors may provide, by resolution, without other notice than such resolution.
-4-
SECTION 3.06. Special Meetings. A special meeting of the Board of Directors may be called by the Chairman of the Board of Directors, by the President of the corporation or the Chief Executive Officer of the corporation and shall be called by the Secretary on the written request of any director. Notice of special meetings of the Board of Directors shall be given to each director at least 48 hours prior to the time of such meeting and shall be given in writing or by electronic transmission. Each such notice shall state the time and place (within or without the State of Delaware), if any, of the meeting but need not state the purposes thereof, except that notice shall be given of any proposed amendment to the by-laws if it is to be adopted at any special meeting or with respect to any other matter where notice is required by statute or by these by-laws.
SECTION 3.07. Quorum. A majority of the Board of Directors shall constitute a quorum for the transaction of business at any meeting of the Board of Directors, and the act of a majority of the directors present at any meeting at which there is a quorum shall be the act of the Board of Directors, except as may be otherwise specifically provided by statute, by the certificate of incorporation or by these by-laws. If a quorum shall not be present at any meeting of the Board of Directors, the directors present thereat may adjourn the meeting from time to time, without notice other than announcement at the meeting, until a quorum shall be present.
SECTION 3.08. Communications. Members of the Board of Directors, or of any committee thereof, may participate in a meeting of such board or committee by means of conference telephone or other communications equipment by means of which all persons participating in the meeting can hear each other, and participation in a meeting pursuant to this Section shall constitute presence in person at such meeting.
SECTION 3.09. Action Without Meeting. Unless otherwise restricted by the certificate of incorporation or these by-laws, any action required or permitted to be taken at any meeting of the Board of Directors, or of any committee thereof as provided in Article IV of these by-laws, may be taken without a meeting, if all the members of the Board of Directors or of such committee, as the case may be, consent thereto in writing or by electronic transmission, and the writing or writings or electronic transmission or transmissions are filed with the minutes of proceedings of the Board of Directors or such committee. Such filing shall be in paper form if the minutes are maintained in paper form and shall be in electronic form if the minutes are maintained in electronic form.
SECTION 3.10. Compensation. Unless otherwise restricted by the certificate of incorporation or these by-laws, the Board of Directors shall have the authority to fix the compensation of directors. The directors may be paid their expenses, if any, of attendance at each meeting of the board of directors and may be paid a fixed sum for attendance at each meeting of the Board of Directors or a stated salary as director. Nothing herein shall preclude any director from serving the corporation in any other capacity and receiving compensation therefor. Members of special or standing committees may be allowed like compensation for attending committee meetings.
-5-
ARTICLE IV
COMMITTEE OF DIRECTORS
SECTION 4.01. Designation, Powers and Name. The Board of Directors may, by resolution passed by a majority of the whole Board of Directors, designate one or more committees, including, if they shall so determine, an Executive Committee, each such committee to consist of one or more of the directors of the corporation. The committee shall have and may exercise such of the powers of the Board of Directors in the management of the business and affairs of the corporation as may be provided in such resolution. The committee may authorize the seal of the corporation to be affixed to all papers that may require it. The Board of Directors may designate one or more directors as alternate members of any committee, who may replace any absent or disqualified member at any meeting of such committee. In the absence or disqualification of any member of such committee or committees, the member or members thereof present at any meeting and not disqualified from voting, whether or not he or they constitute a quorum, may unanimously appoint another member of the Board of Directors to act at the meeting in the place of any such absent or disqualified member. Such committee or committees shall have such name or names and such limitations of authority as may be determined from time to time by resolution adopted by the Board of Directors.
SECTION 4.02. Minutes. Each committee of directors shall keep regular minutes of its proceedings and report the same to the Board of Directors when required.
SECTION 4.03. Compensation. Members of special or standing committees may be allowed compensation for attending committee meetings, if the Board of Directors shall so determine.
ARTICLE V
NOTICE
SECTION 5.01. Methods of Giving Notice. Whenever, under the provisions of applicable statutes, the certificate of incorporation or these by-laws, notice is required to be given to any director, member of any committee, or stockholder, it shall not be necessary that personal notice be given, and such notice may be given in writing, by mail, addressed to such director, member, or stockholder at his or her address as it appears on the records of the corporation or at his or her residence or usual place of business, with postage thereon prepaid, and such notice shall be deemed to be given at the time when the same shall be deposited in the United States mail. Notice also may be given in any other proper form, as authorized by the Delaware General Corporation Law. Notice that is given by facsimile shall be deemed delivered when sent to a number at which any director, member or stockholder has consented to receive such notice. Notice by telegram or cablegram shall be deemed to be given when the same shall be filed. Notice that is given in person or by telephone shall be deemed to be given when the same shall be delivered. Without limiting the manner by which notice otherwise may be given effectively to any director, member or stockholder, any notice given under any provision of these by-laws shall be
-6-
effective if given by a form of electronic transmission consented to by such person. Notice given by electronic mail shall be deemed delivered when directed to an electronic mail address at which such person has consented to receive notice and notice given by a posting on an electronic network together with separate notice to such person of such specific posting shall be deemed delivered upon the later of (a) such posting and (b) the giving of such separate notice. Notice given by any other form of electronic transmission shall be deemed given when directed to any director, member or stockholder in the manner consented to by such director, member or stockholder
SECTION 5.02. Waiver. Whenever any notice is required to be given under the provisions of an applicable statute, the certificate of incorporation, or these by-laws, a waiver thereof in writing, signed by the person or persons entitled to said notice, or a waiver by electronic transmission by the person or persons entitled to said notice, whether before or after the time stated therein, shall be deemed equivalent thereto. Attendance of a person at a meeting shall constitute a waiver of notice of such meeting, except when the person attends a meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened.
ARTICLE VI
OFFICERS
SECTION 6.01. Officers. The officers of the corporation shall be a President, one or more Vice Presidents, any one or more of which may be designated Executive Vice President or Senior Vice President, a Secretary and a Treasurer. The Board of Directors may appoint such other officers and agents, including a Chairman of the Board, a Vice Chairman of the Board, a Chief Executive Officer, Assistant Vice Presidents, Assistant Secretaries, and Assistant Treasurers, in each case as the Board of Directors shall deem necessary, who shall hold their offices for such terms and shall exercise such powers and perform such duties as shall be determined by the Board. Any two or more offices may be held by the same person. The Chairman and Vice Chairman of the Board, if any, shall be elected from among the directors. With the foregoing exceptions, none of the other officers need be a director, and none of the officers need be a stockholder of the corporation.
SECTION 6.02. Election and Term of Office. The officers of the corporation shall be elected annually by the Board of Directors at its first regular meeting held after the annual meeting of stockholders or as soon thereafter as conveniently possible. Each officer shall hold office until his successor shall have been chosen and shall have qualified or until his death or the effective date of his resignation or removal, or until he shall cease to be a director in the case of the Chairman and the Vice Chairman.
SECTION 6.03. Removal and Resignation. Any officer or agent elected or appointed by the Board of Directors may be removed without cause at any time by the Board of Directors. Any officer may resign at any time by giving written notice to the corporation. Any such resignation shall take effect at the date of the receipt of such notice or at any later time specified therein, and unless otherwise specified therein, the acceptance of such resignation shall not be necessary to make it effective.
-7-
SECTION 6.04. Vacancies. Any vacancy occurring in any office of the corporation by death, resignation, removal, or otherwise, may be filled by the Board of Directors for the unexpired portion of the term.
SECTION 6.05. Salaries. The salaries of all officers and agents of the corporation shall be fixed by the Board of Directors or pursuant to its direction; and no officer shall be prevented from receiving such salary by reason of his also being a director.
SECTION 6.06. Chairman of the Board. The Chairman of the Board (if such office is created by the Board) shall preside at all meetings of the Board of Directors or of the stockholders of the corporation. The Chairman shall formulate and submit to the Board of Directors or the Executive Committee matters of general policy for the corporation and shall perform such other duties as usually appertain to the office or as may be prescribed by the Board of Directors or the Executive Committee.
SECTION 6.07. Vice Chairman of the Board. The Vice Chairman of the Board (if such office is created by the Board) shall, in the absence or disability of the Chairman of the Board, perform the duties and exercise the powers of the Chairman of the Board. The Vice Chairman shall perform such other duties as from time to time may be prescribed by the Board of Directors or the Executive Committee or assigned by the Chairman of the Board.
SECTION 6.08. President; Chief Executive Officers. The President and the Chief Executive Officer (if such office is created by the Board), which posts may be held by the same or different persons, shall be the chief executive officers of the corporation and, subject to the control of the Board of Directors, shall in general supervise and control the business and affairs of the corporation. In the absence of the Chairman of the Board or the Vice Chairman of the Board (if such offices are created by the Board), the President or the Chief Executive Officer shall preside at all meetings of the Board of Directors and of the stockholders. Either such person may also preside at any such meeting attended by the Chairman or Vice Chairman of the Board if he is so designated by the Chairman, or in the Chairman’s absence by the Vice Chairman. Both shall have the power to appoint and remove subordinate officers, agents and employees, except those elected or appointed by the Board of Directors. The President and the Chief Executive Officer both shall keep the Board of Directors and the Executive Committee fully informed and shall consult them concerning the business of the corporation. Either may sign certificates for shares of the corporation and any deeds, bonds, mortgages, contracts, checks, notes, drafts, or other instruments that the Board of Directors has authorized to be executed, except in cases where the signing and execution thereof has been expressly delegated by these by-laws or by the Board of Directors to some other officer or agent of the corporation, or shall be required by law to be otherwise executed. Either shall vote, or give a proxy to any other officer of the corporation to vote, all shares of stock of any other corporation standing in the name of the corporation and in general he shall perform all other duties normally incident to the office of President or Chief Executive Officer, as the case may be, and such other duties as may be prescribed by the stockholders, the Board of Directors, or the Executive Committee from time to time.
-8-
SECTION 6.09. Vice Presidents. In the absence of the President and the Chief Executive Officer, or in the event of their inability or refusal to act, the Executive Vice President (or in the event there shall be no Vice President designated Executive Vice President, any Vice President designated by the Board) shall perform the duties and exercise the powers of the President and the Chief Executive Officer. Any Vice President may sign, with the Secretary or Assistant Secretary, certificates for shares of the corporation. The Vice Presidents shall perform such other duties as from time to time may be assigned to them by the President, the Chief Executive Officer or the Board of Directors.
SECTION 6.10. Secretary. The Secretary shall (a) keep the minutes of the meetings of the stockholders, the Board of Directors and committees of directors; (b) see that all notices are duly given in accordance with the provisions of these by-laws and as required by law; (c) be custodian of the corporate records and of the seal of the corporation, and see that the seal of the corporation or a facsimile thereof is affixed to all certificates for shares prior to the issue thereof and to all documents, the execution of which on behalf of the corporation under its seal is duly authorized in accordance with the provisions of these by-laws; (d) keep or cause to be kept a register of the post office address of each stockholder which shall be furnished by such stockholder; (e) sign with the President, the Chief Executive Officer or an Executive Vice President or Vice President, certificates for shares of the corporation, the issue of which shall have been authorized by resolution of the Board of Directors; (f) have general charge of the stock transfer books of the corporation; and (g) in general, perform all duties normally incident to the office of Secretary and such other duties as from time to time may be assigned to him by the President, the Chief Executive Officer or the Board of Directors.
SECTION 6.11. Treasurer. If required by the Board of Directors, the Treasurer shall give a bond for the faithful discharge of his duties in such sum and with such surety or sureties as the Board of Directors shall determine. He shall (a) have charge and custody of and be responsible for all funds and securities of the corporation; (b) receive and give receipts for moneys due and payable to the corporation from any source whatsoever and deposit all such moneys in the name of the corporation in such banks, trust companies, or other depositories as shall be selected in accordance with the provisions of Section 7.03 of these by-laws; (c) prepare, or cause to be prepared, for submission at each regular meeting of the Board of Directors, at each annual meeting of the stockholders, and at such other times as may be required by the Board of Directors, the President or the Chief Executive Officer, a statement of financial condition of the corporation in such detail as may be required; and (d) in general, perform all the duties incident to the office of Treasurer and such other duties as from time to time may be assigned to him by the President, the Chief Executive Officer or the Board of Directors.
SECTION 6.12. Assistant Secretary and Treasurer. The Assistant Secretaries and Assistant Treasurers shall, in general, perform such duties as shall be assigned to them by the Secretary or the Treasurer, respectively, or by the President, the Chief Executive Officer, the Board of Directors, or the Executive Committee. The Assistant Secretaries and Assistant Treasurers shall, in the absence of the Secretary or Treasurer, respectively, perform all functions and duties which such absent officers may delegate, but such delegation shall not relieve the absent officer from the responsibilities and liabilities of his office. The Assistant Secretaries may
-9-
sign, with the President, the Chief Executive Officer or a Vice President, certificates for shares of the corporation, the issue of which shall have been authorized by a resolution of the Board of Directors. The Assistant Treasurers shall respectively, if required by the Board of Directors, give bonds for the faithful discharge of their duties in such sums and with such sureties as the Board of Directors shall determine.
ARTICLE VII
CONTRACTS, CHECKS AND DEPOSITS
SECTION 7.01. Contracts. Subject to the provisions of Section 6.01, the Board of Directors may authorize any officer, officers, agent, or agents, to enter into any contract or execute and deliver any instrument in the name of and on behalf of the corporation, and such authority may be general or confined to specific instances.
SECTION 7.02. Checks. All checks, demands, drafts, or other orders for the payment of money, notes, or other evidences of indebtedness issued in the name of the corporation, shall be signed by such officer or officers or such agent or agents of the corporation, and in such manner, as shall be determined by the Board of Directors.
SECTION 7.03. Deposits. All funds of the corporation not otherwise employed shall be deposited from time to time to the credit of the corporation in such banks, trust companies, or other depositories as the Board of Directors may select.
ARTICLE VIII
CERTIFICATES OF STOCK
SECTION 8.01. Issuance. Each stockholder of this corporation shall be entitled to a certificate or certificates showing the number of shares of capital stock registered in his name on the books of the corporation. The certificates shall be in such form as may be determined by the Board of Directors, shall be issued in numerical order and shall be entered in the books of the corporation as they are issued. They shall exhibit the holder’s name and number of shares and shall be signed by the President, the Chief Executive Officer or a Vice President and by the Secretary or an Assistant Secretary. The same person shall be permitted to sign a single stock certificate in more than one capacity. If any certificate is countersigned (1) by a transfer agent other than the corporation or any employee of the corporation, or (2) by a registrar other than the corporation or any employee of the corporation, any other signature on the certificate may be a facsimile. If the corporation shall be authorized to issue more than one class of stock or more than one series of any class, the designations, preferences, and relative participating, optional, or other special rights of each class of stock or series thereof and the qualifications, limitations, or restrictions of such preferences and rights shall be set forth in full or summarized on the face or back of the certificate which the corporation shall issue to represent such class of stock; provided that, except as otherwise provided by statute, in lieu of the foregoing requirements there may be set forth on the face or back of the certificate which the corporation shall issue to represent such class or series of stock, a statement that the corporation will furnish
-10-
to each stockholder who so requests the designations, preferences and relative, participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations, or restrictions of such preferences and rights. All certificates surrendered to the corporation for transfer shall be canceled and no new certificate shall be issued until the former certificate for a like number of shares shall have been surrendered and canceled, except that in the case of a lost, stolen, destroyed, or mutilated certificate a new one may be issued therefor upon such terms and with such indemnity, if any, to the corporation as the Board of Directors may prescribe. Certificates shall not be issued representing fractional shares of stock.
SECTION 8.02. Lost Certificates. The Board of Directors may direct a new certificate or certificates to be issued in place of any certificate or certificates theretofore issued by the corporation alleged to have been lost, stolen, or destroyed, upon the making of an affidavit of that fact by the person claiming the certificate of stock to be lost, stolen or destroyed. When authorizing such issue of a new certificate or certificates, the Board of Directors may, in its discretion and as a condition precedent to the issuance thereof, require (1) the owner of such lost, stolen, or destroyed certificate or certificates, or his legal representative, to advertise the same in such manner as it shall require, (2) such owner to give the corporation a bond in such sum as it may direct as indemnity against any claim that may be made against the corporation with respect to the certificate or certificates alleged to have been lost, stolen, or destroyed, or (3) both.
SECTION 8.03. Transfers. Upon surrender to the corporation or the transfer agent of the corporation of a certificate for shares duly endorsed or accompanied by proper evidence of succession, assignment, or authority to transfer, it shall be the duty of the corporation to issue a new certificate to the person entitled thereto, cancel the old certificate, and record the transaction upon its books. Transfers of shares shall be made only on the books of the corporation by the registered holder thereof, or by his attorney thereunto authorized by power of attorney and filed with the Secretary of the corporation or the Transfer Agent.
SECTION 8.04. Registered Stockholders. The corporation shall be entitled to treat the holder of record of any share or shares of the corporation’s capital stock as the holder in fact thereof and, accordingly, shall not be bound to recognize any equitable or other claim to or interest in such share or shares on the part of any other person, whether or not it shall have express or other notice thereof, except as otherwise provided by the laws of the State of Delaware.
ARTICLE IX
DIVIDENDS
SECTION 9.01. Declaration. Dividends with respect to the shares of the corporation’s capital stock, subject to the provisions of the certificate of incorporation, if any, may be declared by the Board of Directors at any regular or special meeting, pursuant to applicable law. Dividends may be paid in cash, in property, or in shares of capital stock, subject to the provisions of the certificate of incorporation.
-11-
SECTION 9.02. Reserve. Before payment of any dividend, there may be set aside out of any funds of the corporation available for dividends such sum or sums as the Board of Directors from time to time, in their absolute discretion, think proper as a reserve or reserves to meet contingencies, or for equalizing dividends, or for repairing or maintaining any property of the corporation, or for such other purpose as the Board of Directors shall think conducive to the interest of the corporation, and the Board of Directors may modify or abolish any such reserve in the manner in which it was created.
ARTICLE X
INDEMNIFICATION
SECTION 10.01. Third Party Actions. The corporation shall indemnify any director or officer of the corporation, and may indemnify any other person, who was or is a party or is threatened to be made a party to any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that he is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise, against expenses (including attorneys’ fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with such action, suit, or proceeding if he acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. The termination of any action, suit, or proceeding by judgment, order, settlement, or conviction, or upon a plea of nolo contendere or its equivalent, shall not, of itself, create a presumption that the person did not act in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had reasonable cause to believe that his conduct was unlawful.
SECTION 10.02. Actions By or In the Right of the Corporation. The corporation shall indemnify any director or officer, and may indemnify any other person, who was or is a party or is threatened to be made a party to any threatened, pending, or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise against expenses (including attorneys’ fees) actually and reasonably incurred by him in connection with the defense or settlement of such action or suit if he acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue, or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses as the Court of Chancery or such other court shall deem proper.
-12-
SECTION 10.03. Mandatory Indemnification. To the extent that a present or former director or officer of the corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to in Sections 10.01 and 10.02, or in defense of any claim, issue or matter therein, such person shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection therewith.
SECTION 10.04. Determination of Conduct. Any indemnification under Section 10.01 or 10.02 of this Article X (unless ordered by a court) shall be made by the corporation only as authorized in the specific case upon a determination that indemnification of the present or former director, officer, employee or agent is proper in the circumstances because he has met the applicable standard of conduct set forth in Section 10.01 or 10.02 of this Article X. Such determination shall be made (a) by a majority vote of directors who were not parties to such action, suit or proceeding, even though less than a quorum, or (b) by a committee of such directors designated by majority vote of such directors, even though less than a quorum, or (c) if there are no such directors, or if such directors so direct, by independent legal counsel in a written opinion, or (d) by the stockholders.
SECTION 10.05. Payment of Expenses in Advance. Expenses (including attorneys’ fees) incurred in defending a civil or criminal action, suit, or proceeding may be paid by the corporation in advance of the final disposition of such action, suit, or proceeding upon receipt of an undertaking by or on behalf of the director, officer, employee, or agent to repay such amount if it shall ultimately be determined that he is not entitled to be indemnified by the corporation as authorized in this Article X. Such expenses (including attorneys’ fees) incurred by former directors and officers or other employees and agents may be so paid upon such terms and conditions, if any, as the corporation deems appropriate.
SECTION 10.06. Indemnity Not Exclusive. The indemnification and advancement of expenses provided or granted hereunder shall not be deemed exclusive of any other rights to which those seeking indemnification or advancement of expenses may be entitled under the certificate of incorporation, any other bylaw, agreement, vote of stockholders, or disinterested directors or otherwise, both as to action in his official capacity and as to action in another capacity while holding such office.
SECTION 10.07. Definitions. For purposes of this Article X:
(a) “the corporation” shall include, in addition to the resulting corporation, any constituent corporation (including any constituent of a constituent) absorbed in a consolidation or merger that, if its separate existence had continued, would have had power and authority to indemnify its directors, officers, and employees or agents, so that any person who is or was a director, officer, employee, or agent of such constituent corporation, or is or was serving at the request of such constituent corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise, shall stand in the same position under this Article X with respect to the resulting or surviving corporation as he would have with respect to such constituent corporation if its separate existence had continued;
-13-
(b) “other enterprises” shall include employee benefit plans;
(c) “fines” shall include any excise taxes assessed on a person with respect to any employee benefit plan;
(d) “serving at the request of the corporation” shall include any service as a director, officer, employee, or agent of the corporation that imposes duties on, or involves services by, such director, officer, employee, or agent with respect to an employee benefit plan, its participants or beneficiaries; and
(e) a person who acted in good faith and in a manner he reasonably believed to be in the interest of the participants and beneficiaries of an employee benefit plan shall be deemed to have acted in a manner “not opposed to the best interests of the corporation’’ as referred to in this Article X.
SECTION 10.08. Continuation of Indemnity. The indemnification and advancement of expenses provided or granted hereunder shall, unless otherwise provided when authorized or ratified, continue as to a person who has ceased to be a director, officer, employee, or agent and shall inure to the benefit of the heirs, executors, and administrators of such a person.
ARTICLE XI
MISCELLANEOUS
SECTION 11.01. Seal. The corporate seal, if one is authorized by the Board of Directors, shall have inscribed thereon the name of the corporation, and the words “Corporate Seal, Delaware”. The seal may be used by causing it or a facsimile thereof to be impressed or affixed or otherwise reproduced.
-14-
SECTION 11.02. Books. The books of the corporation may be kept (subject to any provision contained in the statutes) outside the State of Delaware at the offices of the corporation, or at such other place or places as may be designated from time to time by the Board of Directors.
ARTICLE XII
SECTION HEADINGS
The headings contained in these by-laws are for reference purposes only and shall not be construed to be part of and shall not affect in any way the meaning or interpretation of these by-laws.
ARTICLE XIII
AMENDMENT
These by-laws may be altered, amended, or repealed or new by-laws may be adopted by a majority of the number of directors then constituting the Board of Directors at any regular meeting of the Board of Directors without prior notice, or at any special meeting of the Board of Directors if notice of such alteration, amendment, or repeal be contained in the notice of such special meeting.
-15-
Exhibit 4.3 A
NEITHER THIS SECURITY NOR THE SECURITIES INTO WHICH THIS SECURITY IS EXERCISABLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL OPINION OF COUNSEL TO THE TRANSFEROR TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY. THIS SECURITY AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS SECURITY MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN SECURED BY SUCH SECURITIES AS PERMITTED BY LAW AND THE SECURITIES PURCHASE AGREEMENT PURSUANT TO WHICH THE SECURITIES WERE ISSUED.
COMMON STOCK PURCHASE WARRANT
To Purchase Shares of Common Stock of
ALTERIX, INC.
This COMMON STOCK PURCHASE WARRANT (this “Warrant”) certifies that, for services rendered and to be rendered, Logic International Consulting Group, L.L.C. or assignee (the “Holder”), is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the effective date hereof, December 1, 2013 and on or prior to the close of business on the fifth (5th) anniversary of the date of this Warrant (the “Termination Date”), to subscribe for and purchase up to Four Million (4,000,000) shares of common stock (the “Warrant Shares”), from ALTERIX, INC., a Delaware corporation (the “Company”), par value $.01 per share of the Company (the “Common Stocks”), as provided below. The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(a) below, as adjusted.
Section 1. Exercise.
a) Exercise of Warrant/Exercise Price. Exercise of the purchase rights represented by this Warrant may be made at any time or times on or after the closing of a Qualifying Transaction, at an Exercise Price per share equal to Five and No/100 Dollars ($5.00) per share (the “Exercise Price”). This Warrant may be exercised on or before the Termination Date (each, an “Exercise Date”) by delivery to the Company of a duly executed facsimile copy of the Notice of Exercise Form annexed hereto (or such other office or agency of the Company as it may designate by notice in writing to the registered Holder at the address of such Holder appearing on the books of the Company); provided, however, within five (5) Business Days of the date said Notice of Exercise is delivered to the Company, the Holder shall have surrendered this Warrant to the Company and the Company shall have received payment of the aggregate Exercise Price of the shares thereby purchased by wire transfer or cashier’s check drawn on a United States bank.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 1 |

|
Exhibit “A”
b) Cashless Exercise. This Warrant may also be exercised at such time by means of a “cashless exercise” in which the Holder shall be entitled to receive a certificate for the number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by (A), where:
| (A) = | the price of said Common Stock determined by reference to the last reported sale price for the Common Stock on such day on the principal securities exchange on which the Common Stock is listed or admitted to trading or if no such sale takes place on such date, the average of the closing bid and asked prices thereof as officially reported, or, if not so listed or admitted to trading on any securities exchange, the last sale price for the Common Stock on the National Association of Securities Dealers national market system on such date, or, if there shall have been no trading on such date or if the Common Stock shall not be listed on such system, the average of the closing bid and asked prices in the over-the-counter market as furnished by any NASD member firm selected from time to time by the Company for such purpose or, if the Common Stock is not traded, then such price as is reasonably determined by the Company’s Board of Directors (the “Market Value”); |
| (B) = | the Exercise Price of this Warrant, as adjusted; and |
| (X) = | the number of Warrant Shares issuable upon exercise of this Warrant in accordance with the terms of this Warrant by means of a cash exercise rather than a cashless exercise. |
Notwithstanding anything herein to the contrary, on the Termination Date, this Warrant shall be automatically exercised via cashless exercise pursuant to this Section 2(b).
c) Mechanics of Exercise.
i. Authorization of Warrant Shares. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges in respect of the issuance thereof (other than taxes in respect of any transfer occurring contemporaneously with such issuance). The Company covenants that during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full
| Common Stock Purchase Warrant (Alterix, Inc.), Page 2 |

|
authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed.
ii. Delivery of Certificates Upon Exercise. If and to the extent that the Company completes a Public Offering pursuant to the Securities Act of 1933, as amended, and, if applicable to shares of the Company, certificates for shares purchased hereunder shall be transmitted by the transfer agent of the Company to the Holder by crediting the account of the Holder’s prime broker with the Depository Trust Company through its Deposit Withdrawal Agent Commission (“DWAC”) system if the Company is a participant in such system and if the certificates may be issued without a restrictive legend in accordance with applicable federal securities laws, and otherwise by physical delivery to the address specified by the Holder in the Notice of Exercise, within ten (10) Business Days from the delivery to the Company of the Notice of Exercise Form, surrender of this Warrant and payment of the aggregate Exercise Price as set forth above (“Warrant Share Delivery Date”). This Warrant shall be deemed to have been exercised on the date the Exercise Price is received by the Company. The Warrant Shares shall be deemed to have been issued, and Holder or any other person so designated to be named therein shall be deemed to have become a holder of record of such shares for all purposes, as of the date the Warrant has been exercised by payment to the Company of the Exercise Price and all taxes required to be paid by the Holder, if any, pursuant to Section 2(c)(vii) prior to the issuance of such shares, have been paid.
iii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the time of delivery of the certificate or certificates representing Warrant Shares, deliver to Holder a new Warrant evidencing the rights of Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iv. Rescission Rights. If the Company fails to cause its transfer agent to transmit to the Holder a certificate or certificates representing the Warrant Shares pursuant to this Section 2(c)(iv) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 3 |

|
v. Failure to Timely Deliver Certificates Upon Exercise. In addition to any other rights available to the Holder, (A) if the Company or the Company’s transfer agent fails to cause delivery to the Holder of a certificate or certificates representing the Warrant Shares (assuming the valid exercise of this Warrant by the Holder and the satisfaction of all applicable legal and regulatory requirements in connection therewith), or (B) if the Company or its transfer agent fails to deliver such certificates without the restrictive legend (if the Holder shall first have demonstrated to the reasonable satisfaction of the Company and its counsel the Holder’s compliance with all requirements applicable under the securities laws for the removal of any restrictive legend) on or before the Warrant Share Delivery Date (or other evidence reasonably satisfactory to Holder confirming that the Warrant Shares may be freely traded on the Trading Market in question), the Company shall pay to Purchaser, in cash, as partial liquidated damages and not as a penalty, $50 for each Business Day after the Warrant Share Delivery Date until (A) such certificate is delivered with an appropriate legend, or (B) without a restrictive legend (or other evidence reasonably satisfactory to Holder confirming that the Warrant Shares may be freely traded on the Trading Market in question), as the case may be. The Holder acknowledges that if the Company shall at the time of exercise of this Warrant be a participant in the Depository Trust and Clearing Corporation’s clearance and settlement systems, all exercises of this Warrant may be settled on such system without the issuance of physical stock certificates.
vi. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which Holder would otherwise be entitled to purchase upon such exercise, the Company shall round such fractional share up to the next whole number.
vii. Charges, Taxes and Expenses. Issuance of certificates for Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such certificate, all of which taxes and expenses shall be paid by the Company, and such certificates shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event certificates for Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto duly executed by the Holder, and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto.
viii. Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 4 |

|
Section 3. Certain Adjustments.
a) Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (A) pays a stock dividend or otherwise make a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock, (B) subdivides outstanding shares of Common Stock into a larger number of shares, (C) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (D) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding before such event and of which the denominator shall be the number of shares of Common Stock outstanding after such event and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted. If the Company, at any time while this warrant is outstanding, is merged, absorbed, reabsorbed or combined in any way with its parent company or any affiliate or other spin out from the parent entity, the number of warrants owned by Holder in the combined entity will be adjusted to equal the average of the percentage of ownership Holder had in each of the separate pre merged entities. This ownership percentage is determined by a fraction whereby the numerator is the sum of the Warrants owned in the Company plus the warrants owned by Holder in the pre combination parent or affiliate entity, divided by the sum of the total authorized common shares in the Company plus the total authorized shares in the to be combined company. This percentage is then multiplied by the total shares authorized and outstanding in the post combined entity. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
b) Vesting Schedule. These warrants are issued to Holder pursuant to a Service Agreement provided by the Holder to the Company and executed on the same date as this Warrant Agreement. As long as the Service Agreement is in full effect, Warrants shall vest pursuant to the following schedule: 1,000,000 shares immediately, and 1,000,000 shares on each of the next three anniversaries of this Agreement. Provided that in the event of a public offering, or sale or merger into a non related or affiliated entity, these warrants shall immediately vest in their entirety. These Warrants are issued pursuant to a Service Agreement whereby Holder provides management and advisory services to the Company. As such, these warrants become null and void in the event that the Holder or any principal in the Holder’s organization receive additional compensation for serving in an executive or Board capacity in the Company or parent company.
c) Subsequent Equity Sales. Subject to Section 3(e) below, if, commencing upon the closing of a Qualifying Transaction, the Company shall issue any New Securities (as defined in the Purchase Agreement) in a material transaction, at a price per share less than the Exercise Price then in effect pursuant to Section 2 of this Warrant (such lower price, the “Base Share Price” and such issuances collectively, a “Dilutive Issuance”), then, the Exercise Price of these Warrants shall be reduced to equal the Base Share Price. Such adjustment shall be made whenever such Common Stock or Common
| Common Stock Purchase Warrant (Alterix, Inc.), Page 5 |

|
Stock Equivalents are issued, and effective and not just contingent on some future event. For example, reset provisions, floating conversion, exercise or exchange prices or otherwise are not given effect until they are actually exercised and common stock is in fact delivered at a lower price than that specified in Section 2. Note a material transaction is one involving more the 5% of the float of the Company per transaction or equity raise. The Company shall notify the Holder in writing, no later than the Business Day following the issuance of any Common Stock or Common Stock Equivalents subject to this section, indicating therein the applicable issuance price, or of applicable reset price, exchange price, conversion price and other pricing terms (such notice the “Dilutive Issuance Notice”). For purposes of clarification, whether or not the Company provides a Dilutive Issuance Notice pursuant to this Section 3(b) upon the occurrence of any Dilutive Issuance, after the date of such Dilutive Issuance the Holder is entitled to receive Warrant Shares based upon the Base Share Price regardless of whether the Holder accurately refers to the Base Share Price in the Notice of Exercise.
d) Pro Rata Distributions. If the Company, at any time prior to the Termination Date, shall distribute to all holders of Common Stock (and not to Holders of the Warrants) evidences of its indebtedness or assets or rights or warrants to subscribe for or purchase any security other than the Common Stock (which shall be subject to Section 3(b)), then in each such case the Exercise Price shall be adjusted by multiplying the Exercise Price in effect immediately prior to the record date fixed for determination of stockholders entitled to receive such distribution by a fraction of which the denominator shall be the closing bid price of the Common Stock on the then principal Trading Market determined as of the record date mentioned above (if the closing bid price of the Common Stock on the then principal Trading Market shall then be determinable) and otherwise the fair market value per share as determined by the Board of Directors in good faith, and of which the numerator shall be such closing bid price of the Common Stock on the then principal Trading Market on such record date (if the closing bid price of the Common Stock on the then principal Trading Market shall then be determinable) or the price per share used to determine the denominator less the then per share fair market value at such record date of the portion of such assets or evidence of indebtedness so distributed applicable to one outstanding share of the Common Stock as determined by the Board of Directors in good faith. In either case the adjustments shall be described in a statement provided to the Holder of the portion of assets or evidences of indebtedness so distributed or such subscription rights applicable to one share of Common Stock. Such adjustment shall be made whenever any such distribution is made and shall become effective immediately after the record date mentioned above.
| e) | Exempt Issuance. Notwithstanding the foregoing, no adjustments, Alternate Consideration, or notices shall be made, paid, or issued under this Section 3 in respect of an Exempt Issuance. “Exempt Issuance’’ means the issuance of (a) shares of Common Stock or other securities to employees, consultants or directors pursuant to stock option, stock grant, stock purchase or similar plans or arrangements approved by the Board of Directors, including without limitation upon the exercise of options currently outstanding, (b) shares of capital stock issuable upon conversion of shares of preferred stock, |
| Common Stock Purchase Warrant (Alterix, Inc.), Page 6 |

|
| convertible securities, options or warrants issued and outstanding on the date of this Agreement; provided that such securities have not been amended since the date of this Agreement to increase the number of such underlying shares in connection therewith, (c) upon the exercise or conversion of any securities issued hereunder, (d) shares of Common Stock or other securities issued to equipment lessors, banks, financial institutions or similar entities in a transaction approved by the Board of Directors, the principal purpose of which is other than the raising of capital (e) securities issued pursuant to a merger, acquisition or strategic transaction that is approved by the Board of Directors, (f) securities in the Approved Subsequent Financing or the Public Offering, (g) shares of Common Stock or other securities issued as a dividend or other distribution in connection with which an automatic adjustment in the conversion price of the Debenture is made, (h) shares of Common Stock or other securities issued pursuant to any transaction approved by the Board of Directors primarily for the purpose of (i) a joint venture, technology licensing or research and development activity, (ii) distribution or manufacture of the corporation’s products or services, or (iii) any other transaction involving a corporate partner that is primarily for a purpose other than the raising of capital. |
f) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. The number of shares of Common Stock outstanding at any given time shall not include shares of Common Stock owned or held by or for the account of the Company, and the description of any such shares of Common Stock shall be considered on issue or sale of Common Stock. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
g) Notice to Holders.
i. Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to this Section 3, the Company shall promptly mail to each Holder a notice setting forth the Exercise Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
ii. Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution) on the Common Stock; (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock; (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights; (D) the approval of any stockholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, of any compulsory share exchange whereby the Common Stock is converted into
| Common Stock Purchase Warrant (Alterix, Inc.), Page 7 |

|
other securities, cash or property; (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company; then, in each case, the Company shall cause to be mailed to the Holder at its last address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided, however that the failure to mail such notice or any defect therein or in the mailing thereof shall not affect the validity of the corporate action required to be specified in such notice. The Holder is entitled to exercise this Warrant during the 20-day period commencing on the date of such notice to the effective date of the event triggering such notice.
Section 4. Transfer of Warrant.
a) Transferability. Subject to compliance with any applicable securities laws, the conditions set forth in this Warrant along with secured written permission from the Company which may grant permission in its sole discretion, this Warrant and all rights hereunder are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company, together with a written assignment of this Warrant substantially in the form attached hereto duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. A Warrant, if properly assigned, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 8 |

|
c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
d) Transfer Restrictions. If, at the time of the surrender of this Warrant in connection with any transfer of this Warrant, the transfer of this Warrant shall not be registered pursuant to an effective registration statement under the Securities Act and under applicable state securities or blue sky laws, the Company may require, as a condition of allowing such transfer (i) that the Holder or transferee of this Warrant, as the case may be, furnish to the Company a written opinion of counsel (which opinion shall be in form, substance and scope customary for opinions of counsel in comparable transactions) to the effect that such transfer may be made without registration under the Securities Act and under applicable state securities or blue sky laws, (ii) that the holder or transferee execute and deliver to the Company an investment letter in form and substance acceptable to the Company and (iii) that the transferee be an “accredited investor” as defined in Rule 501(a) of Regulation D promulgated under the Securities Act or a qualified institutional buyer as defined in Rule 144A(a) under the Securities Act.
Section 5. Miscellaneous.
a) Title to Warrant. Prior to the Termination Date and subject to compliance with applicable laws and Section 4 of this Warrant, this Warrant and all rights hereunder are transferable, in whole or in part, at the office or agency of the Company by the Holder in person or by duly authorized attorney, upon surrender of this Warrant together with the Assignment Form annexed hereto properly endorsed. The transferee shall sign an investment letter in form and substance reasonably satisfactory to the Company.
b) No Rights as Shareholder Until Exercise. This Warrant does not entitle the Holder to any voting rights or other rights as a shareholder of the Company prior to the exercise hereof. Upon the surrender of this Warrant and the payment of the aggregate Exercise Price (or by means of a cashless exercise), the Warrant Shares so purchased shall be and be deemed to be issued to such Holder as the record owner of such shares as of the close of business on the later of the date of such surrender or payment.
c) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 9 |

|
d) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall be a Saturday, Sunday or a legal holiday, then such action may be taken or such right may be exercised on the next succeeding day not a Saturday, Sunday or legal holiday.
e) Authorized Shares.
The Company covenants that during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed.
h) Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its articles of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (a) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (b) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant, and (c) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof as may be necessary to enable the Company to perform its obligations under this Warrant. These Warrants are issued pursuant to a Service Agreement whereby Holder provides management and advisory services to the Company. As such, these warrants become null and void in the event that the Service Agreement is terminated or the Holder or any principal in the Holder’s organization receive additional compensation for serving in an executive or Board capacity in the Company or parent company.
Before taking any action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
f) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be determined in accordance with the provisions of the Purchase Agreement as applied in the courts of the Commonwealth of Massachusetts.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 10 |

|
g) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, will have restrictions upon resale imposed by state and federal securities laws.
h) Expenses. If the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any Material damages to the Holder, the Company shall pay to Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder. However, in the event that Holder fails to prevail, Holder will be liable to the Company for the Company’s expenses related to the defense of Holder’s action including but not limited to reasonable attorney’s costs.
i) Notices. Any notice, request or other document required or permitted to be given or delivered to the Holder by the Company shall be delivered in accordance with the notice provisions of the Purchase Agreement.
j) Limitation of Liability. No provision hereof, in the absence of any affirmative action by Holder to exercise this Warrant or purchase Warrant Shares, and no enumeration herein of the rights or privileges of Holder, shall give rise to any liability of Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
k) Remedies. Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive the defense in any action for specific performance that a remedy at law would be adequate.
1) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of all Holders from time to time of this Warrant and shall be enforceable by any such Holder or holder of Warrant Shares.
m) Amendment and Waiver. This Warrant may be modified or amended only with the written consent of the Company and the Holder. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice Holder’s rights, powers or remedies, notwithstanding the fact that all rights hereunder terminate on the Termination Date.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 11 |

|
n) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
o) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
p) Registration Rights. The Holder has certain rights with respect to the registration of the Warrant Shares upon exercise of this Warrant, such rights being specifically set forth in the Purchase Agreement.
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first written above.
| ALTERIX, INC. | ||
| By: | /s/ John Masiz | |
| Name: | John Masiz | |
| Title: | Chief Executive Officer | |
| Common Stock Purchase Warrant (Alterix, Inc.), Page 12 |

|
NOTICE OF EXERCISE
TO: ALTERIX, INC.
(1) The undersigned hereby elects to purchase Warrant Shares of BioChemics, Inc. pursuant to the terms of the attached Warrant (only if exercised in full), and tenders herewith payment of the Exercise Price in full, together with all applicable transfer taxes, if any.
(2) Payment shall take the form of (check applicable box):
[ ] lawful money of the United States; or
[ ] the cancellation of such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2(b) of the Warrant, to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2(b).
(3) Please issue a certificate or certificates representing said Warrant Shares in the name of the undersigned or in such other name as is specified below:
The Warrant Shares shall be delivered to the following:
(4) Accredited Investor. The undersigned, and, if applicable, the person or entity identified in subsection 3 above, is an “accredited investor” as defined in Regulation D promulgated under the Securities Act of 1933, as amended.
[SIGNATURE OF HOLDER]
Name of Investing Entity:
Signature of Authorized Signatory of Investing Entity:
Name of Authorized Signatory:
Title of Authorized Signatory:
Date:
ASSIGNMENT FORM
(To assign the foregoing warrant, execute
this form and supply required information.
Do not use this form to exercise the warrant.)
FOR VALUE RECEIVED, the foregoing Warrant and all rights evidenced thereby are hereby assigned to
whose address is
.
Dated: ,
| Holder’s Signature: |
| |||
| Holder’s Address: |
| |||
|
| ||||
Signature Guaranteed:
NOTE: The signature to this Assignment Form must correspond with the name as it appears on the face of the Warrant, without alteration or enlargement or any change whatsoever, and must be guaranteed by a bank or trust company. Officers of corporations and those acting in a fiduciary or other representative capacity should file proper evidence of authority to assign the foregoing Warrant.
Exhibit 4.3 B
Amendment to Common Stock Purchase Warrant
Amendment to Common Stock Purchase Warrant between Logic International Consulting Group, LLC as (“Holder”) and Alterix, Inc., a Delaware Corporation (“Company”), dated this 10th day of June, 2015.
WHEREAS, Company and Holder entered into a Common Stock Purchase Warrant on December 1, 2013, (“Agreement”), a copy of which is attached hereto as Exhibit “A”;
WHEREAS, Section 5(m) of the Warrant requires that any modifications to the Agreement be authorized in writing by Company and Holder;
WHEREAS, the Company and Holder desire to delete Section 3(c) (“Subsequent Equity Sales”) the Warrant as to specific terms and provisions;
WHEREAS, the Company and Holder desire to add a Section 4(e) (“Market Stand-Off Agreement”) to the Warrant as to specific terms and provisions;
IT IS THEREFORE AGREED:
| 1. | Section 3(c) is deleted entirely. |
| 2. | Section 4(e) Market Stand-Off Agreement. The Holder hereby agrees that it will not, without the prior written consent of the managing underwriter, during the period commencing on the date of the final prospectus relating to the registration by the Company of shares of its Common Stock or any other equity securities under the Securities Act on a registration statement on Form S-1 or Form S-3, and ending on the date specified by the Company and the managing underwriter (such period not to exceed one hundred eighty (180) days in the case of the initial public offering, or ninety (90) days in the case of any registration other than the initial public offering, (i) lend; offer; pledge; sell; contract to sell; sell any option or contract to purchase; purchase any option or contract to sell; grant any option, right, or warrant to purchase; or otherwise transfer or dispose of, directly or indirectly, any shares of Common Stock or any securities convertible into or exercisable or exchangeable (directly or indirectly) for Common Stock held immediately before the effective date of the registration statement for such offering or (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of such securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of Common Stock or other securities, in cash, or otherwise. The foregoing provisions shall not apply to the sale of any shares to an |
Amendment to Common Stock Purchase Warrant- Page 1
| underwriter pursuant to an underwriting agreement, or the transfer of any shares to any trust for the direct or indirect benefit of the Holder or the immediate family of the Holder, provided that the trustee of the trust agrees to be bound in writing by the restrictions set forth herein, and provided further that any such transfer shall not involve a disposition for value, and shall be applicable to the Holder only if all officers and directors are subject to the same restrictions and the Company uses commercially reasonable efforts to obtain a similar agreement from all stockholders individually owning more than one percent (1%) of the Company’s outstanding Common Stock. The underwriters in connection with such registration are intended third-party beneficiaries of these provisions and shall have the right, power and authority to enforce the provisions hereof as though they were a party hereto. The Holder further agrees to execute such agreements as may be reasonably requested by the underwriters in connection with such registration that are consistent with these provisions or that are necessary to give further effect thereto. Any discretionary waiver or termination of the restrictions of any or all of such agreements by the Company or the underwriters shall apply pro rata to all holders of the Company’s Common Stock subject to such agreements, based on the number of shares subject to such agreements. |
| 3. | Except for the amendments and modifications set forth paragraphs 1 and 2 in this Amendment, all other provisions of the Agreement are hereby ratified and shall remain in full force and effect. |
| COMPANY: | HOLDER: | |||
| Alterix, Inc. | Logic International Consulting Group, LLC | |||
| a Delaware Corporation | a New York Limited Liability Company | |||
| a subsidiary of BioChemics, Inc. | ||||
| By: /s/ David Staskin | By: /s/ Kavin M. Cassidy | |||
Amendment to Common Stock Purchase Warrant- Page 2
NEITHER THIS SECURITY NOR THE SECURITIES INTO WHICH THIS SECURITY IS EXERCISABLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL OPINION OF COUNSEL TO THE TRANSFEROR TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY. THIS SECURITY AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS SECURITY MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN SECURED BY SUCH SECURITIES AS PERMITTED BY LAW AND THE SECURITIES PURCHASE AGREEMENT PURSUANT TO WHICH THE SECURITIES WERE ISSUED.
COMMON STOCK PURCHASE WARRANT
To Purchase Shares of Common Stock of
ALTERIX, INC.
This COMMON STOCK PURCHASE WARRANT (this “Warrant”) certifies that, for services rendered and to be rendered, Logic International Consulting Group, L.L.C. or assignee (the “Holder”), is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the effective date hereof, December 1, 2013 and on or prior to the close of business on the fifth (5th) anniversary of the date of this Warrant (the “Termination Date”), to subscribe for and purchase up to Four Million (4,000,000) shares of common stock (the “Warrant Shares”), from ALTERIX, INC., a Delaware corporation (the “Company”), par value $.01 per share of the Company (the “Common Stocks”), as provided below. The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(a) below, as adjusted.
Section 1. Exercise.
a) Exercise of Warrant/Exercise Price. Exercise of the purchase rights represented by this Warrant may be made at any time or times on or after the closing of a Qualifying Transaction, at an Exercise Price per share equal to Five and No/100 Dollars ($5.00) per share (the “Exercise Price”). This Warrant may be exercised on or before the Termination Date (each, an “Exercise Date”) by delivery to the Company of a duly executed facsimile copy of the Notice of Exercise Form annexed hereto (or such other office or agency of the Company as it may designate by notice in writing to the registered Holder at the address of such Holder appearing on the books of the Company); provided, however, within five (5) Business Days of the date said Notice of Exercise is delivered to the Company, the Holder shall have surrendered this Warrant to the Company and the Company shall have received payment of the aggregate Exercise Price of the shares thereby purchased by wire transfer or cashier’s check drawn on a United States bank.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 1 |

|
EXHIBIT “A”
b) Cashless Exercise. This Warrant may also be exercised at such time by means of a “cashless exercise” in which the Holder shall be entitled to receive a certificate for the number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by (A), where:
| (A) = | the price of said Common Stock determined by reference to the last reported sale price for the Common Stock on such day on the principal securities exchange on which the Common Stock is listed or admitted to trading or if no such sale takes place on such date, the average of the closing bid and asked prices thereof as officially reported, or, if not so listed or admitted to trading on any securities exchange, the last sale price for the Common Stock on the National Association of Securities Dealers national market system on such date, or, if there shall have been no trading on such date or if the Common Stock shall not be listed on such system, the average of the closing bid and asked prices in the over-the-counter market as furnished by any NASD member firm selected from time to time by the Company for such purpose or, if the Common Stock is not traded, then such price as is reasonably determined by the Company’s Board of Directors (the “Market Value”); |
| (B) = | the Exercise Price of this Warrant, as adjusted; and |
| (X) = | the number of Warrant Shares issuable upon exercise of this Warrant in accordance with the terms of this Warrant by means of a cash exercise rather than a cashless exercise. |
Notwithstanding anything herein to the contrary, on the Termination Date, this Warrant shall be automatically exercised via cashless exercise pursuant to this Section 2(b).
c) Mechanics of Exercise.
i. Authorization of Warrant Shares. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges in respect of the issuance thereof (other than taxes in respect of any transfer occurring contemporaneously with such issuance). The Company covenants that during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full
| Common Stock Purchase Warrant (Alterix, Inc.), Page 2 |

|
authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed.
ii. Delivery of Certificates Upon Exercise. If and to the extent that the Company completes a Public Offering pursuant to the Securities Act of 1933, as amended, and, if applicable to shares of the Company, certificates for shares purchased hereunder shall be transmitted by the transfer agent of the Company to the Holder by crediting the account of the Holder’s prime broker with the Depository Trust Company through its Deposit Withdrawal Agent Commission (“DWAC”) system if the Company is a participant in such system and if the certificates may be issued without a restrictive legend in accordance with applicable federal securities laws, and otherwise by physical delivery to the address specified by the Holder in the Notice of Exercise, within ten (10) Business Days from the delivery to the Company of the Notice of Exercise Form, surrender of this Warrant and payment of the aggregate Exercise Price as set forth above (“Warrant Share Delivery Date”). This Warrant shall be deemed to have been exercised on the date the Exercise Price is received by the Company. The Warrant Shares shall be deemed to have been issued, and Holder or any other person so designated to be named therein shall be deemed to have become a holder of record of such shares for all purposes, as of the date the Warrant has been exercised by payment to the Company of the Exercise Price and all taxes required to be paid by the Holder, if any, pursuant to Section 2(c)(vii) prior to the issuance of such shares, have been paid.
iii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the time of delivery of the certificate or certificates representing Warrant Shares, deliver to Holder a new Warrant evidencing the rights of Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iv. Rescission Rights. If the Company fails to cause its transfer agent to transmit to the Holder a certificate or certificates representing the Warrant Shares pursuant to this Section 2(c)(iv) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 3 |

|
v. Failure to Timely Deliver Certificates Upon Exercise. In addition to any other rights available to the Holder, (A) if the Company or the Company’s transfer agent fails to cause delivery to the Holder of a certificate or certificates representing the Warrant Shares (assuming the valid exercise of this Warrant by the Holder and the satisfaction of all applicable legal and regulatory requirements in connection therewith), or (B) if the Company or its transfer agent fails to deliver such certificates without the restrictive legend (if the Holder shall first have demonstrated to the reasonable satisfaction of the Company and its counsel the Holder’s compliance with all requirements applicable under the securities laws for the removal of any restrictive legend) on or before the Warrant Share Delivery Date (or other evidence reasonably satisfactory to Holder confirming that the Warrant Shares may be freely traded on the Trading Market in question), the Company shall pay to Purchaser, in cash, as partial liquidated damages and not as a penalty, $50 for each Business Day after the Warrant Share Delivery Date until (A) such certificate is delivered with an appropriate legend, or (B) without a restrictive legend (or other evidence reasonably satisfactory to Holder confirming that the Warrant Shares may be freely traded on the Trading Market in question), as the case may be. The Holder acknowledges that if the Company shall at the time of exercise of this Warrant be a participant in the Depository Trust and Clearing Corporation’s clearance and settlement systems, all exercises of this Warrant may be settled on such system without the issuance of physical stock certificates.
vi. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which Holder would otherwise be entitled to purchase upon such exercise, the Company shall round such fractional share up to the next whole number.
vii. Charges, Taxes and Expenses. Issuance of certificates for Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such certificate, all of which taxes and expenses shall be paid by the Company, and such certificates shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event certificates for Warrant Shares are to be issued in a name other than the name of the Holder; this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto duly executed by the Holder, and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto.
viii. Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 4 |

|
Section 3. Certain Adjustments.
a) Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (A) pays a stock dividend or otherwise make a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock, (B) subdivides outstanding shares of Common Stock into a larger number of shares, (C) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (D) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding before such event and of which the denominator shall be the number of shares of Common Stock outstanding after such event and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted. If the Company, at any time while this warrant is outstanding, is merged, absorbed, reabsorbed or combined in any way with its parent company or any affiliate or other spin out from the parent entity, the number of warrants owned by Holder in the combined entity will be adjusted to equal the average of the percentage of ownership Holder had in each of the separate pre merged entities. This ownership percentage is determined by a fraction whereby the numerator is the sum of the Warrants owned in the Company plus the warrants owned by Holder in the pre combination parent or affiliate entity, divided by the sum of the total authorized common shares in the Company plus the total authorized shares in the to be combined company. This percentage is then multiplied by the total shares authorized and outstanding in the post combined entity. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
b) Vesting Schedule. These warrants are issued to Holder pursuant to a Service Agreement provided by the Holder to the Company and executed on the same date as this Warrant Agreement. As long as the Service Agreement is in full effect, Warrants shall vest pursuant to the following schedule: 1,000,000 shares immediately, and 1,000,000 shares on each of the next three anniversaries of this Agreement. Provided that in the event of a public offering, or sale or merger into a non related or affiliated entity, these warrants shall immediately vest in their entirety. These Warrants are issued pursuant to a Service Agreement whereby Holder provides management and advisory services to the Company. As such, these warrants become null and void in the event that the Holder or any principal in the Holder’s organization receive additional compensation for serving in an executive or Board capacity in the Company or parent company.
c) Subsequent Equity Sales. Subject to Section 3(e) below, if, commencing upon the closing of a Qualifying Transaction, the Company shall issue any New Securities (as defined in the Purchase Agreement) in a material transaction, at a price per share less than the Exercise Price then in effect pursuant to Section 2 of this Warrant (such lower price, the “Base Share Price” and such issuances collectively, a (“Dilutive Issuance”), then, the Exercise Price of these Warrants shall be reduced to equal the Base Share Price. Such adjustment shall be made whenever such Common Stock or Common
| Common Stock Purchase Warrant (Alterix, Inc.), Page 5 |

|
Stock Equivalents are issued, and effective and not just contingent on some future event. For example, reset provisions, floating conversion, exercise or exchange prices or otherwise are not given effect until they are actually exercised and common stock is in fact delivered at a lower price than that specified in Section 2. Note a material transaction is one involving more the 5% of the float of the Company per transaction or equity raise. The Company shall notify the Holder in writing, no later than the Business Day following the issuance of any Common Stock or Common Stock Equivalents subject to this section, indicating therein the applicable issuance price, or of applicable reset price, exchange price, conversion price and other pricing terms (such notice the “Dilutive Issuance Notice”). For purposes of clarification, whether or not the Company provides a Dilutive Issuance Notice pursuant to this Section 3(b) upon the occurrence of any Dilutive Issuance, after the date of such Dilutive Issuance the Holder is entitled to receive Warrant Shares based upon the Base Share Price regardless of whether the Holder accurately refers to the Base Share Price in the Notice of Exercise.
d) Pro Rata Distributions. If the Company, at any time prior to the Termination Date, shall distribute to all holders of Common Stock (and not to Holders of the Warrants) evidences of its indebtedness or assets or rights or warrants to subscribe for or purchase any security other than the Common Stock (which shall be subject to Section 3(b)), then in each such case the Exercise Price shall be adjusted by multiplying the Exercise Price in effect immediately prior to the record date fixed for determination of stockholders entitled to receive such distribution by a fraction of which the denominator shall be the closing bid price of the Common Stock on the then principal Trading Market determined as of the record date mentioned above (if the closing bid price of the Common Stock on the then principal Trading Market shall then be determinable) and otherwise the fair market value per share as determined by the Board of Directors in good faith, and of which the numerator shall be such closing bid price of the Common Stock on the then principal Trading Market on such record date (if the closing bid price of the Common Stock on the then principal Trading Market shall then be determinable) or the price per share used to determine the denominator less the then per share fair market value at such record date of the portion of such assets or evidence of indebtedness so distributed applicable to one outstanding share of the Common Stock as determined by the Board of Directors in good faith. In either case the adjustments shall be described in a statement provided to the Holder of the portion of assets or evidences of indebtedness so distributed or such subscription rights applicable to one share of Common Stock. Such adjustment shall be made whenever any such distribution is made and shall become effective immediately after the record date mentioned above.
| e) | Exempt Issuance. Notwithstanding the foregoing, no adjustments, Alternate Consideration, or notices shall be made, paid, or issued under this Section 3 in respect of an Exempt Issuance. “Exempt Issuance” means the issuance of (a) shares of Common Stock or other securities to employees, consultants or directors pursuant to stock option, stock grant, stock purchase or similar plans or arrangements approved by the Board of Directors, including without limitation upon the exercise of options currently outstanding, (b) shares of capital stock issuable upon conversion of shares of preferred stock, |
| Common Stock Purchase Warrant (Alterix, Inc.), Page 6 |

|
| convertible securities, options or warrants issued and outstanding on the date of this Agreement; provided that such securities have not been amended since the date of this Agreement to increase the number of such underlying shares in connection therewith, (c) upon the exercise or conversion of any securities issued hereunder, (d) shares of Common Stock or other securities issued to equipment lessors, banks, financial institutions or similar entities in a transaction approved by the Board of Directors, the principal purpose of which is other than the raising of capital (e) securities issued pursuant to a merger, acquisition or strategic transaction that is approved by the Board of Directors, (f) securities in the Approved Subsequent Financing or the Public Offering, (g) shares of Common Stock or other securities issued as a dividend or other distribution in connection with which an automatic adjustment in the conversion price of the Debenture is made, (h) shares of Common Stock or other securities issued pursuant to any transaction approved by the Board of Directors primarily for the purpose of (i) a joint venture, technology licensing or research and development activity, (ii) distribution or manufacture of the corporation’s products or services, or (iii) any other transaction involving a corporate partner that is primarily for a purpose other than the raising of capital. |
f) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. The number of shares of Common Stock outstanding at any given time shall not include shares of Common Stock owned or held by or for the account of the Company, and the description of any such shares of Common Stock shall be considered on issue or sale of Common Stock. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
g) Notice to Holders.
i. Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to this Section 3, the Company shall promptly mail to each Holder a notice setting forth the Exercise Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
ii. Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution) on the Common Stock; (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock; (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights; (D) the approval of any stockholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, of any compulsory share exchange whereby the Common Stock is converted into
| Common Stock Purchase Warrant (Alterix, Inc.), Page 7 |

|
other securities, cash or property; (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company; then, in each case, the Company shall cause to be mailed to the Holder at its last address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided, however that the failure to mail such notice or any defect therein or in the mailing thereof shall not affect the validity of the corporate action required to be specified in such notice. The Holder is entitled to exercise this Warrant during the 20-day period commencing on the date of such notice to the effective date of the event triggering such notice.
Section 4. Transfer of Warrant.
a) Transferability. Subject to compliance with any applicable securities laws, the conditions set forth in this Warrant along with secured written permission from the Company which may grant permission in its sole discretion, this Warrant and all rights hereunder are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company, together with a written assignment of this Warrant substantially in the form attached hereto duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. A Warrant, if properly assigned, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 8 |

|
c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
d) Transfer Restrictions. If, at the time of the surrender of this Warrant in connection with any transfer of this Warrant, the transfer of this Warrant shall not be registered pursuant to an effective registration statement under the Securities Act and under applicable state securities or blue sky laws, the Company may require, as a condition of allowing such transfer (i) that the Holder or transferee of this Warrant, as the case may be, furnish to the Company a written opinion of counsel (which opinion shall be in form, substance and scope customary for opinions of counsel in comparable transactions) to the effect that such transfer may be made without registration under the Securities Act and under applicable state securities or blue sky laws, (ii) that the holder or transferee execute and deliver to the Company an investment letter in form and substance acceptable to the Company and (iii) that the transferee be an “accredited investor” as defined in Rule 501(a) of Regulation D promulgated under the Securities Act or a qualified institutional buyer as defined in Rule 144A(a) under the Securities Act.
Section 5. Miscellaneous.
a) Title to Warrant. Prior to the Termination Date and subject to compliance with applicable laws and Section 4 of this Warrant, this Warrant and all rights hereunder are transferable, in whole or in part, at the office or agency of the Company by the Holder in person or by duly authorized attorney, upon surrender of this Warrant together with the Assignment Form annexed hereto properly endorsed. The transferee shall sign an investment letter in form and substance reasonably satisfactory to the Company.
b) No Rights as Shareholder Until Exercise. This Warrant does not entitle the Holder to any voting rights or other rights as a shareholder of the Company prior to the exercise hereof. Upon the surrender of this Warrant and the payment of the aggregate Exercise Price (or by means of a cashless exercise), the Warrant Shares so purchased shall be and be deemed to be issued to such Holder as the record owner of such shares as of the close of business on the later of the date of such surrender or payment.
c) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 9 |

|
d) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall be a Saturday, Sunday or a legal holiday, then such action may be taken or such right may be exercised on the next succeeding day not a Saturday, Sunday or legal holiday.
e) Authorized Shares.
The Company covenants that during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed.
h) Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its articles of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (a) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (b) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant, and (c) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof as may be necessary to enable the Company to perform its obligations under this Warrant. These Warrants are issued pursuant to a Service Agreement whereby Holder provides management and advisory services to the Company. As such, these warrants become null and void in the event that the Service Agreement is terminated or the Holder or any principal in the Holder’s organization receive additional compensation for serving in an executive or Board capacity in the Company or parent company.
Before taking any action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 10 |

|
f) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be determined in accordance with the provisions of the Purchase Agreement as applied in the courts of the Commonwealth of Massachusetts.
g) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, will have restrictions upon resale imposed by state and federal securities laws.
h) Expenses. If the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any Material damages to the Holder, the Company shall pay to Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder. However, in the event that Holder fails to prevail, Holder will be liable to the Company for the Company’s expenses related to the defense of Holder’s action including but not limited to reasonable attorney’s costs.
i) Notices. Any notice, request or other document required or permitted to be given or delivered to the Holder by the Company shall be delivered in accordance with the notice provisions of the Purchase Agreement.
j) Limitation of Liability. No provision hereof, in the absence of any affirmative action by Holder to exercise this Warrant or purchase Warrant Shares, and no enumeration herein of the rights or privileges of Holder, shall give rise to any liability of Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
k) Remedies. Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive the defense in any action for specific performance that a remedy at law would be adequate.
l) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of all Holders from time to time of this Warrant and shall be enforceable by any such Holder or holder of Warrant Shares.
m) Amendment and Waiver. This Warrant may be modified or amended only with the written consent of the Company and the Holder. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice Holder’s rights, powers or remedies, notwithstanding the fact that all rights hereunder terminate on the Termination Date.
| Common Stock Purchase Warrant (Alterix, Inc.), Page 11 |

|
n) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
o) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
p) Registration Rights. The Holder has certain rights with respect to the registration of the Warrant Shares upon exercise of this Warrant, such rights being specifically set forth in the Purchase Agreement.
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first written above.
| ALTERIX, INC. | ||
| By: | /s/ John Masiz | |
| Name: | John Masiz | |
| Title: | Chief Executive Officer | |
| Common Stock Purchase Warrant (Alterix, Inc.), Page 12 |

|
NOTICE OF EXERCISE
TO: ALTERIX, INC.
(1) The undersigned hereby elects to purchase Warrant Shares of BioChemics, Inc. pursuant to the terms of the attached Warrant (only if exercised in full), and tenders herewith payment of the Exercise Price in full, together with all applicable transfer taxes, if any.
(2) Payment shall take the form of (check applicable box):
[ ] lawful money of the United States; or
[ ] the cancellation of such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2(b) of the Warrant, to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2(b).
(3) Please issue a certificate or certificates representing said Warrant Shares in the name of the undersigned or in such other name as is specified below:
The Warrant Shares shall be delivered to the following:
(4) Accredited Investor. The undersigned, and, if applicable, the person or entity identified in subsection 3 above, is an “accredited investor” as defined in Regulation D promulgated under the Securities Act of 1933, as amended.
[SIGNATURE OF HOLDER]
Name of Investing Entity:
Signature of Authorized Signatory of Investing Entity:
Name of Authorized Signatory:
Title of Authorized Signatory:
Date:
ASSIGNMENT FORM
(To assign the foregoing warrant, execute
this form and supply required information.
Do not use this form to exercise the warrant.)
FOR VALUE RECEIVED, the foregoing Warrant and all rights evidenced thereby are hereby assigned to
whose address is
.
|
|
Dated: ,
| Holder’s Signature: |
| |||
| Holder’s Address: |
| |||
|
| ||||
Signature Guaranteed:
NOTE: The signature to this Assignment Form must correspond with the name as it appears on the face of the Warrant, without alteration or enlargement or any change whatsoever, and must be guaranteed by a bank or trust company. Officers of corporations and those acting in a fiduciary or other representative capacity should file proper evidence of authority to assign the foregoing Warrant.
Exhibit 4.4
SECURITIES PURCHASE AGREEMENT
This Securities Purchase Agreement (this “Agreement”) is dated as of August 17, 2015, between Alterix Inc., a Delaware corporation (the “Company”), and each purchaser identified on the signature pages hereto (each, including its successors and assigns, a “Purchaser” and collectively, the “Purchasers”).
WHEREAS, the Company is offering Original Issue Discount Convertible Notes with Warrants to acquire up to that number of shares of Common Stock as is determined in accordance with the terms of the Warrants (the “Offering”);
WHEREAS, the Company has engaged Alexander Capital for this Offering; and
WHEREAS, the Company is conducting this Offering and shall conduct future offerings to qualify to list its common stock on a national securities exchange; and
WHEREAS, subject to the terms and conditions set forth in this Agreement and pursuant to Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”), and Rule 506 promulgated thereunder, the Company desires to issue and sell to each Purchaser, and each Purchaser, severally and not jointly, desires to purchase from the Company, securities of the Company as more fully described in this Agreement.
NOW, THEREFORE, IN CONSIDERATION of the mutual covenants contained in this Agreement, and for other good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, the Company and each Purchaser agree as follows:
ARTICLE I
DEFINITIONS
1.1 Definitions. In addition to the terms defined elsewhere in this Agreement: (a) capitalized terms that are not otherwise defined herein have the meanings given to such terms in the Notes (as defined herein), and (b) the following terms have the meanings set forth in this Section 1.1:
“Acquiring Person” shall have the meaning ascribed to such term in Section 4.7.
“Action” shall have the meaning ascribed to such term in Section 3.1(j).
“Affiliate” means any Person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control with a Person, as such terms are used in and construed under Rule 405 under the Securities Act.
“Board of Directors” means the board of directors of the Company.
“Business Day” means any day except any Saturday, any Sunday, any day which is a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Closing” means the closing of the purchase and sale of the Securities pursuant to Section 2.1.
“Closing Date” means the Trading Day on which all of the Transaction Documents have been executed and delivered by the applicable parties thereto, and all conditions precedent to (i) the Purchasers’ obligations to pay the Subscription Amount and (ii) the Company’s obligations to deliver the Securities, in each case, have been satisfied or waived.
“Closing Statement” means the Closing Statement in the form on Annex A attached hereto.
“Commission” means the United States Securities and Exchange Commission.
“Common Stock” means the common stock of the Company, $0.001 par value per share, and any other class of securities into which such securities may hereafter be reclassified or changed.
“Common Stock Equivalents” means any securities of the Company or the Subsidiaries which would entitle the holder thereof to acquire at any time Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Common Stock.
“Conversion Price” shall have the meaning ascribed to such term in the Notes.
“Conversion Shares” shall have the meaning ascribed to such term in the Notes.
“Disclosure Schedules” shall have the meaning ascribed to such term in Section 3.1.
“Effective Date” means the earliest of the date that (a) a registration statement covering the Underlying Shares has been declared effective by the Commission, or (b) all of the Underlying Shares have been sold pursuant to Rule 144 or may be sold pursuant to Rule 144 without the requirement for the Company to be in compliance with the current public information required under Rule 144 and without volume or manner-of-sale restrictions.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“FCPA” means the Foreign Corrupt Practices Act of 1977, as amended.
“GAAP” shall have the meaning ascribed to such term in Section 3.1(h).
“Indebtedness” shall have the meaning ascribed to such term in Section 3.1(x).
“Intellectual Property Rights” shall have the meaning ascribed to such term in Section 3.1(n).
2
“Liens” means a lien, charge, pledge, security interest, encumbrance, right of first refusal, preemptive right or other restriction.
“Material Adverse Effect” shall have the meaning assigned to such term in Section 3.1(b).
“Material Permits” shall have the meaning ascribed to such term in Section 3.1(l).
“Maximum Rate” shall have the meaning ascribed to such term in Section 5.17.
“Notes” means the Original Issue Discount Convertible Notes issued by the Company to the Purchasers hereunder, in the form of Exhibit A attached hereto.
“Original Issue Discount” means 20%.
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Principal Amount” means, as to each Purchaser, the principal amount of the Note, set forth below such Purchaser’s signature block on the signature pages hereto next to the heading “Principal Amount,” in United States Dollars.
“Proceeding” means an action, claim, suit, investigation or proceeding (including, without limitation, an informal investigation or partial proceeding, such as a deposition), whether commenced or threatened.
“Public Information Failure” shall have the meaning ascribed to such term in Section 4.3(b).
“Public Information Failure Payments” shall have the meaning ascribed to such term in Section 4.3(b).
“Purchaser Party” shall have the meaning ascribed to such term in Section 4.10.
“Required Approvals” shall have the meaning ascribed to such term in Section 3.1(e).
“Required Minimum” means, as of any date, the maximum aggregate number of shares of Common Stock then issued or potentially issuable in the future pursuant to the Transaction Documents, including any Underlying Shares issuable upon exercise in full of all Warrants or conversion in full of all Notes, ignoring any conversion or exercise limits set forth therein, and assuming that the Conversion Price is at all times on and after the date of determination 75% of the then Conversion Price on the Trading Day immediately prior to the date of determination.
“Rule 144” means Rule 144 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same effect as such Rule.
3
“Rule 424” means Rule 424 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect as such Rule.
“Securities” means the Notes, the Warrants, the Warrant Shares and the Underlying Shares.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Short Sales” means all “short sales” as defined in Rule 200 of Regulation SHO under the Exchange Act (but shall not be deemed to include the location and/or reservation of borrowable shares of Common Stock).
“Subscription Amount” means, as to each Purchaser, the aggregate amount to be paid for Notes and Warrants, which shall equal the Principal Amount multiplied by 80%, set forth below such Purchaser’s signature block on the signature pages hereto next to the heading “Subscription Amount,” in United States dollars and in immediately available funds.
“Subsidiary” means any subsidiary of the Company, as set forth on Schedule 3.1(a), and shall, where applicable, also include any direct or indirect subsidiary of the Company formed or acquired after the date hereof.
“Trading Day” means a day on which the principal Trading Market is open for trading.
“Trading Market” means any of the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the NYSE MKT (formerly NYSE AMEX), the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the OTC Bulletin Board or the Pink OTC Markets (or any successors to any of the foregoing).
“Transaction Documents” means this Agreement, the Notes, the Warrants, all exhibits and schedules thereto and hereto and any other documents or agreements executed in connection with the transactions contemplated hereunder.
“Transfer Agent” means the Company’s transfer agent with respect to its shares of Common Stock.
“Underlying Shares” means the shares of Common Stock issued and issuable upon conversion or redemption of the Notes and upon exercise of the Warrants.
“Warrants” means, collectively, the Common Stock purchase warrants delivered to the Purchasers at the Closing, which Warrants shall be exercisable immediately following the Closing Date and have a term of exercise equal to five years, in the form of Exhibit B attached hereto.
4
“Warrant Shares” means the shares of Common Stock issuable upon exercise of the Warrants.
ARTICLE II
PURCHASE AND SALE
2.1 Closing. On the Closing Date, upon the terms and subject to the conditions set forth herein, substantially concurrent with the execution and delivery of this Agreement by the parties hereto, the Company agrees to sell, and the Purchasers, severally and not jointly, agree to purchase, the Notes. Each Purchaser shall deliver to the Company, via wire transfer or a certified check, immediately available funds equal to such Purchaser’s Subscription Amount as set forth on the signature page hereto executed by such Purchaser, and the Company shall deliver to each Purchaser its respective Note and a Warrant, as determined pursuant to Section 2.2(a), and the Company and each Purchaser shall deliver the other items set forth in Section 2.2 deliverable at the Closing. Upon satisfaction or waiver of the covenants and conditions set forth in Sections 2.2 and Section 2.3, the Closing shall occur at the offices of Holland & Knight LLP, 10 St. James Avenue, Boston, Massachusetts, or such other location as the parties shall mutually agree.
2.2 Deliveries.
(a) On or prior to the Closing Date, the Company shall deliver or cause to be delivered to each Purchaser the following:
(i) this Agreement duly executed by the Company;
(ii) a Note with a principal amount equal to such Purchaser’s Principal Amount, registered in the name of such Purchaser; and
(iii) a Warrant registered in the name of such Purchaser with an exercise price per share equal to the lesser of i) $1.52 or ii) a 15% premium to the Conversion Price if the Conversion Price is determined based on the pre money IPO valuation of the being less than $75,000,000.00, and to purchase up to a number of shares of Common Stock equal to 75% of the number of shares of Common Stock issuable upon conversion of such Purchaser’s Note at a conversion price per share determined in accordance with the terms of the Note; provided, however, in the event the Company does not consummate its initial public offering or become public in some other manner on or before February 18, 2016, the Warrant coverage shall increase from 75% to 100%.
(b) On or prior to the Closing Date, each Purchaser shall deliver or cause to be delivered to the Company the following:
(i) this Agreement duly executed by the Purchaser; and
(ii) such Purchaser’s Subscription Amount by wire transfer to the account specified in writing by the Company; and
5
2.3 Closing Conditions.
(a) The obligations of the Company hereunder in connection with the Closing are subject to the following conditions being met:
(i) the accuracy in all material respects on the Closing Date of the representations and warranties of the Purchasers contained herein (unless as of a specific date therein in which case they shall be accurate as of such date);
(ii) all obligations, covenants and agreements of each Purchaser required to be performed at or prior to the Closing Date shall have been performed; and
(iii) the delivery by each Purchaser of the items set forth in Section 2.2(b) of this Agreement.
(b) The respective obligations of the Purchasers hereunder in connection with the Closing are subject to the following conditions being met:
(i) the accuracy in all material respects on the Closing Date of the representations and warranties of the Company contained herein (unless as of a specific date therein in which case they shall be accurate as of such date);
(ii) all obligations, covenants and agreements of the Company required to be performed at or prior to the Closing Date shall have been performed;
(iii) the delivery by the Company of the items set forth in Section 2.2(a) of this Agreement;
(iv) there shall have been no Material Adverse Effect with respect to the Company since the date hereof; and
(v) from the date hereof to the Closing Date, trading in the Common Stock shall not have been suspended by the Commission or the Company’s principal Trading Market and, at any time prior to the Closing Date, trading in securities generally as reported by Bloomberg L.P. shall not have been suspended or limited, or minimum prices shall not have been established on securities whose trades are reported by such service, or on any Trading Market, nor shall a banking moratorium have been declared either by the United States or New York State authorities.
ARTICLE III
REPRESENTATIONS AND WARRANTIES
3.1 Representations and Warranties of the Company. Except as set forth in the Disclosure Schedules, which Disclosure Schedules shall be deemed a part hereof and shall qualify any representation or otherwise made herein to the extent of the disclosure contained in the corresponding section of the Disclosure Schedules, the Company hereby makes the following representations and warranties to each Purchaser:
(a) Subsidiaries. All of the direct and indirect subsidiaries of the Company which conduct any operation or which have more than de minimis assets are set forth on Schedule 3.1(a). The Company owns, directly or indirectly, all of the capital stock or other equity interests of each Subsidiary free and clear of any Liens, and all of the issued and outstanding shares of capital stock of each Subsidiary are validly issued and are fully paid, non-assessable and free of preemptive and similar rights to subscribe for or purchase securities.
6
(b) Organization and Qualification. The Company and each of the Subsidiaries is an entity duly incorporated or otherwise organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation or organization, with the requisite power and authority to own and use its properties and assets and to carry on its business as currently conducted. Neither the Company nor any Subsidiary is in violation nor default of any of the provisions of its respective certificate or articles of incorporation, bylaws or other organizational or charter documents. Each of the Company and the Subsidiaries is duly qualified to conduct business and is in good standing as a foreign corporation or other entity in each jurisdiction in which the nature of the business conducted or property owned by it makes such qualification necessary, except where the failure to be so qualified or in good standing, as the case may be, could not have or reasonably be expected to result in: (i) a material adverse effect on the legality, validity or enforceability of any Transaction Document, (ii) a material adverse effect on the results of operations, assets, business, prospects or condition (financial or otherwise) of the Company and the Subsidiaries, taken as a whole, or (iii) a material adverse effect on the Company’s ability to perform in any material respect on a timely basis its obligations under any Transaction Document (any of (i), (ii) or (iii), a “Material Adverse Effect”; and provided, that changes in the trading price of the Common Stock shall not, in and of itself, constitute a Material Adverse Effect) and no Proceeding has been instituted in any such jurisdiction revoking, limiting or curtailing or seeking to revoke, limit or curtail such power and authority or qualification.
(c) Authorization; Enforcement. The Company has the requisite corporate power and authority to enter into and to consummate the transactions contemplated by this Agreement and each of the other Transaction Documents and otherwise to carry out its obligations hereunder and thereunder. The execution and delivery of this Agreement and each of the other Transaction Documents by the Company and the consummation by it of the transactions contemplated hereby and thereby have been duly authorized by all necessary action on the part of the Company and no further action is required by the Company, the Board of Directors or the Company’s stockholders in connection herewith or therewith other than in connection with the Required Approvals. This Agreement and each other Transaction Document to which it is a party has been (or upon delivery will have been) duly executed by the Company and, when delivered in accordance with the terms hereof and thereof, will constitute the valid and binding obligation of the Company enforceable against the Company in accordance with its terms, except: (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by applicable law.
7
(d) No Conflicts. The execution, delivery and performance by the Company of this Agreement and the other Transaction Documents to which it is a party, the issuance and sale of the Securities and the consummation by it of the transactions contemplated hereby and thereby do not and will not: (i) conflict with or violate any provision of the Company’s or any Subsidiary’s certificate or articles of incorporation, bylaws or other organizational or charter documents, (ii) conflict with, or constitute a default (or an event that with notice or lapse of time or both would become a default) under, result in the creation of any Lien upon any of the properties or assets of the Company or any Subsidiary, or give to others any rights of termination, amendment, acceleration or cancellation (with or without notice, lapse of time or both) of, any agreement, credit facility, debt or other instrument (evidencing a Company or Subsidiary debt or otherwise) or other understanding to which the Company or any Subsidiary is a party or by which any property or asset of the Company or any Subsidiary is bound or affected, or (iii) subject to the Required Approvals, conflict with or result in a violation of any law, rule, regulation, order, judgment, injunction, decree or other restriction of any court or governmental authority to which the Company or a Subsidiary is subject (including federal and state securities laws and regulations), or by which any property or asset of the Company or a Subsidiary is bound or affected; except in the case of each of clauses (ii) and (iii), such as could not have or reasonably be expected to result in a Material Adverse Effect.
(e) Filings, Consents and Approvals. The Company is not required to obtain any consent, waiver, authorization or order of, give any notice to, or make any filing or registration with, any court or other federal, state, local or other governmental authority or other Person in connection with the execution, delivery and performance by the Company of the Transaction Documents, other than: (i) the filings required pursuant to Section 4.6 of this Agreement, (ii) the notice and/or application(s) to each applicable Trading Market for the issuance and sale of the Securities and the listing of the Conversion Shares and Warrant Shares for trading thereon in the time and manner required thereby, and (iii) the filing of Form D with the Commission and such filings as are required to be made under applicable state securities laws (collectively, the “Required Approvals”).
(f) Issuance of the Securities. The Securities are duly authorized and, when issued and paid for in accordance with the applicable Transaction Documents, will be duly and validly issued, fully paid and nonassessable, free and clear of all Liens imposed by the Company other than restrictions on transfer provided for in the Transaction Documents. The Underlying Shares, when issued in accordance with the terms of the Transaction Documents, will be validly issued, fully paid and nonassessable, free and clear of all Liens imposed by the Company other than restrictions on transfer provided for in the Transaction Documents. The Company has reserved from its duly authorized capital stock a number of shares of Common Stock for issuance of the Underlying Shares at least equal to the Required Minimum on the date hereof.
(g) Capitalization. Schedule 3.1(g) sets forth the capitalization of the Company. Except as set forth on Schedule 3.1(g), the Company has not issued any capital stock, other than pursuant to the exercise of employee stock options under the Company’s stock option plans, the issuance of shares of Common Stock to employees pursuant to the Company’s employee stock purchase plans and pursuant to the conversion and/or exercise of Common Stock Equivalents outstanding as of the date hereof. Except as set forth on Schedule 3.1(g), no Person has any right of first refusal, preemptive right, right of participation, or any similar right to
8
participate in the transactions contemplated by the Transaction Documents. No further approval or authorization of any stockholder, the Board of Directors or others is required for the issuance and sale of the Securities. Except as set forth on Schedule 3.1(g), there are no stockholders agreements, voting agreements or other similar agreements with respect to the Company’s capital stock to which the Company is a party or, to the knowledge of the Company, between or among any of the Company’s stockholders.
(h) Financial Statements. The Company has delivered to each Purchaser its unaudited financial statements as of March 31, 2015 (collectively, the “Financial Statements”). The Financial Statements have been prepared in accordance with generally accepted accounting principles (“GAAP”) applied on a consistent basis throughout the periods indicated, except that the unaudited Financial Statements may not contain all footnotes required by GAAP. The Financial Statements fairly present in all material respects the financial condition and operating results of the Company as of the dates, and for the periods, indicated therein, subject in the case of the unaudited Financial Statements to normal year-end audit adjustments. Except as set forth in the Financial Statements, the Company has no material liabilities or obligations, contingent or otherwise, other than (i) liabilities incurred in the ordinary course of business subsequent to March 31, 2015; (ii) obligations under contracts and commitments incurred in the ordinary course of business; and (iii) liabilities and obligations of a type or nature not required under GAAP to be reflected in the Financial Statements, which, in all such cases, individually and in the aggregate would not reasonably be expected to have a Material Adverse Effect.
(i) Material Changes; Undisclosed Events, Liabilities or Developments. Since the date of the latest Financial Statements, except as specifically disclosed in a subsequent SEC Report filed prior to the date hereof: (i) there has been no event, occurrence or development that has had or that could reasonably be expected to result in a Material Adverse Effect, (ii) the Company has not incurred any liabilities (contingent or otherwise) other than (A) trade payables and accrued expenses incurred in the ordinary course of business consistent with past practice and (B) liabilities not required to be reflected in the Company’s financial statements pursuant to GAAP or disclosed in filings made with the Commission, (iii) the Company has not altered its method of accounting, (iv) the Company has not declared or made any dividend or distribution of cash or other property to its stockholders or purchased, redeemed or made any agreements to purchase or redeem any shares of its capital stock and (v) except as set forth on Schedule 3.1(i), the Company has not issued any equity securities to any officer, director or Affiliate, except pursuant to existing Company stock option plans. The Company does not have pending before the Commission any request for confidential treatment of information. Except for the issuance of the Securities contemplated by this Agreement or as set forth on Schedule 3.1(i), no event, liability, fact, circumstance, occurrence or development has occurred or exists or is reasonably expected to occur or exist with respect to the Company or its Subsidiaries or their respective businesses, properties, operations, assets or financial condition, that would be required to be disclosed by the Company under applicable securities laws at the time this representation is made or deemed made that has not been publicly disclosed at least one Trading Day prior to the date that this representation is made.
(j) Litigation. Except as set forth on Schedule 3.1(j), there is no action, suit, inquiry, notice of violation, proceeding or investigation pending or, to the knowledge of the Company, threatened against or affecting the Company, any Subsidiary or any of their respective
9
properties before or by any court, arbitrator, governmental or administrative agency or regulatory authority (federal, state, county, local or foreign) (collectively, an “Action”) which (i) adversely affects or challenges the legality, validity or enforceability of any of the Transaction Documents or the Securities or (ii) could, if there were an unfavorable decision, have or reasonably be expected to result in a Material Adverse Effect. Neither the Company nor any Subsidiary, nor any director or officer thereof, is or has been the subject of any Action involving a claim of violation of or liability under federal or state securities laws or a claim of breach of fiduciary duty. Except as set forth on Schedule 3.1(j), there has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation by the Commission involving the Company or any current or former director or officer of the Company. The Commission has not issued any stop order or other order suspending the effectiveness of any registration statement filed by the Company or any Subsidiary under the Exchange Act or the Securities Act.
(k) Compliance. Except as set forth on Schedule 3.1(k), neither the Company nor any Subsidiary: (i) is in default under or in violation of (and no event has occurred that has not been waived that, with notice or lapse of time or both, would result in a default by the Company or any Subsidiary under), nor has the Company or any Subsidiary received notice of a claim that it is in default under or that it is in violation of, any indenture, loan or credit agreement or any other agreement or instrument to which it is a party or by which it or any of its properties is bound (whether or not such default or violation has been waived), (ii) is in violation of any judgment, decree or order of any court, arbitrator or other governmental authority or (iii) is or has been in violation of any statute, rule, ordinance or regulation of any governmental authority, including without limitation all foreign, federal, state and local laws relating to taxes, environmental protection, occupational health and safety, product quality and safety and employment and labor matters, except in each case as could not have or reasonably be expected to result in a Material Adverse Effect.
(l) Regulatory Permits. The Company and the Subsidiaries possess all certificates, authorizations and permits issued by the appropriate federal, state, local or foreign regulatory authorities necessary to conduct their respective businesses as currently conducted, except where the failure to possess such permits could not reasonably be expected to result in a Material Adverse Effect (“Material Permits”), and neither the Company nor any Subsidiary has received any notice of proceedings relating to the revocation or modification of any Material Permit.
(m) Title to Assets. The Company and the Subsidiaries have good and marketable title in fee simple to all real property owned by them and good and marketable title in all personal property owned by them that is material to the business of the Company and the Subsidiaries, in each case free and clear of all Liens, except for the Liens disclosed on Schedule 3.1(m), (ii) Liens as do not materially affect the value of such property and do not materially interfere with the use made and proposed to be made of such property by the Company and the Subsidiaries, and (iii) Liens for the payment of federal, state or other taxes, for which appropriate reserves have been made therefor in accordance with GAAP and, the payment of which is neither delinquent nor subject to penalties. Any real property and facilities held under lease by the Company and the Subsidiaries are held by them under valid, subsisting and enforceable leases with which the Company and the Subsidiaries are in compliance.
10
(n) Intellectual Property. The Company and the Subsidiaries have, or have rights to use, all patents, patent applications, trademarks, trademark applications, service marks, trade names, trade secrets, inventions, copyrights, licenses and other intellectual property rights and similar rights as necessary or required for use in connection with their respective businesses and which the failure to so have could have a Material Adverse Effect (collectively, the “Intellectual Property Rights”).
(o) Insurance. The Company and the Subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as are prudent and customary in the businesses in which the Company and the Subsidiaries are engaged. Neither the Company nor any Subsidiary has any reason to believe that it will not be able to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business without a significant increase in cost.
(p) Transactions With Affiliates and Employees. Except as disclosed on Schedule 3(p), none of the officers or directors of the Company or any Subsidiary and, to the knowledge of the Company, none of the employees of the Company or any Subsidiary is presently a party to any transaction with the Company or any Subsidiary (other than for services as employees, officers and directors), including any contract, agreement or other arrangement providing for the furnishing of services to or by, providing for rental of real or personal property to or from providing for the borrowing of money from or lending of money to, or otherwise requiring payments to or from any officer, director or such employee or, to the knowledge of the Company, any entity in which any officer, director, or any such employee has a substantial interest or is an officer, director, trustee, stockholder, member or partner, in each case in excess of $120,000 other than for: (i) payment of salary or consulting fees for services rendered, (ii) reimbursement for expenses incurred on behalf of the Company and (iii) other employee benefits, including stock option agreements under any stock option plan of the Company.
(q) Private Placement. Assuming the accuracy of the Purchasers’ representations and warranties set forth in Section 3.2, no registration under the Securities Act is required for the offer and sale of the Securities by the Company to the Purchasers as contemplated hereby.
(r) Investment Company. The Company is not, and is not an Affiliate of, and immediately after receipt of payment for the Securities, will not be or be an Affiliate of, an “investment company” within the meaning of the Investment Company Act of 1940, as amended. The Company shall conduct its business in a manner so that it will not become an “investment company” subject to registration under the Investment Company Act of 1940, as amended.
(s) Registration Rights. Except as disclosed on Schedule 3(s), no Person has any right to cause the Company to effect the registration under the Securities Act of any securities of the Company or any Subsidiaries.
(t) Disclosure. Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents, the Company confirms that neither it
11
nor any other Person acting on its behalf has provided any of the Purchasers or their agents or counsel with any information that it believes constitutes or might reasonably constitute material, non-public information. The Company understands and confirms that the Purchasers will rely on the foregoing representation in effecting transactions in securities of the Company. All of the disclosure furnished by or on behalf of the Company to the Purchasers regarding the Company and its Subsidiaries, their respective businesses and the transactions contemplated hereby, including the Disclosure Schedules to this Agreement, is true and correct and does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. The press releases disseminated by the Company during the twelve months preceding the date of this Agreement taken as a whole do not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made and when made, not misleading. The Company acknowledges and agrees that no Purchaser makes or has made any representations or warranties with respect to the transactions contemplated hereby other than those specifically set forth in Section 3.2 hereof.
(u) No Integrated Offering. Assuming the accuracy of the Purchasers’ representations and warranties set forth in Section 3.2, neither the Company, nor any of its Affiliates, nor any Person acting on its or their behalf has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would cause this offering of the Securities to be integrated with prior offerings by the Company for purposes of the Securities Act which would require the registration of any such securities under the Securities Act.
(v) Solvency. The Company has no knowledge of any facts or circumstances which lead it to believe that it will file for reorganization or liquidation under the bankruptcy or reorganization laws of any jurisdiction within one year from the Closing Date. Schedule 3.1(v) sets forth as of the date hereof all outstanding secured and unsecured Indebtedness of the Company or any Subsidiary, or for which the Company or any Subsidiary has commitments. For the purposes of this Agreement, “Indebtedness” means (x) any liabilities for borrowed money or amounts owed in excess of $50,000 (other than trade accounts payable incurred in the ordinary course of business), (y) all guaranties, endorsements and other contingent obligations in respect of indebtedness of others, whether or not the same are or should be reflected in the Company’s consolidated balance sheet (or the notes thereto), except guaranties by endorsement of negotiable instruments for deposit or collection or similar transactions in the ordinary course of business; and (z) the present value of any lease payments in excess of $50,000 due under leases required to be capitalized in accordance with GAAP. Neither the Company nor any Subsidiary is in default with respect to any Indebtedness.
(w) Tax Status. Except for matters that would not, individually or in the aggregate, have or reasonably be expected to result in a Material Adverse Effect, the Company and its Subsidiaries each (i) has made or filed all United States federal, state and local income and all foreign income and franchise tax returns, reports and declarations required by any jurisdiction to which it is subject, (ii) has paid all taxes and other governmental assessments and charges that are material in amount, shown or determined to be due on such returns, reports and declarations and (iii) has no material tax obligations for periods subsequent to the periods to
12
which such returns, reports or declarations apply. There are no unpaid taxes in any material amount claimed to be due by the taxing authority of any jurisdiction, and the officers of the Company or of any Subsidiary know of no basis for any such claim.
(x) No General Solicitation. Neither the Company nor any person acting on behalf of the Company has offered or sold any of the Securities by any form of general solicitation or general advertising. The Company has offered the Securities for sale only to the Purchasers and certain other “accredited investors” within the meaning of Rule 501 under the Securities Act.
(y) Foreign Corrupt Practices. Neither the Company nor any Subsidiary, nor to the knowledge of the Company or any Subsidiary, any agent or other person acting on behalf of the Company or any Subsidiary, has: (i) directly or indirectly, used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to foreign or domestic political activity, (ii) made any unlawful payment to foreign or domestic government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds, (iii) failed to disclose fully any contribution made by the Company or any Subsidiary (or made by any person acting on its behalf of which the Company is aware) which is in violation of law or (iv) violated in any material respect any provision of the FCPA.
(z) No Disagreements with Accountants and Lawyers. There are no disagreements of any kind presently existing, or reasonably anticipated by the Company to arise, between the Company and the accountants and lawyers formerly or presently employed by the Company and the Company is current with respect to any fees owed to its accountants and lawyers which could affect the Company’s ability to perform any of its obligations under any of the Transaction Documents.
(aa) Acknowledgment Regarding Purchasers’ Purchase of Securities. The Company acknowledges and agrees that each of the Purchasers is acting solely in the capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated thereby. The Company further acknowledges that no Purchaser is acting as a financial advisor or fiduciary of the Company (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated thereby and any advice given by any Purchaser or any of their respective representatives or agents in connection with the Transaction Documents and the transactions contemplated thereby is merely incidental to the Purchasers’ purchase of the Securities. The Company further represents to each Purchaser that the Company’s decision to enter into this Agreement and the other Transaction Documents has been based solely on the independent evaluation of the transactions contemplated hereby by the Company and its representatives.
(bb) Office of Foreign Assets Control. Neither the Company nor any Subsidiary nor, to the Company’s knowledge, any director, officer, agent, employee or affiliate of the Company or any Subsidiary is currently subject to any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Treasury Department (“OFAC”).
13
3.2 Representations and Warranties of the Purchasers. Each Purchaser, for itself and for no other Purchaser, hereby represents and warrants as of the date hereof and as of the Closing Date to the Company as follows (unless as of a specific date therein):
(a) Organization; Authority. Such Purchaser is either an individual or an entity duly incorporated or formed, validly existing and in good standing under the laws of the jurisdiction of its incorporated or formed with full right, corporate, partnership, limited liability company or similar power and authority to enter into and to consummate the transactions contemplated by the Transaction Documents and otherwise to carry out its obligations hereunder and thereunder. The execution and delivery of the Transaction Documents and performance by such Purchaser of the transactions contemplated by the Transaction Documents have been duly authorized by all necessary corporate, partnership, limited liability company or similar action, as applicable, on the part of such Purchaser. Each Transaction Document to which it is a party has been duly executed by such Purchaser, and when delivered by such Purchaser in accordance with the terms hereof, will constitute the valid and legally binding obligation of such Purchaser, enforceable against it in accordance with its terms, except: (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by applicable law.
(b) Own Account. Such Purchaser understands that the Securities are “restricted securities” and have not been registered under the Securities Act or any applicable state securities law and is acquiring the Securities as principal for its own account and not with a view to or for distributing or reselling such Securities or any part thereof in violation of the Securities Act or any applicable state securities law, has no present intention of distributing any of such Securities in violation of the Securities Act or any applicable state securities law and has no direct or indirect arrangement or understandings with any other persons to distribute or regarding the distribution of such Securities in violation of the Securities Act or any applicable state securities law (this representation and warranty not limiting such Purchaser’s right to sell the Securities in compliance with applicable federal and state securities laws). Such Purchaser is acquiring the Securities hereunder in the ordinary course of its business.
(c) Purchaser Status. At the time such Purchaser was offered the Securities, it was, and as of the date hereof it is, and on each date on which it exercises any Warrants or converts any Notes it will be either: (i) an “accredited investor” as defined in Rule 501(a)(1), (a)(2), (a)(3), (a)(7) or (a)(8) under the Securities Act or (ii) a “qualified institutional buyer” as defined in Rule 144A(a) under the Securities Act. Such Purchaser is not required to be registered as a broker-dealer under Section 15 of the Exchange Act.
(d) Experience of Such Purchaser. Such Purchaser, either alone or together with its representatives, has such knowledge, sophistication and experience in business and financial matters so as to be capable of evaluating the merits and risks of the prospective investment in the Securities, and has so evaluated the merits and risks of such investment. Such Purchaser is able to bear the economic risk of an investment in the Securities and, at the present time, is able to afford a complete loss of such investment.
14
(e) General Solicitation. Such Purchaser is not purchasing the Securities as a result of any advertisement, article, notice or other communication regarding the Securities published in any newspaper, magazine or similar media or broadcast over television or radio or presented at any seminar or any other general solicitation or general advertisement.
(f) Certain Transactions and Confidentiality. Other than consummating the transactions contemplated hereunder, such Purchaser has not directly or indirectly, nor has any Person acting on behalf of or pursuant to any understanding with such Purchaser, executed any purchases or sales, including Short Sales, of the securities of the Company during the period commencing as of the time that such Purchaser first received a term sheet (written or oral) from the Company or any other Person representing the Company setting forth the material terms of the transactions contemplated hereunder and ending immediately prior to the execution hereof. Notwithstanding the foregoing, in the case of a Purchaser that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of such Purchaser’s assets and the portfolio managers have no direct knowledge of the investment decisions made by the portfolio managers managing other portions of such Purchaser’s assets, the representation set forth above shall only apply with respect to the portion of assets managed by the portfolio manager that made the investment decision to purchase the Securities covered by this Agreement. Other than to other Persons party to this Agreement, such Purchaser has maintained the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of this transaction).
The Company acknowledges and agrees that the representations contained in Section 3.2 shall not modify, amend or affect such Purchaser’s right to rely on the Company’s representations and warranties contained in this Agreement or any express representations and warranties contained in any other Transaction Document or any other document or instrument executed and/or delivered in connection with this Agreement or the consummation of the transaction contemplated hereby.
ARTICLE IV
OTHER AGREEMENTS OF THE PARTIES
4.1 Transfer Restrictions.
(a) The Securities may only be disposed of in compliance with state and federal securities laws. In connection with any transfer of Securities other than pursuant to an effective registration statement or Rule 144, to the Company or to an Affiliate of a Purchaser, the Company may require the transferor thereof to provide to the Company an opinion of corporation counsel selected by the transferor and reasonably acceptable to the Company, the form and substance of which opinion shall be reasonably satisfactory to the Company, to the effect that such transfer does not require registration of such transferred Securities under the Securities Act. As a condition of transfer, any such transferee shall agree in writing to be bound by the terms of this Agreement and shall make the representations set forth in Section 3.2, and then shall have the rights and obligations of a Purchaser under this Agreement.
(b) The Purchasers agree to the imprinting, so long as is required by this Section 4.1, of a legend on any of the Securities in the following form:
NEITHER THIS SECURITY NOR ANY SECURITIES INTO WHICH THIS SECURITY IS CONVERTIBLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL OPINION OF CORPORATE COUNSEL TO THE TRANSFEROR TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY.
15
4.2 Acknowledgment of Dilution. The Company acknowledges that the issuance of the Securities may result in dilution of the outstanding shares of Common Stock, which dilution may be substantial under certain market conditions. The Company further acknowledges that its obligations under the Transaction Documents, including, without limitation, its obligation to issue the Underlying Shares pursuant to the Transaction Documents, are unconditional and absolute and not subject to any right of set off, counterclaim, delay or reduction, regardless of the effect of any such dilution or any claim the Company may have against any Purchaser and regardless of the dilutive effect that such issuance may have on the ownership of the other stockholders of the Company.
4.3 Furnishing of Information; Public Information.
(a) Until the earliest of the time that (i) no Purchaser owns Securities or (ii) the Warrants have expired, and so long as the Company is a reporting company pursuant to the Exchange Act, the Company covenants to maintain the registration of the Common Stock under Section 12(b) or 12(g) of the Exchange Act and to timely file (or obtain extensions in respect thereof and file within the applicable grace period) all reports required to be filed by the Company after the date thereof pursuant to the Exchange Act even if the Company subsequently is no longer then subject to the reporting requirements of the Exchange Act.
(b) Following the date that the Company becomes a reporting company pursuant to the Exchange Act and the Securities are eligible to be resold pursuant to Rule 144 and ending at such time that all of the Securities may be sold without the requirement for the Company to be in compliance with Rule 144(c)(1) and otherwise without restriction or limitation pursuant to Rule 144, the Company shall fail for any reason to satisfy the current public information requirement under Rule 144(c) (a “Public Information Failure”), then, in addition to such Purchaser’s other available remedies, the Company shall pay to a Purchaser, in cash, as partial liquidated damages and not as a penalty, by reason of any such delay in or reduction of its ability to sell the Securities, an amount in cash equal to two percent (2.0%) of the aggregate Subscription Amount of such Purchaser’s Securities on the day of a Public Information Failure and on every thirtieth (30th) day (pro rated for periods totaling less than thirty days) thereafter until the earlier of (a) the date such Public Information Failure is cured and (b) such time that
16
such public information is no longer required for the Purchasers to transfer the Underlying Shares pursuant to Rule 144. The payments to which a Purchaser shall be entitled pursuant to this Section 4.3(b) are referred to herein as “Public Information Failure Payments.” Public Information Failure Payments shall be paid on the earlier of (i) the last day of the calendar month during which such Public Information Failure Payments are incurred and (ii) the third (3rd) Business Day after the event or failure giving rise to the Public Information Failure Payments is cured. In the event the Company fails to make Public Information Failure Payments in a timely manner, such Public Information Failure Payments shall bear interest at the rate of 1.5% per month (prorated for partial months) until paid in full. Nothing herein shall limit such Purchaser’s right to pursue actual damages for the Public Information Failure, and such Purchaser shall have the right to pursue all remedies available to it at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief. The Company shall promptly notify Purchaser of the occurrence of a Public Information Failure.
4.4 Integration. The Company shall not sell, offer for sale or solicit offers to buy or otherwise negotiate in respect of any security (as defined in Section 2 of the Securities Act) that would be integrated with the offer or sale of the Securities in a manner that would require the registration under the Securities Act of the sale of the Securities or that would be integrated with the offer or sale of the Securities for purposes of the rules and regulations of any Trading Market such that it would require shareholder approval prior to the closing of such other transaction unless shareholder approval is obtained before the closing of such subsequent transaction.
4.5 Conversion and Exercise Procedures. Each of the form of Notice of Exercise included in the Warrants and the mandatory conversion feature included in the Notes set forth the totality of the procedures required of the Purchasers in order to exercise the Warrants or convert the Notes. No additional legal opinion, other information or instructions shall be required of the Purchasers to exercise their Warrants or convert their Notes. The Company shall honor exercises of the Warrants and conversions of the Notes and shall deliver Underlying Shares in accordance with the terms, conditions and time periods set forth in the Transaction Documents.
4.6 Securities Laws Disclosure; Publicity. The Company and each Purchaser shall consult with each other in issuing any press releases with respect to the transactions contemplated hereby, and neither the Company nor any Purchaser shall issue any such press release nor otherwise make any such public statement without the prior consent of the Company, with respect to any press release of any Purchaser, or without the prior consent of each Purchaser, with respect to any press release of the Company, which consent shall not unreasonably be withheld or delayed, except if such disclosure is required by law, in which case the disclosing party shall promptly provide the other party with prior notice of such public statement or communication. Notwithstanding the foregoing, the Company shall not publicly disclose the name of any Purchaser, or include the name of any Purchaser in any filing with the Commission or any regulatory agency or Trading Market, without the prior written consent of such Purchaser, except: (a) as required by federal securities laws, (b) to the extent requested by the Commission and (c) to the extent such disclosure is required by law or Trading Market regulations, in which case the Company shall provide the Purchasers with prior notice of such disclosure permitted under this clauses (b) and (c).
17
4.7 Shareholder Rights Plan. No claim will be made or enforced by the Company or, with the consent of the Company, any other Person, that any Purchaser is an “Acquiring Person” under any control share acquisition, business combination, poison pill (including any distribution under a rights agreement) or similar anti-takeover plan or arrangement in effect or hereafter adopted by the Company, or that any Purchaser could be deemed to trigger the provisions of any such plan or arrangement, by virtue of receiving Securities under the Transaction Documents or under any other agreement between the Company and the Purchasers.
4.8 Non-Public Information. Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents, the Company covenants and agrees that neither it, nor any other Person acting on its behalf, will provide any Purchaser or its agents or counsel with any information that the Company believes constitutes material non-public information, unless prior thereto such Purchaser shall have entered into a written agreement with the Company regarding the confidentiality and use of such information. The Company understands and confirms that each Purchaser shall be relying on the foregoing covenant in effecting transactions in securities of the Company.
4.9 Use of Proceeds. Except as set forth on Schedule 4.9 attached hereto, the Company shall use the net proceeds from the sale of the Securities hereunder for working capital purposes and expenses related to an initial public offering and shall not use such proceeds: (a) for the satisfaction of any portion of the Company’s debt (other than payment of trade payables in the ordinary course of the Company’s business and prior practices), (b) for the redemption of any Common Stock or Common Stock Equivalents, (c) for the settlement of any outstanding litigation or (d) in violation of FCPA or OFAC regulations.
4.10 Indemnification of Purchasers. Subject to the provisions of this Section 4.10, the Company will indemnify and hold each Purchaser and its directors, officers, shareholders, members, partners, employees and agents (and any other Persons with a functionally equivalent role of a Person holding such titles notwithstanding a lack of such title or any other title), each Person who controls such Purchaser (within the meaning of Section 15 of the Securities Act and Section 20 of the Exchange Act), and the directors, officers, shareholders, agents, members, partners or employees (and any other Persons with a functionally equivalent role of a Person holding such titles notwithstanding a lack of such title or any other title) of such controlling persons (each, a “Purchaser Party”) harmless from any and all losses, liabilities, obligations, claims, contingencies, damages, costs and expenses, including all judgments, amounts paid in settlements, court costs and reasonable attorneys’ fees and costs of investigation that any such Purchaser Party may suffer or incur as a result of or relating to (a) any breach of any of the representations, warranties, covenants or agreements made by the Company in this Agreement or in the other Transaction Documents or (b) any action instituted against the Purchaser Parties in any capacity, or any of them or their respective Affiliates, by any stockholder of the Company who is not an Affiliate of such Purchaser Party, with respect to any of the transactions contemplated by the Transaction Documents (unless such action is based upon a breach of such Purchaser Party’s representations, warranties or covenants under the Transaction Documents or any agreements or understandings such Purchaser Party may have with any such stockholder or any violations by such Purchaser Party of state or federal securities laws or any conduct by such Purchaser Party which constitutes fraud, gross negligence, willful misconduct or malfeasance). If any action shall be brought against any Purchaser Party in respect of which indemnity may be
18
sought pursuant to this Agreement, such Purchaser Party shall promptly notify the Company in writing, and the Company shall have the right to assume the defense thereof with counsel of its own choosing reasonably acceptable to the Purchaser Party. Any Purchaser Party shall have the right to employ separate counsel in any such action and participate in the defense thereof, but the fees and expenses of such counsel shall be at the expense of such Purchaser Party except to the extent that (i) the employment thereof has been specifically authorized by the Company in writing, (ii) the Company has failed after a reasonable period of time to assume such defense and to employ counsel or (iii) in such action there is, in the reasonable opinion of counsel, a material conflict on any material issue between the position of the Company and the position of such Purchaser Party, in which case the Company shall be responsible for the reasonable fees and expenses of no more than one such separate counsel. The Company will not be liable to any Purchaser Party under this Agreement (y) for any settlement by a Purchaser Party effected without the Company’s prior written consent, which shall not be unreasonably withheld or delayed; or (z) to the extent, but only to the extent that a loss, claim, damage or liability is attributable to any Purchaser Party’s breach of any of the representations, warranties, covenants or agreements made by such Purchaser Party in this Agreement or in the other Transaction Documents. The indemnification required by this Section 4.10 shall be made by periodic payments of the amount thereof during the course of the investigation or defense, in a commercially reasonable manner. The indemnity agreements contained herein shall be in addition to any cause of action or similar right of any Purchaser Party against the Company or others and any liabilities the Company may be subject to pursuant to law.
4.11 Reservation and Listing of Securities.
(a) The Company shall maintain a reserve from its duly authorized shares of Common Stock for issuance pursuant to the Transaction Documents in such amount as may then be required to fulfill its obligations in full under the Transaction Documents.
(b) If, on any date, the number of authorized but unissued (and otherwise unreserved) shares of Common Stock is less than the Required Minimum on such date, then the Board of Directors shall use commercially reasonable efforts to amend the Company’s certificate or articles of incorporation to increase the number of authorized but unissued shares of Common Stock to at least the Required Minimum at such time, as promptly as practicable and in any event not later than the 120th day after such date.
(c) The Company shall, if applicable: (i) in the time and manner required by the principal Trading Market, prepare and file with such Trading Market an additional shares listing application covering a number of shares of Common Stock at least equal to the Required Minimum on the date of such application, (ii) take all steps necessary to cause such shares of Common Stock to be approved for listing or quotation on such Trading Market as soon as commercially reasonable thereafter, (iii) provide to the Purchasers evidence of such listing or quotation and (iv) maintain the listing or quotation of such Common Stock on any date at least equal to the Required Minimum on such date on such Trading Market or another Trading Market.
19
4.12 Equal Treatment of Purchasers; Limitation on Additional Debt.
(a) No consideration (including any modification of any Transaction Document) shall be offered or paid to any Person to amend or consent to a waiver or modification of any provision of this Agreement unless the same consideration is also offered to all of the parties to this Agreement. Further, the Company shall not make any payment of principal or interest on the Notes in amounts which are disproportionate to the respective principal amounts outstanding on the Notes at any applicable time. For clarification purposes, this provision constitutes a separate right granted to each Purchaser by the Company and negotiated separately by each Purchaser, and is intended for the Company to treat the Purchasers as a class and shall not in any way be construed as the Purchasers acting in concert or as a group with respect to the purchase, disposition or voting of Securities or otherwise.
(b) The Company shall not, without the prior written consent of the Purchasers holding at least a majority in principal amount of the Notes then outstanding, issue any convertible promissory note or similar instrument from the date of this Agreement to the date on which all of the Notes have been either paid in full or converted to Common Stock, provided that this provision shall not limit the right of the Company to issue Notes pursuant to the terms and conditions of this Agreement for cash consideration, in the aggregate, of up to $6,000,000.00.
4.13 Short Sales and Confidentiality After the Date Hereof. Each Purchaser, severally and not jointly with the other Purchasers, represents and covenants that neither it, nor any Affiliate acting on its behalf or pursuant to any understanding with it, has executed any purchases or sales, including Short Sales, of any of the Company’s securities during the period commencing with the execution of this Agreement and ending at such time that the transactions contemplated by this Agreement are first publicly announced. Each Purchaser, severally and not jointly with the other Purchasers, represents and covenants that until such time as the transactions contemplated by this Agreement are publicly disclosed by the Company, such Purchaser will maintain the confidentiality of the existence and terms of this transaction and the information included in the Transaction Documents and the Disclosure Schedules.
4.14 Form D; Blue Sky Filings. The Company agrees to timely file a Form D with respect to the Securities as required under Regulation D and to provide a copy thereof, promptly upon request of any Purchaser. The Company shall take such action as the Company shall reasonably determine is necessary in order to obtain an exemption for, or to qualify the Securities for, sale to the Purchasers at the Closing under applicable securities or “Blue Sky” laws of the states of the United States, and shall provide evidence of such actions promptly upon request of any Purchaser.
4.15 Initial Public Offering. The Company shall complete an initial public offering on or before February 18, 2015, the registration statement for which shall, if permitted by the managing underwriter, include the Underlying Shares. Each Purchaser agrees and acknowledges that the Purchasers shall not be permitted to distribute their securities through such public offering. If the Company is unable to include at least 25% of the shares of Common Stock issuable on conversion of the Notes in the registration statement for such public offering, the Warrant coverage shall increase from 75% to 100%.
20
ARTICLE V
MISCELLANEOUS
5.1 Termination. This Agreement may be terminated by any Purchaser, as to such Purchaser’s obligations hereunder only and without any effect whatsoever on the obligations between the Company and the other Purchasers, by written notice to the other parties, if the Closing has not been consummated on or before September 30, 2015; provided, however, that such termination will not affect the right of any party to sue for any breach by any other party (or parties).
5.2 Fees and Expenses. Each party shall pay the fees and expenses of its advisers, counsel, accountants and other experts, if any, and all other expenses incurred by such party incident to the negotiation, preparation, execution, delivery and performance of this Agreement. The Company shall pay all Transfer Agent fees (including, without limitation, any fees required for same-day processing of any instruction letter delivered by the Company and any conversion or exercise notice delivered by a Purchaser), stamp taxes and other taxes and duties levied in connection with the delivery of any Securities to the Purchasers.
5.3 Entire Agreement. The Transaction Documents, together with the exhibits and schedules thereto, contain the entire understanding of the parties with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, oral or written, with respect to such matters, which the parties acknowledge have been merged into such documents, exhibits and schedules.
5.4 Notices. Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be in writing and shall be deemed given and effective on the earliest of: (a) the date of transmission, if such notice or communication is delivered via e-mail or facsimile at the e-mail address or facsimile number set forth on the signature pages attached hereto at or prior to 5:30 p.m. (New York City time) on a Trading Day, (b) the next Trading Day after the date of transmission, if such notice or communication is delivered via e-mail or facsimile at the e-mail address or facsimile number set forth on the signature pages attached hereto on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (c) the second (2nd) Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service or (d) upon actual receipt by the party to whom such notice is required to be given. The address for such notices and communications shall be as set forth on the signature pages attached hereto.
5.5 Amendments; Waivers. No provision of this Agreement may be waived, modified, supplemented or amended except in a written instrument signed, in the case of an amendment, by the Company and the Purchasers holding at least a majority in interest of the Securities then outstanding or, in the case of a waiver, by the party against whom enforcement of any such waived provision is sought. No waiver of any default with respect to any provision, condition or requirement of this Agreement shall be deemed to be a continuing waiver in the future or a waiver of any subsequent default or a waiver of any other provision, condition or requirement hereof, nor shall any delay or omission of any party to exercise any right hereunder in any manner impair the exercise of any such right.
21
5.6 No Short Sales. For as long as any Purchaser holds Securities, neither the Purchaser nor any of its Affiliates nor any entity managed or controlled by each such Purchaser will, directly or indirectly, or cause or assist any Person to (x) enter into any Short Sale or (y) trade in derivative securities to the same effect. For instance, no Purchaser shall engage in any Short Sale which would prevent the Company from exercising its rights under Section 6 of the Note.
5.7 Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their successors and permitted assigns. The Company may not assign this Agreement or any rights or obligations hereunder without the prior written consent of each Purchaser (other than by merger). Any Purchaser may assign any or all of its rights under this Agreement to any Person to whom such Purchaser assigns or transfers any Securities, provided that such transferee agrees in writing to be bound, with respect to the transferred Securities, by the provisions of the Transaction Documents that apply to the “Purchasers.”
5.8 No Third-Party Beneficiaries. This Agreement is intended for the benefit of the parties hereto and their respective successors and permitted assigns and is not for the benefit of, nor may any provision hereof be enforced by, any other Person, except as otherwise set forth in Section 4.10 and this Section 5.8.
5.9 Governing Law. All questions concerning the construction, validity, enforcement and interpretation of the Transaction Documents shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Agreement and any other Transaction Documents (whether brought against a party hereto or its respective affiliates, directors, officers, shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein (including with respect to the enforcement of any of the Transaction Documents), and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient venue for such proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted by law.
22
5.10 Survival. The representations and warranties contained herein shall survive the Closing and the delivery of the Securities until the earlier of (i) one year following the Closing Date and (ii) the date the Notes are no longer outstanding.
5.11 Execution. This Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to each other party, it being understood that the parties need not sign the same counterpart. In the event that any signature is delivered by facsimile transmission or by e-mail delivery of a “.pdf” format data file, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such facsimile or “.pdf” signature page were an original thereof.
5.12 Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially reasonable efforts to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision, covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable.
5.13 Rescission and Withdrawal Right. Notwithstanding anything to the contrary contained in (and without limiting any similar provisions of) any of the other Transaction Documents, whenever any Purchaser exercises a right, election, demand or option under a Transaction Document and the Company does not timely perform its related obligations within the periods therein provided, then such Purchaser may rescind or withdraw, in its sole discretion from time to time upon written notice to the Company, any relevant notice, demand or election in whole or in part without prejudice to its future actions and rights.
5.14 Replacement of Securities. If any certificate or instrument evidencing any Securities is mutilated, lost, stolen or destroyed, the Company shall issue or cause to be issued in exchange and substitution for and upon cancellation thereof (in the case of mutilation), or in lieu of and substitution therefor, a new certificate or instrument, but only upon receipt of evidence reasonably satisfactory to the Company of such loss, theft or destruction and an indemnification relating thereto. The applicant for a new certificate or instrument under such circumstances shall also pay any reasonable third-party costs (including customary indemnity) associated with the issuance of such replacement Securities.
5.15 Remedies. In addition to being entitled to exercise all rights provided herein or granted by law, including recovery of damages, each of the Purchasers and the Company will be entitled to specific performance under the Transaction Documents. The parties agree that monetary damages may not be adequate compensation for any loss incurred by reason of any breach of obligations contained in the Transaction Documents and hereby agree to waive and not to assert in any action for specific performance of any such obligation the defense that a remedy at law would be adequate.
23
5.16 Payment Set Aside. To the extent that the Company makes a payment or payments to any Purchaser pursuant to any Transaction Document or a Purchaser enforces or exercises its rights thereunder, and such payment or payments or the proceeds of such enforcement or exercise or any part thereof are subsequently invalidated, declared to be fraudulent or preferential, set aside, recovered from, disgorged by or are required to be refunded, repaid or otherwise restored to the Company, a trustee, receiver or any other Person under any law (including, without limitation, any bankruptcy law, state or federal law, common law or equitable cause of action), then to the extent of any such restoration the obligation or part thereof originally intended to be satisfied shall be revived and continued in full force and effect as if such payment had not been made or such enforcement or setoff had not occurred.
5.17 Usury. To the extent it may lawfully do so, the Company hereby agrees not to insist upon or plead or in any manner whatsoever claim, and will resist any and all efforts to be compelled to take the benefit or advantage of, usury laws wherever enacted, now or at any time hereafter in force, in connection with any claim, action or proceeding that may be brought by any Purchaser in order to enforce any right or remedy under any Transaction Document. Notwithstanding any provision to the contrary contained in any Transaction Document, it is expressly agreed and provided that the total liability of the Company under the Transaction Documents for payments in the nature of interest shall not exceed the maximum lawful rate authorized under applicable law (the “Maximum Rate”), and, without limiting the foregoing, in no event shall any rate of interest or default interest, or both of them, when aggregated with any other sums in the nature of interest that the Company may be obligated to pay under the Transaction Documents exceed such Maximum Rate. It is agreed that if the maximum contract rate of interest allowed by law and applicable to the Transaction Documents is increased or decreased by statute or any official governmental action subsequent to the date hereof, the new maximum contract rate of interest allowed by law will be the Maximum Rate applicable to the Transaction Documents from the effective date thereof forward, unless such application is precluded by applicable law. If under any circumstances whatsoever, interest in excess of the Maximum Rate is paid by the Company to any Purchaser with respect to indebtedness evidenced by the Transaction Documents, such excess shall be applied by such Purchaser to the unpaid principal balance of any such indebtedness or be refunded to the Company, the manner of handling such excess to be at such Purchaser’s election.
5.18 Independent Nature of Purchasers’ Obligations and Rights. The obligations of each Purchaser under any Transaction Document are several and not joint with the obligations of any other Purchaser, and no Purchaser shall be responsible in any way for the performance or non-performance of the obligations of any other Purchaser under any Transaction Document. Nothing contained herein or in any other Transaction Document, and no action taken by any Purchaser pursuant hereto or thereto, shall be deemed to constitute the Purchasers as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Purchasers are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by the Transaction Documents. Each Purchaser shall be entitled to independently protect and enforce its rights, including, without limitation, the rights arising out of this Agreement or out of the other Transaction Documents, and it shall not be necessary for any other Purchaser to be joined as an additional party in any proceeding for such purpose. Each Purchaser has been represented by its own separate legal counsel in its review and negotiation of the Transaction Documents.
24
5.19 Liquidated Damages. The Company’s obligations to pay any partial liquidated damages or other amounts owing under the Transaction Documents is a continuing obligation of the Company and shall not terminate until all unpaid partial liquidated damages and other amounts have been paid notwithstanding the fact that the instrument or security pursuant to which such partial liquidated damages or other amounts are due and payable shall have been canceled.
5.20 Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day, then such action may be taken or such right may be exercised on the next succeeding Business Day.
5.21 Construction. The parties agree that each of them and/or their respective counsel have reviewed and had an opportunity to revise the Transaction Documents and, therefore, the normal rule of construction to the effect that any ambiguities are to be resolved against the drafting party shall not be employed in the interpretation of the Transaction Documents or any amendments thereto. In addition, each and every reference to share prices and shares of Common Stock in any Transaction Document shall be subject to adjustment for reverse and forward stock splits, stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the date of this Agreement.
5.22 WAIVER OF JURY TRIAL. IN ANY ACTION, SUIT, OR PROCEEDING IN ANY JURISDICTION BROUGHT BY ANY PARTY AGAINST ANY OTHER PARTY, THE PARTIES EACH KNOWINGLY AND INTENTIONALLY, TO THE GREATEST EXTENT PERMITTED BY APPLICABLE LAW, HEREBY ABSOLUTELY, UNCONDITIONALLY, IRREVOCABLY AND EXPRESSLY WAIVES FOREVER TRIAL BY JURY.
(Signature Pages Follow)
25
IN WITNESS WHEREOF, the parties hereto have caused this Securities Purchase Agreement to be duly executed by their respective authorized signatories as of the date first indicated above.
| ALTERIX INC. | Address for Notice: | |||||||||
| By: | /s/ Patrick T. Mooney |
Fax; | ||||||||
| Name: | Patrick T. Mooney | Email: | ||||||||
| Title: | Chief Executive Officer | |||||||||
with a copy to (which shall not constitute notice):
Holland & Knight LLP
10 St. James Avenue
Boston, MA 02116
Attention: Thomas L. Barrette, Esq.
Tel. No.: 617-305-2134
Fax No.: 617-523-6850
E-mail: [email protected]
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK
SIGNATURE PAGE FOR PURCHASER FOLLOWS]
1
IN WITNESS WHEREOF, the undersigned have caused this Securities Purchase Agreement to be duly executed by their respective authorized signatories as of the date first indicated above.
Name of Purchaser:
Signature of Authorized Signatory of Purchaser:
Name of Authorized Signatory:
Title of Authorized Signatory:
Email Address of Authorized Signatory:
Facsimile Number of Authorized Signatory:
Address for Notice to Purchaser:
Address for Delivery of Securities to Purchaser
(if not same as address for notice):
Subscription Amount (dollar amount paid for the Notes): $
Principal Amount (Subscription Amount/0.80): $
Conversion Shares (Principal Amount / $1.32): Conversion Shares
Warrant Shares: (Conversion Shares *0.75): Warrant Shares
2
EXHIBIT A
NEITHER THIS SECURITY NOR THE SECURITIES INTO WHICH THIS SECURITY IS CONVERTIBLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”) AND APPLICABLE STATE SECURITIES LAWS, AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL OPINION OF CORPORATE COUNSEL TO THE TRANSFEROR TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY.
Original Issue Date: August , 2015
$ .00
ORIGINAL ISSUE DISCOUNT CONVERTIBLE NOTE
DUE AUGUST , 2016
THIS ORIGINAL ISSUE DISCOUNT CONVERTIBLE NOTE is one of a series of a duly authorized and validly issued Original Issue Discount Convertible Notes of Alterix Inc., a Delaware corporation (the “Company”), having its principal place of business at 100 Cummings Center, Suite 463E, Beverly, MA 01915, designated as its Original Issue Discount Convertible Note due August [*], 2016 (this Note, the “Note” and, collectively with the other Notes of such series, the “Notes”).
FOR VALUE RECEIVED, the Company promises to pay to [*] or its registered assigns (the “Holder”), or shall have paid pursuant to the terms hereunder, the principal sum of $[*] on August [*], 2016 (the “Maturity Date”) or such earlier date as this Note is required or permitted to be repaid as provided hereunder. This Note is subject to the following additional provisions:
Section 1. Definitions. For the purposes hereof, in addition to the terms defined elsewhere in this Note, (a) capitalized terms not otherwise defined herein shall have the meanings set forth in the Purchase Agreement and (b) the following terms shall have the following meanings:
“Bankruptcy Event” means any of the following events: (a) the Company or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X) thereof commences a case or other proceeding under any bankruptcy, reorganization, arrangement, adjustment of debt, relief of debtors, dissolution, insolvency or liquidation or similar law of any jurisdiction relating to the Company or any Significant Subsidiary thereof, (b) there is commenced against the Company or any Significant Subsidiary thereof any such case or
3
proceeding that is not dismissed within 60 days after commencement, (c) the Company or any Significant Subsidiary thereof is adjudicated insolvent or bankrupt or any order of relief or other order approving any such case or proceeding is entered, (d) the Company or any Significant Subsidiary thereof suffers any appointment of any custodian or the like for it or any substantial part of its property that is not discharged or stayed within 60 calendar days after such appointment, (e) the Company or any Significant Subsidiary thereof makes a general assignment for the benefit of creditors, (f) the Company or any Significant Subsidiary thereof calls a meeting of its creditors with a view to arranging a composition, adjustment or restructuring of its debts or (g) the Company or any Significant Subsidiary thereof, by any act or failure to act, expressly indicates its consent to, approval of or acquiescence in any of the foregoing or takes any corporate or other action for the purpose of effecting any of the foregoing.
“Beneficial Ownership Limitation” shall have the meaning set forth in Section 4(e).
“Business Day” means any day except any Saturday, any Sunday, any day which is a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Buy-In” shall have the meaning set forth in Section 4(d)(v).
“Common Stock” means the common stock of the Company, par value $0.001 per share.
“Conversion” shall have the meaning ascribed to such term in Section 4.
“Conversion Date” shall have the meaning set forth in Section 4(a).
“Conversion Price” shall have the meaning set forth in Section 4(c).
“Conversion Schedule” means the Conversion Schedule in the form of Schedule 1 attached hereto.
“Conversion Shares” means, collectively, the shares of Common Stock issuable upon conversion of this Note in accordance with the terms hereof.
“Event of Default” shall have the meaning set forth in Section 5(a).
“New York Courts” shall have the meaning set forth in Section 6(d).
“Note Register” shall have the meaning set forth in Section 2.
“Notice of Conversion” shall have the meaning set forth in Section 4(a).
“Original Issue Date” means the date of the first issuance of the Notes, regardless of any transfers of any Note and regardless of the number of instruments which may be issued to evidence such Notes.
4
“Purchase Agreement” means the Securities Purchase Agreement, dated as of August [*], 2015 among the Company and the original Holders, as amended, modified or supplemented from time to time in accordance with its terms.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Share Delivery Date” shall have the meaning set forth in Section 4(d)(ii).
“Trading Day” means a day on which the principal Trading Market is open for trading.
“Trading Market” means any of the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the NYSE MKT (formerly NYSE AMEX), the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the OTC Bulletin Board or the Pink OTC Markets (or any successors to any of the foregoing).
Section 2. Original Issue Discount; Prepayment. The Company acknowledges and agrees that this Note has been issued at an original issue discount. No regularly scheduled interest payments shall be made on this Note. All payments hereunder will be paid to the Person in whose name this Note is registered on the records of the Company regarding registration and transfers of this Note (the “Note Register”).
Section 3. Registration of Transfers and Exchanges.
a) Different Denominations. This Note is exchangeable for an equal aggregate principal amount of Notes of different authorized denominations, as requested by the Holder surrendering the same; provided, that the minimum principal amount of any replacement Note shall be $50,000. No service charge will be payable for such registration of transfer or exchange.
b) Investment Representations. This Note has been issued subject to certain investment representations of the original Holder set forth in the Purchase Agreement and may be transferred or exchanged only in compliance with the Purchase Agreement and applicable federal and state securities laws and regulations to successor Holders who provide the same investment representations to the Company.
c) Reliance on Note Register. Prior to due presentment for transfer to the Company of this Note, the Company and any agent of the Company may treat the Person in whose name this Note is duly registered on the Note Register as the owner hereof for the purpose of receiving payment as herein provided and for all other purposes, whether or not this Note is overdue, and neither the Company nor any such agent shall be affected by notice to the contrary.
Section 4. Conversion.
a) Voluntary Conversion. At any time after the Original Issue Date until this Note is no longer outstanding, this Note shall be convertible, in whole or in part, into shares of Common Stock at the option of the Holder, at any time and from time to time (subject to the
5
conversion limitations set forth in Section 4(e) hereof). The Holder shall effect conversions by delivering to the Company a Notice of Conversion, the form of which is attached hereto as Annex A (each, a “Notice of Conversion”), specifying therein the principal amount of this Note to be converted and the date on which such conversion shall be effected (such date, the “Conversion Date”). If no Conversion Date is specified in a Notice of Conversion, the Conversion Date shall be the date that such Notice of Conversion is deemed delivered hereunder. To effect conversions hereunder, the Holder shall not be required to physically surrender this Note to the Company unless the entire principal amount of this Note, plus all accrued and unpaid interest thereon, has been so converted. Conversions hereunder shall have the effect of lowering the outstanding principal amount of this Note in an amount equal to the applicable conversion. The Holder and the Company shall maintain records showing the principal amount(s) converted and the date of such conversion(s). The Holder, and any assignee by acceptance of this Note, acknowledge and agree that, by reason of the provisions of this paragraph, following conversion of a portion of this Note, the unpaid and unconverted principal amount of this Note may be less than the amount stated on the face hereof.
b) Mandatory Conversion. In the event that the Company completes an underwritten public offering of its Common Stock with gross proceeds to the Company of not less than $10 million, the aggregate principal amount of this Note shall be converted, automatically and without any further action on the part of the Holder, the Company or any other Person, into that number of shares of Common Stock as is equal to the quotient obtained by dividing (i) the aggregate principal amount of this Note by (ii) the Conversion Price.
c) Conversion Price. The Conversion Price in effect on any Conversion Date shall be equal to the lesser of (A) (i) $1.32 or (B) (i) the per share price of the Common Stock representing the pre-money valuation immediately prior to any shares sold in the Company’s initial public offering, multiplied by (ii) 80%. In the event the Company (i) pays a stock dividend or otherwise makes a distribution or distributions payable in shares of Common Stock on shares of Common Stock or any Common Stock Equivalents (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon conversion of, or payment of interest on, the Notes), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of a reverse stock split) outstanding shares of Common Stock into a smaller number of shares or (iv) issues, in the event of a reclassification of shares of the Common Stock, any shares of capital stock of the Company, then the Conversion Price shall be adjusted by multiplying the Conversion Price by a fraction of which the numerator shall be the number of shares of Common Stock (excluding any treasury shares of the Company) outstanding immediately before such event, and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event. Any adjustment made pursuant to this Section shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
d) Mechanics of Conversion.
i. Conversion Shares Issuable Upon Conversion of Principal Amount. The number of Conversion Shares issuable upon a conversion hereunder shall be determined by the quotient obtained by dividing (x) the outstanding principal amount of this Note to be converted by (y) the Conversion Price.
6
ii. Delivery of Certificate Upon Conversion. Not later than five (5) Trading Days after each Conversion Date (the “Share Delivery Date”), the Company shall deliver, or cause to be delivered, to the Holder (A) a certificate or certificates representing the Conversion Shares which, on or after the Effective Date, shall be free of restrictive legends and trading restrictions (other than those which may then be required by federal securities laws or the Purchase Agreement) representing the number of Conversion Shares being acquired upon the conversion of this Note and (B) a bank check in the amount of any accrued and unpaid interest (if the Company has elected or is required to pay accrued interest in cash). On or after the Effective Date, the Company shall use commercially reasonable efforts to deliver any certificate or certificates required to be delivered by the Company under this Section 4(d) electronically through the Depository Trust Company or another established clearing corporation performing similar functions.
iii. Failure to Deliver Certificates. If, in the case of any Notice of Conversion, such certificate or certificates are not delivered to or as directed by the applicable Holder by the Share Delivery Date, the Holder shall be entitled to elect by written notice to the Company at any time on or before its receipt of such certificate or certificates, to rescind such Conversion, in which event the Company shall promptly return to the Holder any original Note delivered to the Company and the Holder shall promptly return to the Company the Common Stock certificates issued to such Holder pursuant to the rescinded Notice of Conversion.
iv. Obligation Absolute; Partial Liquidated Damages. The Company’s obligations to issue and deliver the Conversion Shares upon conversion of this Note in accordance with the terms hereof are absolute and unconditional, irrespective of any action or inaction by the Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery of any judgment against any Person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination, or any breach or alleged breach by the Holder or any other Person of any obligation to the Company or any violation or alleged violation of law by the Holder or any other Person, and irrespective of any other circumstance which might otherwise limit such obligation of the Company to the Holder in connection with the issuance of such Conversion Shares; provided, however, that such delivery shall not operate as a waiver by the Company of any such action the Company may have against the Holder. Nothing herein shall limit a Holder’s right to pursue actual damages or declare an Event of Default pursuant to Section 5 hereof for the Company’s failure to deliver Conversion Shares within the period specified herein and the Holder shall have the right to pursue all remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief. The exercise of any such rights shall not prohibit the Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
v. Compensation for Buy-In on Failure to Timely Deliver Certificates Upon Conversion. In addition to any other rights available to the Holder, if the Company fails for any reason to deliver to the Holder such certificate or certificates by the Share Delivery Date pursuant to Section 4(d)(ii), and if after such Share Delivery Date the Holder is required by its
7
brokerage firm to purchase (in an open market transaction or otherwise), or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Conversion Shares which the Holder was entitled to receive upon the conversion relating to such Share Delivery Date (a “Buy-In”), then the Company shall (A) pay in cash to the Holder (in addition to any other remedies available to or elected by the Holder) the amount, if any, by which (x) the Holder’s total purchase price (including any brokerage commissions) for the Common Stock so purchased exceeds (y) the product of (1) the aggregate number of shares of Common Stock that the Holder was entitled to receive from the conversion at issue multiplied by (2) the actual sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions) and (B) at the option of the Holder, either reissue (if surrendered) this Note in a principal amount equal to the principal amount of the attempted conversion (in which case such conversion shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued if the Company had timely complied with its delivery requirements under Section 4(d)(ii). For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted conversion of this Note with respect to which the actual sale price of the Conversion Shares (including any brokerage commissions) giving rise to such purchase obligation was a total of $10,000 under clause of the immediately preceding sentence, the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver certificates representing shares of Common Stock upon conversion of this Note as required pursuant to the terms hereof.
vi. Reservation of Shares Issuable Upon Conversion. The Company covenants that it will at all times reserve and keep available out of its authorized and unissued shares of Common Stock for the sole purpose of issuance upon conversion of this Note and payment of interest on this Note, each as herein provided, free from preemptive rights or any other actual contingent purchase rights of Persons other than the Holder (and the other holders of the Notes), not less than such aggregate number of shares of the Common Stock as shall (subject to the terms and conditions set forth in the Purchase Agreement) be issuable the conversion of the then outstanding principal amount of this Note and payment of interest hereunder. The Company covenants that all shares of Common Stock that shall be so issuable shall, upon issue, be duly authorized, validly issued, fully paid and nonassessable and, if a registration statement is then effective under the Securities Act, shall be registered for public resale in accordance with such registration statement.
vii. Fractional Shares. No fractional shares or scrip representing fractional shares shall be issued upon the conversion of this Note. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such conversion, the Company shall at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Conversion Price or round up to the next whole share.
viii. Transfer Taxes and Expenses. The issuance of certificates for shares of the Common Stock on conversion of this Note shall be made without charge to the
8
Holder hereof for any documentary stamp or similar taxes that may be payable in respect of the issue or delivery of such certificates, provided that, the Company shall not be required to pay any tax that may be payable in respect of any transfer involved in the issuance and delivery of any such certificate upon conversion in a name other than that of the Holder of this Note so converted and the Company shall not be required to issue or deliver such certificates unless or until the Person or Persons requesting the issuance thereof shall have paid to the Company the amount of such tax or shall have established to the satisfaction of the Company that such tax has been paid. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Conversion.
Section 5. Events of Default.
a) “Event of Default” means, wherever used herein, any of the following events (whatever the reason for such event and whether such event shall be voluntary or involuntary or effected by operation of law or pursuant to any judgment, decree or order of any court, or any order, rule or regulation of any administrative or governmental body):
i. any default in the payment of (A) the principal amount of any Note or (B) interest, liquidated damages and other amounts owing to a Holder on any Note, as and when the same shall become due and payable (whether on a Conversion Date or the Maturity Date or by acceleration or otherwise) which default, solely in the case of an interest payment or other default under clause (B) above, is not cured within fifteen (15) Trading Days;
ii. the Company shall fail to observe or perform any other material covenant or agreement contained in the Notes which failure is not cured, if possible to cure, within the earlier to occur of (A) fifteen (15) Trading Days after notice of such failure sent by the Holder or by any other Holder to the Company and thirty (30) Trading Days after the Company has become or should reasonably have become aware of such failure;
iii. any representation or warranty made in this Note or any other Transaction Documents shall be untrue or incorrect in any material respect as of the date when made or deemed made;
iv. the Company or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X) shall be subject to a Bankruptcy Event; or
v. following the date the Company initially becomes a reporting company pursuant to the Exchange Act and its shares of Common Stock are listed on a Trading Market, the Common Stock shall subsequently not be eligible for listing or quotation for trading on a Trading Market and shall not be eligible to resume listing or quotation for trading thereon within five (5) Trading Days.
b) Remedies Upon Event of Default. If any Event of Default occurs and is continuing before the Maturity Date, the outstanding principal amount of this Note, plus liquidated damages, interest and other amounts owing in respect thereof through the date of acceleration, shall become, at the Holder’s election, immediately due and payable in cash. Commencing five (5) Trading Days after the occurrence of any Event of Default that results in the eventual acceleration of this Note, the interest rate on this Note shall accrue at an interest rate
9
equal to the lesser of 15% per annum or the maximum rate permitted under applicable law. Upon the payment in full, the Holder shall promptly surrender this Note to or as directed by the Company. In connection with such acceleration described herein, the Holder need not provide, and the Company hereby waives, any presentment, demand, protest or other notice of any kind, and the Holder may immediately and without expiration of any grace period enforce any and all of its rights and remedies hereunder and all other remedies available to it under applicable law. Such acceleration may be rescinded and annulled by Holder at any time prior to payment hereunder and the Holder shall have all rights as a holder of the Note until such time, if any, as the Holder receives full payment pursuant to this Section 5(b). No such rescission or annulment shall affect any subsequent Event of Default or impair any right consequent thereon.
Section 6. Miscellaneous.
a) Notices. Any and all notices or other communications or deliveries to be provided by the Holder hereunder, including, without limitation, any Notice of Conversion, shall be in writing and delivered personally, by e-mail, facsimile, or sent by a nationally recognized overnight courier service, addressed to the Company, at the address set forth above, or such other e-mail address, facsimile number or address as the Company may specify for such purposes by notice to the Holder delivered in accordance with this Section 6(a). Any and all notices or other communications or deliveries to be provided by the Company hereunder shall be in writing and delivered personally, by e-mail or facsimile, or sent by a nationally recognized overnight courier service addressed to each Holder at the e-mail address, facsimile number or address of the Holder appearing on the signature pages attached to the Purchase Agreement. Any notice or other communication or deliveries hereunder shall be deemed given and effective on the earliest of (i) the date of transmission, if such notice or communication is delivered via e-mail or facsimile at the email address or facsimile number set forth on the signature pages attached to the Purchase Agreement prior to 5:30 p.m. (New York City time) on any date, (ii) the next Trading Day after the date of transmission, if such notice or communication is delivered via e-mail or facsimile at the e-mail address or facsimile number set forth on the signature pages attached to the Purchase Agreement on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (iii) the second Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service or (iv) upon actual receipt by the party to whom such notice is required to be given.
b) Absolute Obligation. Except as expressly provided herein, no provision of this Note shall alter or impair the obligation of the Company, which is absolute and unconditional, to pay the principal of, liquidated damages and accrued interest, as applicable, on this Note at the time, place, and rate, and in the coin or currency, herein prescribed. This Note is a direct debt obligation of the Company.
c) Lost or Mutilated Note. If this Note shall be mutilated, lost, stolen or destroyed, the Company shall execute and deliver, in exchange and substitution for and upon cancellation of a mutilated Note, or in lieu of or in substitution for a lost, stolen or destroyed Note, a new Note for the principal amount of this Note so mutilated, lost, stolen or destroyed, but only upon receipt of evidence of such loss, theft or destruction of such Note, and of the ownership hereof, reasonably satisfactory to the Company.
10
d) Governing Law. All questions concerning the construction, validity, enforcement and interpretation of this Note shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflict of laws thereof. Each party agrees that all legal proceedings concerning the interpretation, enforcement and defense of the transactions contemplated by any of the Transaction Documents (whether brought against a party hereto or its respective Affiliates, directors, officers, shareholders, employees or agents) shall be commenced in the state and federal courts sitting in the City of New York, Borough of Manhattan (the “New York Courts”). Each party hereto hereby irrevocably submits to the exclusive jurisdiction of the New York Courts for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein (including with respect to the enforcement of any of the Transaction Documents), and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of such New York Courts, or such New York Courts are improper or inconvenient venue for such proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Note and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted by applicable law. Each party hereto hereby irrevocably waives, to the fullest extent permitted by applicable law, any and all right to trial by jury in any legal proceeding arising out of or relating to this Note or the transactions contemplated hereby. If any party shall commence an action or proceeding to enforce any provisions of this Note, then the prevailing party in such action or proceeding shall be reimbursed by the other party for its attorney’s fees and other costs and expenses incurred in the investigation, preparation and prosecution of such action or proceeding.
e) Waiver. Any waiver by the Company or the Holder of a breach of any provision of this Note shall not operate as or be construed to be a waiver of any other breach of such provision or of any breach of any other provision of this Note. The failure of the Company or the Holder to insist upon strict adherence to any term of this Note on one or more occasions shall not be considered a waiver or deprive that party of the right thereafter to insist upon strict adherence to that term or any other term of this Note on any other occasion. Any waiver by the Company or the Holder must be in writing.
f) Severability. If any provision of this Note is invalid, illegal or unenforceable, the balance of this Note shall remain in effect, and if any provision is inapplicable to any Person or circumstance, it shall nevertheless remain applicable to all other Persons and circumstances. If it shall be found that any interest or other amount deemed interest due hereunder violates the applicable law governing usury, the applicable rate of interest due hereunder shall automatically be lowered to equal the maximum rate of interest permitted under applicable law. The Company covenants (to the extent that it may lawfully do so) that it shall not at any time insist upon, plead, or in any manner whatsoever claim or take the benefit or advantage of, any stay, extension or usury law or other law which would prohibit or forgive the Company from paying all or any portion of the principal of or interest on this Note as contemplated herein, wherever enacted, now or at any time hereafter in force, or which may affect the covenants or the performance of this Note, and the Company (to the extent it may
11
lawfully do so) hereby expressly waives all benefits or advantage of any such law, and covenants that it will not, by resort to any such law, hinder, delay or impede the execution of any power herein granted to the Holder, but will suffer and permit the execution of every such as though no such law has been enacted.
g) Next Business Day. Whenever any payment or other obligation hereunder shall be due on a day other than a Business Day, such payment shall be made on the next succeeding Business Day.
h) Headings. The headings contained herein are for convenience only, do not constitute a part of this Note and shall not be deemed to limit or affect any of the provisions hereof.
IN WITNESS WHEREOF, the Company has caused this Note to be duly executed by a duly authorized officer as of the date first above indicated.
| Alterix Inc. | ||||
| By: |
| |||
| Name: | Patrick T. Mooney | |||
| Title: | Chief Executive Officer | |||
12
ANNEX A
NOTICE OF CONVERSION
The undersigned hereby elects to convert principal under the Original Issue Discount Convertible Note due August [*], 2016 of Alterix Inc., a Delaware corporation (the “Company”), into shares of common stock (the “Common Stock”), of the Company according to the conditions hereof, as of the date written below. If shares of Common Stock are to be issued in the name of a person other than the undersigned, the undersigned will pay all transfer taxes payable with respect thereto and is delivering herewith such certificates and opinions as reasonably requested by the Company in accordance therewith. No fee will be charged to the holder for any conversion, except for such transfer taxes, if any.
Conversion calculations:
Date to Effect Conversion:
Principal Amount of Note to be Converted:
Number of shares of Common Stock to be issued:
Cash to be paid to Holder:
Signature:
Name:
Address for Delivery of Common Stock Certificates:
Or
DWAC Instructions:
Broker No: \
Account No:
Schedule 1
CONVERSION SCHEDULE
The Original Issue Discount Convertible Notes due on August [*], 2016 in the aggregate principal amount of $[*] are issued by Alterix Inc., a Delaware corporation. This Conversion Schedule reflects conversions made under Section 4 of the above referenced Note.
Dated:
| Date of Conversion (or for first entry, Original Issue Date) |
Amount of Conversion | Aggregate Principal Amount Remaining Subsequent to Conversion (or original Principal Amount) |
Company Attest | |||
EXHIBIT B
NEITHER THIS SECURITY NOR THE SECURITIES FOR WHICH THIS SECURITY IS EXERCISABLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”) AND APPLICABLE STATE SECURITIES LAWS, AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL OPINION OF CORPORATE COUNSEL TO THE TRANSFEROR TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY.
COMMON STOCK PURCHASE WARRANT
ALTERIX INC.
| Warrant Shares: [*] | Original Issue Date: August [*], 2015 | |||
THIS COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received [*] or its assigns (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth and in the Securities Purchase Agreement between the Company and the Holder (the “Purchase Agreement”), at any time on or after the Original Issue Date and on or prior to the close of business on the fifth anniversary of the Original Issue Date (the “Termination Date”) but not thereafter, to subscribe for and purchase from Alterix Inc., a Delaware corporation (the “Company”), up to [*] shares (as subject to adjustment hereunder, the “Warrant Shares”) of Common Stock. The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b).
Section 1. Definitions. Capitalized terms used and not otherwise defined herein shall have the meanings set forth in the Original Issue Discount Convertible Notes (the “Notes”), dated August [*], 2015, issued by the Company to the purchasers pursuant to the Purchase Agreement.
Section 2. Exercise.
a) Exercise of Warrant. Exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company (or such other office or agency of the Company as the Company may designate by notice in writing to the registered Holder at the address of the Holder appearing on the books of the Company) of a duly executed facsimile copy of the Notice of Exercise form annexed hereto and within three (3) Trading Days of the date said Notice of Exercise is delivered to the Company, the Company shall have
received payment of the aggregate Exercise Price of the shares thereby purchased by wire transfer to an account designated by the Company or cashier’s check drawn on a United States bank or, if available, pursuant to the cashless exercise procedure specified in Section 2(c) below. If the amount of payment received by the Company is less than the aggregate Exercise Price of the shares being purchased, the Holder shall make payment of the deficiency within three (3) Trading Days following notice thereof. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall automatically reduce the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company shall deliver any objection to any Notice of Exercise Form within two (2) Business Days of receipt of such notice. The Holder and any assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a portion of the Warrant Shares hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less than the amount stated on the face hereof.
b) Exercise Price. The exercise price per share of the Common Stock under this Warrant shall be the lesser of i) $1.52 or ii) a 15% premium to the Conversion Price if the Conversion Price is determined based on the pre money IPO valuation of the being less than $75 million (the “Exercise Price”);
c) Cashless Exercise. In connection with a Cashless Exercise, this Warrant shall represent the right to subscribe for and acquire the number of Warrant Shares equal to (i) the number of Warrant Shares specified by the Holder in its Notice of Exercise (the “Total Number”) less (ii) the number of Warrant Shares equal to the quotient obtained by dividing (A) the product of the Total Number and the applicable existing Exercise Price by (B) the Fair Market Value. “Fair Market Value” shall mean: (1) if the Warrant Shares are listed on the NYSE MKT (formerly NYSE AMEX), the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the OTC Bulletin Board or the Pink OTC Markets (or any successors to any of the foregoing), the last reported sale price of the Warrant Shares on such exchange or Nasdaq on the date for which the determination is being made; or (2) if the Warrant Shares are not so listed, “Fair Market Value” shall be determined in good faith by the Board of Directors of the Company.
d) Mechanics of Exercise.
i. Delivery of Certificates Upon Exercise. Certificates for shares purchased hereunder shall be transmitted by the Transfer Agent to the Holder by crediting the account of the Holder’s prime broker with The Depository Trust Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to or resale of the Warrant Shares by the Holder and such Warrant Shares have been sold
or (B) the shares are eligible for resale by the Holder without volume or manner-of-sale limitations pursuant to Rule 144, and otherwise by physical delivery to the address specified by the Holder in the Notice of Exercise by the date that is five (5) Trading Days after the latest of (A) the delivery to the Company of the Notice of Exercise, (B) surrender of this Warrant (if required), and (C) payment of the aggregate Exercise Price as set forth above (including by cashless exercise, if permitted) (such date, the “Warrant Share Delivery Date”). The Warrant Shares shall be deemed to have been issued, and Holder or any other person so designated to be named therein shall be deemed to have become a holder of record of such shares for all purposes, as of the date the Warrant has been exercised in accordance with the requirements of the preceding sentence and with payment to the Company of the Exercise Price (or by cashless exercise, if permitted) and all taxes required to be paid by the Holder, if any, pursuant to Section 2(d)(vi) prior to the issuance of such shares, having been paid.
ii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the certificate or certificates representing Warrant Shares, deliver to the Holder a new Warrant evidencing the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iii. Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder a certificate or the certificates representing the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
iv. Compensation for Buy-In on Failure to Timely Deliver Certificates Upon Exercise. In addition to any other rights available to the Holder, if the Company fails to cause the Transfer Agent to transmit to the Holder a certificate or the certificates representing the Warrant Shares pursuant to an exercise on or before the Warrant Share Delivery Date, and if after such date the Holder is required by its broker to purchase (in an open market transaction or otherwise) or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a “Buy-In”), then the Company shall (A) pay in cash to the Holder the amount, if any, by which (x) the Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased exceeds (y) the amount obtained by multiplying (1) the number of Warrant Shares that the Company was required to deliver to the Holder in connection with the exercise at issue times (2) the price at which the sell order giving rise to such purchase obligation was executed, and (B) at the option of the Holder, either reinstate the portion of the Warrant and equivalent number of Warrant Shares for which such exercise was not honored (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued had the Company timely complied with its exercise and delivery obligations hereunder subject to payment of the Exercise Price therefor. For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of shares of Common Stock with an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the immediately preceding sentence the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice
indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver certificates representing shares of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof.
v. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round to the nearest whole share.
vi. Charges, Taxes and Expenses. Issuance of certificates for Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such certificate, and such certificates shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event certificates for Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise.
vii. Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
Section 3. Certain Adjustments.
a) Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend or otherwise makes a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
b) Issuance of Additional Shares of Common Stock.
i. Until the Company consummates its initial public offering, in the event the Company shall issue any Additional Shares of Common Stock (as defined below), at a price per share less than the Exercise Price then in effect or without consideration, then the Exercise Price upon each such issuance shall be adjusted to that price determined by multiplying the Exercise Price then in effect by a fraction:
(A) the numerator of which shall be equal to the sum of (x) the number of shares of outstanding Common Stock (assuming full exercise, conversion or exchange of all options, warrants and other securities which are convertible into or exercisable or exchangeable for, and any right to subscribe for, shares of Common Stock) immediately prior to the issuance of such Additional Shares of Common Stock plus (y) the number of shares of Common Stock (rounded to the nearest whole share) which the aggregate consideration for the total number of such Additional Shares of Common Stock so issued would purchase at a price per share equal to the Exercise Price then in effect, and
(B) the denominator of which shall be equal to the number of shares of outstanding Common Stock (assuming full exercise, conversion or exchange of all options, warrants and other securities which are convertible into or exercisable or exchangeable for, and any right to subscribe for, shares of Common Stock) immediately after the issuance of such Additional Shares of Common Stock.
ii. “Additional Shares of Common Stock” means all shares of Common Stock issued by the Company after the date hereof, except: (i) securities issued (other than for cash) in connection with a merger, acquisition, or consolidation, (ii) securities issued pursuant to the conversion or exercise of convertible or exercisable securities issued or outstanding on or prior to the date of the Purchase Agreement or issued pursuant to the Purchase Agreement (so long as the conversion or exercise price in such securities are not amended to lower such price and/or adversely affect the Holders), (iii) the Warrant Shares, (iv) securities issued in connection with bona fide strategic license agreements or other partnering arrangements so long as such issuances are not for the purpose of raising capital, (v) Common Stock issued or the issuance or grants of options to purchase Common Stock to the Company’s employees, directors, consultants or advisors, and (vi) any warrants issued to any placement agent and its designees for the transactions contemplated by the Purchase Agreement.
c) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
d) Notice to Holder.
i. Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall promptly mail to the Holder a notice setting forth the Exercise Price after such adjustment and any resulting adjustment to the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment.
ii. Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, or any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be mailed to the Holder at its last address as it shall appear upon the Warrant Register of the Company, at least 10 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to mail such notice or any defect therein or in the mailing thereof shall not affect the validity of the corporate action required to be specified in such notice. To the extent that any notice provided hereunder constitutes, or contains, material, non-public information regarding the Company or any of the Subsidiaries, the Company shall simultaneously publicly disclose such notice. The Holder shall remain entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice except as may otherwise be expressly set forth herein.
e) Voluntary Adjustment By Company. The Company may at any time during the term of this Warrant reduce the then current Exercise Price to any amount and for any period of time deemed appropriate by the Board of Directors of the Company.
f) Adjustment for Number of Warrant Shares. In the event that the Company is unable to include at least 25% of the Conversion Shares in the IPO registration, then the number of Warrant Shares will be recalculated as follows:
New Number of Warrant Shares = Principal Amount divided by the Exercise Price.
Section 4. Transfer of Warrant.
a) Transferability. Subject to compliance with any applicable securities laws and the conditions set forth in Section 4(d) hereof, this Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon
surrender of this Warrant at the principal office of the Company or its designated agent, together with a written assignment of this Warrant substantially in the form attached hereto duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer, but only after such transferee agrees to be bound by the provisions of this Agreement. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. The Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the Original Issue Date and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
d) Transfer Restrictions. The Warrant may only be disposed of in compliance with state and federal securities laws and shall not transferred unless the Warrant is (i) registered pursuant to an effective registration statement under the Securities Act and under applicable state securities or blue sky laws or (ii) eligible for resale without volume or manner-of-sale restrictions or current public information requirements pursuant to Rule 144.
e) Representation by the Holder. The Holder, by the acceptance hereof, represents and warrants that it is acquiring this Warrant and, upon any exercise hereof, will acquire the Warrant Shares issuable upon such exercise, for its own account and not with a view to or for distributing or reselling such Warrant Shares or any part thereof in violation of the Securities Act or any applicable state securities law, except pursuant to sales registered or exempted under the Securities Act.
Section 5. Miscellaneous.
a) No Rights as Stockholder Until Exercise. This Warrant does not entitle the Holder to any voting rights, dividends or other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2(d)(i), except as expressly set forth in Section 3.
b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day, then, such action may be taken or such right may be exercised on the next succeeding Business Day.
d) Authorized Shares.
The Company covenants that, during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue).
Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its articles of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
Before taking any action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body having jurisdiction thereof.
e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of the Transaction Documents shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof.
f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered and the Holder does not utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder or Company shall operate as a waiver of such right or otherwise prejudice the Holder’s or Company’s rights, powers or remedies, notwithstanding the fact that all rights hereunder terminate on the Termination Date. If either party willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the other, the first party shall pay to the other party such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by the affected party in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
h) Notices. Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be in writing and shall be deemed given and effective on the earliest of: (a) the date of transmission, if such notice or communication is delivered via email or facsimile at the email address or facsimile number set forth on the signature pages attached to the Purchase Agreement at or prior to 5:30 p.m. (New York City time) on a Trading Day, (b) the next Trading Day after the date of email or facsimile transmission, if such notice or communication is delivered via email or facsimile at the email address or facsimile number set forth on the signature pages attached to the Purchase Agreement on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (c) the second (2nd) Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service or (d) upon actual receipt by the party to whom such notice is required to be given. The address for such notices and communications shall be as set forth on the signature page attached to the Purchase Agreement.
i) Limitation of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability of the Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
j) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
k) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
l) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company and the Holder.
m) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
| ALTERIX INC. | ||||
| By: |
| |||
| Name: | Patrick T. Mooney | |||
| Title: | Chief Executive Officer | |||
NOTICE OF EXERCISE
| TO: | ALTERIX INC. |
(1) The undersigned hereby elects to purchase Warrant Shares of the Company pursuant to the terms of the attached Warrant (only if exercised in full), and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any.
(2) Payment shall take the form of (check applicable box): [ ] lawful money of the United States; or [ ] if permitted the cancellation of such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2(c), to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2(c).
(3) Please issue a certificate or certificates representing said Warrant Shares in the name of the undersigned or in such other name as is specified below:
|
| ||
The Warrant Shares shall be delivered to the following DWAC Account Number or by physical delivery of a certificate to:
|
| ||
|
| ||
|
| ||
(4) Accredited Investor. The undersigned is an “accredited investor” as defined in Regulation D promulgated under the Securities Act of 1933, as amended, and that the aforesaid Shares are being acquired for the account of the undersigned for investment and not with a view to, or for resale, in connection with the distribution thereof, and that the undersigned has no present intention of distributing or reselling such shares.
[SIGNATURE OF HOLDER]
| Name of Investing Entity: | ||
| Signature of Authorized Signatory of Investing Entity: | ||
| Name of Authorized Signatory: | ||
| Title of Authorized Signatory: | ||
| Date: |
| |
ASSIGNMENT FORM
(To assign the foregoing warrant, execute
this form and supply required information.
Do not use this form to exercise the warrant.)
FOR VALUE RECEIVED, [ all of or [ shares of the foregoing Warrant and all rights evidenced thereby are hereby assigned to whose address is .
Dated: ,
| Holder’s Signature: |
| |
| Holder’s Address: |
| |
|
|
NOTE: The signature to this Assignment Form must correspond with the name as it appears on the face of the Warrant, without alteration or enlargement or any change whatsoever, and must be guaranteed by a bank or trust company. Officers of corporations and those acting in a fiduciary or other representative capacity should file proper evidence of authority to assign the foregoing Warrant.
Exhibit 10.5
Alterix Inc.
EMPLOYMENT AGREEMENT
THIS EMPLOYMENT AGREEMENT (the “Agreement”), by and between Alterix Inc., a Delaware corporation (“Alterix”), and Patrick Mooney, M.D. (the “Executive”) is effective as of June 11, 2015 (the “Effective Date”). Alterix and the Executive are sometimes referred to herein individually as a “Party” and collectively as the “Parties.” In consideration of the mutual covenants and agreements contained herein and other good and valuable consideration, the receipt and sufficiency of which the Parties hereby acknowledge, the Parties, intending to be legally bound, agree as follows:
1. Employment. Alterix shall employ the Executive, and the Executive shall accept such employment with Alterix, upon the terms and conditions set forth in this Agreement for the period beginning on the Effective Date and ending as provided in Section 4 (the “Employment Period”).
2. Position and Duties.
(a) Location; Title; Duties. During the Employment Period, the Executive shall serve Alterix in the capacity of Chief Executive Officer, be based in the greater Philadelphia, PA, metropolitan area, have such duties, responsibilities and authorities consistent with responsibilities generally associated with the position of Chief Executive Officer as may be augmented or revised from time to time by the Board of Directors and generally to carry out the policies and goals of Alterix (collectively, the “Duties”). Executive may have to travel in order to properly perform the Duties. Executive shall use the Executive’s best efforts to perform the Duties and promote the interests of Alterix, devote the Executive’s full business time, attention and effort to the performance of the Duties and refrain from acts or omissions detrimental to Alterix or harmful to its interests. While employed by Alterix, the Executive shall not engage in any business activity that conflicts with the Employee’s duties to Alterix.
(b) Outside Activities. Notwithstanding the foregoing, nothing in this Agreement shall prevent or be deemed to prohibit Executive from spending time on and participating in Executive’s personal and family investments, industry, civic, or charitable and other non-profit affairs, and as a board member or advisor to no more than two other companies; provided, however, that such participation and time expended in such activities do not materially interfere with the responsibilities as an employee of Alterix in accordance with this Agreement.
3. Compensation. In consideration for the performance of the Duties as well as other obligations under this Agreement, Executive shall receive the following compensation.
(a) Base Salary. Alterix shall pay the Executive an annual base salary of Four Hundred Eighty Thousand Dollars ($480,000.00) per year, to be paid in equal monthly installments in accordance with Alterix’s standard practices and policies in effect from time to time, provided that such salary shall not begin to be paid until the month in which Alterix receives proceeds from the sale, or series of related sales, of shares of stock, or promissory notes convertible into shares of stock, which total $3,000,000.00 or more in the aggregate. Prior to such month, Alterix shall pay the Executive a monthly stipend of Ten Thousand Dollars ($10,000.00) per month for any
month during which the Executive performs services for the Company until the month in which Alterix receives proceeds from the sale, or series of related sales, of shares of stock, or promissory notes convertible into shares of stock which total $500,000 or more in the aggregate. Commencing with the month in which Alterix receives the $500,000 in proceeds described in the preceding sentence, Alterix shall pay the Executive a monthly stipend of $20,000 per month. If, prior to the Company meeting the $3,000,000 threshold described above, it has received proceeds from the sale, or series of related sales, of shares of stock, or promissory notes convertible into shares of stock, which total $1,500,000 or more in the aggregate, Alterix shall pay the Executive a monthly stipend of $30,000 per month commencing with the month in which the aggregate proceeds received equal or exceed $1,500,000.
(b) Annual Bonus. Alterix shall pay the Executive an annual bonus in an amount up to One Hundred Percent (100%) of his base salary based on the Company’s financial performance in each calendar year. For the purpose of determining Executive’s annual bonus, the Company’s financial performance shall be measured by the degree to which the Company achieved objectives reasonably and realistically established by the Board of Directors in good faith, with the advice and consent of the Executive, prior to the commencement of each year. The annual bonus shall be paid no later than February 15 of the year immediately following the year on which the annual bonus is based.
(c) IPO Bonus. Alterix shall pay the Executive a one-time bonus within 180 days of the closing of an initial public offering of shares by Alterix (the “IPO”). The amount of such bonus shall be determined by multiplying (i) the amount set forth on the cover page of the final prospectus filed by Alterix with the United States Securities and Exchange Commission in connection with the IPO (the “Final Prospectus”) by (ii) the number of shares set forth as “Common stock to be outstanding after the offering” in the subsection titled “The Offering” in the Prospectus Summary section of the Final Prospectus by (iii) 0.9%. In the event the underwriters of the IPO exercise their overallotment option the Executive will be paid an additional bonus. That additional bonus will be calculated in the same manner as the provided above, except that the number of shares in item (ii) will be the number of shares purchased by the underwriters in their exercise of the overallotment option.
(d) Benefits. Executive shall be entitled to participate in such benefit plans as Alterix provides to its employees generally from time to time in accordance with Alterix policies and subject to the terms and conditions of such plans. Alterix agrees to provide Executive with family medical, dental and vision insurance, and long and short term disability insurance the benefits of which will guarantee Executive his full base salary during any short or long term disability.
(e) Car Allowance. Alterix shall pay the Executive a monthly car allowance of Eight Hundred Dollars ($800.00) on a monthly basis.
(f) Expenses. Alterix shall reimburse the Executive for all reasonable business travel, business development, business entertainment, mobile telephone and other expenses incurred by him in the course of performing the Duties. Expenses should be consistent with the policies established by Alterix from time to time with respect to travel and other business expenses, subject to Alterix’s requirements with respect to reporting and documentation of such expenses.
- 2 -
(g) Paid Vacation. Executive shall be entitled to take up to four (4) weeks paid vacation per calendar year. If Executive does not use some or all of his four (4) weeks vacation in a given year, he may, at his discretion, roll up to two (2) weeks of vacation time over into the following year and/or obtain payment from Alterix for unused vacation time at the rate of his base annual salary.
(h) Equity. Pursuant to the contemplated 2015 Equity Incentive Plan of Alterix (the “Plan”), a copy of which Executive will be given the opportunity to review, Executive shall receive an Incentive Stock Option (“Option”) providing terms for the purchase of 3,461,540 shares of Common Stock, which number shall be subject to adjustment in the event of a stock split, reverse stock split, stock dividend or similar event, at 100% of the then Fair Market Value, as each of those terms are defined in the Plan. The grant of Incentive Stock Option shall be made pursuant to the standard form of Stock Option Grant Notice and Agreement under the Plan. The Incentive Stock Option Grant Notice will include a vesting schedule as follows: (i) 692,308 shares vest on closing of the IPO, (ii) 692,308 shares vest on the date the first patient is enrolled in the Phase 2B/3 clinical trial of the Alterix ibuprofen product knows as AX-IBU-01, (iii) 692,308 shares vest on the date the first patient is enrolled in the Phase 1/2 clinical trial of the Alterix benfotiamine product knows as AX-DN-01, and (iv) 1,384,616 shares vest on the submission of a New Drug Application for AX-IBU-01 to the United States Food and Drug Administration. Executive will not be permitted to sell any shares issued pursuant to the Option, or any other shares owned by Executive, unless Executive has obtained the prior written approval of the Board of Directors of Alterix and has complied with all other applicable policies of Alterix, including the Alterix Insider Trading Policy as in effect at the applicable time and all applicable laws and regulations. Once issued and executed the Grant Notice will supersede and replace this paragraph, which will become null and void. In addition to the Option, the Company shall also grant to the Executive 1,384,616 shares of Common Stock pursuant to a Restricted Stock Agreement. The Company acknowledges and agrees that such shares shall not be subject to vesting and Executive Acknowledges and agrees that the grant of the shares to him will be a taxable event.
4. Term.
(a) Employment Period. The Employment Period shall continue from the Effective Date for a period of three (3) years unless terminated earlier upon the occurrence of the following events:
(i) the Executive’s death or “Permanent Disability” (defined as the expiration of a continuous period of 90 days during which the Executive is unable to perform the Duties due to physical or mental incapacity);
(ii) the Agreement is terminated for Cause (as defined below) by Alterix;
(iii) the Agreement is terminated for Good Reason (as defined below) by Executive at any time upon thirty (30) days’ prior written notice to Alterix;
- 3 -
(iv) the Agreement is terminated by Alterix at any time without Cause upon thirty (30) days’ prior written notice to the Executive; or
(v) the Agreement is terminated by the Executive without Good Reason at any time upon thirty (30) days’ prior written notice to Alterix.
(b) Definitions. For purposes of this Agreement:
(i) “Cause” shall mean only the following:
(A) the deliberate failure or refusal by the Executive to perform his Duties (other than any such failure resulting from the Executive’s incapacity due to illness, physical or mental incapacity) as expressly directed in writing by Alterix’s Board of Directors that results in actual and material demonstrable financial harm to Alterix which deliberate failure or refusal has not been cured within fifteen (15) business days after Alterix’s Board of Directors provides written notice to Executive of its belief that Executive has deliberately failed or refused to perform his duties as directed in writing by the Board of Directors;
(B) the Executive’s deliberate breach of this Agreement that results in actual and material demonstrable financial harm to Alterix that has been communicated to him in writing which deliberate breach has not been cured within fifteen (15) business days after written notice of such alleged breach is given to Executive by Alterix’s Board of Directors;
(C) the Executive’s gross intentional misconduct or gross negligence with respect to the performance of the Duties that results in actual and material demonstrable financial harm to Alterix which gross intentional misconduct or gross negligence has been communicated to him in writing and has not been cured within fifteen (15) business days after written notice of such alleged gross intentional misconduct or gross negligence;
(D) the conviction of the Executive, or plea of guilty or nolo contendere, with respect to any felony, any act of fraud, theft, or financial dishonesty with respect to Alterix or any of its affiliates, customers or business partners; or
(E) alcohol abuse or illegal substance abuse by the Executive that results in actual and material demonstrable financial harm to Alterix that has been communicated to him in writing and has not been cured within fifteen (15) business days after written notice of such alleged alcohol abuse or illegal substance abuse.
(ii) Termination for “Cause” under this Agreement shall not occur unless, prior to the date Executive’s employment is terminated:
(A) the Company has clear evidence that Executive has, in fact, engaged in conduct constituting “Cause” as defined herein; and
- 4 -
(B) the Company furnishes Executive in writing at the time of termination all of the reasons for Executive’s termination for “Cause” and describing in complete detail all of the facts and evidence that the support the Executive’s termination for “Cause.”
(iii) “Good Reason” shall exist upon
(A) mutual written agreement by the Executive and Alterix;
(B) the relocation of Executive’s office such that the Executive’s daily commute is increased by at least 75 miles round-trip without the written consent of the Executive;
(C) reduction of the Executive’s annual base salary without the prior consent of the Executive;
(D) material diminution of the Executive’s duties, title, position, or responsibilities; or
(E) material breach by Alterix of any of the terms of this Agreement, which breach is not substantially cured or rectified, or in process of being substantially cured or rectified, if such breach is curable or rectifiable, within fifteen (15) business days after Executive gives written notice of such breach to Alterix.
(c) Disability. In the event of any dispute regarding the existence of the Executive’s Permanent Disability hereunder, the matter will be resolved by an independent physician qualified to practice medicine in Philadelphia, Pennsylvania with greater than fifteen (15) years practice experience who is on the staff of a university hospital and has no prior knowledge of the Executive and no prior engagement by, or other relationship with, Alterix. The independent physician will be selected by the mutual agreement of Executive’s physician and a physician of Alterix’s choosing. For this purpose, the Executive will submit to all appropriate medical examinations.
(d) Payments Upon Termination of Employment.
(i) Upon the termination of the Executive’s employment with Alterix, the Executive shall be entitled to receive all earned or accrued but unpaid salary, accrued vacation and reimbursement of approved business expenses as well as all amounts or benefits to which the Executive is entitled under any applicable the employee benefit plan in which the Executive was a participant during his employment with Alterix, in accordance with the terms of that plan and in accordance with Alterix’s usual payroll practices.
(ii) In addition to the foregoing, upon the termination of the Executive’s employment with Alterix for any reason other than Executive’s death or Permanent Disability or for Cause, or if Executive terminates his employment with Good Reason, the Executive shall also be entitled to receive a lump sum equal to the Executive’s base salary and benefits set forth in Section 3(a), (c) and (d) hereof for a period of 24 months, and a lump sum equal to the annual bonus he received for the preceding year pursuant to Section 3(b) above.
- 5 -
(iii) The payments to which Executive is entitled under Section 4(d)(ii) shall be payable within thirty (30) days upon the occurrence of each of: (1) the execution of a document acknowledging Executive’s obligations under this Agreement, and (2) the return of all property of Alterix in Executive’s possession.
(iv) Alterix will withhold all federal, state, local and other taxes and amounts that it determines are required by law on all amounts paid pursuant to this Section.
(e) Automatic Renewal. Unless otherwise terminated prior to the expiration of the original Employment Period, this Agreement shall automatically renew upon the expiration of the original Employment Period for successive two (2) year terms unless either Party gives written notice of its intention not to renew this Agreement at least One Hundred Eighty (180) days prior to the date of expiration, provided that the terms of this Agreement addressing the rights and obligations of the Parties with respect to the termination of the Executive’s employment shall survive the expiration of this Agreement.
5. Proprietary Information, Assignment of Developments and Non-Competition Agreement. As a condition of the Executive’s employment with Alterix and a condition of any and all of Alterix’s obligations under this Agreement and the Purchase Agreement, the Executive shall enter into the accompanying Proprietary Information, Assignment of Developments and Non-Competition Agreement, a form of which is attached as Exhibit A (the “IP & Non-Competition Agreement”) and comply with his obligations thereunder. Notwithstanding any other provision of this Agreement, if the Executive materially breaches the IP & Non-Competition Agreement, Alterix shall be relieved of any and all obligations under this Agreement.
6. Section 409A. Notwithstanding any other provision herein to the contrary, to the extent that any payment to be made to the Executive, whether pursuant to this Agreement or otherwise, is determined to constitute “nonqualified deferred compensation” within the meaning of and subject to Section 409A of the Internal Revenue Code of 1986, as amended (“Section 409A”) such payment shall not be made prior to the date that is the earlier of (i) six months and one day after the Executive’s separation from service with Alterix and any parent, affiliate or subsidiary of Alterix and (ii) the Executive’s death. The terms of this Section 6 shall apply only if the Executive is a “specified employee” (within the meaning of Section 409A) on the date of such separation from service, and shall only apply to the extent the delay of such payment is necessary to prevent such payment from being subject to interest, penalties and/or additional tax imposed pursuant to Section 409A.
7. Executive and Alterix Representations. The Executive hereby represents and warrants to Alterix that (i) the execution, delivery and performance of this Agreement by the Executive does not and will not conflict with, breach, violate or cause a default under any contract, agreement, instrument, order, judgment or decree to which the Executive is a party or by which he is bound, and (ii) upon the execution and delivery of this Agreement by Alterix, this Agreement and the IP & Non-Competition Agreement shall be a valid and binding obligation of the Executive,
- 6 -
enforceable in accordance with its terms. Alterix hereby represents and warrants to the Executive that (i) the execution, delivery and performance of this Agreement by Alterix does not and will not conflict with, breach, violate or cause a default under any contract, agreement, instrument, order, judgment or decree to which Alterix is a party or by which it is bound and (ii) upon the execution and delivery of this Agreement by the Executive, this Agreement shall be a valid and binding obligation of Alterix, enforceable in accordance with its term.
8. Arbitration. The Parties agree that, except for any rights that any party may have to apply to a court of competent jurisdiction for preliminary injunctive relief, all disputes shall be submitted solely and exclusively to final and binding arbitration before a single, neutral arbitrator before the American Arbitration Association (“AAA”), in accordance with the AAA’s National Rules for the Resolution of Commercial Disputes then in effect. Such arbitration shall proceed in Philadelphia, Pennsylvania, and the Demand for Arbitration shall only be filed with the AAA after the initiating party provides the other party(s) with at least thirty (30) days’ advance notice of the contemplated demand. Judgment upon the award rendered by the arbitrator may be entered in any court of competent jurisdiction. Alterix shall advance the arbitration filing fee, and all other AAA administrative fees. The parties agree that this arbitration provision and any arbitration award rendered hereunder, shall be subject to the Pennsylvania Uniform Arbitration Act, 42 P.S. §§ 7301-7320 and that in all other respects, the arbitrator is bound to apply Massachusetts law in determining any dispute under this Agreement. The parties agree that the scope of discovery, and the discovery methods that may be used in arbitration, shall be the same as provided in the Massachusetts Rules of Civil Procedure. The parties agree further that, notwithstanding any case law, regulation, statute, amendment, ordinance or other source to the contrary, and without any further limitation of any rights, any payment due to Executive under Section 3 and/or 4(d) of this Agreement shall be deemed to be “wages” within the meaning of the Massachusetts Wage Act, Mass. G.L. c. 149 § 148 and that Executive shall be entitled to all relief afforded under the MWA as the MWA exists as of the Effective Date and as it may be subsequently amended if Alterix breaches this Agreement. The foregoing notwithstanding, in the event that either party shall institute litigation in court for purposes of seeking preliminary injunctive relief, then all claims, counterclaims or causes of action that one party may have against the other shall be resolved in such litigation and the foregoing requirement that the parties shall resolve their disputes by mandatory arbitration shall be null and void.
9. Notices. Any notice or communication required or permitted under this Agreement shall be in writing and shall be delivered by hand, delivered by nationally recognized overnight courier service or sent by e-mail. Any such notice or communication shall be deemed to have been given (i) if by hand, when delivered, (ii) if sent by nationally recognized overnight courier service, upon confirmation of delivery by such service, (iii) if sent by e-mail upon confirmation of receipt by the recipient in each case, to the following address, or e-mail address, or to such other address or addresses or e-mail address or addresses as such party may subsequently designate to the other parties by notice given in accordance with this Section 8.
- 7 -
| If to Alterix: | Alterix Inc. | |
| 100 Cummings Center, Suite 463E | ||
| Beverly, MA 01915 | ||
| Attention: Harry McCoy | ||
| Email: [email protected] | ||
| With a copy to: | Holland & Knight LLP | |
| 10 St. James Avenue | ||
| Boston, MA 02116 | ||
| Attn: Thomas L. Barrette, Esq. | ||
| Email: [email protected] | ||
| If to the Executive: | see signature page | |
10. Miscellaneous.
(a) Severability. Whenever possible, each provision of this Agreement will be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable law or rule in any jurisdiction, such invalidity, illegality or unenforceability will not affect any other provision or any other jurisdiction, but this Agreement will be reformed, construed and enforced in such jurisdiction as if such invalid, illegal or unenforceable provision had never been contained herein.
(b) Complete Agreement. This Agreement and the IP & Non-Competition Agreement embody the complete agreement and understanding among the Parties with respect to the terms and conditions of Executive’s employment and supersede and preempt any prior understandings, agreements or representations by or among the Parties, written or oral, which may have related to the terms and conditions of Executive’s employment.
(c) Successors and Assigns. This Agreement is intended to bind and inure to the benefit of and be enforceable by the Executive, Alterix and their respective heirs, successors and permitted assigns. The Executive may not assign his rights or delegate his obligations hereunder. Alterix may assign all or any part of this Agreement to any third party that shall acquire Alterix, or any parent of Alterix, in a merger, acquire a majority of the capital stock of Alterix, or of any parent of Alterix, or purchase all or substantially all of Alterix’s assets (or all or substantially all of the assets of the portion of Alterix’s business that the Executive’s employment was most associated with), provided, however, Alterix shall provide the Executive with prior written notice thereof.
(d) Choice of Law; Jurisdiction. This Agreement shall be governed, construed and enforced in accordance with the laws of the Commonwealth of Massachusetts . All suits, actions or other proceedings seeking to enforce, or otherwise arising in connection with, this Agreement shall be brought in the state or federal courts located in Philadelphia, Pennsylvania . Each of the Parties irrevocably consents to the exclusive jurisdiction of the foregoing courts in such matters and irrevocably waives any objection such Party may otherwise have against such jurisdiction.
- 8 -
(e) Amendment and Waiver. The provisions of this Agreement may be amended or waived only with the express, prior, written consent of Alterix and the Executive, and no course of conduct or failure or delay in enforcing the provisions of this Agreement shall affect the validity, binding effect or enforceability of this Agreement.
(f) Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument representing the agreement of the parties hereto.
(g) Electronic Document. A copy of this Agreement or signature page hereto signed and transmitted by facsimile machine, as an attachment to an e-mail or by other electronic means (collectively an “Electronic Document”), shall be treated as an original document. The signature of any party thereon, for purposes hereof, is to be considered an original signature, and the Electronic Document transmitted is to be considered to have the same binding effect as an original signature on an original document. No party shall raise the use of an Electronic Document or the fact that a signature was transmitted through the use of a facsimile machine, e-mail or other electronic means as a defense to the enforcement of this Agreement or any amendment or other document executed in compliance with this Agreement.
[Signature Page Follows]
- 9 -
IN WITNESS WHEREOF, the Parties hereto have executed this Agreement as of the date first above written.
| ALTERIX INC. | ||
| By: | /s/ Harry G. McCoy | |
| Name: Harry G. McCoy | ||
| Title: Chairman | ||
| EXECUTIVE: |
| /s/ Patrick Mooney |
| Patrick Mooney, M.D. |
| 625 Clinton Ave. Haddonfield, NJ 08033 |
| Address |
Signature Page to Employment Agreement
Exhibit A
Form of IP & Non-Competition Agreement
attached
Exhibit A
Alterix Inc.
PROPRIETARY INFORMATION, ASSIGNMENT OF DEVELOPMENTS
AND NON-COMPETITION AGREEMENT
THIS PROPRIETARY INFORMATION, ASSIGNMENT OF DEVELOPMENTS AND NON-COMPETITION AGREEMENT (“Agreement”), by and between Alterix Inc., a Delaware corporation (“Alterix”), and Patrick Mooney, M.D. (the “Employee”), is effective as of June 11, 2015 (the “Effective Date”).
WHEREAS Alterix and Employee have executed an Employment Agreement as of the Effective Date; and
WHEREAS a condition of the Employment Agreement was the execution of this Agreement.
NOW THEREFORE, in consideration for the execution of the Employment Agreement and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Alterix and the Employee agree as follows:
1. Employment Agreement. The parties agree that the terms of Employee’s engagement with Alterix shall be governed by the Employment Agreement referenced above save for those issues specifically addressed in this Agreement. The Employee acknowledges that this Agreement does not change any of the terms of the Employment Agreement. The Employee further acknowledges that the nature of Alterix’s business is such that protection of its proprietary and confidential information is critical to Alterix’s survival and success.
2. Ownership and Non-Disclosure of Proprietary and Confidential Information.
(a) Proprietary Information of Alterix. The Employee agrees that all information and know-how, whether or not in writing, of a private, secret or confidential nature concerning Alterix’s business or financial affairs (collectively, “Proprietary Information”) is and will be the exclusive property of Alterix. By way of illustration, but not limitation, Proprietary Information includes any and all information regarding clinical trials, discoveries, inventions, products, product improvements, product enhancements, processes, methods, techniques, formulas, compositions, compounds, negotiation strategies and positions, projects, developments, plans (including business and marketing plans), research data, clinical data, financial data (including sales costs, profits, pricing methods), personnel data, computer programs (including software used pursuant to a license agreement), customer, prospect and supplier lists, and contacts at or knowledge of customers or prospective customers of Alterix. The Employee will not disclose any Proprietary Information to any person or entity other than employees of Alterix or use the same for any purposes (other than in the performance of his/her duties as an employee of Alterix) without written approval by an officer of Alterix, either during or after his/her employment with Alterix. While employed by Alterix, the Employee will use the Employee’s best efforts to prevent unauthorized publication or disclosure of any Proprietary Information.
(b) Use of Proprietary Information. The Employee agrees that all files, documents, letters, memoranda, reports, records, data, sketches, drawings, models, laboratory notebooks, program listings, computer equipment or devices, computer programs or other written, photographic, or other tangible or intangible material containing Proprietary Information, whether created by the Employee or others, which will come into his/her custody or possession, will be and are the exclusive property of Alterix to be used by the Employee only in the performance of his/her duties for Alterix and will not be copied or removed from Alterix premises except in the pursuit of the business of Alterix. All such materials or copies thereof and all tangible property of Alterix in the custody or possession of the Employee will be delivered to Alterix, upon the earlier of (i) a request by Alterix or (ii) termination of his/her employment. After such delivery, the Employee will not retain any such materials or copies thereof or any such tangible property.
(c) Proprietary Information of Third Parties. The Employee agrees that his/her obligation not to disclose or to use information and materials of the types set forth in paragraphs 2(a) and 2(b) above, and his/her obligation to return materials and tangible property, set forth in paragraph 2(b) above, also extends to such types of information, materials and tangible property of customers of Alterix or suppliers to Alterix or other third parties who may have disclosed or entrusted the same to Alterix or to the Employee in the course of Alterix’s business.
3. Developments.
(a) Disclosure. The Employee has and will make full and prompt disclosure to Alterix of all discoveries, inventions, improvements, enhancements, processes, methods, techniques, developments, software, and works of authorship, whether patentable or not, which are created, made, conceived or reduced to practice by him/her or under his/her direction or jointly with others during his/her employment by Alterix, whether or not during normal working hours or on the premises of Alterix (all of which are collectively referred to in this Agreement as “Developments”).
(b) Assignment. The Employee agrees to assign and does hereby assign to Alterix (or any person or entity designated by Alterix) all his/her right, title and interest in and to all Developments and all related patents, patent applications, copyrights and copyright applications whether created prior to or after the Effective Date. The Employee understands that, to the extent this Agreement will be construed in accordance with the laws of any state which precludes a requirement in an employee agreement to assign certain classes of inventions made by an employee, this paragraph 3(b) will be interpreted not to apply to any invention which a court rules and/or Alterix agrees falls within such classes. The Employee also hereby waives all claims to moral rights in any Developments.
(c) Cooperation. The Employee agrees to cooperate fully with Alterix, both during and after his employment with Alterix, with respect to the procurement, maintenance and enforcement of copyrights, patents and other intellectual property rights (both in the United States and foreign countries) relating to Developments. The Employee will sign all papers, including, without limitation, copyright applications, patent applications, declarations, oaths, formal assignments, assignments of priority rights, and powers of attorney, which Alterix may
2
deem necessary or desirable in order to protect its rights and interests in any Development. The Employee further agrees that if Alterix is unable, after reasonable effort, to secure the signature of the Employee on any such papers, any executive officer of Alterix will be entitled to execute any such papers as the agent and the attorney-in-fact of the Employee, and the Employee hereby irrevocably designates and appoints each executive officer of Alterix as his/her agent and attorney-in-fact to execute any such papers on his/her behalf, and to take any and all actions as Alterix may deem necessary or desirable in order to protect its rights and interests in any Development, under the conditions described in this sentence.
4. Other Agreements.
The Employee represents that, except as the Employee has disclosed in writing to Alterix, the Employee is not bound by the terms of any agreement with any previous employer or other party to refrain from using or disclosing any trade secret or confidential or proprietary information in the course of his/her employment with Alterix, to refrain from competing, directly or indirectly, with the business of such previous employer or any other party or to refrain from soliciting employees, customers or suppliers of such previous employer or other party. The Employee further represents that his/her performance of all the terms of this Agreement and the performance of his/her duties as an employee of Alterix do not and will not conflict with or breach any agreement with any prior employer or other party to which the Employee is a party (including without limitation any nondisclosure or non-competition agreement), and that the Employee will not disclose to Alterix or induce Alterix to use any confidential or proprietary information or material belonging to any previous employer or others.
5. Obligations to Third Parties.
The Employee acknowledges that Alterix from time to time may have agreements with other persons or with federal, state or local governments, or agencies thereof, which impose obligations or restrictions on Alterix regarding inventions made during the course of work under such agreements or regarding the confidential nature of such work. The Employee agrees to be bound by all such obligations and restrictions which are made known to the Employee and to take all action necessary to discharge the obligations of Alterix under such agreements.
6. Non-Competition and Non-Solicitation.
While the Employee is employed by Alterix and for a period of two years after the termination or cessation of such employment for any reason, the Employee will not directly or indirectly:
(a) Engage or assist others in engaging in any business or enterprise (whether as owner, partner, officer, director, employee, consultant, investor, lender or otherwise, except as the holder of not more than 1% of the outstanding stock of a publicly-held company) that is competitive with Alterix’s business, including but not limited to any business or enterprise that develops, manufactures, markets, licenses, sells or provides any product or service that competes with any product or service developed, manufactured, marketed, licensed, sold or provided, or planned to be developed, manufactured, marketed, licensed, sold or provided, by Alterix while the Employee was employed by Alterix; or
3
(b) Either alone or in association with others, solicit, divert or take away, or attempt to divert or take away, the business or patronage of any of the clients, customers, or business partners of Alterix which were contacted, solicited, or served by Alterix during the 12-month period prior to the termination or cessation of the Employee’s employment with Alterix; or
(c) Either alone or in association with others (i) solicit, induce or attempt to induce, any employee or independent contractor of Alterix to terminate his/her employment or other engagement with Alterix, or (ii) hire, or recruit or attempt to hire, or engage or attempt to engage as an independent contractor, any person who was employed or otherwise engaged by Alterix at any time during the term of the Employee’s employment with Alterix; provided, that this clause (ii) will not apply to the recruitment or hiring or other engagement of any individual whose employment or other engagement with Alterix has been terminated for a period of six months or longer.
(d) If the Employee violates the provisions of any of the preceding paragraphs of this Section 6, the Employee will continue to be bound by the restrictions set forth in such paragraph until a period of two years has expired without any violation of such provisions. Further, the Employee agrees that the duration of the non-competition and non-solicitation obligations described in this Section 6 shall be extended and their expirations tolled upon the filing of any lawsuit challenging the validity or enforceability of this Agreement until the lawsuit is finally resolved and all rights of appeal have expired.
7. Miscellaneous.
(a) Equitable Remedies and Other Remedies. The restrictions contained in this Agreement are necessary for the protection of the business and goodwill of Alterix and are considered by the Employee to be reasonable for such purpose. The Employee agrees that any breach of this Agreement is likely to cause Alterix substantial and irrevocable damage which is difficult to measure. Therefore, in the event of any such breach or threatened breach, the Employee agrees that Alterix, in addition to such other remedies which may be available, will have the right to obtain an injunction from a court restraining such a breach or threatened breach and the right to specific performance of the provisions of this Agreement and the Employee hereby waives the adequacy of a remedy at law as a defense to such relief.
(b) Return of Property. The Employee agrees to immediately deliver to Alterix all Proprietary Information and other information relating to Alterix’s business that is in the Employee’s possession or under the Employee’s control upon termination of the Employee’s employment with Alterix.
(c) Disclosure of this Agreement. The Employee hereby authorizes Alterix to notify others, including but not limited to customers of Alterix and any of the Employee’s future employers or prospective business associates, of the terms and existence of this Agreement and the Employee’s continuing obligations to Alterix hereunder.
4
(d) Successors and Assigns. This Agreement will be binding upon and inure to the benefit of both parties and their respective successors and assigns, including any entity with which, or into which, Alterix may be merged or which may succeed to Alterix’s assets or business, provided, however, that the obligations of the Employee are personal and will not be assigned by him or her. The Employee expressly consents to be bound by the provisions of this Agreement for the benefit of Alterix or any subsidiary or affiliate thereof to whose employ the Employee may be transferred without the necessity that this Agreement be re-signed at the time of such transfer.
(e) Severability. In case any provision of this Agreement will be invalid, illegal or otherwise unenforceable, the validity, legality and enforceability of the remaining provisions will in no way be affected or impaired thereby.
(f) Waivers. No delay or omission by Alterix in exercising any right under this Agreement will operate as a waiver of that or any other right. A waiver or consent given by Alterix on any one occasion is effective only in that instance and will not be construed as a bar to or waiver of any right on any other occasion.
(g) Governing Law; Venue. This Agreement will be governed by and construed in accordance with the laws of the Commonwealth of Massachusetts (without reference to the conflicts of laws provisions thereof). Any action, suit, or other legal proceeding which is commenced to resolve any matter arising under or relating to any provision of this Agreement will be commenced only in a court of the Commonwealth of Massachusetts (or, if appropriate, a federal court located within the Commonwealth of Massachusetts), and Alterix and the Employee each consents to the jurisdiction of such a court. Alterix and the Employee each hereby irrevocably waive any right to a trial by jury in any action, suit or other legal proceeding arising under or relating to any provision of this Agreement.
(h) Entire Agreement; Amendment. This Agreement supersedes all prior agreements, written or oral, between the Employee and Alterix relating to the subject matter of this Agreement. This Agreement may not be modified, changed or discharged in whole or in part, except by an agreement in writing signed by the Employee and Alterix. The Employee agrees that any change or changes in his/her duties, salary or compensation after the signing of this Agreement will not affect the validity or scope of this Agreement.
(i) Interpretation. If any restriction set forth in Section 6 is found by any court of competent jurisdiction to be unenforceable because it extends for too long a period of time or over too great a range of activities or in too broad a geographic area, it will be interpreted to extend only over the maximum period of time, range of activities, or geographic area as to which it may be enforceable
(j) Captions. The captions of the sections of this Agreement are for convenience of reference only and in no way define, limit or affect the scope or substance of any section of this Agreement.
(k) Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument representing the agreement of the parties hereto.
5
(l) Electronic Document. A copy of this Agreement or signature page hereto signed and transmitted by facsimile machine, as an attachment to an e-mail or by other electronic means (collectively an “Electronic Document”), shall be treated as an original document. The signature of any party thereon, for purposes hereof, is to be considered an original signature, and the Electronic Document transmitted is to be considered to have the same binding effect as an original signature on an original document. No party shall raise the use of an Electronic Document or the fact that a signature was transmitted through the use of a facsimile machine, e-mail or other electronic means as a defense to the enforcement of this Agreement or any amendment or other document executed in compliance with this Agreement.
[Signature page follows.]
6
The signature of the Employee below certifies that he has read, understands and agrees with the terms and conditions of this Agreement, effective as of the first day of the Employee’s service to Alterix.
| Alterix Inc. | ||
| By: |
| |
| Name: |
| |
| Title: |
| |
| Address: | ||
| EMPLOYEE | ||
|
| ||
| Name: | Patrick Mooney, M.D. | |
| Address: | ||
Exhibit 10.6
Alterix Inc.
EMPLOYMENT AGREEMENT
THIS EMPLOYMENT AGREEMENT (the “Agreement”), by and between Alterix Inc., a Delaware corporation (“Alterix”), and David R. Staskin, M.D. (the “Executive”) is effective as of September 1, 2015 (the “Effective Date”). Alterix and the Executive are sometimes referred to herein individually as a “Party” and collectively as the “Parties.” In consideration of the the mutual covenants and agreements contained herein and other good and valuable consideration, the receipt and sufficiency of which the Parties hereby acknowledge, the Parties, intending to be legally bound, agree as follows:
1. Employment. Alterix shall employ the Executive, and the Executive shall accept such employment with Alterix, upon the terms and conditions set forth in this Agreement for the period beginning on the Effective Date and ending as provided in Section 4 (the “Employment Period”).
2. Position and Duties.
(a) Location; Title; Duties. During the Employment Period, the Executive shall serve Alterix in the capacity of Chief Strategy Officer, be based in the greater Boston, Massachusetts, have such duties, responsibilities and authorities consistent with responsibilities generally associated with the position of Chief Strategy Officer as may be augmented or revised from time to time by the Chief Executive Officer and generally to carry out the policies and goals of Alterix (collectively, the “Duties”). Executive may have to travel to in order to properly perform the Duties. Executive shall use the Executive’s best efforts to perform the Duties and promote the interests of Alterix, devote such of the Executive’s business time, attention and effort to the performance of the Duties as is reasonably required and refrain from acts or omissions detrimental to Alterix or harmful to its interests. While employed by Alterix, the Executive shall not engage in any business activity that conflicts with the Employee’s duties to Alterix.
(b) Outside Activities. Notwithstanding the foregoing, nothing in this Agreement shall prevent or be deemed to prohibit Executive from spending time on and participating in Executive’s other business pursuits; provided, however, that such participation and time expended in such activities do not materially interfere with the responsibilities as an employee of Alterix in accordance with this Agreement.
3. Compensation. In consideration for the performance of the Duties as well as other obligations under this Agreement, Executive shall receive the following compensation.
(a) Salary. Alterix shall pay the Executive a monthly salary in the amount of not less than $30,000, which will be calculated and paid in accordance with Alterix’s standard practices and policies in effect from time to time, provided that such salary shall not begin to be paid until the month in which Alterix closes an initial public offering of its common stock.

(b) Annual Bonus. Alterix shall pay the Executive an annual bonus in an amount up to be determined by the Board of Directors in good faith. The annual bonus shall be paid no later than February 15 of the year immediately following the year on which the annual bonus is based.
(c) Benefits. Executive shall be entitled to participate in such benefit plans as Alterix provides to its employees generally from time to time in accordance with Alterix policies and subject to the terms and conditions of such plans.
(d) Expenses. Alterix shall reimburse the Executive for all reasonable business travel, business development, customer entertainment, mobile telephone and other expenses incurred by him in the course of performing the Duties. Expenses should be consistent with the policies established by Alterix from time to time with respect to travel and other business expenses, subject to Alterix’s requirements with respect to reporting and documentation of such expenses.
(e) Option. Pursuant to the contemplated 2015 Equity Incentive Plan of Alterix (the “Plan”), a copy of which Executive will be given the opportunity to review, Executive shall receive a fully vested Stock Option (“Option”) providing terms for the purchase of 1,384,616 shares of Common Stock, which number shall be subject to adjustment in the event of a stock split, reverse stock split, stock dividend or similar event, at 100% of the then Fair Market Value, as each of those terms are defined in the Plan. The grant of Stock Option shall be made pursuant to the standard form of Stock Option Grant Notice and Agreement under the Plan. The Stock Option Grant Notice will provide that the option is exercisable in full on the date of grant. Executive will not be permitted to sell any shares issued pursuant to the Option, or any other shares owned by Executive, unless Executive has obtained the prior written approval of the Board of Directors of Alterix and has complied with all other applicable policies of Alterix, including the Alterix Insider Trading Policy as in effect at the applicable time and all applicable laws and regulations. Once issued and executed the Grant Notice will supersede and replace this paragraph, which will become null and void.
4. Term.
(a) Employment Period. The Employment Period shall continue from the Effective Date until the first to occur of the following events:
(i) the Executive’s death or “Permanent Disability” (defined as the expiration of a continuous period of 90 days during which the Executive is unable to perform the Duties due to physical or mental incapacity);
(ii) the Agreement is terminated for Cause (as defined below) by Alterix;
(iii) the Agreement is terminated for Good Reason (as defined below) by Executive at any time upon thirty (30) days’ prior written notice to Alterix;

- 2 -
(iv) the Agreement is terminated by Alterix at any time without Cause upon thirty (30) days’ prior written notice to the Executive; or
(v) the Agreement is terminated by the Executive without Good Reason at any time upon thirty (30) days’ prior written notice to Alterix.
(b) Definitions. For purposes of this Agreement:
(i) “Cause” shall mean (1) the failure or refusal by the Executive to perform his Duties (other than any such failure resulting from the Executive’s incapacity due to illness, physical or mental incapacity) which, if capable of cure, has not been cured within fifteen (15) business days after written notice of such breach delivered; (2) the Executive’s breach of this Agreement or any other agreement with or policy of Alterix applicable to him that has been communicated to him in writing which, if capable of cure, has not been cured within five (5) business days after written notice of such breach; (3) the Executive’s willful negligence, misconduct or gross negligence with respect to the performance of the Duties; (4) the conviction of the Executive, or plea of guilty or nolo contendere, with respect to any felony, any act of fraud, theft, or financial dishonesty with respect to Alterix or any of its affiliates, customers or business partners, or any other crime involving fraud, theft, or financial dishonesty; or (5) alcohol or substance abuse by the Executive.
(ii) “Good Reason” shall exist upon (1) mutual written agreement by the Executive and Alterix; (2) the relocation of Executive’s office such that the Executive’s daily commute is increased by at least 75 miles round-trip without the written consent of the Executive; (3) material reduction of the Executive’s annual base salary without the prior consent of the Executive unless associated with either a company wide salary reductions or repeated and material failure of the Executive to complete the Duties; (4) material diminution of the Executive’s duties, title, position, or responsibilities without good reason; (5) material breach by Alterix of any of the terms of this Agreement, which breach is not substantially cured or rectified, or in process of being substantially cured or rectified, if such breach is curable or rectifiable, within five or (5) business days after Executive gives written notice of such breach to Alterix.
(c) Disability. In the event of any dispute regarding the existence of the Executive’s Permanent Disability hereunder, the matter will be resolved by an independent physician qualified to practice medicine in Boston, Massachusetts with greater than fifteen (15) years practice experience who is on the staff of a university hospital and has no prior knowledge of the Executive and no prior engagement by or other relationship by Alterix, which physician shall be selected by Alterix. For this purpose, the Executive will submit to all appropriate medical examinations.
(d) Payments Upon Termination of Employment.
(i) Upon the termination of the Executive’s employment with Alterix the Executive shall be entitled to receive all earned or accrued but unpaid salary, accrued

- 3 -
vacation and reimbursement of approved business expenses as well as all amounts or benefits to which the Executive is entitled under any applicable the employee benefit plan in which the Executive was a participant during his employment with Alterix, in accordance with the terms of that plan.
(ii) In addition to the foregoing, if at any time prior to the first anniversary of the Effective Date the Executive’s employment with Alterix is terminated by Alterix without Cause or by the Executive with Good Reason, the Executive shall also be entitled, upon the occurrence of each of: (1) the execution of a document acknowledging Executive’s obligations under this Agreement, (2) the return of all property of Alterix and (3) the execution of a Release by Executive substantially similar to that attached as Exhibit A and the occurrence of the Release Effective Date, to continue to receive pro rata payments of the Executive’s then current salary, in accord with Alterix’s current payroll practices, for the period beginning with the termination date and ending 60 days after the Termination Date (the “Continuation Period”).
(iii) Alterix will withhold all federal, state, local and other taxes and amounts that it determines are required by law on all amounts paid pursuant to this Section.
5. Proprietary Information. Assignment of Developments and Non-Competition Agreement. As a condition of the Executive’s employment with Alterix and a condition of any and all of Alterix’s obligations under this Agreement and the Purchase Agreement, the Executive shall enter into the accompanying Proprietary Information, Assignment of Developments and Non-Competition Agreement, a form of which is attached as Exhibit B (the “IP & Non-Competition Agreement”) and comply with his obligations thereunder. Notwithstanding any other provision of this Agreement, if the Executive breaches the IP & Non-Competition Agreement, Alterix shall be relieved of any and all obligations under this Agreement.
6. Section 409A. Notwithstanding any other provision herein to the contrary, to the extent that any payment to be made to the Executive, whether pursuant to this Agreement or otherwise, is determined to constitute “nonqualified deferred compensation” within the meaning of and subject to Section 409A of the Internal Revenue Code of 1986, as amended (“Section 409A”) such payment shall not be made prior to the date that is the earlier of (i) six months and one day after the Executive’s separation from service with Alterix and any parent, affiliate or subsidiary of Alterix and (ii) the Executive’s death. The terms of this Section 6 shall apply only if the Executive is a “specified employee” (within the meaning of Section 409A) on the date of such separation from service, and shall only apply to the extent the delay of such payment is necessary to prevent such payment from being subject to interest, penalties and/or additional tax imposed pursuant to Section 409A.
7. Executive and Alterix Representations. The Executive hereby represents and warrants to Alterix that (i) the execution, delivery and performance of this Agreement by the Executive does not and will not conflict with, breach, violate or cause a default under any contract, agreement, instrument, order, judgment or decree to which the Executive is a party or by which he is bound, and (ii) upon the execution and delivery of this Agreement by Alterix, this Agreement and the IP & Non-Competition Agreement shall be a valid and

- 4 -
binding obligation of the Executive, enforceable in accordance with its terms. Alterix hereby represents and warrants to the Executive that (i) the execution, delivery and performance of this Agreement by Alterix does not and will not conflict with, breach, violate or cause a default under any contract, agreement, instrument, order, judgment or decree to which Alterix is a party or by which it is bound and (ii) upon the execution and delivery of this Agreement by the Executive, this Agreement shall be a valid and binding obligation of Alterix, enforceable in accordance with its terms.
8. Arbitration. The Parties agree that, except for any rights that any party may have to apply to a court of competent jurisdiction for injunctive relief, all disputes shall be submitted solely and exclusively to final and binding arbitration before a single, neutral arbitrator before the American Arbitration Association (“AAA”), in accordance with the AAA’s National Rules for the Resolution of Commercial Disputes then in effect except as limited by this section. Such arbitration shall proceed in Boston, Massachusetts, and the Demand for Arbitration shall only be filed with the AAA after the initiating party provides the other party(s) with at least ten (10) business days’ advance notice of the contemplated demand and, at either party’s request, the parties have a reasonable period of time, not to exceed sixty (60) days, to mediate the dispute under the auspices of a recognized mediation service or by agreement of the parties. Judgment upon the award rendered by the arbitrator may be entered in any court of competent jurisdiction. Alterix shall advance the arbitration filing fee and all other AAA administrative fees. The parties agree that this arbitration provision and any arbitration award rendered hereunder, shall be subject to the Massachusetts Uniform Arbitration Act, and that, in all other respects, the arbitrator is bound to apply Massachusetts law in determining any dispute under this Agreement. The parties agree that the arbitration shall be conducted in as expeditious and efficient manner as is practical and that the parties shall not engage in any form of discovery as is provided in the Massachusetts Rules of Civil Procedure or any other form of protracted pre-arbitration procedure. The parties agree further that, notwithstanding any case law, regulation, statute, amendment, ordinance or other source to the contrary, and without any further limitation of any rights, any payment due to Executive under Section 3 and/or 4(d) of this Agreement shall be deemed to be “wages” within the meaning of the Massachusetts Wage Act, Mass. G.L. c. 149 § 148 and that Executive shall be entitled to all relief afforded under the MWA as the MWA exists as of the Effective Date and as it may be subsequently amended if Alterix breaches this Agreement.
9. Notices. Any notice or communication required or permitted under this Agreement shall be in writing and shall be delivered by hand, delivered by nationally recognized overnight courier service or sent by e-mail. Any such notice or communication shall be deemed to have been given (i) if by hand, when delivered, (ii) if sent by nationally recognized overnight courier service, upon confirmation of delivery by such service, (iii) if sent by e-mail upon confirmation of receipt by the recipient in each case, to the following address, or e-mail address, or to such other address or addresses or e-mail address or addresses as such party may subsequently designate to the other parties by notice given in accordance with this Section 9.

- 5 -
| If to Alterix: | Alterix Inc. | |
| 100 Cummings Center, Suite 463E | ||
| Beverly, MA 01915 | ||
| Attention: Patrick T. Mooney, M.D. | ||
| Email: [email protected] | ||
| With a copy to: | Holland & Knight LLP | |
| 10 St. James Avenue | ||
| Boston, MA 02116 | ||
| Attn: Thomas L. Barrette, Esq. | ||
| Email: [email protected] | ||
| If to the Executive: | see signature page | |
10. Miscellaneous.
(a) Severability. Whenever possible, each provision of this Agreement will be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable law or rule in any jurisdiction, such invalidity, illegality or unenforceability will not affect any other provision or any other jurisdiction, but this Agreement will be reformed, construed and enforced in such jurisdiction as if such invalid, illegal or unenforceable provision had never been contained herein.
(b) Complete Agreement. This Agreement and the IP & Non-Competition Agreement embody the complete agreement and understanding among the Parties with respect to the terms and conditions of Executive’s employment and supersede and preempt any prior understandings, agreements or representations by or among the Parties, written or oral, which may have related to the terms and conditions of Executive’s employment.
(c) Successors and Assigns. This Agreement is intended to bind and inure to the benefit of and be enforceable by the Executive, Alterix and their respective heirs, successors and permitted assigns. The Executive may not assign his rights or delegate his obligations hereunder. Alterix may assign all or any part of this Agreement to any third party that shall acquire Alterix, or any parent of Alterix, in a merger, acquire a majority of the capital stock of Alterix, or of any parent of Alterix, or purchase all or substantially all of Alterix’s assets (or all or substantially all of the assets of the portion of Alterix’s business that the Executive’s employment was most associated with), provided, however, Alterix shall provide the Executive with prior written notice thereof.
(d) Choice of Law; Jurisdiction. This Agreement shall be governed, construed and enforced in accordance with the laws of the Commonwealth of Massachusetts. All suits, actions or other proceedings seeking to enforce, or otherwise arising in connection with, this Agreement shall be brought in the state or federal courts located in Boston, Massachusetts. Each of the Parties irrevocably consents to the exclusive jurisdiction of the foregoing courts in such matters and irrevocably waives any objection such Party may otherwise have against such jurisdiction. This Section 10(d) shall be construed to be consistent with and to carry out the purposes of Section 8 above regarding the resolution of any disputes through arbitration.

- 6 -
(e) Amendment and Waiver. The provisions of this Agreement may be amended or waived only with the express, prior, written consent of Alterix and the Executive, and no course of conduct or failure or delay in enforcing the provisions of this Agreement shall affect the validity, binding effect or enforceability of this Agreement.
(f) Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument representing the agreement of the parties hereto.
(g) Electronic Document. A copy of this Agreement or signature page hereto signed and transmitted by facsimile machine, as an attachment to an e-mail or by other electronic means (collectively an “Electronic Document”), shall be treated as an original document. The signature of any party thereon, for purposes hereof, is to be considered an original signature, and the Electronic Document transmitted is to be considered to have the same binding effect as an original signature on an original document. No party shall raise the use of an Electronic Document or the fact that a signature was transmitted through the use of a facsimile machine, e-mail or other electronic means as a defense to the enforcement of this Agreement or any amendment or other document executed in compliance with this Agreement.
[Signature Page Follows]

- 7 -
IN WITNESS WHEREOF, the Parties hereto have executed this Agreement as of the date first above written.
| ALTERIX INC. | ||||
| By: | /s/ Patrick Mooney | |||
| Name: | Patrick Mooney | |||
| Title: | CEO | |||
| EXECUTIVE: |
| /s/ David R. Staskin, M.D. |
| David R. Staskin, M.D. |
| Unit 34B |
| 85 East India Row |
| Boston, MA 02110
|
Signature Page to Employment Agreement
Exhibit A
Form of Release
1. For and in consideration of the severance payments and other benefits provided in the Employment Agreement, effective as of the Effective Date (the “Employment Agreement”), by and between Alterix Inc., a Delaware corporation (“Alterix”) and me, and other good and valuable consideration, I, for and on behalf of myself and my heirs, administrators, executors, and assigns, effective the date hereof, do hereby fully and forever release, remise and discharge Alterix, any predecessor or successor entity, its successors and assigns, and the direct and indirect parents, subsidiaries, affiliates of Alterix, together with their respective officers, directors, partners, shareholders, members, managers, employees and agents (collectively, the “Group”), from any and all Claims (as defined below) which I had, may have had, or now have against Alterix and/or any other member of the Group, for or by reason of any matter, cause or thing whatsoever, including any claim arising out of or attributable to my employment or the termination of my employment with Alterix, including but not limited to Claims of breach of contract, wrongful termination, unjust dismissal, defamation, libel or slander, or under any federal, state or local law dealing with discrimination based on age, race, sex, national origin, handicap, religion, disability or sexual preference. This release of Claims includes, but is not limited to, all Claims arising under Title VII of the Civil Rights Act, the Americans with Disabilities Act, the Civil Rights Act of 1991, the Family Medical Leave Act, the Equal Pay Act, Massachusetts General Laws, Chapter 151B, the Massachusetts Wage Act, and any and all applicable federal statutes and regulations, and any and all applicable statutes, regulations and common law of the Commonwealth of Massachusetts, and any and all other federal, state, and local laws, statutes, regulations, and common law, and any other purported restriction on an employer’s right to terminate the employment of employees. As used in this Release, the term “Claims” shall include all claims, covenants, warranties, promises, undertakings, actions, suits, causes of action, obligations, debts, attorneys’ fees, accounts, judgments, losses and liabilities, of whatsoever kind or nature, in law, equity or otherwise.
2. I specifically release all Claims under the Age Discrimination in Employment Act (the “ADEA”) relating to my employment and its termination.
3. I represent that I have not filed or permitted to be filed against the Group, individually or collectively, any lawsuits and I covenant and agree that I will not do so at any time hereafter with respect to the subject matter of this Release and Claims released pursuant to this Release (including, without limitation, any Claims relating to the termination of my employment), except as may be necessary to enforce this Release, to obtain benefits described in or granted under this Release, or to seek a determination of the validity of the waiver of my rights under the ADEA. Except as otherwise provided in the preceding sentence, I will not voluntarily participate in any judicial proceeding of any nature or description against any member of the Group that in any way involves the allegations and facts that I could have raised against any member of the Group as of the date hereof.
4. I am specifically agreeing to the terms of this Release because Alterix has agreed to pay to me money and other benefits to which I am not otherwise entitled under Alterix’s policies, and has provided such other good and valuable consideration as specified herein. Alterix has

Exhibit A – Page 1
agreed to provide this money and other benefits because of my agreement to accept it in full settlement of all possible Claims I might have or ever had, and because of my execution of this Release.
6. Upon termination of my employment, I agree to return to Alterix all Alterix property, including without limitation, proprietary information, reports, files, memoranda, records, computer hardware, software, credit cards, door and file keys, computer access codes or disks and instructional manuals, and other physical or personal property which I received or prepared or helped prepare in connection with my employment with Alterix, and that I will not retain any copies, duplicates, reproductions or excerpts thereof.
7. I acknowledge that I have read this Release in its entirety, fully understand its meaning and am executing this Release voluntarily and of my own free will with full knowledge of its significance. I acknowledge and warrant that I have had the opportunity to consider for twenty-one (21) days the terms and provisions of this Release and that I have been advised by Alterix to consult with an attorney prior to executing this Release. I shall have the right to revoke this Release for a period of seven (7) days following my execution of this Release, by giving written notice of such revocation to Alterix. This Release shall not become effective until the eighth day following my execution of it (the “Release Effective Date”).
8. I agree to maintain the confidentiality of this Release, and to refrain from disclosing or making reference to its terms except as required by law or with my accountant or attorney.
9. I agree that I shall not make, or cause to be made, any statement or communicate any information (whether oral or written) that disparages or reflects negatively on Alterix or any member of the Group.
10. Alterix shall be entitled to have the provisions of this Release specifically enforced through injunctive relief, without having to prove the inadequacy of the available remedies at law, and without being required to post bond or security, it being acknowledged and agreed that such breach will cause irreparable injury to Alterix and that money damages will not provide an adequate remedy to Alterix. In addition, in the event that I breach any of the provisions of this Release (and in addition to any other legal or equitable remedies Alterix may have), Alterix shall be entitled to cease making any of the payments or providing any of the benefits referred to in paragraph 1 above, recover any payments referred to in paragraph 1 previously paid to me, and recover as permitted by applicable law the reasonable costs and attorneys’ fees incurred in seeking relief for any such breach. Moreover, I understand and agree that if I breach any provisions of this Release, in addition to any other legal or equitable remedy Alterix may have, I shall reimburse Alterix for all its reasonable attorneys’ fees and costs incurred by it arising out of any such breach. The remedies set forth in this paragraph shall not apply to any challenge to the validity of the waiver and release of my rights under the ADEA. In the event I challenge the validity of the waiver and release of my rights under the ADEA, then Alterix’s right to attorneys’ fees and costs shall be governed by the provisions of the ADEA, so that Alterix may recover such fees and costs if the lawsuit is brought by me in bad faith. Any such action permitted to Alterix by this paragraph, however, shall not affect or impair any of my obligations under this Release, including without limitation, the release of claims in paragraph 1 hereof. I further agree that nothing herein shall preclude Alterix from recovering attorneys’ fees, costs or any other remedies specifically authorized under applicable law.

Exhibit A – Page 2
11. In the event that any one or more of the provisions of this Release is held to be invalid, illegal or unenforceable, the validity, legality and enforceability of the remaining provisions will not in any way be affected or impaired thereby. Moreover, if any one or more of the provisions contained in this Release is held to be excessively broad as to duration, scope, activity or subject, such provisions will be construed by limiting and reducing them so as to be enforceable to the maximum extent compatible with applicable law.
12. Nothing herein shall be deemed to constitute an admission of wrongdoing by Alterix or any member of the Group. Neither this Release nor any of its terms shall be used as an admission or introduced as evidence as to any issue of law or fact in any proceeding, suit or action, other than an action to enforce this Release.
13. The terms of this Release and all rights and obligations of the parties thereto, including its enforcement, shall be interpreted and governed by the laws of the Commonwealth of Massachusetts, without regard to the choice of law provisions of Massachusetts law, to the extent such provisions require the application of the laws of any other jurisdiction.

|
|
|
| [Executive] |
| Date Sep 1st, 2015 |
Exhibit A – Page 3
Exhibit B
Form of IP & Non-Competition Agreement
attached

Exhibit B
Exhibit 10.7
Alterix Inc.
PROPRIETARY INFORMATION, ASSIGNMENT OF DEVELOPMENTS
AND NON-COMPETITION AGREEMENT
THIS PROPRIETARY INFORMATION, ASSIGNMENT OF DEVELOPMENTS AND NON-COMPETITION AGREEMENT (“Agreement”), by and between Alterix Inc., a Delaware corporation (“Alterix”), and Patrick Mooney, M.D. (the “Employee”), is effective as of June 11, 2015 (the “Effective Date”).
WHEREAS Alterix and Employee have executed an Employment Agreement as of the Effective Date; and
WHEREAS a condition of the Employment Agreement was the execution of this Agreement.
NOW THEREFORE, in consideration for the execution of the Employment Agreement and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Alterix and the Employee agree as follows:
1. Employment Agreement. The parties agree that the terms of Employee’s engagement with Alterix shall be governed by the Employment Agreement referenced above save for those issues specifically addressed in this Agreement. The Employee acknowledges that this Agreement does not change any of the terms of the Employment Agreement. The Employee further acknowledges that the nature of Alterix’s business is such that protection of its proprietary and confidential information is critical to Alterix’s survival and success.
2. Ownership and Non-Disclosure of Proprietary and Confidential Information.
(a) Proprietary Information of Alterix. The Employee agrees that all information and know-how, whether or not in writing, of a private, secret or confidential nature concerning Alterix’s business or financial affairs (collectively, “Proprietary Information”) is and will be the exclusive property of Alterix. By way of illustration, but not limitation, Proprietary Information includes any and all information regarding clinical trials, discoveries, inventions, products, product improvements, product enhancements, processes, methods, techniques, formulas, compositions, compounds, negotiation strategies and positions, projects, developments, plans (including business and marketing plans), research data, clinical data, financial data (including sales costs, profits, pricing methods), personnel data, computer programs (including software used pursuant to a license agreement), customer, prospect and supplier lists, and contacts at or knowledge of customers or prospective customers of Alterix. The Employee will not disclose any Proprietary Information to any person or entity other than employees of Alterix or use the same for any purposes (other than in the performance of his/her duties as an employee of Alterix) without written approval by an officer of Alterix, either during or after his/her employment with Alterix. While employed by Alterix, the Employee will use the Employee’s best efforts to prevent unauthorized publication or disclosure of any Proprietary Information.
(b) Use of Proprietary Information. The Employee agrees that all files, documents, letters, memoranda, reports, records, data, sketches, drawings, models, laboratory notebooks, program listings, computer equipment or devices, computer programs or other written, photographic, or other tangible or intangible material containing Proprietary Information, whether created by the Employee or others, which will come into his/her custody or possession, will be and are the exclusive property of Alterix to be used by the Employee only in the performance of his/her duties for Alterix and will not be copied or removed from Alterix premises except in the pursuit of the business of Alterix. All such materials or copies thereof and all tangible property of Alterix in the custody or possession of the Employee will be delivered to Alterix, upon the earlier of (i) a request by Alterix or (ii) termination of his/her employment. After such delivery, the Employee will not retain any such materials or copies thereof or any such tangible property.
(c) Proprietary Information of Third Parties. The Employee agrees that his/her obligation not to disclose or to use information and materials of the types set forth in paragraphs 2(a) and 2(b) above, and his/her obligation to return materials and tangible property, set forth in paragraph 2(b) above, also extends to such types of information, materials and tangible property of customers of Alterix or suppliers to Alterix or other third parties who may have disclosed or entrusted the same to Alterix or to the Employee in the course of Alterix’s business.
3. Developments.
(a) Disclosure. The Employee has and will make full and prompt disclosure to Alterix of all discoveries, inventions, improvements, enhancements, processes, methods, techniques, developments, software, and works of authorship, whether patentable or not, which are created, made, conceived or reduced to practice by him/her or under his/her direction or jointly with others during his/her employment by Alterix, whether or not during normal working hours or on the premises of Alterix (all of which are collectively referred to in this Agreement as “Developments”).
(b) Assignment. The Employee agrees to assign and does hereby assign to Alterix (or any person or entity designated by Alterix) all his/her right, title and interest in and to all Developments and all related patents, patent applications, copyrights and copyright applications whether created prior to or after the Effective Date. The Employee understands that, to the extent this Agreement will be construed in accordance with the laws of any state which precludes a requirement in an employee agreement to assign certain classes of inventions made by an employee, this paragraph 3(b) will be interpreted not to apply to any invention which a court rules and/or Alterix agrees falls within such classes. The Employee also hereby waives all claims to moral rights in any Developments.
(c) Cooperation. The Employee agrees to cooperate fully with Alterix, both during and after his employment with Alterix, with respect to the procurement, maintenance and enforcement of copyrights, patents and other intellectual property rights (both in the United States and foreign countries) relating to Developments. The Employee will sign all papers, including, without limitation, copyright applications, patent applications, declarations, oaths, formal assignments, assignments of priority rights, and powers of attorney, which Alterix may
2
deem necessary or desirable in order to protect its rights and interests in any Development. The Employee further agrees that if Alterix is unable, after reasonable effort, to secure the signature of the Employee on any such papers, any executive officer of Alterix will be entitled to execute any such papers as the agent and the attorney-in-fact of the Employee, and the Employee hereby irrevocably designates and appoints each executive officer of Alterix as his/her agent and attorney-in-fact to execute any such papers on his/her behalf, and to take any and all actions as Alterix may deem necessary or desirable in order to protect its rights and interests in any Development, under the conditions described in this sentence.
4. Other Agreements.
The Employee represents that, except as the Employee has disclosed in writing to Alterix, the Employee is not bound by the terms of any agreement with any previous employer or other party to refrain from using or disclosing any trade secret or confidential or proprietary information in the course of his/her employment with Alterix, to refrain from competing, directly or indirectly, with the business of such previous employer or any other party or to refrain from soliciting employees, customers or suppliers of such previous employer or other party. The Employee further represents that his/her performance of all the terms of this Agreement and the performance of his/her duties as an employee of Alterix do not and will not conflict with or breach any agreement with any prior employer or other party to which the Employee is a party (including without limitation any nondisclosure or non-competition agreement), and that the Employee will not disclose to Alterix or induce Alterix to use any confidential or proprietary information or material belonging to any previous employer or others.
5. Obligations to Third Parties.
The Employee acknowledges that Alterix from time to time may have agreements with other persons or with federal, state or local governments, or agencies thereof, which impose obligations or restrictions on Alterix regarding inventions made during the course of work under such agreements or regarding the confidential nature of such work. The Employee agrees to be bound by all such obligations and restrictions which are made known to the Employee and to take all action necessary to discharge the obligations of Alterix under such agreements.
6. Non-Competition and Non-Solicitation.
While the Employee is employed by Alterix and for a period of two years after the termination or cessation of such employment for any reason, the Employee will not directly or indirectly:
(a) Engage or assist others in engaging in any business or enterprise (whether as owner, partner, officer, director, employee, consultant, investor, lender or otherwise, except as the holder of not more than 1% of the outstanding stock of a publicly-held company) that is competitive with Alterix’s business, including but not limited to any business or enterprise that develops, manufactures, markets, licenses, sells or provides any product or service that competes with any product or service developed, manufactured, marketed, licensed, sold or provided, or planned to be developed, manufactured, marketed, licensed, sold or provided, by Alterix while the Employee was employed by Alterix; or
3
(b) Either alone or in association with others, solicit, divert or take away, or attempt to divert or take away, the business or patronage of any of the clients, customers, or business partners of Alterix which were contacted, solicited, or served by Alterix during the 12-month period prior to the termination or cessation of the Employee’s employment with Alterix; or
(c) Either alone or in association with others (i) solicit, induce or attempt to induce, any employee or independent contractor of Alterix to terminate his/her employment or other engagement with Alterix, or (ii) hire, or recruit or attempt to hire, or engage or attempt to engage as an independent contractor, any person who was employed or otherwise engaged by Alterix at any time during the term of the Employee’s employment with Alterix; provided, that this clause (ii) will not apply to the recruitment or hiring or other engagement of any individual whose employment or other engagement with Alterix has been terminated for a period of six months or longer.
(d) If the Employee violates the provisions of any of the preceding paragraphs of this Section 6, the Employee will continue to be bound by the restrictions set forth in such paragraph until a period of two years has expired without any violation of such provisions. Further, the Employee agrees that the duration of the non-competition and non-solicitation obligations described in this Section 6 shall be extended and their expirations tolled upon the filing of any lawsuit challenging the validity or enforceability of this Agreement until the lawsuit is finally resolved and all rights of appeal have expired.
7. Miscellaneous.
(a) Equitable Remedies and Other Remedies. The restrictions contained in this Agreement are necessary for the protection of the business and goodwill of Alterix and are considered by the Employee to be reasonable for such purpose. The Employee agrees that any breach of this Agreement is likely to cause Alterix substantial and irrevocable damage which is difficult to measure. Therefore, in the event of any such breach or threatened breach, the Employee agrees that Alterix, in addition to such other remedies which may be available, will have the right to obtain an injunction from a court restraining such a breach or threatened breach and the right to specific performance of the provisions of this Agreement and the Employee hereby waives the adequacy of a remedy at law as a defense to such relief.
(b) Return of Property. The Employee agrees to immediately deliver to Alterix all Proprietary Information and other information relating to Alterix’s business that is in the Employee’s possession or under the Employee’s control upon termination of the Employee’s employment with Alterix.
(c) Disclosure of this Agreement. The Employee hereby authorizes Alterix to notify others, including but not limited to customers of Alterix and any of the Employee’s future employers or prospective business associates, of the terms and existence of this Agreement and the Employee’s continuing obligations to Alterix hereunder.
4
(d) Successors and Assigns. This Agreement will be binding upon and inure to the benefit of both parties and their respective successors and assigns, including any entity with which, or into which, Alterix may be merged or which may succeed to Alterix’s assets or business, provided, however, that the obligations of the Employee are personal and will not be assigned by him or her. The Employee expressly consents to be bound by the provisions of this Agreement for the benefit of Alterix or any subsidiary or affiliate thereof to whose employ the Employee may be transferred without the necessity that this Agreement be re-signed at the time of such transfer.
(e) Severability. In case any provision of this Agreement will be invalid, illegal or otherwise unenforceable, the validity, legality and enforceability of the remaining provisions will in no way be affected or impaired thereby.
(f) Waivers. No delay or omission by Alterix in exercising any right under this Agreement will operate as a waiver of that or any other right. A waiver or consent given by Alterix on any one occasion is effective only in that instance and will not be construed as a bar to or waiver of any right on any other occasion.
(g) Governing Law; Venue. This Agreement will be governed by and construed in accordance with the laws of the Commonwealth of Massachusetts (without reference to the conflicts of laws provisions thereof). Any action, suit, or other legal proceeding which is commenced to resolve any matter arising under or relating to any provision of this Agreement will be commenced only in a court of the Commonwealth of Massachusetts (or, if appropriate, a federal court located within the Commonwealth of Massachusetts), and Alterix and the Employee each consents to the jurisdiction of such a court. Alterix and the Employee each hereby irrevocably waive any right to a trial by jury in any action, suit or other legal proceeding arising under or relating to any provision of this Agreement.
(h) Entire Agreement; Amendment. This Agreement supersedes all prior agreements, written or oral, between the Employee and Alterix relating to the subject matter of this Agreement. This Agreement may not be modified, changed or discharged in whole or in part, except by an agreement in writing signed by the Employee and Alterix. The Employee agrees that any change or changes in his/her duties, salary or compensation after the signing of this Agreement will not affect the validity or scope of this Agreement.
(i) Interpretation. If any restriction set forth in Section 6 is found by any court of competent jurisdiction to be unenforceable because it extends for too long a period of time or over too great a range of activities or in too broad a geographic area, it will be interpreted to extend only over the maximum period of time, range of activities, or geographic area as to which it may be enforceable
(j) Captions. The captions of the sections of this Agreement are for convenience of reference only and in no way define, limit or affect the scope or substance of any section of this Agreement.
(k) Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument representing the agreement of the parties hereto.
5
(l) Electronic Document. A copy of this Agreement or signature page hereto signed and transmitted by facsimile machine, as an attachment to an e-mail or by other electronic means (collectively an “Electronic Document”), shall be treated as an original document. The signature of any party thereon, for purposes hereof, is to be considered an original signature, and the Electronic Document transmitted is to be considered to have the same binding effect as an original signature on an original document. No party shall raise the use of an Electronic Document or the fact that a signature was transmitted through the use of a facsimile machine, e-mail or other electronic means as a defense to the enforcement of this Agreement or any amendment or other document executed in compliance with this Agreement.
[Signature page follows.]
6
The signature of the Employee below certifies that he has read, understands and agrees with the terms and conditions of this Agreement, effective as of the first day of the Employee’s service to Alterix.
| Alterix Inc. | ||
| By: | /s/ Harry G. McCoy | |
| Name: | Harry G. McCoy | |
| Title: | Chairman | |
| Address: Suite 463E | ||
| Beverly, MA 01915 | ||
| EMPLOYEE | ||
| /s/ Patrick Mooney | ||
| Name: | Patrick Mooney, M.D. | |
| Address: | 625 Clinton Ave. | |
| Haddonfield, NJ 08033 | ||
Exhibit 10.9
LEASE
STREAMWOOD
ASSOCIATES/HADDONFIELD, LLC
T/A
30 WASHINGTON AVENUE
(“LANDLORD”)
AND
ALTERIX, INC.
(“TENANT”)
TABLE OF CONTENTS
| PAGE | ||||||
| 1. |
PREMISES |
1 | ||||
| 2. |
TERM |
1 | ||||
| 3. |
RENT |
1 | ||||
| 4. |
PROPORTIONATE AREA |
2 | ||||
| 5. |
LATE PAYMENT |
2 | ||||
| 6. |
USE |
2 | ||||
| 7. |
ALTERATIONS AND TRADE FIXTURES |
3 | ||||
| 8. |
MECHANIC’S LIENS |
3 | ||||
| 9. |
CONDITION OF PREMISES |
4 | ||||
| 10. |
BUILDING SERVICES |
4 | ||||
| 11. |
ASSIGNMENT AND SUBLETTING |
4 | ||||
| 12. |
ACCESS TO PREMISES |
5 | ||||
| 13. |
REPAIRS |
5 | ||||
| 14. |
INDEMNIFICATION AND LIABILITY INSURANCE |
6 | ||||
| 15. |
NEGATIVE COVENANTS OF TENANT |
7 | ||||
| 16. |
FIRE OR OTHER CASUALTY |
7 | ||||
| 17. |
SUBORDINATION AND NON-DISTURBANCE |
8 | ||||
| 18. |
[INTENTIONALLY OMITTED] |
9 | ||||
| 19. |
CONDEMNATION |
9 | ||||
| 20. |
ESTOPPEL CERTIFICATE |
9 | ||||
| 21. |
DEFAULT |
9 | ||||
| 22. |
REMEDIES |
10 | ||||
| 23. |
REQUIREMENT OF STRICT PERFORMANCE |
11 | ||||
| 24. |
LANDLORD’S OBLIGATIONS |
11 | ||||
| 25. |
SUCCESSORS |
12 | ||||
| 26. |
GOVERNING LAW |
12 | ||||
| 27. |
SEVERABILITY |
12 | ||||
| 28. |
CAPTIONS |
12 | ||||
| 29. |
GENDER |
12 | ||||
i
| 30. |
NOTICES |
12 | ||||
| 31. |
SIGNS |
13 | ||||
| 32. |
ENTIRE AGREEMENT |
13 | ||||
| 33. |
ADDITIONAL SCHEDULES |
13 | ||||
| 34. |
MISCELLANEOUS |
14 | ||||
| 35. |
QUIET ENJOYMENT |
14 | ||||
| 36. |
ENVIRONMENTAL |
14 | ||||
ii
LEASE
This Lease Agreement, made this day of September 2015, by and between STREAMWOOD ASSOCIATES/HADDONFIELD, LLC T/A 30 WASHINGTON AVENUE (C/O The Streamwood Company, 30 Washington Avenue Suite A-1, Haddonfield, New Jersey 08033) hereinafter called the “Landlord” and ALTERIX, INC. presently doing business at 625 Clinton Avenue, Haddonfield, NJ 08033 hereinafter called the “Tenant”.
WITNESSETH AS FOLLOWS;
| 1. | PREMISES |
Landlord, for and in consideration of the rent to be paid and the covenants and agreements to be performed by Tenant, as hereinafter set forth, does hereby lease, demise let unto Tenant that space (hereinafter called the “Premises”) situate on the first floor, being Suite F-1 and F-2 as designated on the first floor plan of Roominders consisting of 1,287 usable square feet plus 222 square feet of common area for a total of 1,509 rentable square feet and situated at 30 Washington Avenue, Haddonfield, New Jersey together with the right to use in common with the other Tenants in the Building, all common areas including parking as may be limited herein, in Paragraph 6 (b), located rear to building and the non-exclusive use of the walks, access roads and land surrounding the building and the common areas in the building (sometimes collectively referred to together as “Land and Buildings”.
| 2. | TERM |
(a) The term of this Lease shall be two (2) years, and shall commence November 1, 2015 and shall terminate at 12:00 Noon on October 31, 2017, unless extended as permitted herein. See Additional Provisions — Exhibit “E”.
(b) Option To Renew: Tenant shall have two (2) options to renew this Lease for successive terms of two (2) years each under the same rent terms, conditions and provisions as herein provided (with a 3% yearly increase to base rent as reflected in Section 3. b. hereof). The renewal option, to be effective, must be exercised by Tenant, by written notice of such exercise mailed to the Landlord no less than (120) days prior to the commencement of any such renewal term. Any such notice of exercise or any such option shall be mailed to Landlord pursuant to the notice provisions hereinafter contained.
| 3. | RENT |
(a) Base Rent: Tenant shall pay to Landlord, without set off or deduction, except as otherwise permitted herein, by check made payable to 30 WASHINGTON AVENUE at its offices C/O The Streamwood Company, 30 Washington Avenue, Suite A-1, Haddonfield, New Jersey 08033, or such other place as Landlord directs, as yearly Minimum Annual Rent, also referred to as Base Rent, of $32,020.98 for the first year only, payable in equal monthly installments of $2,668.42 on the first business day of each month in advance during the term of this Lease. TENANT’S SECURITY DEPOSIT IS $5,336.84.
1
| Square Feet |
Year |
Date |
Yearly | Monthly | Per Sq Ft | |||||
| 1509 |
1 | 11/112015 | $32,020.98 | $2,668.42 | $21.22 | |||||
| 1509 |
2 | 11/1/2016 | $32,981.61 | $2,748.47 | $21.86 | |||||
| 1509 |
Option 1 – Year 1 | 11/1/2017 | $33,971.06 | $2,830.92 | $22.51 | |||||
| 1509 |
Option 1 – Year 2 | 11/1/2018 | $34,990.19 | $2,915.85 | $23.19 | |||||
| 1509 |
Option 1 - Year 3 | 11/1/2019 | $36,039,90 | $3,003.32 | $23.88 | |||||
| 1509 |
Option 1 - Year 4 | 11/1/2020 | $37,121.09 | $3,093.42 | $24.60 | |||||
| 1509 |
Option 1 - Year 5 | 11/1/2021 | $38,234.72 | $3,186.23 | $25.34 |
(b) Adjustment to Base Rent — Beginning with the second full year of the Lease, and with each successive year thereafter, the annual Base Rent for each full year within the rental term shall be increased by three (3%) percent per annum.
(c) In addition the Tenant shall pay the Landlord its proportionate share of all increases in real estate taxes and fire and casualty and liability insurance from base year 2013 (base year taxes $71,012.50, base year insurance $8,641.00) during the term of this Lease on an annual basis beginning with the commencement date of the Lease. (See Exhibit “H” of this Lease) Landlord shall provide Tenant with evidence of such increases and receipt confirming payment for Landlord, Tenant shall within thirty (30) days of said notice pay to the Landlord Tenant’s proportionate share of such increases in their monthly rent.
4. PROPORTIONATE AREA: For all purposes of this Lease, the Tenant’s proportionate area attributable to the Premises shall be 1,509 square feet, which is 6.71% of the total area of the first floor, second floor and lower level of entire building.
| 5. | LATE PAYMENT |
Rent is due and payable on or before the first day of each month. Rent received after the tenth (10th) of the month is subject to a late charge of five (5%) which charge must accompany rent.
| 6. | USE |
(a) Tenant shall use and occupy the Premises for office space and related purposes. No machinery, equipment or other thing that could cause unusual vibration or noise shall be installed or placed therein. Tenant shall not use or occupy the Premises for any other purpose or business without the prior written consent of Landlord. Tenant shall observe and comply with the Rules and Regulations attached hereto as Exhibit “B” and may be amended from time to time provided such amendments do not materially adversely affect Tenant’s use of the Premises, as well as all applicable laws and governmental regulations and to all reasonable requirements of the insurer of the Premises or the Building or property which the Premises are a part. All such Rules and Regulations shall apply to Tenant and its employees, agents, licensees, invitees, subtenants and contractors. Tenant shall not subject any portion of the floor to greater loading than that portion of the Premises is designed to carry and which Landlord has advised Tenant.
2
(b) Tenant will have the use of parking spaces in common parking lot in rear of the Building plus the non-exclusive, periodic use of delivery spaces for deliveries only.
| 7. | ALTERATIONS AND TRADE FIXTURES |
Tenant will, during the term and all continuations hereof, keep, and at the expiration hereof, peaceably surrender possession of the Premises in as good order and condition as existed at the inception of this Lease, reasonable wear and tear and damage by fire, elements or casualty excepted, and will, at the expiration of said term, or any continuation thereof, deliver the keys at the office of said Landlord. Tenant shall have the right to make any reasonable alterations, additions, or improvements with Landlord’s prior written consent which consent shall not be unreasonably withheld and all alterations, additions or improvements made by either of the parties hereto upon the Premises, except moveable and detached or detachable office furniture, and moveable partitions, and moveable machinery and equipment installed at Tenant’s expense, shall be the property of Landlord, and shall remain upon and be surrendered with the termination of this Lease. Any damages caused by or arising from the Tenant’s removal of its property from the Premises shall be restored and repaired at Tenant’s expense.
Any property or fixtures which remain upon the Premises after the expiration of the Lease shall be deemed abandoned by Tenant and Landlord may take possession of same and dispose of in any reasonable manner without any further liability of Landlord to Tenant. Any cost associated with the removal of such property shall be payable by Tenant.
All labor and materials furnished by or on behalf of Tenant under or pursuant to this Lease shall be first class, not less than the caliber and quality which exists in the quality which exists in the Premises and by contractors approved in writing by Landlord, which approval shall not be unreasonably withheld, and shall be accomplished at times so as not to materially disturb the activities of other tenants. Tenant shall not install any alterations, additions, or improvements in such manner as to comprise the structural integrity of the Premises or any part thereof. The labor and materials shall be installed in complete conformity to all applicable statues, codes, ordinances and regulations.
| 8. | MECHANIC’S LIENS |
If an mechanics’ or other lien shall be filed against the Premises or the Building for labor or material furnished or to be furnished at the request of the Tenant, then Tenant shall at its expense cause such lien to be discharges of record by payment, bond or otherwise, within thirty (30) days after the filing thereof. If Tenant shall fail to cause such lien to be discharged of record within such thirty (30) day period, Landlord may cause investigation as to the validity thereof or as to any offsets or defenses thereto. Any reasonable cost to Landlord for removal of such lien will be charged to Tenant as additional rent and payable on the first day of the month next following the payment by Landlord. Tenant shall indemnify and hold Landlord harmless against any and all claims, costs, damages, liabilities and expenses (including reasonable attorney fees) which may be brought or imposed against or incurred by Landlord by reason of any such lien or its discharge.
3
| 9. | CONDITION OF PREMISES |
Tenant acknowledges and agrees that, except as expressly set forth in this Lease, there has been no representations or warranties made by or on behalf of Landlord with respect to the Premises. Landlord represents and warrants that Tenant’s proposed use of the Premises for office space is permitted under all applicable laws and zoning ordinances. if Landlord is required to complete any work in order to place them in a condition for occupancy by Tenant then the specifications for any such new construction shall be as set forth on the attached Exhibit “E”. The taking of possession of the Premises by Tenant shall conclusively establish that the Premises were at such time in satisfactory condition, order and repair.
| 10. | BUILDING SERVICES |
(a) Landlord shall provide and pay for the following services and facilities.
| (1) | Air Conditioning, ventilating and heating for leased and common areas. |
| (2) | Electric current in such reasonable quality as may be required by Tenant For the operation of the lighting fixtures, typical office computer needs and electrical outlets existing upon the Premises as of the date Tenant takes possession of the Premises. |
| (3) | Janitorial and utilities of the common areas including cleaning and maintenance of common areas, restrooms, lobbies, hallways and elevators. |
| (4) | Janitorial three times a week for Tenant’s leased space. |
| (5) | Exterior lighting; landscaping; snow, ice and debris removal. |
| (6) | Water and sewer service. |
| 11. | ASSIGNMENT AND SUBLETTING |
(a) Subject to Exhibit E, Tenant shall have no right to assign or sublet all or any part of the Premises without the prior written approval of Landlord which approval shall not be unreasonably withheld. On any approved assignment or subletting of all or any part of the Premises, (I) Landlord shall receive from Tenant all profit derived by Tenant from the assignment or subletting, and (2) The sub-tenant or assignee shall be required to enter into a written Agreement with Landlord whereby it agrees to be bound by all terms and conditions of the Lease and to pay all rents due directly to Landlord.
(b) The written approval of Landlord to one or more sublettings or assignments shall not operate as a waiver of Landlord’s right to approve any further sublettings and assignments as provided herein.
4
(c) Notwithstanding the foregoing, Landlord’s consent shall not be required in connection with an assignment or subletting to a parent, subsidiary, or affiliate of the Tenant, or corporation of which Tenant is a majority shareholder, or in connection with a merger, consolidation or similar corporate transaction in which Tenant engages so long as either the Tenant or the surviving entity remain primarily liable and the assignee or sublessee enters into an agreement with Landlord agreeing to be bound by all of the terms and conditions of this Lease.
| 12. | ACCESS TO PREMISES |
Landlord, its employees and agents upon prior reasonable notice to Tenant (except in cases of emergency) shall have the right to enter the Premises, with a representative of Tenant present, at all reasonable times for the purpose of examining or inspecting the same, showing the same to prospective purchasers, mortgagees or tenants of the Building, and making such alterations, repairs, improvements or additions to the Premises or to the Building as may be necessary, provided that Landlord uses its best efforts to complete such alteration, repair, improvement or addition with minimum disturbance to Tenant’s operations. If representatives of Tenant shall not be present to open and permit entry into the Premises at any time when such entry is necessary, Landlord may enter by a master key (or forcibly in the event of an emergency) without liability to Tenant except for damages to the Premises or inventory, fixtures or equipment caused by the negligence of Landlord or Landlord’s agent and without such entry constituting an eviction of Tenant or termination of this Lease.
| 13. | REPAIRS |
(a) Tenant, at its sole cost and expense and throughout the term of this Lease, shall maintain the interior of the Premises in clean condition (including carpeting) free of accumulation of dirt and rubbish. Other than this obligation and Tenant’s obligation to repair any damage caused by the misuse or negligence of Tenant, its agents or employees, Landlord shall keep and maintain the Premises and all common areas in good order and condition. When used in this Article 13, the term “repairs” shall include replacements and renewals when necessary. All repairs made by Landlord or Tenant shall utilize materials and equipment which are at least equal in quality to those originally used in constructing the Building and the Premises.
(b) Landlord, throughout the term of this Lease and at Landlord’s sole cost and expense, shall make all necessary repairs to the footings and foundations and the structural steel columns and girders forming a part of the Premises, together with the roof, HVAC system, other mechanical and electrical systems and other capital items. Tenants shall pay the cost of any repairs made pursuant to this paragraph if same are occasioned by the negligent act or omission of Tenant, its employees or invitees.
(c) Landlord shall not be liable by reason of any injury to or interference with Tenant’s business arising from the making of any repairs, alterations, additions or improvements in or to the Premises or the Building or to any appurtenances or equipment therein except as may have occurred by reason of the willful misconduct or improper or negligent acts of Landlord and/or omissions of Landlord. There shall be no abatement of rent because of such repairs, alterations, additions or improvements, except as set forth in this paragraph or “Fire and other Casualty” Section hereof.
5
(d) Except as provided in Paragraph 16 or 18, upon termination of this lease Tenant shall return the Premises to Landlord broom clean and in as good order as existed at the inception of this Lease, reasonable wear and tear and damage by fire, elements or casualty excepted.
(e) In the event the Landlord, following written notice from the Tenant, fails to make repairs required of it within three (3) days, the Tenant may proceed to make such repairs and charge the Landlord accordingly or offset such charge against rent.
| 14. | INDEMNIFICATION AND LIABILITY INSURANCE |
(a) Tenant, shall indemnify, hold harmless and defend Landlord from and against any and all costs, expenses (including reasonable counsel fees), liabilities, losses, damages, suits, actions, fines, penalties, claims or demands of any kind and asserted by or on behalf of any person or governmental authority, arising out of or in any way caused by the Tenant’s possession of the Premises, and Landlord shall not be liable to Tenant on account of (i) any failure by Tenant to perform any of the agreements, terms, covenants or conditions of this Lease required to be performed by Tenant, (ii) any failure by Tenant to comply with any statues, ordinances, regulations or orders of any governmental authority where compliance by Tenant is required, or (iii) any accident, death or personal injury, or damage to property, which shall occur in or about the Premises except as the same may be caused by the willful misconduct or wrongful or negligent act or omission of Landlord, its employees, invitees or agents.
(b) Landlord shall indemnify, hold harmless, and defend Tenant from and against any and all costs, expenses (including reasonable counsel fees), liabilities, losses, damages, suits, actions, fines, penalties, claims, or demands of any kind and asserted by or on behalf of any person or governmental authority, arising out of or in any way connected with Landlord’s ownership of the Land and Building, and Tenant shall not be liable to Landlord on account of (i) any failure by Landlord to perform any of the agreements, terms, covenants, or conditions of this Lease required to be performed by Landlord, (ii) any failure by Landlord to comply with any statues, ordinances, regulations, or orders of any governmental authority, or (iii) any accident, death or personal injury, or damage to property, which shall occur in or about the Land and Building except as the same may be caused by the willful misconduct or wrongful or negligent act of Tenant, its employees or agents.
(c) During the term of this Lease or arty renewal thereof, Tenant shall obtain and promptly pay all premiums for comprehensive general liability insurance against claims for personal injury, death or property damage occurring upon, in or about Premises demised to Tenant, with minimum limits of $1,000,000.00 combined bodily injury and property damage with such insurance company or companies as shall be reasonably satisfactory to Landlord from time to time, and all such policies and renewals thereof shall name the Landlord as an additional insured. On or before the Commencement Date of the term of this Lease, and thereafter not less than fifteen (15) days prior to the expiration dates of said policy of policies, Tenant shall provide copies of policies or certificates of insurance evidencing coverage required by this Lease.
(d) During the term of this Lease or any renewal thereof, Landlord shall obtain and promptly pay all premiums for comprehensive general liability insurance against claims for
6
personal injury, death or property damage occurring upon, in or about Premises and common areas, with minimum limits of $1,000,000.00 combined bodily injury and property damage. On or before the Commencement Date of the term of this Lease, and thereafter not less than fifteen (15) days prior to the expiration dates of said policy of policies, Landlord shall provide copies of policies or certificates of insurance evidencing coverage required by this Lease.
(e) All policies of insurance identified in this Lease shall (to the extent possible) provide: (i) that no material change or cancellation of said policies shall be made without thirty (30) days prior written notice to Landlord, (ii) that any loss shall be payable notwithstanding any act or negligence of the Landlord which might otherwise result in the forfeiture of said insurance, and (iii) that the insurance company issuing the same shall have no right of subrogation against the Landlord or Tenant, as the case may be.
| 15. | NEGATIVE COVENANTS OF TENANT |
Tenant agrees that it will not do or suffer to be done, any act, matter or thing whereby the fire insurance or any other insurance now in force or hereafter to be placed on the Premises or any part thereof, or on the Building of which the Premises is a part, shall become void or suspended, or whereby the same shall be rated as a more hazardous risk than at the date when Tenant received possession hereunder. In case of a breach of this covenant, Tenant agrees to pay to Landlord as additional rent, any and all increase or increases of premiums on insurance carried by Landlord on the Premises, or any part thereof, or on the building of which the Premises is a part, directly caused by the said act, matter or thing.
| 16. | FIRE OR OTHER CASUALTY |
(a) If the Premises are damaged by fire or other casualty, the damages shall be repaired by and at the expense of Landlord and the rent and other charges until such repairs shall be made shall be apportioned from the date of such fire or other casualty according to the part of the Premises that is usable by Tenant. Landlord agrees to repair such damage within a reasonable period of time after the occurrence of fire or other damage, except that Tenant shall be responsible to repair and replace its own furniture, furnishings, equipment and any alteration or improvement installed by Tenant, Landlord shall not be liable for any inconvenience or annoyance to Tenant or injury to the business of Tenant resulting from such damage or the repair thereof. All repairs shall be performed with due diligence and in a good and workmanlike manner.
(b) If the Premises are rendered substantially untenantable by fire or other casualty, and the Premises cannot be fully restored within 120 days of the date of casualty, Tenant shall have the option to terminate the Lease upon written notice to Landlord.
(c) If the Premises, in the opinion of Landlord’s licensed architect or engineer, are rendered substantially untenantable by reason of such fire or other casualty, and less than six (6) months remain on the Lease term from the date of loss, including any options to renew, then Landlord shall have the right to be exercised by notice in writing delivered to the Tenant within thirty (30) days from and after said occurrence, to elect to terminate this Lease, and, in such event, this Lease and the tenancy hereby created shall cease as of the date of said occurrence, the rent to be adjusted as of said date.
7
(d) Landlord shall procure and maintain the following insurance coverage during the term and all renewal periods at Landlord’s expense, subject to reimbursement as set forth in this Lease: “all-risk” (also known as “causes of loss — special form”) property damage insurance on all buildings and common area improvements, to the extent insurable, for the full replacement cost of such buildings and improvements. Such insurance shall be issued for periods of not less than one (1) year by companies having an AM Best rating of A- or better (or equivalent) and authorized to issue such insurance in the state in which the Premises is located. The policy or policies shall provide that any loss will be paid notwithstanding any act or negligence of Landlord, Tenant or any other tenant or occupant. Upon request by Tenant, Landlord shall deliver to Tenant copies of certificates evidencing the policies (ACORD or equivalent standard form). The certificates of insurance shall provide that no lapse, cancellation or material modification of the policy shall be effective until at least twenty (20) days after mailing of written notice thereof to Tenant. All loss proceeds payable under any insurance required of Landlord under this Section (d) shall be disbursed and utilized solely for the purpose of repairing and restoring the buildings and improvements.
(e) In the event that the insurance proceeds obtained as a result of fire or other casualties set forth within this paragraph 16, are not sufficient to fully repair and restore the Premises, then the Tenant has the right to contribute the deficiency for purposes of such repair or restoration. In the event that the Premises are totally destroyed by fire or other casualty, and the Tenant does not put up any money related to rebuilding, and the Landlord rebuilds the Premises, the Tenant shall have the right of first refusal with respect to that portion of the new building which otherwise would have been occupied by Tenant on the same terms and conditions as provided, herein.
| 17. | SUBORDINATION AND NON-DISTURBANCE |
This Lease is subject and subordinate to all mortgages now or hereafter affecting or covering the Premises and all or any part of the Building and to all renewals, modifications, replacements or extensions thereof. Notwithstanding the aforesaid subordination, in the event of the foreclosure of any such mortgage, (a) this Lease shall not terminate, and (b) the peaceful possession of Tenant shall not be disturbed, provided that Tenant is not in default under any of the terms and conditions of this Lease. Tenant agrees to attorn to and to recognize the mortgagee or the purchaser at foreclosure sale as Tenant’s landlord for the balance of the term of this Lease. Tenant hereby agrees, however, that such mortgagee or the purchaser at foreclosure sale shall not be (i) liable for any act or omission of Landlord, (ii) subject to any offsets or defenses which Tenant might have against Landlord, (iii) bound by any rent or additional rent which Tenant may have paid to Landlord for modification of this Lease made without its consent. The aforesaid subordination, non-disturbance and attornment provisions shall be self-operative; however Tenant agrees to promptly execute any other agreement submitted by Landlord in confirmation or acknowledgment of same.
8
| 18. | [INTENTIONALLY OMITTED] |
| 19. | CONDEMNATION |
(a) if the whole of the Premises shall be condemned or taken either permanently or temporarily for any public or quasi-public use of purpose, under any statute or by right of eminent domain, or by private purchase in lieu thereof, then in that event the term of this Lease shall cease and terminate from the date when possession is taken thereunder pursuant to such termination and any rent paid for a period thereafter shall be refunded. In the event of a partial condemnation, the rental paid by the Tenant to the Landlord shall be reduced, such reduction to be proportionate to the partial taking, such partial condemnation not to otherwise affect this Lease unless the partial taking materially adversely affects the operation of Tenant in Tenant’s sole, but reasonable discretion in which event the Tenant shall have the right to terminate this lease upon forty five (45) days prior written notice to landlord. In the event more than 15% of the Premises or more than 15% of the Building containing same shall be so taken (or if more than fifteen percent (15%) of the parking areas are taken and not promptly replaced with contiguous parking areas) then Tenant or Landlord may elect to terminate this Lease from the date when possession is taken thereunder pursuant to such proceedings or purchase or, upon mutual agreement of the parties, Landlord shall repair and restore, at its own expense, the portion not taken and thereafter the rent shall be reduced proportionately to the portion of the Premises taken.
(b) In the event of any total or partial taking of the Premises or the Building, Landlord shall be entitled to receive the entire award in such proceeding for the Building and land and Tenant shall make a separate application for Tenant’s fixtures, equipment and moving expenses under the then applicable New Jersey eminent domain code.
| 20. | ESTOPPEL CERTIFICATE |
Tenant shall, at any time and from time to time, within fifteen (15) days after written request by Landlord, execute, acknowledge and deliver to Landlord, or its mortgagee or trustee, a statement in writing duly executed by Tenant (i) certifying that this Lease is in full force and effect (if that be the case) without modification or amendment (or, if there have been any modifications or amendments, that this Lease is in full force and effect as modified and amended and setting forth the modifications and amendments), (ii) certifying the dates to which Base Rent and additional rent have been paid, and (iii) either certifying that to the knowledge of the Tenant no default exists under this Lease or specifying each such default; it being the intention and agreement of Landlord and Tenant that any such statement by Tenant may be relied upon by a prospective purchaser or a prospective or current mortgagee of the Building, or by others, in any matter affecting the Premises.
| 21. | DEFAULT |
The occurrence of any of the following shall constitute a default and breach of this Lease by Tenant.
(a) failure of Tenant to accept possession of the Premises within ninety (90) days after the date of commencement providing the Premises is ready for occupancy;
9
(b) failure by Tenant to pay, when due, any installment of rent hereunder or any additional rent or any such other sum herein required to be paid by Tenant, following the expiration of thirty (30) days written notice from Landlord to Tenant;
(c) a failure by Tenant to observe and perform any other provisions or covenants of this Lease to be observed or performed by Tenant, where such failure continues for thirty (30) days after written notice thereof from Landlord to Tenant provided, however, that if the nature of the default is such that the same cannot reasonably be cured within such thirty (30) day period, Tenant shall not be deemed to be in default if Tenant shall within such period commence such cure and thereafter diligently prosecute the same to completion;
(d) the filing of a petition by or against Tenant for adjudication as a bankrupt or insolvent or for its reorganization or for the appointment pursuant to any local, state or federal bankruptcy or insolvency law of a receiver or trustee of Tenant’s property; or an assignment by Tenant for the benefit of creditors; or the taking of possession of the property of Tenant by any local, state or federal governmental officer or agency of court appointed official for the dissolution or liquidation of Tenant or for the operating, either temporary or permanent, of Tenant’s business; provided, however, that if any such action is commenced against Tenant the same shall not constitute a default if Tenant causes the same to be dismissed within sixty (60) days after the filing of same.
| 22. | REMEDIES |
Upon the occurrence of any such event of default set forth above:
(a) Landlord may (but shall not be required to) perform for the account of Tenant any such default of Tenant and immediately recover as additional rent any expenditure made and the amount of any obligations incurred in connection therewith plus interest at the rate of 2% per annum over the PNC prime rate (as it may be adjusted from time to time) from the date of such expenditure;
(b) Landlord, at its option, may serve notice upon Tenant that this Lease and the then unexpired term hereof and all renewal options shall cease and expire and become absolutely void on the date specified in such notice, to be not less than five (5) days after the date of such notice without any right on the part of the Tenant to save the forfeiture by payment of any sum due or by the performance of any terms provisions, covenant, agreement or condition broken; and, thereupon and at the expiration of the time limit in such notice, (not to exceed thirty (30) days) this Lease and the term hereof granted, as well as the right, title and interest of the Tenant hereunder, shall wholly cease and expire and become void in the same manner and with the same force and effect (except as to Tenant’s liability) as if the date fixed in such notice were the date herein granted for expiration of the term of this Lease. Thereupon, Tenant shall immediately quit and surrender to Landlord the Premises, and Landlord may enter into and repossess the Premises by summary proceedings, detained, ejectment or otherwise and remove all occupants thereof and, at landlord’s option, any property thereon without being liable to indictment, prosecution or damages therefore. No such expiration or termination of this Lease shall relieve Tenant of its liability and obligations under this Lease, whether or not the Premises shall be relet. Landlord may accelerate ail base rent for the balance of the term of this Lease and declare the same to be immediately due and payable, subject to the ability of the Landlord to make a good faith effort to mitigate damages.
10
(c) Landlord may, at any time after the occurrence of any event of default, following notice of termination and the expiration of any period provided therein re-enter and repossess the Premises and any part thereof and attempt in its own name, as agent for Tenant or on its own behalf, to relet all or any part of such Premises for and upon such terms to anyone; for the purpose of such reletting, Landlord may decorate or make reasonably necessary repairs, changes, alterations or additions which shall be charged to and be payable by Tenant as additional rent hereunder, as well as any reasonable brokerage and legal fees expended by Landlord, and any sums collected by Landlord from any new Tenant obtained on account of the Tenant shall be credited against the balance of the rent due hereunder as aforesaid. Tenant shall pay to Landlord monthly, on the days when the rent would have been payable under this Lease, the amount due hereunder less the amount obtained by Landlord from such new Tenant.
(d) Landlord shall have the right of injunction, in the event of a breach or threatened breach by Tenant of any of the agreements, conditions, covenants or terms hereof, to restrain the same and the right to invoke any remedy allowed by law or in equity, whether or not other remedies, indemnity or reimbursements are herein provided. The right and remedies given to Landlord in this Lease are distinct, separate and cumulative remedies; and no one of them, whether or not exercised by Landlord, shall be deemed to be in exclusion of any of the others.
(e) In addition to all remedies provided herein or by law, the non-prevailing party shall pay to the prevailing party, reasonable attorneys’ fees and court costs incurred as a result of litigation related to such breach.
| 23. | REQUIREMENT OF STRICT PERFORMANCE |
The failure or delay on the part of either party to enforce or exercise at any time any of the provisions, rights or remedies in the Lease shall in no way be construed to be a waiver thereof, nor in any way to affect the validity of this Lease or any part hereof, or the right of the party to thereafter enforce each and every such provision, right or remedy. No waiver of any breach of this Lease shall be held to be a waiver of any other of subsequent breach. The receipt by Landlord of rent at a time when the rent is in default under this Lease shall not be construed as a waiver of such default. The receipt by Landlord of a lesser amount than the rent due shall not be construed to be other than a payment on account of the rent then due, nor shall any statement on Tenant’s check or any letter accompanying Tenant’s check be deemed an accord and satisfaction, and Landlord may accept such payment without prejudice to Landlord’s right to recover the balance of the rent due or to pursue any other remedies provided in this Lease. No act or thing done by Landlord or Landlord’s agents or employees during the term of this Lease shall be deemed an acceptance of a surrender of the Premises, and no agreement to accept such surrender shall be valid unless in writing and signed by Landlord.
| 24. | LANDLORD’S OBLIGATIONS |
Landlord agrees to maintain the Premises as a first class building. Landlord’s obligations hereunder shall be binding upon Landlord only for the period of time that Landlord is in
11
ownership of the Building; and, upon termination of that ownership, Tenant, except as to any obligations which have then matured, shall look solely to Landlord’s successor in interest in the building for the satisfaction of each and every obligation of Landlord hereunder.
| 25. | SUCCESSORS |
The respective rights and obligations provided in this Lease shall bind and inure to the benefit of the parties hereto, their legal representatives, heirs, successors and assigns; provided, however, that no rights shall inure to the benefit of any successors of Tenant unless Landlord’s written consent for the transfer to such successor has first been obtained as provided in paragraph 11 hereof.
| 26. | GOVERNING LAW |
This Lease shall be construed, governed and enforced in accordance with the laws of the State of New Jersey.
| 27. | SEVERABILITY |
If any provisions of this Lease shall be held to be invalid, void or unenforceable, the remaining provisions hereof shall in no way be affected or impaired and such remaining provisions shall remain in full force and effect.
| 28. | CAPTIONS |
Marginal captions, titles or Exhibits and Riders and the table of contents in this Lease are for convenience and reference only, and are in no way to be construed as defining, limiting or modifying the scope or intent of the various provisions of this Lease.
| 29. | GENDER |
As used in this Lease, the word “person” shall mean and include, where appropriate, an individual, corporation, partnership or other entity; the plural shall be substituted for the singular, and the singular for the plural, where appropriate; and the words of any gender shall mean to include any other gender.
| 30. | NOTICES |
Wherever in this Lease it shall be required or permitted that notice or demand be given or served by either party to this Lease to or on the other party, such notice or demand shall be deemed to have been duly given or served if in writing and either personally served or forwarded by Registered or Certified Mail, postage prepaid, or by overnight delivery; and addressed as follows:
| To Landlord: |
Streamwood Associates/Haddonfield, LLC | |
| C/O The Streamwood Company | ||
| 30 Washington Avenue | ||
| Suite A-1 | ||
| Haddonfield, NJ 08033 |
12
| To Tenant: |
Alterix, Inc. | |
| Attention: Patrick Mooney | ||
| 30 Washington Avenue, Suite F-1 | ||
| Haddonfield, New Jersey 08033 |
Each such mailed notice shall be deemed to have been given to or served upon the party to which addressed two days after the date the same is deposited in the United States Registered or Certified Mail, postage prepaid, and properly addressed in the manner above provided. Personal or overnight delivery shall be deemed complete when delivered. Either party hereto may change its address to which said notices shall be delivered or mailed by giving written notice of such change to the other party hereto as herein provided.
| 31. | SIGNS |
Tenant will be permitted one exterior door plus established directory signs. Tenant shall have the right to place a sign reasonably acceptable to Landlord in space provided by Landlord. Tenant shall not post any sign or other listing in the windows or upon the exterior of the Building. Landlord will have sign painted and installed at Tenant’s expense.
| 32. | ENTIRE AGREEMENT |
This Lease, including the Exhibits and any Riders hereto, contains all the agreements, conditions, understandings, representations and warranties made between the parties hereto with respect to the subject matter hereof, and may not be modified orally or in any manner other than by an agreement in writing signed by both parties hereto or their respective successors in interest.
| 33. | ADDITIONAL SCHEDULES |
The following additional schedules are attached hereto and made a part of this Lease:
| Exhibit A: |
Tenant Estoppel | |
| Exhibit B: |
Rules and Regulations | |
| Exhibit C: |
Sign Criteria | |
| Exhibit D: |
Parking Spaces | |
| Exhibit E: |
Additional Provisions | |
| Exhibit F: |
Sketch of Premises and Entire Building | |
| Exhibit G: |
Percentages of Leased Space | |
| Exhibit H: |
Tax & Insurance Increases — Example |
13
| 34. | MISCELLANEOUS |
(a) Landlord will not unreasonably withhold or delay Landlord’s consent whenever such consent is required to be given.
(b) Landlord shall comply with all laws, rules, ordinances and regulations pertaining to Landlord’s ownership and operation of Premises and the Building.
| 35. | QUIET ENJOYMENT |
The Landlord represents and warrants that it is the owner of the Premises, and that the Tenant, upon payment of rents and performance of the conditions, covenants, promises and agreements to be performed by it herein contained, shall and may peaceably possess and enjoy the Premises and common areas during the term of this lease without any interruption or disturbance.
| 36. | ENVIRONMENTAL |
The Landlord represents and warrants that, to its knowledge, there are no adverse environmental conditions affecting the Premises or the building or the property on which the building is located.
14
IN WITNESS WHEREOF, the parties hereto have duly executed this Lease and have initialed the Exhibits and any Riders hereto, the day and year first above written.
| LANDLORD: | ||
| Streamwood Associates/Haddonfield, LLC | ||
| By: | /s/ John F. Leonard | |
| John F. Leonard | ||
| Managing Partner | ||
| TENANT: | ||
| ALTERIX, INC. NC. | ||
| By: | /s/ Patrick Mooney | |
| Patrick Mooney | ||
| Corporate Representative | ||
15
Exhibit 10.10
INTELLECTUAL PROPERTY PURCHASE AGREEMENT
THIS INTELLECTUAL PROPERTY PURCHASE AGREEMENT (the “Agreement”) is entered into as of October 24, 2015 (the “Effective Date”) by and between BioChemics, Inc., a Delaware corporation having an address at 300 Rosewood Drive, Suite 103, Danvers MA 01923 (“BioChemics”) and Inpellis, Inc., a Delaware corporation having an address at 30 Washington Avenue, Suite F, Haddonfield, NJ 08033 (“Inpellis”).
RECITALS
WHEREAS, BioChemics owns certain assets, rights, intellectual property and know-how related to designing, developing and using transdermal, intra-dermal and topical drug delivery technology (the “BioChemics Intellectual Property”); and
WHEREAS, BioChemics desires to sell to Inpellis, and Inpellis desires to purchase from BioChemics, full ownership of certain of the BioChemics Intellectual Property and joint ownership of certain of the BioChemics Intellectual Property; and
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants contained herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereto agree as follows:
| 1. | Definitions and Interpretation |
The following words have the following meaning when used in this Agreement.
1.1 “Affiliate” means any corporation, company, partnership, joint venture or other entity which Controls, is Controlled by, or is under common Control with a Party (as hereinafter defined), as the case may be. For the purpose of this definition, “Control” of an entity means the ownership, directly or indirectly, of more than fifty percent (50%) of the outstanding voting securities or capital stock of such entity, or the legal power to direct or cause the direction of the general management and policies of the entity in question. For purposes of this definition, BioChemics shall not be considered an Affiliate of Inpellis.
1.2 “BioChemics Intellectual Property” means (a) Know-How (as hereinafter defined) that relates to the BioChemics VALE (Vaso Active Lipid Encapsulated) technology and the Products Controlled by BioChemics as of the Effective Date or that is or was either conceived by BioChemics independently at any time, whether or not patents are applied for or issued to BioChemics and (b) Patents or Joint Patents owned by BioChemics that claim or are directed to be the foregoing Know-How.
1.3 “Commercialize” or Commercialization” shall mean any and all activities, excluding Development or manufacturing, necessary or desirable to realize and maximize commercial sales of each of the Products in accordance with applicable law, including distributing, importing, transporting, customs clearance, export, warehousing, packing, handling and delivering to
customers, as well as offering for sale and sales, marketing, promoting and reimbursement related activities, including booking sales. When used as a verb “Commercialize” means to engage in Commercialization
1.4 “Confidential Information” of a Party means trade secrets or confidential or proprietary information, whether written, oral or in any other form, designated as such in writing (e-mail is sufficient) by such Party, including by letter or by the use of an appropriate proprietary stamp or legend, prior to or at the time any such trade secret or confidential or proprietary information is disclosed by such Party to the other Party. Confidential Information disclosed in oral form shall be deemed Confidential Information only to the extent that it is confirmed in writing to the other Party within twenty (20) days after the date of oral disclosure.
1.5 “Develop” or “Development” shall mean all activities relating to research and development in connection with seeking obtaining and/or maintaining any Regulatory Approval (as hereinafter defined) of the Products including without limitation all pre-clinical research and development activities, all human clinical studies and all other activities relating to seeking, obtaining and/or maintaining any Regulatory Approval from FDA or other Regulatory Authority (as hereinafter defined).
1.6 “FDA” means the United States Food and Drug Administration, or any successor agency thereto having the administrative authority to regulate the marketing of human pharmaceutical products or biological therapeutic products, delivery systems and devices in the United States of America.
1.7 “Field” shall mean the prescriptive prophylactic or therapeutic treatment of pain in humans other than in the skin (‘non-dermal pain”) with the products and actives specified in Schedule 1.15.
1.8 Reserved.
1.9 “Joint Patents” means patents and patent application owned by BioChemics that are not Patents as defined in Section 1.14 below and any substitutions, extensions, additions, reissues, reexaminations, renewals, divisions, continuations, continuations-in-part or supplementary protection certificates thereof, and all foreign counterparts of any of the foregoing, existing as of the consummation of the Agreement (the “Closing”, and the date upon which the Closing occurs, the “Closing Date”). The Joint Patents are set forth on Schedule 1.9.
1.10 “Know-How” means all know-how, show-how, proprietary technical and non-technical information, trade secrets, formulae, techniques, sketches, drawings, materials, models, inventions, designs, specifications, processes, apparatus, equipment, databases, research, experimental work, development, pharmacology and clinical data, software programs and applications, software source documents, and any related type of proprietary intellectual property right or other information other than the Patents and Joint Patents.
2
1.11 “NDA” means a New Drug Application as defined in the U.S. Food, Drug and Cosmetics Act and the regulations promulgated thereunder and any corresponding or equivalent foreign application or registration.
1.12 “Party” means BioChemics or Inpellis. If either Party assigns this Agreement to any of its Affiliates in accordance with and subject to Section 7.8, “Party” shall include such Affiliate of such Party.
1.13 “Patent Authority” means a governmental, intergovernmental, or government-authorized body responsible for receiving, examining, issuing, extending or maintaining patents.
1.14 “Patents” mean patents and patent application owned by BioChemics relating to or that cover, in whole or part, the composition, use, manufacture, distribution, marketing, promotion, sale, administration or formulation of the Products and any substitutions, extensions, additions, reissues, reexaminations, renewals, divisions, continuations, continuations-in-part or supplementary protection certificates thereof, and all foreign counterparts of any of the foregoing, existing as of the consummation of the Agreement (the “Closing”, and the date upon which the Closing occurs, the “Closing Date”). The Patents are set forth on Schedule 1.14.
1.15 “Products” shall mean the pharmaceutical products set forth on Schedule 1.15.
1.16 “Regulatory Approval” means any approvals (including price and reimbursement approvals), licenses, registrations, or authorizations of a Regulatory Authority.
1.17 “Regulatory Authority” means any national, supra-national, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity, whose approval or authorization is necessary for, or to whom notice must be given prior to, the manufacture, distribution, use, import, transport and/or sale of a Product in such jurisdiction.
1.18 “Third Party” means any Party other than the Parties and their Affiliates.
In this Agreement headings are for convenience only and do not affect interpretation, and unless the context indicates a contrary intention:
(a) if a word or phrase is given a defined meaning, any other part of speech or grammatical form of that word or phrase has a corresponding meaning;
(b) a Section, schedule, attachment or Exhibit to this Agreement forms a part of this Agreement, but if there is inconsistency between this Agreement and any schedule, attachment or Exhibit to it, this Agreement shall prevail unless the Parties have agreed otherwise in writing;
(c) a reference to a document (including this Agreement) is to that document as varied, novated, ratified or replaced from time to time;
(d) a reference to a statute includes its delegated legislation, and a reference to a statute or delegated legislation or a provision of either includes consolidations, amendments, reenactments and replacements;
3
(e) a reference to “includes” in any form is not a word of limitation;
(f) the captions and headings of clauses contained in this Agreement preceding the text of the Sections, sections, subsections and paragraphs hereof are inserted solely for convenience and ease of reference only and shall not constitute any part of this Agreement, or have any effect on its interpretation or construction;
(g) references to days shall mean calendar days, unless otherwise specified;
(h) ambiguities and uncertainties, if any, shall not be interpreted against either Party, irrespective of which Party may be deemed to have caused the ambiguity or uncertainty to exist; and
(i) this Agreement has been prepared in the English language and the English language shall control its interpretation. In addition, all notices required or permitted to be given hereunder, and all written, electronic, oral or other communications between the Parties regarding this Agreement shall be in the English language.
| 2. | Sale to Inpellis |
2.1 BioChemics Sale of Patents to Inpellis. BioChemics hereby sells, transfers, conveys, assign and delivers to Inpellis for the consideration specified below, all right, title and interest in (a) the Patents, (b) all Know-How and other intellectual property of BioChemics directly related to the Patents and (c) all Know-How and other intellectual property of BioChemics directly related to the Products.
2.2 BioChemics Sale of Joint Patents to Inpellis. BioChemics hereby sells, transfers, conveys, assign and delivers to Inpellis for the consideration specified below, an undivided joint ownership interest in (a) the Joint Patents, (b) all know-how and other intellectual property of BioChemics directly related to the Joint Patents and (c) all know-how and other intellectual property of BioChemics related to the Products not conveyed pursuant to Section 2.1 above.
2.3 No Implied Rights. No right under any other intellectual property of BioChemics is conveyed by implication under this Agreement. All such rights are or shall be conveyed only as expressly provided in the terms of this Agreement. For the avoidance of doubt, it is understood and agreed that the transfers to Inpellis pursuant to this Agreement do not convey any right to practice the BioChemics Intellectual Property in or for any purpose related to any products other than the Products.
2.4 Consideration. Inpellis has paid BioChemics $750,000.00 on the Effective Date in full consideration for the conveyances provided for in this Agreement and BioChemics acknowledges receipt of such payment.
2.5 Further Assurances. At any time and from time to time after the Effective Date, at the request of Inpellis and without further consideration, BioChemics shall execute and deliver such other instruments of sale, transfer, conveyance and assignment and take such actions as Inpellis may reasonably request to more effectively transfer, convey and assign to Inpellis, and to confirm Inpellis’s rights to, title in and ownership of, the property conveyed pursuant to this Agreement.
4
| 3. | Options to Purchase or License |
3.1 Discoveries. In the event of the discovery or development of a new drug compound, molecule or entity and/or new drug delivery or transport mechanisms (“Discovery”) by BioChemics, written notice of such Discovery will be provided to Inpellis with sufficient detail to enable evaluation of the commercial application and potential of such Discovery. Such notice and its content shall constitute Confidential Information under Section 5.1 of this Agreement. Inpellis will, within the later of: (i) thirty (30) days of its receipt of the aforementioned notice, or (ii) its receipt from BioChemics of further information requested as Inpellis deems required for its assessment of its commercial interest in licensing or purchasing such Discovery, provide to BioChemics a notice of its intent to license or purchase such Discovery or to decline to proceed with any prospective license or purchase of same. If Inpellis elects to proceed then BioChemics and Inpellis will jointly secure an independent valuation expert to appraise and value the Discovery in its then current state. Inpellis will then provide a reasonable development and commercialization plan to BioChemics for the transfer of the Discovery and related physical assets or manifestations to Inpellis by license or sale, as the Parties may agree, and upon such terms as the Parties may agree. BioChemics may neither offer nor disclose the Discovery to other potential licensees or purchasers until the conclusion of the notice period(s) to Inpellis and any subsequent negotiations have ended unsuccessfully.
3.2 Additional Products. In the event that Inpellis wishes to utilize the BioChemics Intellectual Property or drug transport or delivery systems or mechanisms developed by BioChemics after the Effective Date in conjunction with drugs to relieve non-dermal pain, which drugs are not part of the Products, then Inpellis shall provide written notice of its desire to use such drug(s) in and BioChemics shall, within thirty (30) days of receipt of such notice, propose to Inpellis in writing reasonable financial licensing terms and conditions for Inpellis’s use of such drugs in conjunction with the BioChemics Intellectual Property for use within the Field. The Parties shall use all reasonable efforts to negotiate and agree upon such terms and conditions as soon as practicable after BioChemics makes it proposal. If the Parties are not able to reach agreement within 30 days of the date on which BioChemics makes the initial proposal, then they shall retain a mutually agreeable independent expert to determine the terms and conditions. Each Party shall pay one-half of the costs of retaining such expert
3.3 BioChemics Right to Repurchase. BioChemics shall, until December 31, 2017, have the right to buy back from Inpellis the rights to the Caine Products (as defined in Schedule 1.15) conveyed pursuant to this Agreement for a payment of $750,000.00 in immediately available funds, provided that Inpellis shall retain joint ownership of that property for the treatment of shingles.
3.4 BioChemics Right of First Offer. In the event Inpellis determines to sell any of the property conveyed to it under this Agreement, it shall notify BioChemics of such determination. BioChemics shall notify Inpellis within 10 days of receiving such notice whether BioChemics
5
wishes to negotiate to purchase such property. Upon receipt of such notice from BioChemics, Inpellis shall, prior to Inpellis selling such property to another party, negotiate in good faith with BioChemics for a period not to exceed 30 days from the date of the notice from BioChemics to sell such property to BioChemics.
| 4. | Representations and Warranties |
4.1 Mutual Representations, Warranties and Covenants. Each Party represents, warrants and covenants as of the Effective Date to the other that:
(a) It is duly organized and validly existing under the laws of its jurisdiction of incorporation or formation, and has all requisite corporate power and authority to carry out its business as presently conducted and as proposed to be conducted.
(b) It is duly authorized to execute and deliver this Agreement and to perform its obligations hereunder, and the person or persons executing this Agreement on its behalf has been duly authorized to do so by all requisite corporate action.
(c) This Agreement is legally binding upon it, enforceable in accordance with its terms, except as limited by (i) applicable bankruptcy, insolvency, reorganization, moratorium, fraudulent conveyance, or other laws of general application relating to or affecting the enforcement of creditors’ rights generally, or (ii) laws relating to the availability of specific performance, injunctive relief, or other equitable remedies. The execution, delivery and performance of this Agreement by it does not conflict with, or result in the breach of the terms of, any agreement, or instrument, to which it is a Party or by which it may be bound, nor violate any material law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
(d) no consent, approval, authorization or order of any court or governmental agency or governmental body or Third Party is required for execution and delivery by such Party of this Agreement.
4.2 BioChemics Representations and Warranties. BioChemics represents and warrants to Inpellis that:
(a) the rights granted to Inpellis hereunder do not conflict with rights granted by BioChemics to any Third Party.
(b) To BioChemics’s knowledge, the use of the property conveyed pursuant to this Agreement does not infringe any issued patents of any Third Party.
(c) There are no agreements with, assignments by, restrictions, liens, or encumbrances on, disputes with, or proceedings or claims against, BioChemics or its Affiliates relating to, affecting or limiting BioChemics’s rights with respect to the property conveyed under this Agreement.
6
(d) Schedules 1.9 and 1.14 together identify all of the pending patent applications and unexpired patents that are Patents or Joint Patents owned by BioChemics as of the Effective Date.
(e) None of the patents or patent applications owned by BioChemics and set forth on either Schedule 1.9 or Schedule 1.14 is, (i) subject to a pending interference action, opposition action, re-examination proceeding, litigation or other similar action by a Third Party challenging such patents or patent applications, other than actions by Patent Authorities in connection with the prosecution of patent applications, or (ii) has been abandoned, or has been asserted to be invalid or unenforceable in a communication to BioChemics or is subject to any inventorship proceeding or dispute.
4.3 Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY PROVIDED IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION AND EXTENDS NO WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING WARRANTIES OF DESIGN, MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR ARISING FROM A COURSE OF DEALING, USAGE OR TRADE PRACTICES, IN ALL CASES WITH RESPECT THERETO. WITHOUT LIMITING THE GENERALITY OF THE FOREGOING, BOTH PARTIES ACKNOWLEDGE AND DISCLAIM ANY WARRANTY AS TO THE USEFULNESS OR COMMERCIAL SUCCESS OF ANY PRODUCT OR THE ACHIEVEMENT OF DESIRED GOALS THROUGH USE OF BIOCHEMICS INTELLECTUAL PROPERTY.
4.4 Limitation of Liability. EXCEPT FOR LIABILITY FOR BREACH OF SECTION 5 (CONFIDENTIALITY) AND WITHOUT PREJUDICE TO THE OBLIGATION OF EITHER PARTY TO INDEMNIFY THE OTHER IN RESPECT OF CLAIMS BY A THIRD PARTY UNDER SECTION 6, NEITHER PARTY SHALL BE ENTITLED TO RECOVER FROM THE OTHER PARTY ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL, PUNITIVE OR OTHER INDIRECT DAMAGES IN CONNECTION WITH THIS AGREEMENT; PROVIDED, HOWEVER THAT THIS SECTION 4.4 SHALL NOT BE CONSTRUED TO LIMIT DAMAGES AWARDED SPECIFICALLY IN RESPECT OF EITHER PARTY’S GROSS NEGLIGENCE OR WILLFUL MISCONDUCT.
| 5. | Confidentiality |
5.1 Confidentiality; Exceptions. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing, the Parties agree that each Party shall keep confidential and shall not publish or otherwise disclose or use for any purpose other than for the purpose of exercising its retained rights hereunder, or performing obligations hereunder, or as otherwise provided for in this Agreement, any confidential and proprietary information and materials of the other Party (collectively, “Confidential Information”). Notwithstanding the foregoing, Confidential Information shall not include any information to the extent that it can be established by written documentation by the receiving party that such information:
(a) was already known to the receiving party, at the time of disclosure, other than under an obligation of confidentiality (except to the extent such obligation has expired or an exception is applicable under the relevant agreement pursuant to which such obligation established);
7
(b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving party in breach of this Agreement;
(d) was independently discovered or developed by the receiving party without reference to or use of Confidential Information of the disclosing party as demonstrated by documented evidence; or
(e) was disclosed to the receiving party, other than under an obligation of confidentiality (except to the extent such obligation has expired or an exception is applicable under the relevant agreement pursuant to which such obligation established), by a Third Party who had no obligation to the disclosing party not to disclose such information to others.
The obligations set forth in this Section 5.1 shall remain in effect for five years.
5.2 Authorized Disclosure. Except as expressly provided otherwise in this Agreement, each Party may use and disclose Confidential Information of the other Party as follows: (i) under appropriate confidentiality provisions substantially equivalent to those in this Agreement (but for a confidentiality period that is reasonable and customary under the applicable circumstances) in connection with the performance of its obligations or as reasonably necessary or useful in the exercise of its rights under this Agreement; (ii) to the extent such disclosure is reasonably necessary in prosecuting or maintaining any Patent or other intellectual property right in accordance with this Agreement, prosecuting or defending litigation related to this Agreement, complying with applicable governmental regulations with respect to performance under this Agreement (including to comply with the applicable rules of any public stock exchange upon which the stock of such Party or its Affiliate is listed), provided that the Party seeking to disclose Confidential Information of the other Party uses commercially reasonable efforts, consistent with typical practice in the pharmaceutical industry, to secure confidential treatment thereof, as applicable; (iii) to the extent such disclosure is otherwise required by applicable law, provided, however, that if a Party is required by applicable law or court order to make any such disclosure of the other Party’s Confidential Information it will, except where impracticable for necessary disclosures (for example, in the event of medical emergency), give reasonable advance notice to the other Party of such disclosure requirement and, in each of the foregoing, (but not to the extent inappropriate in the case of prosecution and maintenance of Patents), will use its reasonable efforts to seek confidential treatment of such Confidential Information required to be disclosed and limit disclosure of the Confidential Information to only that part necessary to comply with the request; any disclosure of Confidential Information as permitted in the foregoing sentence shall not alter the confidential nature of such Confidential Information for all other purposes; (iv) in communication with advisors (including financial advisors, lawyers and accountants) or actual or bona fide potential investors or acquirers, or actual or bona fide potential licensees or sublicensees related to Products, or approved or permitted contractors, service providers, vendors and the like used (or to be used) in connection with activities hereunder, each on a need to know basis, and in each case under standard confidentiality obligations (subject to the allowances for term of
8
confidentiality provided in subsection (i) above, except with respect to disclosures to actual or bona fide potential investors and acquirers receiving any technical data or information related to the Licensed Products that is Confidential Information of the other Party shall be subject to obligations of confidentiality for a period of at least five (5) years after such disclosure, or (v) to the extent mutually agreed to by the Parties. In addition to the foregoing, with respect to complying with the disclosure requirements of the U.S. Securities and Exchange Commission (“SEC”) or similar regulatory bodies or the rules of an applicable public stock exchange, in connection with any required disclosure of material information related to this Agreement, the Parties shall consult with one another concerning the information to be disclosed and secure confidential treatment thereof where practicable. If time does not permit such discussion, or if after such discussion between counsel, the Party desiring to make the disclosure still believes such Party is required by applicable Law or applicable stock exchange rule to make such disclosure, it may do so, upon written notice to the other Party. For clarity, nothing in this Section 5.2 shall prevent any Party from making disclosures required by applicable law.
| 6. | Indemnification |
6.1 Indemnification by Inpellis. Inpellis agrees to indemnify, defend and hold harmless BioChemics and its Affiliates and their officers, directors, shareholders, employees and agents (“BioChemics Indemnitees”) from and against any and all claims, costs, expenses, damages and liabilities, including reasonable attorney’s fees (“Losses”) to which the BioChemics Indemnitees may become subject as a result of any claim, demand, action or other proceeding by any Third Party (a) arising out of the research, use, testing, exploitation, development, commercialization, import, export, sale, offer for sale, and any transfer of any Products developed or commercialized by Inpellis, its Affiliates and/or licensees, or (b) from the breach of any of Inpellis’s obligations hereunder or (c) alleging infringement of Third Party intellectual property rights by use of Inpellis intellectual property in the research, development, manufacture, Commercialization, use, import, export, sale, offer for sale and/or any transfer of any of the Products, except to the extent such Losses result from (i) the negligence or willful misconduct of BioChemics or any of the BioChemics Indemnitees; (ii) breach of this Agreement by BioChemics or any of the BioChemics Indemnitees; (iii) any claim by a Third Party alleging that the grant of rights by BioChemics to Inpellis under this Agreement violates or conflicts with the terms of any license or other grant of rights by BioChemics to such Third Party; or (iv) any claims by a Third Party alleging infringement of Third Party intellectual property rights in the development of the Products or commercialization, use, import, export, sale, offer for sale and/or any transfer of the Products.
6.2 Indemnification by BioChemics. BioChemics shall indemnify, defend and hold harmless Inpellis and its Affiliates and their officers, directors, shareholders, employees and agents (“Inpellis Indemitees”) from and against any and all Losses to which the Inpellis Indemnitees may become subject as a result of any claim, demand, action or other proceeding by any Third Party (i) alleging infringement of Third Party intellectual property rights in the research, development, commercialization, use, import, export, sale, offer for sale and/or any transfer of the Products or (ii) breach of this Agreement by BioChemics, except to the extent such Losses result from (i) the negligence or willful misconduct of Inpellis or any of the Inpellis Indemnitees or, (ii) breach of this Agreement by Inpellis or any of the Inpellis Indemnitees.
9
6.3 Apportionment of Fault. If any Losses occur by reason of or result from the joint fault of Inpellis and BioChemics, liability for such Losses under 6.1 and 6.2 shall be apportioned between Inpellis and BioChemics according to the relative fault of Inpellis and BioChemics.
6.4 Claim for Indemnification. Whenever any Claim shall arise for indemnification under this Section 6, the Inpellis Indemnitees and the BioChemics Indemnitees entitled to indemnification (the “Indemnified Party”) shall promptly notify the other Party (the “Indemnifying Party”) in writing of the Claim and, when known, the facts constituting the basis for the Claim. The Indemnified Party’s failure to notify the Indemnifying Party will not relieve the Indemnifying Party from any liability to such Indemnified Party under this Section 6 except to the extent any liability results from the failure to timely notify the Indemnifying Party. The Indemnifying Party shall promptly assume, and have the right to control, the defense and settlement thereof at its own expense. The Indemnified Party shall not settle or compromise any Claim by a Third Party for which it is entitled to indemnification under this Section 6 without the prior written consent of the Indemnifying Party, unless the Indemnifying Party is in breach of its obligation to defend hereunder. In no event shall either the Indemnified Party or Indemnifying Party settle any Claim without the prior written consent of the Indemnified Party if such settlement does not include a release from liability on such Claim or if such settlement would involve undertaking an obligation other than the payment of money by the settling party that would bind or impair the non-settling party, or result in any patent or trademark of the other party being rendered invalid or unenforceable, or if such settlement contains an admission that any patent is invalid or unenforceable.
| 7. | Miscellaneous Provisions |
7.1 Dispute Resolution. If any dispute arises between the Parties out of or in connection with this Agreement, the Parties shall first attempt to resolve such dispute in accordance with the dispute resolution procedures set forth on Exhibit 7.1 hereto.
7.2 Governing Law; Waiver of Jury Trial. This Agreement shall be governed in all respects by the laws of the Commonwealth of Massachusetts, USA, without regard to its choice of law provisions. BIOCHEMICS AND INPELLIS EACH KNOWINGLY, VOLUNTARILY AND INTENTIONALLY WAIVE ANY RIGHT EACH MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION AMONG THEM AND/OR THEIR AFFILIATES BASED HEREON OR ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT OR ANY COURSE OF CONDUCT, COURSE OF DEALING, STATEMENTS (WHETHER ORAL OR WRITTEN) OR ACTIONS OF THE OTHER PARTIES IN CONNECTION HEREWITH.
7.3 Consent to Jurisdiction. BioChemics and Inpellis each irrevocably consent that any action or proceeding among the Parties and/or their Affiliates, under, arising out of or in any manner relating to this Agreement shall be brought in the United States District Court for the District of Massachusetts. BioChemics and Inpellis hereby each expressly and irrevocably assent and submit to the personal jurisdiction of any such court in any such action or proceeding. BioChemics and Inpellis each further irrevocably consent to the service of summons, notice, or other process relating to any such action or proceeding by delivery thereof by hand or by mail in the manner
10
provided for in Section 7.11 of this Agreement and consent that it may be served with any process or paper by registered mail or by personal service within or without the Commonwealth of Massachusetts, as the case may be, in accordance with applicable law. BioChemics and Inpellis each waive any objection, claim or defense which it may have at any time to the laying of venue of any such action or proceeding in any such court; irrevocably waive any claim that any such action or proceeding brought in any such court has been brought in an inconvenient forum; and further irrevocably waive the right to object, with respect to any such action or proceeding brought in any such court, that such court does not have jurisdiction over such Party.
7.4 Equitable Relief. Notwithstanding Paragraph B (7) of Exhibit 7.1, each Party hereto acknowledges that the remedies at law of the other Party for a breach or threatened breach of this Agreement would be inadequate and, in recognition of this fact, any Party to this Agreement, without posting any bond, and in addition to all other remedies that may be available, shall be entitled to seek equitable relief in the form of specific performance, a temporary restraining order, a temporary or permanent injunction or any other equitable remedy in a court of competent jurisdiction that may then be available.
7.5 Entire Agreement; Modification. This Agreement (including the Exhibits hereto) represents the final expression of the Parties’ agreement and a complete and exclusive statement with respect to all of its respective terms. This Agreement supersedes all prior and contemporaneous agreements and communications. No trade customs, courses of dealing or courses of performance by the Parties shall be relevant to modify, supplement or explain any term(s) used in this Agreement. This Agreement may only be modified or supplemented in a writing and signed by the Parties to this Agreement.
7.6 Relationship Between the Parties. The Parties’ relationship, as established by this Agreement, is solely that of independent contractors. This Agreement does not create any partnership, joint venture or similar business relationship between the Parties. Neither Party is a legal representative of the other Party; neither Party can assume or create any obligation, representation, warranty or guarantee, express or implied, on behalf of the other Party for any purpose whatsoever.
7.7 Non-Waiver. The failure of a Party to insist upon strict performance of any provision of this Agreement or to exercise any right arising out of this Agreement shall neither impair that provision or right nor constitute a waiver of that provision or right, in whole or in part, in that instance or in any other instance. Any waiver by a Party of a particular provision or right shall be in writing, shall be as to a particular matter and, if applicable, for a particular period of time and shall be signed by such Party.
7.8 Assignment. Except as expressly provided hereunder, neither this Agreement nor any rights or obligations hereunder may be assigned or otherwise transferred by either Party without the prior written consent of the other Party (which consent shall not be unreasonably withheld); provided, however, that (a) either Party may assign this Agreement, and its rights and obligations hereunder, to an Affiliate, provided that such Party shall remain liable and responsible to the other Party for the performance and observance of all such duties and obligations by such Affiliate; and (b) either Party may assign this Agreement, and its rights and obligations hereunder, to a Third Party in
11
connection with the transfer or sale of all or substantially all of the business of such Party to which this Agreement relates, whether by merger, sale of stock, sale of assets or otherwise, provided that the assigning party shall remain liable and responsible to the non-assigning party for the performance and observance of all such duties and obligations by such Third Party. The rights and obligations of the Parties under this Agreement shall be binding upon and inure to the benefit of the successors and permitted assigns of the Parties. Any assignment not in accordance with this Agreement shall be void.
7.9 No Third Party Beneficiaries. This Agreement is neither expressly nor impliedly made for the benefit of any Party other than those executing it.
7.10 Severability. If, for any reason, any part of this Agreement is adjudicated invalid, unenforceable or illegal by a court of competent jurisdiction, such adjudication shall not affect or impair, in whole or in part, the validity, enforceability or legality of any remaining portions of this Agreement. All remaining portions shall remain in full force and effect as if the original Agreement had been executed without the invalidated. unenforceable or illegal part.
7.11 Notices. Any notice to be given under this Agreement must be in writing and delivered either (a) in person, (b) by any method of mail (postage prepaid) requiring return receipt, (c) by overnight courier confirmed thereafter to the Party to be notified at its address(es) given below, or at any address such Party has previously designated by prior written notice to the other, or (d) by sending it by facsimile to the facsimile number of the other Party as stated below. Notice shall be deemed sufficiently given for all purposes upon the earlier of (x) the date of actual receipt; (y) if mailed, five business days after the date of postmark; or (z) if delivered by overnight courier, the next business day the overnight courier regularly makes deliveries.
if to Inpellis. notices must be addressed to:
Inpellis, Inc.
30 Washington Avenue
Suite F
Haddonfield, NJ 08033
Attn: Chief Executive Officer
Facsimile: (978) 750-0085
If to BioChemics, notices must be addressed to:
BioChemics, Inc.
300 Rosewood Drive
Suite 103
Danvers, MA. 01923
Attn: Chief Executive Officer
Facsimile: (978) 750-0085
12
7.12 Force Majeure. Except for the obligation to make payment when due, each Party shall be excused from liability for the failure or delay in performance of any obligation under this Agreement by reason of any event beyond such Party’s reasonable control including but not limited to acts of God, fire, flood, explosion, earthquake, or other natural forces, war, civil unrest, accident, destruction or other casualty, any lack or failure of transportation facilities, any lack or failure of supply of raw materials, any strike or labor disturbance, or any other event similar to those enumerated above. Such excuse from liability shall be effective only to the extent and duration of the event(s) causing the failure or delay in performance and provided that the Party has not caused such event(s) to occur. Notice of a Party’s failure or delay in performance due to force majeure must be given to the other Party within ten days after its occurrence. All delivery dates under this Agreement that have been affected by force majeure shall be tolled for the duration of such force majeure.
7.13 No Use of Names. Except as otherwise provided herein, nothing contained in this Agreement shall be construed as conferring any right on either Party to use in advertising, publicity or other promotional activities any name, trade name, trademark or other designation of the other Party, including any contraction, abbreviation or simulation of any of the foregoing, unless the express written permission of such other Party has been obtained.
7.16 Counterparts. This Agreement may be executed in two counterparts, each of which shall be deemed an original document, and both of which, together with this writing, shall be deemed one instrument.
[SIGNATURE PAGE FOLLOWS]
13
IN WITNESS WHEREOF, the Parties hereto have duly executed this Agreement.
| BioChemics, Inc. | ||
| By: | /s/ Marshall S. Sterman | |
| Name: | Marshall S. Sterman | |
| Title: | Chief Executive Officer | |
| Date: | October 24, 2015 | |
| Inpellis, Inc. | ||
| By: | /s/ Patrick T. Mooney, M.D. | |
| Name: | Patrick T. Mooney, M.D. | |
| Title: | Chief Executive Officer | |
| Date: | October 24, 2015 | |
14
SCHEDULE 1.15
Products
Transdermal Ibuprofen
Transdermal Benfothiamine
Transdermal Gabapentin
Transdermal Celecoxib
Transdermal Rofecoxib
Transdermal Lidocaine and any similar products derived from the same chemical including, without limitation, Benzocaine, Dibucaine, Oxybuprocaine, Proparacaine, Proxymetacaine, Tetracaine, Amethocaine, Xylocaine and Lignocaine (collectively, the “Caine Products”)
15
SCHEDULE 1.14
Patents
| Attorney Ref. No. |
Title |
Application Information | ||
| 3326/109AU (Australian Patent Application) |
IBUPROFEN FOR TOPICAL ADMINISTRATION | Appl No.: 2009291755 Filed September 10, 2009 (Priority 61/095,672, Filed September 10, 2008) | ||
| 3326/109CA (Canadian Patent Application) | IBUPROFEN FOR TOPICAL ADMINISTRATION | Appl No.: 2,749,941 Filed: September 10, 2009 (Priority 61/095,672, Filed September 10, 2008) | ||
| 3326/109EP (European Patent Application) | IBUPROFEN FOR TOPICAL ADMINISTRATION | Appl No.: 09792433.6 Filed: September 10, 2009 Publication No. EP 2373346, Published October 12, 2011 | ||
| 3326/109WO (PCT Patent Application) | IBUPROFEN FOR TOPICAL ADMINISTRATION | Appl No.: PCT/US2009/056568 Filed September 10, 2009 (Priority: 61/095,672, Filed September 10, 2008) Publication No. WO 2010/030821, Published March 18, 2010 | ||
| 3326/112 (U.S. Utility Application) |
IBUPROFEN FOR TOPICAL ADMINISTRATION | Appl No.: 13/604,040 Filed September 5, 2012 (Priority: 61/095,672 Filed September 10, 2008) CON of 12,557,490 filed Sept. 10, 2009 (3326/109) Publication No. US 2012-0329875 A1, Published December 27, 2012 | ||
| 3326/117 | TOPICAL AND TRANSDERMAL IBUPROFEN-CONTAINING COMPOSITION | Appl No.: Filed: | ||
16
Schedule 1.9
Joint Patents
| ATTORNEY REF. NO. |
TITLE |
APPLICATION INFORMATION | ||
| 3326/110
(US Utility Application) |
TRANSDERMAL DRUG DELIVERY USING AN OSMOLYTE AND VASOACTIVE AGENT | App. No. 12/584,841 Filed: September 22, 2009 (Priority: 61/099, 129, Filed September 22, 2008) | ||
| Publication No. US 2010/0076035 A1, | ||||
| Published March 25, 2010 | ||||
| 3326/110EP
(European Patent Application) |
TRANSDERMAL DRUG DELIVERY USING AN OSMOLYTE AND VASOACTIVE AGENT | Appl. No.: 09740777.9 Filed: September 22, 2009 (Priority. 61/099,129, Filed September 22, 2008) | ||
| Publication No. EP 2207536, | ||||
| Published July 21, 2010 | ||||
| 3326/110MX
(Mexican Patent Application) |
TRANSDERMAL DRUG DELIVERY USING AN OSMOLYTE AND VASOACTIVE AGENT | Appl. No.: MX/A/2010/004169 Filed: September 22, 2009 (Priority: 61/099,129, Filed September 22, 2008) | ||
| 3326/110WO
(PCT Patent Application) |
TRANSDERMAL DRUG DELIVERY USING AN OSMOLYTE AND VASOACTIVE AGENT | Appl. No.: PCT/US2009/057916 Filed: September 22, 2009 (Priority: 61/099,129, Filed September 22, 2008) | ||
| Publication No. WO 2010/034019, | ||||
| Published March 25, 2010 | ||||
| 3326/116WO (PCT Application) |
TOPICAL FORMULATIONS AND METHODS FOR DRUG DELIVERY | Appl. No.: PCT/US14/29240 Files: 14-Mar-2014 | ||
| (Priority: 61/790,126 filed March 15, 2013) | ||||
| Publication No. WO 2014/144752, | ||||
| Published September 18, 2014 | ||||
17
| ATTORNEY REF. NO. |
TITLE |
APPLICATION INFORMATION | ||
| 484P005-CA (Canadian Patent Application) | SOLUTION-BASED TRANSDERMAL DRUG DELIVERY SYSTEM | Appl. No. 2,360,590 Filed: October 23, 2001 | ||
| 484P005-US
(US Patent Application) |
SOLUTION-BASED TRANSDERMAL DRUG DELIVERY SYSTEM | Appl. No. 09/698,483 Filed: October 27, 2000 | ||
| 484P005-Australia
(Australian Patent Application) |
SOLUTION-BASED TRANSDERMAL DRUG DELIVERY SYSTEM | Appl. No.: 2001179417 Filed: October 15, 2001 | ||
| 484009-Australia
(Australian Patent Application) |
METHODS OF DEVICE-ASSISTED DRUG DELIVERY | Appl. No.: 2005286822 Filed: September 20, 2005 | ||
| 484P009-Canada (Canadian Patent Application) |
METHODS OF DEVICE-ASSISTED DRUG DELIVERY | Appl. No.: 2,569,285 Filed: September 20, 2005 | ||
| 484P009-Brazil (Brazilian Patent Application) |
METHODS OF DEVICE-ASSISTED DRUG DELIVERY | Appl. No.: P10513446-3 Filed: September 20, 2005 | ||
| 484P009-India (Indian Patent Application) |
METHODS OF DEVICE-ASSISTED DRUG DELIVERY | Appl. No.: 7601/DelNp/2006 Filed: December 16, 2006 | ||
| 484P009-Mexico lx/a
(Mexican Patent Application) |
METHODS OF DEVICE-ASSISTED DRUG DELIVERY | Appl. No.: 2007/001222 Filed: January 30, 2007 | ||
| 484P011-US (US Patent Application) |
BIFUNCTIONAL SYNTHETIC MOLECULES | Appl. No. 11/820,172 Filed: June 18, 2007 | ||
| 484P011-US (Canadian Patent Application) | BIFUNCTIONAL SYNTHETIC MOLECULES | Appl. No.: 2,690,357 Filed: June 17, 2008 | ||
18
| ATTORNEY REF. NO. |
TITLE |
APPLICATION INFORMATION | ||
| 484P011-Europe (European Patent Application) | BIFUNCTIONAL SYNTHETIC MOLECULES | Appl. No.: 8768535.9 Filed: June 17, 2008
2167104-Pub March 31, 2010 | ||
| 484P011-Hong Kong
(Hong Kong Patent Application) |
BIFUNCTIONAL SYNTHETIC MOLECULES | Appl. No.: HK1142815 Filed: December 17, 2010 | ||
| 484P011-India (India Patent Application) |
BIFUNCTIONAL SYNTHETIC MOLECULES | Appl. No.: 551/DELNP/2009 Filed: December 29, 2009 | ||
| 484P011-Mexico (Mexican Patent Application) |
BIFUNCTIONAL SYNTHETIC MOLECULES | Appl. No.: lx/a2009013759 Filed: December 15, 2009 | ||
19
EXHIBIT 7.1
Alternative Dispute Resolution Procedures
A. METHOD OF INVOKING ADR PROCEDURES
1. These procedures may be invoked by any Party by giving written notice to the other of the dispute and designating one or more persons (collectively, the “DESIGNEE”) to act on behalf of the disputing Party regarding the dispute. The other Party shall be required to respond to the disputing Party’s notice within ten (10) business days by designating in writing its own Designee. A Party may choose to represent itself, or if it appoints a Designee, its officers may nonetheless attend such meetings.
2. The Parties, each acting through its Designee, shall meet at a mutually acceptable time and place within ten (10) business days after the non-disputing Party designates its Designee to the others. At that meeting, the Parties shall attempt in good faith to negotiate a resolution of the dispute, or failing that, to agree on a method for resolving the claim or dispute.
3. If, within ten (10) business days after the first meeting or within such longer period of time as the Parties may mutually agree, the Parties have not succeeded in negotiating a resolution of the claim or dispute or agreeing on a dispute resolution mechanism, they shall submit the dispute to mediation in accordance with the procedures set forth herein.
4. The Parties will jointly appoint a mutually acceptable mediator to mediate the dispute. If the Parties are unable to agree on a mutually acceptable mediator within five (5) business days after the conclusion of the negotiations described in Paragraph 3 above, then the Parties shall select a neutral Person from American Arbitration Association (“AAA”) in New York, New York, with the assistance of AAA, unless the Parties agree otherwise in finding a mutually acceptable mediator.
5. The non-prevailing Party in the dispute shall be responsible for the payment of the fees and costs of the mediator, and any fees and costs of AAA.
6. The Parties agree to participate in good faith in the mediation and negotiations related thereto for a period of thirty (30) days from appointment of a mediator by any of the Parties or the AAA.
7. The Parties agree that the mediation period may be extended for an additional thirty (30) days beyond the initial thirty (30) day period upon agreement of the Parties. Either Party may terminate the mediation at any time after the initial thirty (30) days or when any agreed upon extension has expired.
20
B. MEDIATION PROCEDURES
1. The mediator shall be neutral and impartial.
2. The mediator shall control the procedural aspects of the mediation. The Parties will cooperate fully with the mediator.
a. The mediator is free to meet and communicate separately with each Party.
b. The mediator will decide when to hold joint meetings with the Parties and when to hold separate meetings. There shall be no stenographic record of any meeting. Formal rules of evidence will not apply.
3. Each Party may be represented by more than one person, including an attorney.
4. The process will be conducted expeditiously.
5. The mediator will not transmit information received from any Party to another Party or any third person unless authorized to do so by the Party transmitting the information.
6. The entire process is confidential. The Parties and the mediator will not disclose information regarding the process, including settlement terms, to third persons, unless the Parties otherwise agree. The process shall be treated as a compromise negotiation for purposes of the applicable rules of evidence. Further, the Parties will not disclose the existence of a dispute or information regarding the mediation to third persons including, without limitation, the media.
7. The Parties will refrain from pursuing administrative and/or judicial remedies during the mediation process, except as otherwise expressly provided in the agreement which incorporates these procedures. The Parties agree that any and all statutes of limitation or periods of time for taking action shall be tolled during the time period that the Parties are engaged in mediation.
8. Unless all Parties and the mediator otherwise agree in writing:
a. The mediator will be disqualified as a witness, consultant or expert in any pending or future investigation, action or proceeding relating to the subject matter of the mediation (including any investigation, action or proceeding which involves persons not Parties to this mediation);
b. The mediator, at the conclusion of the mediation, will immediately either destroy and certify destruction of, or return to the providing Party, any and all documents and information in the mediator’s possession, whether or not the mediation was successful; and
c. The mediator will not be subpoenaed in any such investigation, action or preceding and all Parties will oppose any effort to have the mediator subpoenaed.
9. The mediator, if a lawyer, may freely express views to the Parties on the legal issues of the dispute.
21
10. The mediator shall not be liable for any act or omission in connection with the mediation.
11. The mediator may withdraw at any time by written notice to the Parties (i) for overriding personal reasons, (ii) if the mediator believes that a Party is not acting in good faith, or (iii) if the mediator concludes that further mediation efforts would not be useful.
22
Exhibit 10.11
JOINT OWNERSHIP AGREEMENT
This Joint Ownership Agreement (this “Agreement”) is effective as of October 24, 2015 (the “Effective Date”) by and between BioChemics, Inc., a Delaware corporation having an address at 300 Rosewood Drive, Suite 103, Danvers MA 01923 (“BioChemics”) and Inpellis, Inc., a Delaware corporation having an address at 30 Washington Avenue, Suite F, Haddonfield, NJ 08033 (“Inpellis”). BioChemics and Inpellis are each herein a “Party” and collectively, the “Parties.”
WHEREAS, BioChemics and Inpellis jointly own the intellectual property described in Section 2.2 of the Intellectual Property Purchase Agreement (the “Purchase Agreement”) entered into by BioChemics and Inpellis on the same date as the Effective Date (the “Joint IP”); and
WHEREAS, the Parties wish to clarify their respective rights and obligations with respect to the Joint IP and its commercialization.
NOW, THEREFORE, in consideration of the promises and the mutual covenants contained herein, the Parties agree as follows:
ARTICLE 1 - RIGHTS OF THE PARTIES
1.1 Inpellis hereby agrees that it will use the Joint IP only in connection with the Products, as listed on Schedule 1.1 hereto. BioChemics hereby agrees that it will not use the Joint IP in connection with Products, but may use the Joint IP for any other purpose. The Parties acknowledge and agree that their respective use shall include, but not be limited to, the development and commercialization of drugs using the Joint IP and the prosecution and maintenance of patents relating to the Joint IP. Inpellis will manage the prosecution and maintenance of all patents relating to the Joint IP. BioChemics shall be copied on all patent-related correspondence. BioChemics and Inpellis shall use reasonable efforts to assure full cooperation of its employees/inventors in the preparation, filing, prosecution, and maintenance of such patents. Inpellis agrees not to abandon any patent or patent application prosecution or maintenance relating to the Joint IP without first notifying BioChemics in writing at least sixty (60) days in advance of any applicable deadline. Inpellis will obtain estimates for BioChemics of the costs of all prosecution and maintenance activities hereunder, and shall give BioChemics an opportunity to disapprove prior to committing Inpellis to such expense. Such decision by BioChemics shall be made, in writing to Inpellis, no longer than 30 days from the date of the estimate. Deviations from this time frame will only be granted upon consultation and agreement by the parties.
1.2 Payment of all fees and costs relating to all Joint IP patent filing, prosecution and maintenance, shall timely be effected by Inpellis; provided, however, that Inpellis will obtain estimates of all such fees and costs. Inpellis will bear all such costs and fees in accordance with licensing agreement, including reasonable attorney’s fees, incurred in the filing, prosecution and maintenance of patents and patent applications for the Joint IP in the United States unless otherwise agreed upon by the Parties.
1.3 Except as may otherwise be set forth in this Agreement, neither Party shall have any obligation to pay royalties to, provide an accounting to, or otherwise make payment to, the other Party in connection with such Party’s use of the Joint IP in accordance with the terms of this Agreement.
1
ARTICLE 2 - TERMINATION AND ABANDONMENT
2.1 Unless earlier terminated as provided below, this Agreement shall terminate with the expiration of the last to expire patent issued on the Joint IP, on abandonment by all Parties of all patents and patent applications relating to the Joint IP, or upon the termination of all license agreements relating to the Joint IP, whichever shall last occur.
2.2 In the event that either BioChemics or Inpellis desires to abandon any interest in any patent or patent application relating to the Joint IP (an “Abandoning Party), the Abandoning Party shall notify the other Parties in writing at least sixty (60) days in advance of such abandonment and in advance of any applicable patent filing deadline or maintenance fee payment date so as to allow said other Party the opportunity to continue to pursue such patent applications or patent maintenance. Upon conclusion of said sixty (60) day notice period, the Abandoning Party shall abandon and shall be deemed to have abandoned and assigned all its right, title and interest in and to the noticed patent matter in favor of the non-abandoning Party, including without limitation all rights to any proceeds or royalties relating to the abandoned patent matter. Thereafter, the non-abandoning Party may abandon its interests in the Joint IP at its sole discretion and without notice; provided, however, that such abandonment shall be with notice to the inventors given not less than sixty (60) days prior to any applicable patent filing deadline or maintenance fee payment date.
2.3 If any Party shall cease to carry on its business, dissolve, have a receiver appointed to carry on all or part of its business, become insolvent or become subject to an order for relief under any bankruptcy or other receivership law, the other Party shall have the right to terminate this Agreement by written notice to such Party.
ARTICLE 4 - ASSIGNMENT
4.1 Each Party’s ownership rights in and to the Joint IP may be assigned without the approval of the other Party, except as may be provided in the Purchase Agreement.
ARTICLE 5 - INFRINGEMENT
5.1 In the event that there is an infringement by a third party of the Joint IP, a Party having such knowledge shall notify the other Party in writing to that effect. Upon receipt of any such notice, the Parties shall meet to discuss the substance of such alleged infringement and the potential remedies available to deal with such alleged infringement and the risks associated with such remedies. The Parties may by mutual assent share the responsibility, cost and expense of obtaining a discontinuance of such infringement, or bringing a suit against the third party infringer upon such terms as the Parties shall agree. In the absence of such mutual agreement, the right, but not the obligation, to obtain a discontinuance of such infringement or bring suit against the third party infringer shall first be held by Inpellis for a period of sixty (60) days from the date of said written notice, then by BioChemics for a period of thirty (30) days. In each instance, any Party
2
choosing to exercise its right shall do so at its own cost and expense and shall have the right to join the other Parties as necessary party plaintiffs. Nothing herein shall obligate any Party to obtain a discontinuance of infringement or to bring suit against a third party infringer without such Party’s consent.
5.2 As to any Party bringing a suit in accordance with Section 5.1 (the “Prosecuting Party”), no settlement, consent judgment or other voluntary final disposition of any such action may be entered into without the prior written consent of the other Parties, which consent shall not unreasonably be withheld. Any recovery of damages by the Prosecuting Party for each such suit shall be applied first in satisfaction of any expenses and legal fees of the Prosecuting Party relating to such action, and the balance remaining from any such recovery shall be equally between BioChemics and Inpellis, unless otherwise agreed upon by the Parties.
5.3 Each Party covenants and agrees not to file or participate in, directly or indirectly, any action, suit or proceeding before any court or other regulatory body the purpose of which is to invalidate or limit the scope of any of the rights inherent in the Joint IP during the term of this Agreement.
ARTICLE 6 - INDEMNITY
Each Party agrees to indemnify, hold harmless, and defend at such Party’s own cost and expense, the other Party and its directors, trustees, shareholders, officers, employees, students and agents against any and all claims or actions arising out of damage or injury (including death) to persons or property caused by or sustained in connection with conditions created by the exercise of any rights and services provided under this Agreement where the indemnifying Party is found by a court of competent jurisdiction to have been factually and legally responsible for said condition.
ARTICLE 7 - WARRANTY
7.1 Each Party warrants that it has the right to enter into this Agreement.
7.2 EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT, EACH OF THE PARTIES, AND THEIR RESPECTIVE DIRECTORS, OFFICERS, EMPLOYEES, AND AFFILIATES MAKES NO REPRESENTATIONS AND EXTENDS NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, VALIDITY OF INTELLECTUAL PROPERTY RIGHTS AND PATENT CLAIMS, ISSUED OR PENDING, AND THE ABSENCE OF LATENT OR OTHER DEFECTS, WHETHER OR NOT DISCOVERABLE. NOTHING IN THIS AGREEMENT SHALL BE CONSTRUED AS A REPRESENTATION MADE OR WARRANTY GIVEN BY ANY PARTY THAT THE PRACTICE, USE, COMMERCIALIZATION OR LICENSE OF THE INVENTION SHALL NOT INFRINGE THE INTELLECTUAL PROPERTY RIGHTS OF ANY THIRD PARTY. IN NO EVENT SHALL ANY PARTY, OR THEIR RESPECTIVE DIRECTORS, OFFICERS, EMPLOYEES AND AFFILIATES BE LIABLE FOR INCIDENTAL OR CONSEQUENTIAL DAMAGES OF ANY KIND, INCLUDING ECONOMIC DAMAGE OR INJURY TO PROPERTY AND LOST PROFITS, REGARDLESS OF WHETHER ANY PARTY
3
SHALL BE ADVISED, SHALL HAVE OTHER REASON TO KNOW, OR IN FACT SHALL KNOW OF THE POSSIBILITY, OF SUCH DAMAGES AND NEITHER SHALL ANY PARTY, OR THEIR RESPECTIVE TRUSTEES, DIRECTORS, OFFICERS, EMPLOYEES AND AFFILIATES BE LIABLE FOR PUNITIVE DAMAGES OF ANY KIND.
ARTICLE 8 - MISCELLANEOUS
8.1 No Party shall use or permit to be used the name (or any adaptation thereof) of any other Party or of any staff member, officer, employee or student of any other Party in any advertising, promotional or sales literature, publicity or in any document employed to obtain financing, except as may be required under SEC securities registration requirements, without the prior written approval of the Party or individual whose name is to be used.
8.2 This Agreement shall be governed by and construed and interpreted in accordance with the laws of the Commonwealth of Massachusetts.
8.3 If any provision of this Agreement is or becomes invalid or ruled illegal by any court of competent jurisdiction or is deemed unenforceable under then current applicable law from time to time in effect during the term hereof, it is the intention of the Parties that the remainder of this Agreement shall not be effected thereby. It is further the intention of the Parties that in lieu of each such provision which is invalid, illegal or unenforceable, there shall be substituted or added as part of this Agreement a provision which shall be as similar as possible in economic and business objectives as intended by the Parties to such invalid, illegal or enforceable provision, but shall be valid, legal and enforceable.
8.4 Any notice required or permitted to be given to the Parties hereto shall be deemed to have been properly given if delivered, in writing, in person or mailed by first-class certified mail return receipt requests to the following addresses, or such other addresses as may be designed in writing by the Parties from time to time during the term of this Agreement.
If to BioChemics:
300 Rosewood Drive
Suite 103
Danvers, MA 01923
Attn: Chief Executive Officer
If to Inpellis:
30 Washington Avenue
Suite F
Haddonfield, NJ 08033
Attn: Chief Executive Officer
The Parties acknowledge that this Agreement sets forth the entire Agreement and understanding of the Parties as to the subject matter hereof, and shall not be subject to any change or modification except by the execution of a written instrument subscribed to by the Parties.
4
9.2 No Party shall be liable for any failure to perform as required by this Agreement, to the extent such failure to perform is caused by any reason beyond such Party’s control, or by reason of any of the following: labor disturbances or disputes of any kind, accidents, failure to obtain any required governmental approval, civil disorders, acts of aggression, acts of God, energy or other conservation measures, failure of utilities, mechanical breakdowns, material shortages, disease, or similar occurrences.
IN WITNESS WHEREOF, the Parties have duly executed this Agreement as of the day and year first above written.
| BioChemics, Inc. | ||
| Signature | /s/ Marshall S. Sterman | |
| Name: | Marshall S. Sterman | |
| Title: | President | |
| Inpellis, Inc. | ||
| Signature | /s/ Patrick T. Mooney, M.D. | |
| Name: | Patrick T. Mooney, M.D. | |
| Title: | President and Chief Executive Officer | |
5
SCHEDULE 1.1
Products
Transdermal Ibuprofen
Transdermal Benfothiamine
Transdermal Gabapentin
Transdermal Celecoxib
Transdermal Rofecoxib
Transdermal Lidocaine and any similar products derived from the same chemical including, without limitation, Benzocaine, Dibucaine, Oxybuprocaine, Proparacaine, Proxymetacaine, Tetracaine, Amethocaine, Xylocaine and Lignocaine (collectively, the “Caine Products”)
6
Exhibit 23.1
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM’S CONSENT
We consent to the inclusion in this Registration Statement of Alterix Inc. on Form S-1 of our report dated April 8, 2015, which includes an explanatory paragraph as to the Company’s ability to continue as a going concern with respect to our audits of the financial statements of Alterix Inc. as of December 31, 2014 and 2013 and for the years ended December 31, 2014 and 2013, which report appears in the Prospectus, which is part of this Registration Statement. We also consent to the reference to our Firm under the heading “Experts” in such Prospectus.
/s/ Marcum LLP
Marcum LLP
New York, NY
November 10, 2015
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- VinFast Auto (VFS) Appoints Tham Chee Soon to its Board, Ngan Wan Sing Winston Resigns
- Cleveland-Cliffs (CLF) Reports Q1, Announces $1.5B Share Buyback
- Massimo Group (MAMO) Expands Partnership with Global Omnichannel Retailer for Youth Series Vehicles
Create E-mail Alert Related Categories
SEC FilingsRelated Entities
S1Sign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share